Summary
Mycoplasma genitalium is a flask-shaped bacterium associated with inflammatory urogenital syndromes. Local immune responses are characterized by induction of cytokines and neutrophil recruitment. Extensive antigenic variation, regulated by a unique sigma factor, is associated with persistent infection.
Keywords: mycoplasma, genitalium, immunity, recombination, regulation
Abstract
Mycoplasma genitalium is increasingly appreciated as a common cause of sexually transmitted disease syndromes, including urethritis in men and cervicitis, endometritis, pelvic inflammatory disease, and possibly preterm birth, tubal factor infertility, and ectopic pregnancy in women. Despite these disease associations, which parallel those of Chlamydia trachomatis and Neisseria gonorrhoeae, the mechanisms by which this pathogen elicits inflammation, causes cellular damage, and persists in its only natural host (humans) are unique and are not fully understood. The purpose of this review is to briefly provide a historical background on the discovery, microbiology, and recognition of M. genitalium as a pathogen, and then summarize the recent advances in our understanding of the molecular biology and pathogenesis of this unique urogenital organism. Collectively, the basic scientific discussions herein should provide a framework for understanding the clinical and epidemiological outcomes described in the accompanying articles in this supplemental issue.
M. genitalium (MG) is a fastidious and slow growing STD pathogen. This species was first cultured from the urethral exudates of 2 of 13 men with nongonococcal urethritis in 1981 [1], using a broth medium (SP-4) developed for other mycoplasmas. Growth was detected after 50 days of incubation, indicated by a color change in the medium due to the fermentation of glucose. After subsequent subcultures in broth and on solid media, two strains, G37 and M30, were single- colony cloned on soft agar (0.4%) and further characterized. The organism’s small size (approximately 0.6 × 0.3 µm), ability to pass through 0.3-µm filters, absence of a cell wall, resistance to penicillin, fried-egg appearance of colonies on soft agar plates, and inability to revert to cell wall–containing bacteria, collectively confirmed their identification as mycoplasmas.
Anti-G37 serum cross-reacted with M30, yet both strains failed to react with antisera against other Mycoplasma species, confirming the relatedness between G37 and M30 and their divergence from other, previously described mycoplasmas. These newly described strains were further distinguished from other Mycoplasma species by their inability to hydrolyze urea and arginine and their ability to be inhibited by thallium acetate, which is used for selective growth of other mycoplasmas, including the closely related nongenital species, Mycoplasma pneumoniae. These distinguishing characteristics, as well as their unique flask shape (Figure 1), led to the official recognition of a novel mycoplasma species, M. genitalium, with G37 designated as the type strain [4]. This strain has since been maintained at the American Type Culture Collection and in several laboratories worldwide.
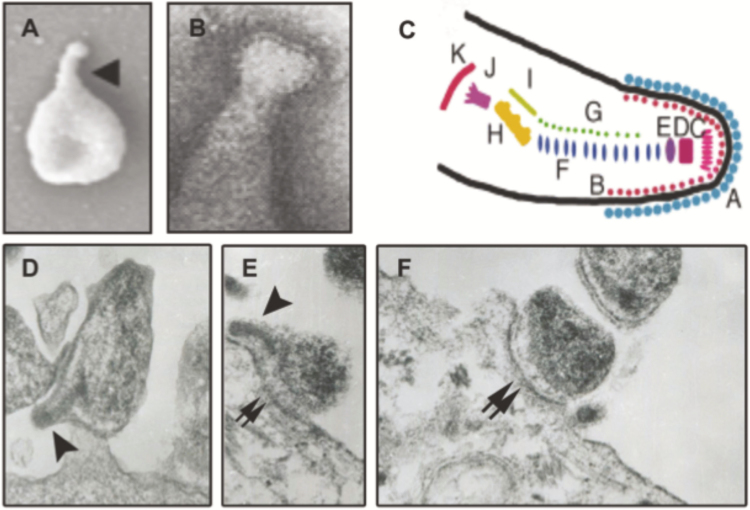
Figure 1.
