Summary
Here we demonstrate a mechanism targeting CD4+ T cells in which an autoantibody against CD4 induces CD4+ T-cell death and prevents CD4+ T-cell recovery in persons with chronic human immunodeficiency virus type 1 infection receiving virus-suppressive antiretroviral therapy.
Keywords: HIV, B cells, antibody responses, autoreactive anti-CD4 antibodies
Abstract
Increased mortality and morbidity occur among human immunodeficiency virus (HIV)–infected patients in whom CD4+ T-cell counts do not increase despite viral suppression with antiretroviral therapy (ART). Here we identified an underlying mechanism. Significantly elevated plasma levels of anti-CD4 immunoglobulin G (IgG) were found in HIV-positive immunologic nonresponders (ie, HIV-positive individuals with CD4+ T-cell counts of ≤350 cells/μL), compared with levels in HIV-positive immunologic responders (ie, HIV-positive individuals with CD4+ T-cell counts of ≥500 cells/μL) and healthy controls. Higher plasma level of anti-CD4 IgG correlated with blunted CD4+ T-cell recovery. Furthermore, purified anti-CD4 IgG from HIV-positive immunologic nonresponders induced natural killer (NK) cell–dependent CD4+ T-cell cytolysis and apoptosis through antibody-dependent cell-mediated cytotoxicity (ADCC) in vitro. We also found that anti-CD4 IgG–mediated ADCC exerts greater apoptosis of naive CD4+ T cells relative to memory CD4+ T cells. Consistently, increased frequencies of CD107a+ NK cells and profound decreases of naive CD4+ T cells were observed in immunologic nonresponders as compared to responders and healthy controls ex vivo. These data indicate that autoreactive anti-CD4 IgG may play an important role in blunted CD4+ T-cell reconstitution despite effective ART.
The advent of antiretroviral therapy (ART) has dramatically improved survival and slowed disease progression in human immunodeficiency virus (HIV)–infected individuals [1]. ART suppresses HIV replication, improves immune function, restores peripheral CD4+ T-cell counts, and decreases morbidity and mortality [2, 3]. However, in a substantial number of patients, CD4+ T-cell counts are not restored to levels observed in healthy controls, despite prolonged HIV suppression with ART [4]. Aviremic ART recipients whose peripheral CD4+ T-cell counts rebound to ≥500 cells/μL can be defined as immunologic responders, while patients with CD4+ T-cell counts that remain at ≤350 cells/μL despite effective viral suppression are defined as immunologic nonresponders [5]. Notably, increased levels of inflammation, cardiovascular disease risk, neurologic dysfunction, malignancy, and liver disease are observed in nonresponders [6–8].
Potential mechanisms of poor CD4+ T-cell reconstitution after viral suppression with ART in HIV disease have been extensively explored, including persistent immune activation, presence of lymphoid fibrosis, thymic insufficiency, and gut mucosal dysfunction leading to microbial translocation and inflammation [5, 9–11]. However, a mechanism specific to direct CD4+ T-cell destruction in patients with virologic suppression is not known.
In the current study, we examined the potential role of anti-CD4 immunoglobulin G (IgG) in insufficient recovery of the CD4+ T-cell count. Here, in the setting of ART and long-term virologic suppression, we found that plasma levels of anti-CD4 IgG were increased in nonresponders relative to those in responders and healthy controls. In vitro, anti-CD4 IgG purified from plasma of nonresponders mediated CD4+ T-cell cytolysis and apoptosis. Thus, our results suggest that anti-CD4 autoantibodies may constitute an important mechanism of blunted immune restoration in HIV-infected patients with virologic suppression.
MethodS
Study Subjects
Three study groups, healthy controls, HIV-positive responders, and HIV-positive nonresponders were included in the present study. The clinical characteristics are shown in Table 1. All HIV-positive responders and HIV-positive nonresponders had been receiving ART and had had an undetectable plasma HIV-1 RNA load (ie, <40 copies/mL) for at least 2 years. Long-term nonprogressors were HIV-infected patients who had maintained undetectable or low levels of plasma HIV RNA (ie, <5000 copies/mL) without ART for >10 years [12]. All participants provided written informed consent. This study was approved by the institutional review board from the Medical University of South Carolina and the University of Alabama at Birmingham.
Table 1.
Clinical Characteristics of Study Participants
| Characteristic | Healthy Control (n = 17) | HIV-Positive Responders (n = 26) | HIV-Positive Nonresponders (n = 22) | P (Responders vs Nonresponders) |
| Sex ratio, female:male | 12:5 | 7:19 | 5:17 | >.99 |
| Age, y | 38 (33–55) | 43 (30–55) | 47 (40–53) | .25 |
| CD4+ T-cell count, cells/µL | 782 (534–982) | 655 (558–804) | 259 (231–287) | <.0001 |
| ART duration, y | … | 4 (3.8–6) | 6 (2.5–8) | .26 |
| Nadir CD4+ T-cell count, cells/µL | … | 381 (226–591) | 54 (14–155) | <.0001 |
Data are median (interquartile range).
Flow Cytometry
Peripheral blood mononuclear cells (PBMCs) were isolated over a Ficoll-Hypaque cushion (GE, Pittsburgh, PA) from ethylenediaminetetraacetic acid–containing blood specimens. Plasma was isolated, aliquoted, and stored at −80°C before use. Antibodies were incubated with PBMCs at 4°C for 30 minutes for surface staining and for 30 minutes for intracellular staining after membrane permeabilization (Fixation/Permeabilization Solution Kit; BD Pharmingen, San Jose, CA). The following fluorochrome-labeled monoclonal antibodies (clones) from BD were used: anti-CD4 (RPA-T4), anti-CD3 (OKT3), anti-CD8 (RPA-T8), anti-CD45RA (HI100), anti-CD107a (H4A3), anti–interferon γ (IFN-γ; B27), anti-CD38 (HIT2), anti-HLA-DR (G46-6), annexin V, and isotype control antibodies. Ghost Red 780 was purchased from Tonbo Biosciences (San Diego, CA). Cells were collected in a BD FACSVerse Flow Cytometer (BD Biosciences), and data were analyzed by FlowJo software (version 10.0.8).