Mycoplasma genitalium structure and interactions with human reproductive tract epithelial cells. A, B, Curved terminal organelle (arrowhead in A) is a dominant feature of the cell body of M. genitalium in scanning electron micrographs [2] (A) and is covered with a “nap” containing the attachment proteins MgpB and MgpC, as observed by transmission electron microscopy [1] (B). Owing to substantial genetic, functional, and morphological homology, the complex structure of the terminal organelle is inferred to be similar to that of Mycoplasma pneumoniae. (C) M. pneumoniae terminal organelle structure, as seen with electron cryotomography [3]; letters indicate different component protein complexes identified in M. pneumoniae, which are presumed to correlate with homologous proteins in M. genitalium. (D–F) Interaction of M. genitalium with cultured human ectocervical epithelial cells. The terminal organelle of M. genitalium with its electron dense core is clearly visible in (D and E), (arrowheads) and is involved in attachment to host epithelial cells. The intimate interaction between M. genitalium and epithelial cells surface and M. genitalium is not limited to the terminal organelle and often involves adherence throughout the cell body (double arrows in E and F). Images in A–C reproduced with permission from the Microbiology Society [2] (A), Elsevier [1] (B), and Wiley and Sons [3] (C).
The detection of M. genitalium in a subset of men with nongonococcal urethritis in 1981 was intriguing but not sufficient to determine whether M. genitalium was associated with, and possibly the cause of, urethritis. Unfortunately the fastidious nature of M. genitalium was an impediment to determining the association of the organism with human disease. The characterization of mgpB [5], the first M. genitalium gene to be sequenced, lead to the identification of M. genitalium–specific DNA targets and the development of 2 polymerase chain reaction tests described in 1991 [6, 7]. Shortly thereafter, epidemiological studies confirmed that M. genitalium was associated with urethritis [8, 9]. As they say, the rest is history, with the development of several additional laboratory-developed and commercial nucleic acid amplification tests that have since confirmed the role of M. genitalium in several reproductive tract disease syndromes in both men and women.
After the isolation of G37 and M30 in 1981, no additional M. genitalium strains could be isolated from the genital tract until the 1990s, when Jensen et al [10] discovered that coculture of genital tract specimens with Vero cells (and detection of growth by quantitative polymerase chain reaction) enhanced M. genitalium growth in vitro and enabled the recovery of new isolates from male urethral swab and urine specimens. As a result of this technique, collections of geographically distinct M. genitalium strains have accumulated in several laboratories, including those of Jensen et al [11] and P. A. T. (unpublished data), which have greatly facilitated the evaluation of genomic diversity, strain differences, and antibiotic susceptibility profiles of this species [12, 13].
Several typing methods have been developed to differentiate M. genitalium strains, the most widely used being the detection of single-nucleotide polymorphisms in the semiconserved 5′ region of the mgpB gene [14]. This typing system has been used to document the sexual transmission of M. genitalium [15], the persistence of strains among longitudinally infected individuals [16–18], and the selection of azithromycin-resistant strains in individuals treated with this antibiotic [12, 13]. These studies have highlighted the genetic heterogeneity of the organism, although many strains maintained at American Type Culture Collection are indistinguishable from G37 by several strain typing systems [14, 19, 20]. Because the laboratory-adapted G37 strain can easily outgrow other less-adapted isolates of M. genitalium, the isolation of clinical strains with strain types identical to G37 should be interpreted with caution.
Perhaps the most intriguing feature of M. genitalium is its predominant slightly curved terminal organelle (Figure 1), a feature shared with, but morphologically distinct from, other pathogenic Mycoplasma species [2]. Required for adhesion, motility, and involved in cell division, the terminal organelle is composed of a complex array of proteins that have homologues in M. pneumoniae, suggesting similar functions. This structure is remarkably complex, containing surface-exposed proteins visible in electron micrographs as a “nap” at the tip of the organelle [1], a terminal button and 2 parallel rods making up the electron-dense core, and an electron-dense bowl complex proximal to the cell body (Figure 1c.). Adding to the complexity of this terminal organelle is the basal structure thought to be the motor providing energy for motility. The surface-exposed proteins at the tip of the organelle (MgpB and MgpC) mediate attachment to eukaryotic cells, are required for motility, and undergo phase and antigenic variation (see below). The exact mechanism of motility mediated by the terminal organelle is unclear, but has been attributed to the catch, binding, and release activity of the MgpB/MgpC complex in a centipede-like motion [21].
A MINIMAL GENOME
With approximately 580 kb of DNA (the smallest in any organism capable of axenic growth), M. genitalium type strain G37 was the second bacterial genome to be fully sequenced. Initially sequenced by the J. Craig Venter Institute in 1995 using shotgun Sanger sequencing [22], this strain was resequenced in 2005 by 454 Life Sciences [23] to demonstrate their novel pyrosequencing technology. These efforts, combined with the 2012 sequencing and annotation of 4 additional strains from Japan, Denmark, and Australia [24], have provided essential data for understanding the genomic structure, energy metabolism, and evolution of M. genitalium. For example, the circular, double-stranded DNA genome of M. genitalium contains the full genetic complement of the organism, because no plasmids or extrachromosomal DNA have yet been discovered.