Enzyme-Linked Immunosorbent Assay (ELISA) for Detection of Anti–Nuclear Antigen and Anti–Double-Stranded DNA (dsDNA) IgG
Plasma levels of anti-dsDNA IgG were quantified using a commercial kit according to the manufacturer’s protocol (Immuno-Biological Laboratories, Minneapolis, MN). Anti–nuclear antigen IgG detection was performed by ELISA, using Hep-2 laryngeal carcinoma cells (ATCC, Manassas, VA) lysate as the coating antigens.
ELISA Development for Detection of Anti-CD4 IgG and Anti-CD8 IgG
Human soluble CD4 protein (sCD4; Progenics, Tarrytown, NY) or human soluble CD8B/P37/LEU2 protein (sCD8; Sino Biological, Beijing, China) were diluted at a concentration of 16 μg/mL, added to microtiter wells, and incubated at 4°C overnight. Microwells were washed 3 times with phosphate-buffered saline (PBS) wash buffer (ie, PBS with 0.1% Tween 20) and then blocked with PBS containing 3% bovine serum albumin (BSA) for 120 minutes at 37°C. Plasma was diluted 1:40 in PBS containing 3% BSA, and 100 μL of the dilution was added to the wells. The plate was incubated at room temperature for 60 minutes. Biotin-labeled goat anti-human IgG was added at a 1:5000 dilution in PBS containing 3% BSA. The plate was then incubated for 60 minutes at room temperature. Horseradish peroxidase–conjugated streptavidin was added at a 1:1000 dilution in PBS containing 3% BSA and then incubated for 30 minutes at room temperature. After washing, 100 μL of 2,2'-azino-di(3-ethylbenzthiazoline-6-sulfonate) was added and incubated for 30 minutes, and 405-nm emission was read within 30 minutes.
Antibody Affinity Purification
Total IgG was purified from plasma of nonresponders by using protein A/G agarose beads in accordance with the manufacturer’s instructions (Pierce, Pittsburgh, PA). Anti-CD4–specific IgG from plasma of nonresponders was purified using NHS Mag Sepharose (GE Healthcare, Wauwatosa, WI). sCD4 protein was covalently coupled to NHS magnetic beads. Plasma samples and binding buffer were mixed at a 1:1 ratio in the presence of 2 M urea and incubated at 4°C for 4 hours in a column with sCD4 immobilized on magnetic beads. The unbound fraction was removed using a magnetic tube rack. To purity high-affinity antibodies, the column was washed extensively with 50 mM Tris/150 mM NaCl in the presence of 2 M urea. Antigen-specific polyclonal IgG was eluted sequentially with 0.1 M glycine/HCl buffer plus 2 M urea at pH 2.9. The purified IgG was concentrated using ultracentrifugal filters (Amicon, EMD Millipore, MA), and the IgG concentration was assessed by quantitative ELISA. Human IgG (ThermoFisher, Rockford, IL) and the human monoclonal anti-CD4 antibody zanolimumab (HuMax-CD4; Genmab) were used to generate standard curves.
To prepare negative controls, purified anti-CD4 IgG from plasma of nonresponders was pretreated with sCD4 at a concentration ratio of 1:2 at 4ºC for 30 minutes (control 1), and anti-CD4 IgG-depleted total IgG from nonresponders was prepared by sCD4 protein-coupled NHS magnetic beads, using NHS Mag Sepharose (control 2). A human monoclonal anti-CD4 antibody, zanolimumab, was used as a positive control.
Antibody-Dependent Cell-Mediated Cytotoxicity (ADCC)
Antibody-Dependent NK Cell Activation
CD107a and IFN-γ intracellular staining in NK cells was performed to assess NK cell cytotoxicity. Briefly, PBMCs from aviremic HIV-positive ART recipients were used to isolate NK cells, using the NK Cell Enrichment Kit; and CD4+ T cells, using the CD4+ T-Cell Enrichment Kit (StemCell, Vancouver, Canada). The purities of CD4+ T cells were >95%, and the purities of NK cells were >94%. Purified NK cells were cocultured with CD4+ T cells at 1:1 ratio in 96-well U-bottomed plates (Corning, NY) in the presence of anti-CD4 IgG and controls. PE-conjugated anti-CD107a, monensin (2.6 μg/mL; BD), and brefeldin A (5 μg/mL; BD) were added and incubated 15 minutes. Next, plates were spun at 250 × g for 4 minutes and incubated for 6 hours at 37°C. After incubation, cells were surface stained with antibodies, permeabilized, intracellularly stained, and analyzed by flow cytometry.
Antibody-Dependent CD4+T-Cell Activation, Cytotoxicity, and Apoptosis
NK cells were cocultured with CD4+ T cells at a 3:1 ratio in 96-well V-bottomed plates (Corning, NY) in the presence of anti-CD4 IgG and controls. Cells were incubated for 15 minutes, spun at 300 × g for 1 minute, and incubated for 6 hours at 37°C. After incubation, cells were surface stained and fixed with 2% paraformaldehyde solution containing a constant number of flow cytometry particles (5 × 104 particles/mL; AccuCount blank particles, 5.3 μm; Spherotech, Lake Forest, IL). A constant number of particles (2.5 × 103) were counted during cytometry acquisition, to normalize the number of CD4+ T cells. The cytolysis percentage was calculated using the formula: [(number of CD3+ T cells in the presence of medium alone) − (number of CD3+ T cells in the presence of anti-CD4 IgG)]/(number of CD3+ T cells in the presence of medium alone) × 100. Cell apoptosis was analyzed by annexin V binding.
Statistical Analysis
The differences in continuous measurements were compared using the Mann-Whitney U test (unpaired) and the Friedman paired nonparametric test (paired). In the prespecified hypothesis, we were interested in the comparisons of nonresponders versus responders or healthy controls; therefore, P values from comparing nonresponders to each control group were not adjusted for multiple comparisons [13]. The same approach was applied to the comparisons of immune parameters induced by anti-CD4 IgG and control antibodies. To explore associations between pairs of continuous variables, Spearman rank correlation was used. A multivariable linear regression model with log transformation was used to analyze anti-CD4 IgG, using SAS (version 9.3, Cary, NC). All tests were 2-sided, and P values of ≤.05 were considered to denote statistical significance.
RESULTS
All patients (26 responders and 22 nonresponders) were receiving virologically suppressive ART. There was no difference in sex, age, and years of ART between responders and nonresponders. However, nadir CD4+ T-cell counts were significantly lower in nonresponders compared to responders (Table 1).
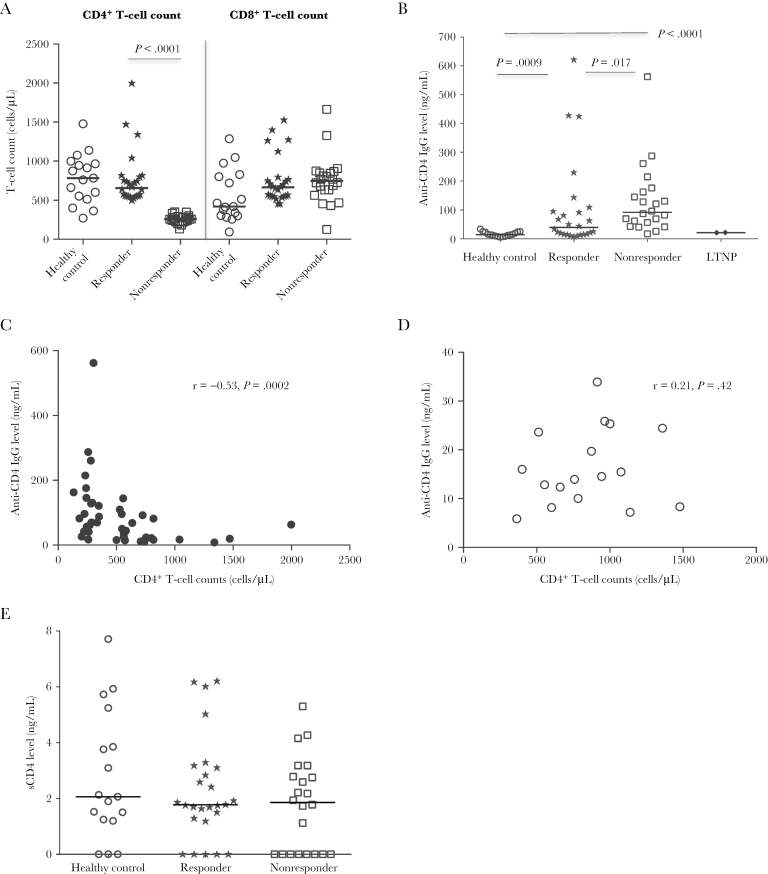
Elevated Plasma Anti-CD4 IgG Levels Are Present in HIV-Infected Subjects After ART and Correlate With Blunted CD4+ T-Cell Recovery In Vivo
Elevated plasma levels of autoreactive antibodies have been previously observed in untreated HIV and simian immunodeficiency virus infections [14–17], and levels of these autoantibodies decrease markedly after ART [18]. A previous study showed that self-reactive repertoires of immunoglobulin M and IgG in responders differed significantly from those of nonresponders [19]. These results suggest that autoantibodies may differ in nonresponders and responders and may be impacted by treatment. To investigate the association of autoantibody production with incomplete immune reconstitution, we quantified plasma levels of CD4-specific autoreactive IgG in a cross-sectional study and correlated them with peripheral CD4+ T-cell counts. When we stratified the ART recipients by CD4+ T-cell count (responders versus nonresponders), we found that peripheral CD8+ T-cell counts were similar between the 2 HIV-positive study groups, suggesting that CD4+ T-cell depletion but not CD8+ T-cell count characterizes nonresponders (Figure 1A). Moreover, plasma anti-CD4 IgG levels were significantly higher in nonresponders relative to those in responders and healthy controls (Figure 1B), and the difference in plasma anti-CD4 IgG level between responders and nonresponders was still significant after controlling for nadir CD4+ T-cell count, age, and sex, which are thought to potentially be involved in CD4+ T-cell decline in HIV disease (P = .04) [20–22]. Both findings suggest the independence of anti-CD4 autoantibodies as a correlate of immune discordance in the 2 HIV-positive study groups. In addition, we found that 3 outliers among responders (ie, those with the highest plasma anti-CD4 IgG levels) had autoimmune diseases and higher levels of both current and nadir CD4+ T-cell counts, suggesting that a high level of plasma anti-CD4 antibodies does not always result in CD4+ T-cell depletion in patients. Furthermore, plasma anti-CD4 IgG levels were inversely correlated with peripheral CD4+ T-cell counts (r = −0.53; P = .0002; Figure 1C) in HIV-infected subjects but not in healthy controls (r = 0.21; P = .42; Figure 1D).
Figure 1.
Increased plasma anti-CD4 immunoglobulin G (IgG) levels in aviremic antiretroviral therapy (ART)–recipient immunologic nonresponders. A, Median absolute numbers of peripheral CD4+ and CD8+ T cells, assessed by flow cytometry, in healthy controls, responders, and nonresponders. B, Median plasma levels of anti-CD4 IgG in healthy controls, responders, nonresponders, and long-term nonprogressors (LTNPs). C and D, Correlations between plasma levels of anti-CD4 IgG and peripheral CD4+ T-cell counts in all ART-recipient aviremic human immunodeficiency virus (HIV)–infected subjects (C) and healthy controls (D). E, Median plasma levels of soluble CD4 (sCD4) antigen in healthy controls, responders, and nonresponders. Statistical analyses were performed using the Mann-Whitney U test (unpaired).