Similar to other mycoplasmas and their reduced genomes, the average guanine-cytosine content of the M. genitalium genome is low, at approximately 31%. Of the approximately 517 protein-coding open reading frames, roughly 21% are classified as hypothetical genes and lack significant similarity to other bacterial proteins in the National Center for Biotechnology Information database [22]. Similar to other mycoplasmas, M. genitalium uses a modified genetic code in which UGA encodes a tryptophan rather than the common translational stop [25]. Through evolutionary reductions in genome size, M. genitalium has marked metabolic restrictions as the genome lacks almost all enzymes required for biosynthesis of amino acids, de novo nucleic acid synthesis, and fatty acid biosynthesis. Thus, like many mycoplasmas, M. genitalium relies on several metabolic growth factors obtained from the host and exemplifies the highly evolved host cell-pathogen interactions that facilitate persistence in urogenital tissues.
Although complete phylogenetic analyses have yet to be reported, genomic structure seems to be similar among M. genitalium strains worldwide [24]. Despite substantial gaps in our understanding of M. genitalium pathogenesis, several inferences can be made from its genomic composition. Genomic heterogeneity is primarily contained in hypervariable regions of adjacent genes encoding MgpB and MgpC and in 9 partial, noncoding genomic loci termed MgPar sites. Remarkably, 4.7% of the M. genitalium genome is allocated to the mgpB/mgpC operon and its homologous MgPar sites, highlighting the importance of this system for survival in reproductive tract tissues (see below). Of evolutionary interest, each open reading frame discovered in M. genitalium is also encoded in the genome of the human respiratory pathogen M. pneumoniae [26]. Despite the genomic similarities, functional studies of putative virulence factors and their interaction with the host are needed to understand the establishment, persistence, and pathogenesis of M. genitalium infections in reproductive tract tissues.
HOST IMMUNITY TO INFECTION
In men, urogenital infection is associated with urethral inflammation and discharge (urethritis), which is composed primarily of polymorphonuclear leukocytes. Studies of female reproductive tract syndromes (namely cervicitis) have demonstrated the inflammatory capacity of M. genitalium both clinically and with models of the lower reproductive tract epithelium. In short, adhesion of M. genitalium to host epithelial cells (Figure 1) elicits acute inflammatory signals via highly expressed innate immune sensors including Toll-like receptors 2 and 6 [27]; the binding of these receptors to M. genitalium and its lipoproteins results in NF-κB activation and acute induction of genes involved in host defense [28]. These proinflammatory signals include potent chemokines that ultimately result in leukocyte recruitment to the site of infection [29, 30].
Lacking the discovery of any specific toxins or secreted virulence factors, it is primarily via the large number of surface-exposed lipoproteins that M. genitalium stimulates the cells lining the reproductive tract epithelium. Metabolic byproducts of M. genitalium and of the local host response include reactive oxygen species and nitric oxide that may have a cytopathological impact on the epithelium. Importantly, recruited leukocytes are robust potentiators of the local inflammatory response; in fact, M. genitalium induces very potent proinflammatory responses from monocytes/macrophages that are common to female reproductive tract tissues [31]. In persistently infected women, neutrophils are a prominent component of the proinflammatory response to M. genitalium, which seems to be chronic, varies in severity over time, and is ablated with microbiological cure [29].
M. genitalium infection elicits systemic [32] and mucosal [33] antibody responses in humans and in experimentally infected primates [34, 35]. Unfortunately, development of serologic tests has been challenging due to the difficulty in distinguishing between past and current infection, discerning potential cross-reactions with other mycoplasmas, and identifying the optimal antigens (purified lipid-associated membrane proteins, whole-cell proteins, and/or purified peptides) and immunoassay format (enzyme immunoassays, Western blot analyses) [32, 36]. For a primary M. genitalium infection, the local immune response without doubt precedes the generation of specific antibodies, but the relative importance of epithelial cell responses and cell-mediated immunity to generation of a robust antibody response are unknown. Nonetheless, both men and women infected with M. genitalium develop specific antibodies, primarily against 2 prominent outer membrane proteins, MgpB and MgpC.
Seroconversion to these antigens has been corroborated in several small animal models of infection and in nonhuman primates [33–35, 37] and mice [38]. Importantly, specific antibodies are measurable in lower genital tract secretions from women, implying that they are present at the site of infection and that M. genitalium must actively circumvent this response to survive. Although antigenic variation may facilitate survival (discussed below), the role of antibodies and cell-mediated immunity in clearance requires further study. Clearance without documented antibiotic treatment has been observed in several studies [39–41]; evaluation of the processes mediating clearance, including the host immune response, is critical to understanding the natural history of M. genitalium infections. In addition, we still lack unequivocal evidence as to whether or not antibody responses protect from subsequent exposures to M. genitalium.