To define whether increased anti-CD4 IgG levels in nonresponders and responders may be due to increased antigen availability, plasma sCD4 levels were analyzed. Interestingly, plasma sCD4 levels were similar among the 3 study groups (Figure 1E), suggesting that differences in plasma anti-CD4 IgG levels in nonresponders and the other study groups were not due to soluble antigen availability in peripheral blood. To determine whether the presence of anti-CD4 antibodies in nonresponders represented a specific anti-CD4 response or was a generalized phenomenon due to polyclonal B-cell activation, we assayed for multiple other autoantibodies, including anti-CD8 IgG, anti–nuclear antigen, and anti–dsDNA IgG, in plasma of healthy controls and patients. Notably, unlike anti-CD4 IgG, plasma levels of anti-CD8 IgG, anti-dsDNA IgG, and anti–nuclear antigen were similar among healthy controls, responders, and nonresponders (Supplemental Figure 1A–1C), and none were correlated with peripheral CD4+ or CD8+ T-cell counts in HIV-infected subjects (data not shown). These results suggest that the mechanism eliciting anti-CD4 IgG production may be different from those of other autoantibodies in treated HIV disease.
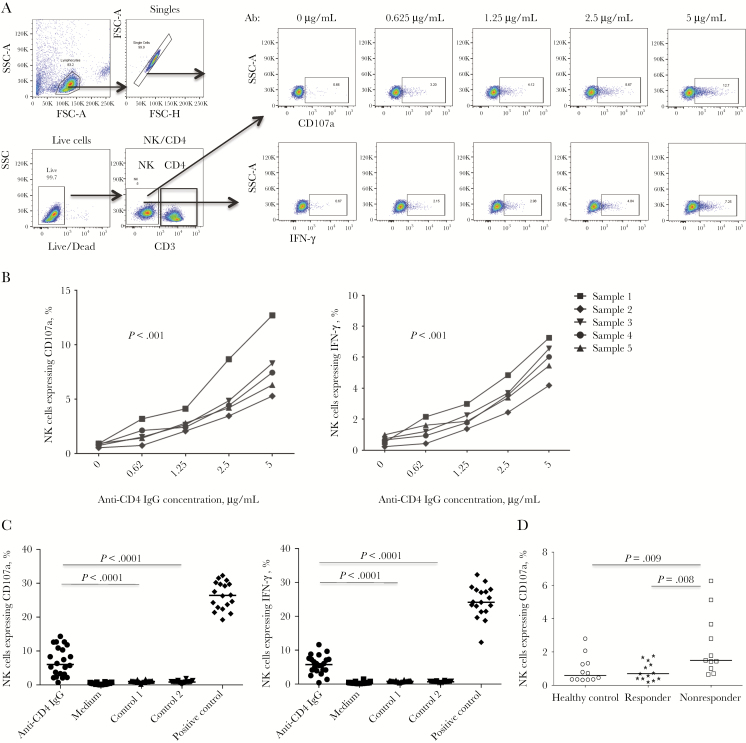
Anti-CD4 IgG of Immunologic Nonresponders Induce NK Cell Activation
Our recent work has shown that purified NK cells from nonresponders induced uninfected CD4+ T-cell death in vitro, suggesting that NK cells may be involved in CD4+ T-cell depletion and reconstitution during ART [23]. To further analyze the potential impact of anti-CD4 antibodies in nonresponders, we purified anti-CD4 IgG from plasma of nonresponders and analyzed their impact on NK cell–mediated cytotoxicity. To identify the CD4 specificity of anti-CD4 IgG from nonresponders, we used purified anti-CD4 IgG pretreated with sCD4 or anti-CD4 IgG-depleted total IgG from nonresponders as a negative control. Equal concentrations of negative controls and zanolimumab were used. The percentage of NK cells expressing IFN-γ or CD107a was analyzed for antibody-dependent NK cell activation in vitro. Notably, purified anti-CD4 IgG from nonresponders but not the same concentration of negative control induced significant NK cell activation and cytotoxicity (IFN-γ+ or CD107a+) in a dose-dependent pattern (Figure 2A and 2C). Consistently, we found an increased frequency of CD107a+ NK cells from nonresponders as compared to responders and controls ex vivo (Figure 2D). These results suggest that anti-CD4 autoantibodies from nonresponders have the ability to induce NK activation and cytotoxicity.
Figure 2.
Anti-CD4 antibody-dependent natural killer (NK) cell activation. CD4+ T cells were cultured with purified anti-CD4 immunoglobulin G (IgG) from nonresponders or control antibodies and cocultured with NK cells at a ratio of 1:1. Intracellular CD107a and interferon γ (IFN-γ) expression in NK cells was analyzed by flow cytometry. A, Dot plots from a representative donor show CD107a and IFN-γ expression in NK cells in response to different concentrations of anti-CD4 antibodies from nonresponders. B, Percentages of NK cells expressing IFN-γ and CD107a in response to different concentrations of anti-CD4 IgG from 5 different nonresponders in vitro. Analyses were performed by the Friedman paired nonparametric test. C, Median percentages of NK cells expressing IFN-γ and CD107a in a mixed culture of CD4+ T cells and NK cells in the presence of anti-CD4 IgG from nonresponders, anti-CD4 IgG from nonresponders pretreated with sCD4 (control 1), anti-CD4 IgG–depleted total IgG from nonresponders (control 2), and zanolimumab (positive control) at 5 μg/mL in vitro. Analyses were performed using the Mann-Whitney U test (unpaired). D, Median percentages of NK cells expressing CD107a in healthy controls, responders, and nonresponders ex vivo. Analyses were performed using the Mann-Whitney U test (unpaired). FSC, forward scatter; SSC, side scatter.
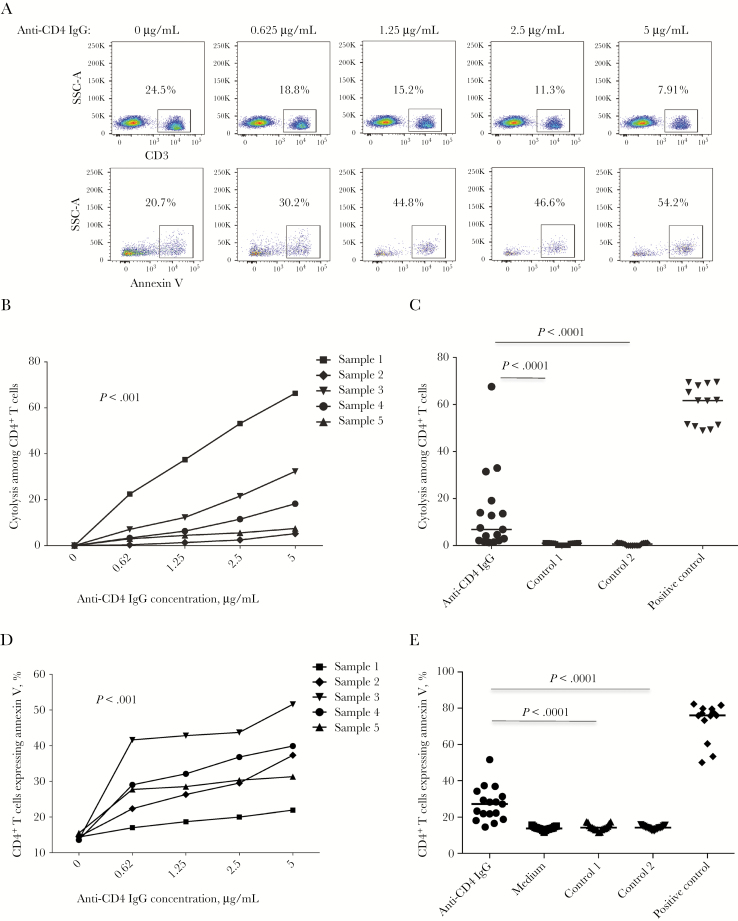
Anti-CD4 IgG From Immunologic Nonresponders Mediate CD4+T Cell Cytolysis and Apoptosis Through ADCC
We next assessed whether anti-CD4 IgG from nonresponders can induce ADCC and death of primary CD4+ T cells. To quantitate the absolute CD4+ T-cell count in the mixed culture including NK cells (CD3−) and CD4+ T cells (CD3+), we stained cells for CD3 but not CD4 to avoid any possibility that purified anti-CD4 antibodies could bind and block surface CD4 on CD4+ T cells. Notably, anti-CD4 IgG from nonresponders but not from negative controls induced CD4+ T-cell cytolysis and apoptosis in vitro (Figure 3). The results suggest that anti-CD4 IgG from nonresponders can mediate CD4+ T-cell death through ADCC.
Figure 3.
Anti-CD4 antibody dependent natural killer (NK) cell–mediated cytolysis to primary CD4+ T cells. CD4+ T cells were cultured with purified anti-CD4 immunoglobulin G (IgG) and control antibodies and cocultured with NK cells at a ratio of 1:3 in vitro, and the CD4+ T-cell cytolysis percentage was analyzed by flow cytometry. A, Dot plots from 1 representative donor were showing CD4+ T-cell frequencies and annexin V binding in response to different concentrations of anti-CD4 IgG from nonresponders. B, Percentages of CD4+ T cells undergoing cytolysis in response to different concentrations of anti-CD4 IgG from 5 different nonresponders through antibody-dependent cell-mediated cytotoxicity (ADCC). Analyses were performed by the Friedman paired nonparametric test. C, Median percentages of CD4+ T cells undergoing cytolysis in response to anti-CD4 IgG from nonresponders and controls at 5 μg/mL in vitro. Analyses were performed using the Mann-Whitney U test (unpaired). D, Percentages of CD4+ T cells undergoing apoptosis in response to different concentrations of anti-CD4 IgG from 5 different nonresponders through ADCC. Analyses were performed by the Friedman paired nonparametric test. E, Percentages of CD4+ T cells undergoing apoptosis in response to anti-CD4 IgG from nonresponders and controls at 5 μg/mL in vitro. Analyses were performed using the Mann-Whitney U test (unpaired). SSC, side scatter.
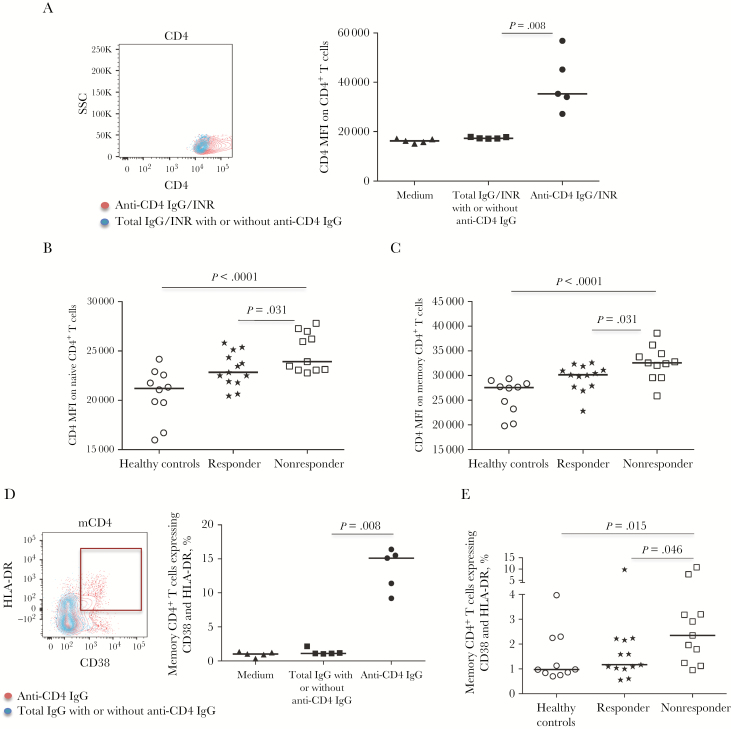
Anti-CD4 IgG From Immunologic Nonresponders Mediate CD4+T Cell Activation
Increased surface CD4 expression and coexpression of CD38 and HLA-DR on CD4+ T cells are related to T-cell activation [10, 24]. To determine whether anti-CD4 IgG from nonresponders can activate CD4+ T cells, CD4+ T cells were cultured with anti-CD4 IgG or controls (Figure 4A–4E). Notably, anti-CD4 IgG increased CD4 expression on total CD4+ T cells (Figure 4A) and coexpression of CD38 and HLA-DR on memory CD4+ T cells (Figure 4D) in vitro. Parallel to CD4+ T-cell activation in vitro, nonresponders had increased CD4 expression on naive (Figure 4B) and memory (Figure 4C) CD4+ T cells and increased coexpression of CD38 and HLA-DR on memory CD4+ T cells (Figure 4E), compared with responders and healthy controls ex vivo. However, neither the CD4 mean fluorescent intensity (MFI) nor the percentages of coexpression of CD38 and HLA-DR on CD4+ T cells were increased by zanolimumab (data not shown), suggesting a difference in the capacity to activate CD4+ T cells or in the binding properties (eg, affinity, avidity, or binding site) between zanolimumab and anti-CD4 IgG. Together, these data suggest that anti-CD4 IgG may play a role in CD4+ T-cell activation in HIV-positive nonresponders.