PERSISTENCE AND IMMUNE EVASION
Despite the infiltration of neutrophils, production of inflammatory mediators, and induction of local and systemic antibodies, M. genitalium has a remarkable ability to evade the host immune responses leading to chronic urogenital infections. For example, among Kenyan female sex workers, M. genitalium persisted for ≥7 months in 21% of these women, and up to 2 years in 2 women [41]. Combined with the observed persistence of M. genitalium in men [18], chimpanzee [34], macaque [37], and mouse models of infection [42], these studies indicate that urogenital persistence is common in both male and female reproductive tract tissues, possibly by antigenic and phase variation of their surface-exposed proteins as detailed below.
The discovery of the MgPar sequences containing multiple partial copies of the mgpB and mgpC genes in the M. genitalium genome suggested that gene variation of the surface-exposed proteins, MgpB (also known as MgPa and P140) and MgpC (also known as P110) [22, 43], may occur. Recent studies have confirmed 2 types of gene variation in M. genitalium: antigenic variation, which results in the expression of MgpB and MgpC variants with differing amino acid sequences, and phase variation, during which cells lose the ability to adhere to cultured cells and bind to red blood cells (hemadsorption). The experimental demonstration that both types of gene variation occur by recombination in vitro and in vivo, as well as the mechanism, regulation, and repercussions of this process, are the focus of the following discussion.
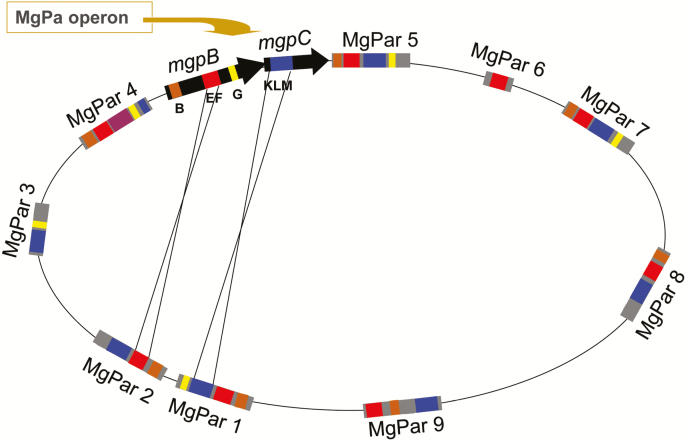
The complete mgpB and mgpC genes are present in a single expression site and consist of conserved regions interspersed with variable regions B, EF, G, and KLM. Homologous copies of the variable regions are present in multiple MgPar sites distributed around the chromosome (Figure 2 and [16, 17, 44, 45]). Antigenic variants arise when the variable regions of the expression site exchange sequences through segmental recombination with ≥1 of the MgPar sites. These recombination events are reciprocal; changes in the mgpBC sequence are accompanied by corresponding changes in the MgPar sites [16, 17], distinguishing mgpBC/MgPar recombination from antigenic variation systems in other bacteria, in which recombination is typically unidirectional (termed gene conversion) [46]. Furthermore, genetic diversity is achieved by recombination between different MgPar sites followed by subsequent mgpBC/MgPar recombination [17]. Phase variants, which do not express the MgpB and/or MgpC proteins and are therefore nonadherent, arise by multiple recombination mechanisms. For example, recombination between the expression site and the MgPar sites can involve 2 variable regions and intervening conserved sequences, resulting in the translocation of the conserved mgpBC sequences to the participating MgPar site, leaving an incomplete mgpBC operon ([47, 48]; P.A.T., unpublished data).
Figure 2.
Schematic of the Mycoplasma genitalium genome showing the MgPa operon containing the adjacent mgpB and mgpC genes, the 9 homologous MgPar sequences and the mechanism of segmental reciprocal exchange between mgpBC and the MgPar sites. Variable regions B (brown), EF (red), and G (yellow) in mgpB and variable region KLM in mgpC (blue) are separated by conserved regions (black) found only in the mgpB and mgpC genes. Truncated but divergent copies of these variable regions are also found in the MgPar sites, separated by adenine-thymine-rich sequences found only in the MgPar sites (gray). Reciprocal recombination between short stretches of the mgpBC genes and the MgPar sites (thin black lines) results in antigenic and phase variation of the MgpB and MgpC proteins and replacement of sequences in the MgPar sites with those previously in the mgpBC expression sites, in a process termed segmental reciprocal recombination.