Figure 4.
CD4+ T-cell activation in response to purified anti-CD4 immunoglobulin G (IgG) from immunologic nonresponders. A, Overlapped dot plots (1 representative donor) and the median CD4 mean fluorescence intensity (MFI) expression on CD4+ T cells treated with anti-CD4 IgG (red) and anti-CD4 IgG–depleted total IgG (blue) in vitro. B and C, Median CD4 MFI expression on naive CD4+ T cells (B) and memory CD4+ T cells (C) in healthy controls, responders, and nonresponders ex vivo. D, Overlapped dot plots (1 representative donor) and median percentages of CD38 and HLA-DR coexpression on memory CD4+ T cells treated with anti-CD4 IgG (red) and anti-CD4 IgG–depleted total IgG in vitro. E, Median percentages of CD38 and HLA-DR coexpression on memory CD4+ T cells in healthy controls, responders, and nonresponders ex vivo. Analyses were performed using the Mann-Whitney U test (unpaired). SSC, side scatter.
Naive CD4+T Cells Were Predominantly Depleted in Immunologic Nonresponders
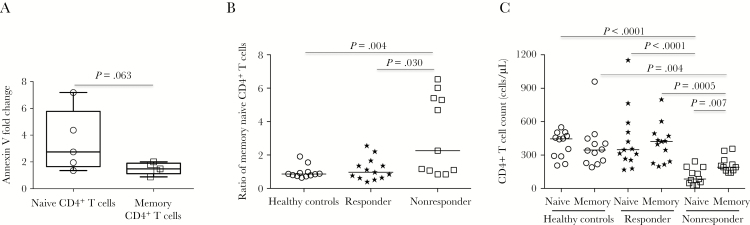
Next, we compared apoptosis of naive (CD3+CD4+CD8−CD45RA+CD27+) and memory (CD3+CD4+CD8−CD45RA−) CD4+ T cells in response to anti-CD4 IgG in vitro. Interestingly, although anti-CD4 IgG from nonresponders induced apoptosis of both CD4+ T-cell subsets, apoptotic induction by anti-CD4 IgG was greater on naive as compared to memory CD4+ T cells (Figure 5A). Moreover, the ratio of memory to naive CD4+ T cells was significantly increased and naive CD4+ T-cell counts were lower than memory CD4+ T-cell counts in nonresponders as compared to responders and controls ex vivo (Figure 5B–5C). These results are consistent with previous reports that naive CD4+ T cells are predominantly depleted in nonresponders [25–27].
Figure 5.
Memory and naive CD4+ T cells. A, Comparison of apoptotic induction between naive CD4+ and memory CD4+ T cells by purified anti-CD4 immunoglobulin G (IgG) from plasma of 5 different nonresponders in antibody-dependent cell-mediated cytotoxicity assays in vitro. Data are median fold change (interquartile range) from anti-CD4 IgG to anti-CD4 IgG–depleted total IgG. B, Median ratio of memory CD4+ T cells to naive CD4+ T cells in healthy controls, responders, and nonresponders ex vivo. C, Median absolute counts of memory CD4+ and naive CD4+ T cells in healthy controls, responders, and nonresponders ex vivo. Analyses were performed using the Mann-Whitney U test (unpaired).
DISCUSSION
In the current study, we found that the presence of anti-CD4 antibodies characterizes nonresponders, as well as evidence suggesting that these specific autoantibodies could play a role in the mechanisms leading to long-term low recovery of the CD4+ T-cell count during virologically successful ART.
Plasma autoanti-CD4 IgG levels vary widely in both responders and nonresponders (Figure 1B), suggesting that this autoantibody may only account for a fraction of peripheral CD4+ T-cell counts in patients. Other contributors, such as the levels of chronic T-cell activation and turnover, inflammation, and parameters related to nadir CD4+ T-cell and naive CD4+ T-cell counts (eg, thymus dysfunction and irreversible damage to secondary lymphoid tissues), may account for the remaining variance. In addition, the inverse relationship between plasma anti-CD4 IgG and CD4+ T-cell counts does not indicate a causal relation in vivo, and elevated plasma anti-CD4 IgG may result from CD4+ T-cell activation (Figure 4).
Autoantibodies are present at low levels even in healthy individuals, but most of them have low affinity and no pathological activity [28]. In HIV infection, polyclonal B-cell activation and elevated autoantibody levels are present as early as the acute phase and persist during chronic infection [29, 30]. Most but not all of this activation is reversed after ART [18]. Several studies have shown negative correlations between plasma or serum autoantibodies against CD4+ T cells and CD4+ T-cell count or disease progression [31, 32]. However, these studies investigated total antibodies with specificities to diverse surface proteins of CD4+ T cells, rather than those specific to the CD4 protein. Moreover, anti-CD4 antibodies in HIV-infected patients were discovered early in the 1990s [33–35]. These studies, however, did not define any role of anti-CD4 antibody in HIV pathogenesis. Notably, HIV-positive subjects in the 1990s were most likely untreated and highly viremic, while nonresponders in the current study are aviremic and receiving ART yet have CD4+ T-cell counts of <350 cells/µL. Therefore, the different results regarding anti-CD4 IgG of the studies from pre-ART era [36, 37] and the present study may be due to the differences in patients’ status. Furthermore, autoimmune diseases are often observed in HIV-infected individuals after ART initiation, suggesting that immune reconstitution by ART may promote autoantibody production and the development of autoimmune diseases [38, 39]. Indeed, our study provides clear experimental evidence that, even in the context of viral control, anti-CD4 IgG are not only present but have all of the properties required to mediate CD4+ T-cell loss by ADCC.
Notably, even in the absence of detectable plasma viral RNA after long-term ART, HIV still actively replicates in the B-cell follicles of lymph nodes and/or tissues in certain patients, most likely nonresponders [40–42]. As a consequence, HIV proteins (eg, gp120), CD4 antigens, or released HIV protein-bound CD4 (eg, gp120-CD4) may accumulate in lymph nodes and induce anti-CD4 antibody production in the presence of residual elevated inflammation and B-cell activation after ART initiation [5, 43], a possibility that requires investigation in future studies.
In summary, we show that anti-CD4 IgG may participate in a thus far undescribed mechanism limiting CD4+ T-cell reconstitution in patients with HIV infection during ART and provide evidence of its presence in vivo. Our findings elucidate possible new strategies to control antibody-mediated CD4+ T-cell death and thereby prevent a blunted recovery in the CD4+ T-cell count after ART.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. We thank the AIDS Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health, for providing human soluble CD4 recombinant protein (Progenics); and Dr Paul Parren (Genmab), for providing human monoclonal anti-CD4 antibody (zanolimumab).
Financial support. This work was supported by the National Institutes of Health (NIH; grants AI091526 [to W. J.], AI128864 [to W. J.], AG045973 [to J. W.], and P30 AI027767 to Michael S. Saag and S. L. H.]), the Beijing Key Laboratory for HIV/AIDS Research (BZ0089), the National Science Foundation of China–NIH Biomedical Collaborative Research Program (81761128001), and the 12th Five Year Research Project of People’s Liberation Army (CWS11J160).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
REFERENCE
- 1. Mocroft A, Vella S, Benfield TL, et al. Changing patterns of mortality across Europe in patients infected with HIV-1. EuroSIDA Study Group. Lancet 1998; 352:1725–30. [DOI] [PubMed] [Google Scholar]
- 2. Battegay M, Nüesch R, Hirschel B, Kaufmann GR. Immunological recovery and antiretroviral therapy in HIV-1 infection. Lancet Infect Dis 2006; 6:280–7. [DOI] [PubMed] [Google Scholar]
- 3. May MT, Sterne JA, Costagliola D, et al. ; Antiretroviral Therapy (ART) Cohort Collaboration. HIV treatment response and prognosis in Europe and North America in the first decade of highly active antiretroviral therapy: a collaborative analysis. Lancet 2006; 368:451–8. [DOI] [PubMed] [Google Scholar]
- 4. Kelley CF, Kitchen CM, Hunt PW, et al. Incomplete peripheral CD4+ cell count restoration in HIV-infected patients receiving long-term antiretroviral treatment. Clin Infect Dis 2009; 48:787–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lederman MM, Calabrese L, Funderburg NT, et al. Immunologic failure despite suppressive antiretroviral therapy is related to activation and turnover of memory CD4 cells. J Infect Dis 2011; 204:1217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Battegay M, Nüesch R, Hirschel B, Kaufmann GR. Immunological recovery and antiretroviral therapy in HIV-1 infection. Lancet Infect Dis 2006; 6:280–7. [DOI] [PubMed] [Google Scholar]
- 7. Baker JV, Peng G, Rapkin J, et al. ; Terry Beirn Community Programs for Clinical Research on AIDS (CPCRA). CD4+ count and risk of non-AIDS diseases following initial treatment for HIV infection. AIDS 2008; 22:841–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lapadula G, Cozzi-Lepri A, Marchetti G, et al. ; ICONA Foundation Study. Risk of clinical progression among patients with immunological nonresponse despite virological suppression after combination antiretroviral treatment. AIDS 2013; 27:769–79. [DOI] [PubMed] [Google Scholar]
- 9. Diaz A, Alós L, León A, et al. ; Study Group of Lymphoid Tissue immunopathogenesis in HIV infection. Factors associated with collagen deposition in lymphoid tissue in long-term treated HIV-infected patients. AIDS 2010; 24:2029–39. [DOI] [PubMed] [Google Scholar]
- 10. Hunt PW, Martin JN, Sinclair E, et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis 2003; 187:1534–43. [DOI] [PubMed] [Google Scholar]
- 11. Teixeira L, Valdez H, McCune JM, et al. Poor CD4 T cell restoration after suppression of HIV-1 replication may reflect lower thymic function. AIDS 2001; 15:1749–56. [DOI] [PubMed] [Google Scholar]
- 12. Jiang Y, Zhou F, Tian Y, et al. Higher NK cell IFN-γ production is associated with delayed HIV disease progression in LTNPs. J Clin Immunol 2013; 33:1376–85. [DOI] [PubMed] [Google Scholar]
- 13. Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology 1990; 1:43–6. [PubMed] [Google Scholar]
- 14. Kuwata T, Nishimura Y, Whitted S, et al. Association of progressive CD4(+) T cell decline in SIV infection with the induction of autoreactive antibodies. PLoS Pathog 2009; 5:e1000372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bonsignori M, Wiehe K, Grimm SK, et al. An autoreactive antibody from an SLE/HIV-1 individual broadly neutralizes HIV-1. J Clin Invest 2014; 124:1835–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Levesque MC, Moody MA, Hwang KK, et al. Polyclonal B cell differentiation and loss of gastrointestinal tract germinal centers in the earliest stages of HIV-1 infection. PLoS Med 2009; 6:e1000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Massabki PS, Accetturi C, Nishie IA, da Silva NP, Sato EI, Andrade LE. Clinical implications of autoantibodies in HIV infection. AIDS 1997; 11:1845–50. [DOI] [PubMed] [Google Scholar]
- 18. Nilssen DE, Øktedalen O, Brandtzaeg P. Intestinal B cell hyperactivity in AIDS is controlled by highly active antiretroviral therapy. Gut 2004; 53:487–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stahl D, Lacroix-Desmazes S, Misra N, et al. ; ANRS SEROCO study group. Alterations of self-reactive antibody repertoires in HIV disease: an insight into the role of T cells in the selection of autoreactive B cells. Immunol Lett 2005; 99:198–208. [DOI] [PubMed] [Google Scholar]
- 20. Asher I, Guri KM, Elbirt D, et al. Characteristics and outcome of patients diagnosed with HIV at older age. Medicine (Baltimore) 2016; 95:e2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Negredo E, Massanella M, Puig J, et al. Nadir CD4 T cell count as predictor and high CD4 T cell intrinsic apoptosis as final mechanism of poor CD4 T cell recovery in virologically suppressed HIV-infected patients: clinical implications. Clin Infect Dis 2010; 50:1300–8. [DOI] [PubMed] [Google Scholar]
- 22. Meier A, Chang JJ, Chan ES, et al. Sex differences in the toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat Med 2009; 15:955–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Luo Z, Li Z, Martin L, et al. Increased natural killer cell activation in HIV-infected immunologic non-responders correlates with CD4+ T cell recovery after antiretroviral therapy and viral suppression. PLoS One 2017; 12:e0167640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wiegers GJ, Stec IE, Klinkert WE, Reul JM. Glucocorticoids regulate TCR-induced elevation of CD4: functional implications. J Immunol 2000; 164:6213–20. [DOI] [PubMed] [Google Scholar]
- 25. Teixeira L, Valdez H, McCune JM, et al. Poor CD4 T cell restoration after suppression of HIV-1 replication may reflect lower thymic function. AIDS 2001; 15:1749–56. [DOI] [PubMed] [Google Scholar]
- 26. Zeng M, Southern PJ, Reilly CS, et al. Lymphoid tissue damage in HIV-1 infection depletes naïve T cells and limits T cell reconstitution after antiretroviral therapy. PLoS Pathog 2012; 8:e1002437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schacker TW, Brenchley JM, Beilman GJ, et al. Lymphatic tissue fibrosis is associated with reduced numbers of naive CD4+ T cells in human immunodeficiency virus type 1 infection. Clin Vaccine Immunol 2006; 13:556–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Elkon K, Casali P. Nature and functions of autoantibodies. Nat Clin Pract Rheumatol 2008; 4:491–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shirai A, Cosentino M, Leitman-Klinman SF, Klinman DM. Human immunodeficiency virus infection induces both polyclonal and virus-specific B cell activation. J Clin Invest 1992; 89:561–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lane HC, Masur H, Edgar LC, Whalen G, Rook AH, Fauci AS. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N Engl J Med 1983; 309:453–8. [DOI] [PubMed] [Google Scholar]
- 31. Müller C, Kukel S, Bauer R. Relationship of antibodies against CD4+ T cells in HIV-infected patients to markers of activation and progression: autoantibodies are closely associated with CD4 cell depletion. Immunology 1993; 79:248–54. [PMC free article] [PubMed] [Google Scholar]
- 32. Ardman B, Mayer K, Bristol J, Ryan M, Settles M, Levy E. Surface immunoglobulin-positive T lymphocytes in HIV-1 infection: relationship to CD4+ lymphocyte depletion. Clin Immunol Immunopathol 1990; 56:249–58. [DOI] [PubMed] [Google Scholar]
- 33. Chams V, Jouault T, Fenouillet E, Gluckman JC, Klatzmann D. Detection of anti-CD4 autoantibodies in the sera of HIV-infected patients using recombinant soluble CD4 molecules. AIDS 1988; 2:353–61. [DOI] [PubMed] [Google Scholar]
- 34. Martin L, Idziorek T, Lehen A, Gluckman JC, Klatzmann D. Anti-CD4 autoantibodies in HIV-infected individuals: detection by two immunoenzymatic techniques. AIDS 1994; 8:389–90. [PubMed] [Google Scholar]
- 35. Callahan LN, Roderiquez G, Mallinson M, Norcross MA. Analysis of HIV-induced autoantibodies to cryptic epitopes on human CD4. J Immunol 1992; 149:2194–202. [PubMed] [Google Scholar]
- 36. Chams V, Idziorek T, Klatzmann D. Biological properties of anti-CD4 autoantibodies purified from HIV-infected patients. AIDS 1991; 5:565–9. [PubMed] [Google Scholar]
- 37. Sekigawa I, Groopmen JE, Allan JD, et al. Characterization of autoantibodies to the CD4 molecule in human immunodeficiency virus infection. Clin Immunol Immunopathol 1991; 58:145–53. [DOI] [PubMed] [Google Scholar]
- 38. Sheikh V, Dersimonian R, Richterman AG, et al. Graves’ disease as immune reconstitution disease in HIV-positive patients is associated with naive and primary thymic emigrant CD4(+) T-cell recovery. AIDS 2014; 28:31–9. [DOI] [PubMed] [Google Scholar]
- 39. Zandman-Goddard G, Shoenfeld Y. HIV and autoimmunity. Autoimmun Rev 2002; 1:329–37. [DOI] [PubMed] [Google Scholar]
- 40. Estaquier J, Hurtrel B. [Mesenteric lymph nodes, a sanctuary for the persistance of HIV. Escape mechanisms]. Med Sci (Paris) 2008; 24:1055–60. [DOI] [PubMed] [Google Scholar]
- 41. Folkvord JM, Armon C, Connick E. Lymphoid follicles are sites of heightened human immunodeficiency virus type 1 (HIV-1) replication and reduced antiretroviral effector mechanisms. AIDS Res Hum Retroviruses 2005; 21:363–70. [DOI] [PubMed] [Google Scholar]
- 42. Heesters BA, Lindqvist M, Vagefi PA, et al. Follicular dendritic cells retain infectious HIV in cycling endosomes. PLoS Pathog 2015; 11:e1005285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Luo Z, Ma L, Zhang L, et al. Key differences in B cell activation patterns and immune correlates among treated HIV-infected patients versus healthy controls following influenza vaccination. Vaccine 2016; 34:1945–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.