Experimental primate models of infection have provided opportunities to study the immune response, mgpBC/MgPar recombination, selection of MgpB and MgpC variants, and M. genitalium persistence. In a female pig-tailed macaque inoculated cervically, serum and cervical antibodies induced by the G37 inoculum were less reactive with an MgpB variant that appeared and became predominant after 8 weeks of infection, consistent with a role for antigenic variation in immune avoidance in vivo [35]. The selection of mgpC variants is also supported by the accumulation of mgpC sequence variants in urethral cultures over 11 weeks of infection in chimpanzees, coincident with development of antibodies [49]. In both primate models, the emerging variant sequences were consistent with replacement of the mgpBC sequences in the inoculating strain with an MgPar-specific sequence. Unfortunately, current studies on chimpanzees must be performed with archived specimens from these animals [49], which are no longer available for M. genitalium research. However, the pig-tailed macaque model is a promising alternative for studying the persistence, clearance, and specific antibody responses that could be exploited in future experiments to examine bacterial-host interactions, pathogenesis, and the dynamics of antigenic variation and immune evasion.
The frequency of mgpBC/MgPar variation, estimated to be >1.25 × 10–4 events per genome per generation, is similar to that observed in the notoriously antigenically variable Neisseria gonorrhoeae [50]. This finding begs the question of how such variation is achieved in M. genitalium, which lacks many of the recombination enzymes found in other bacteria. Homologues of only a few recombination genes, including those that encode RecA (a central recombination enzyme), RuvA and RuvB (required for DNA strand exchange), and RecU (required for resolution of DNA recombination intermediates) were identified in the G37 genome and were subsequently shown to be essential for mgpBC/MgPar recombination [51, 52]. Significantly, both antigenic and phase variation were decreased in a recA mutant, adding further support for the evidence that both variant types derive predominantly from recombination events, rather than from point mutations.
GENE REGULATION IN M. GENITALIUM
Sequencing of the M. genitalium genome has revealed few potential regulatory genes that are important for responding to the different metabolic environments and niches encountered in its human host. Although recombination is one of the most highly regulated systems in bacteria, proteins controlling this system in M. genitalium were not identified until 2014, when Burgos and Totten [53] revealed that MG428 is a positive regulator of mgpBC/MgPar recombination. MG428 coordinates the expression of key recombination genes, including recA, ruvA, and ruvB, as well as other novel proteins required for recombination [53, 54]. The homology of MG428 to alternative sigma factors, the binding of purified MG428 protein to core RNA polymerase, and the presence of the unique promoter sequences upstream of MG428-activated genes, collectively confirmed that MG428 is an alternative sigma factor [53, 54].
The identification of MG428 as a sigma factor was surprising because, until this discovery, mycoplasmas were presumed to lack alternative sigma factors and other means of globally controlling gene expression. Alternative sigma factors, as identified in other organisms, coordinately control the expression of genes involved in a defined physiological process, usually in response to an external stimulus, by binding to and directing RNA polymerase to a subset of genes containing promoter sequences targeted by the sigma factor. Identification of environmental signals that affect MG428 activity and mgpBC/MgPar recombination is needed to understand the activation of phase and antigenic variation in vivo.
The M. genitalium RecA protein is expressed as 3 distinct isoforms, which may represent a novel mechanism of regulation of recombination not found in other bacteria [53]. Although only the full-length isoform is active in recombination, the 2 N-terminally truncated isoforms, produced by alternative translation initiation, are hypothesized to posttranscriptionally regulate RecA activity, thereby fine-tuning the regulation of antigenic and phase variation in M. genitalium. Taken together, these results indicate that recombination leading to antigenic and phase variation in M. genitalium is complex and tightly regulated. The identification of environmental factors that modulate the activity of MG428, the full extent of the Mg428 regulon, and the function of the RecA isoforms in mgpBC/MgPar recombination are crucial for understanding the pathogenesis of this human pathogen.
SUMMARY AND PRIORITIES FOR THE FUTURE
Clearly much remains to be learned about the molecular pathogenesis of M. genitalium and the factors that enable the intimate interactions with host cells of the urogenital tract, long-term survival in vivo, evasion of the host immune response, and transmission. The recombination enzymes required for mgpBC/MgPar recombination, the extent of the regulon controlling this process, and environmental factors enhancing recombination are essential for understanding the pathogenesis and unique molecular strategies used by M. genitalium to persist at reproductive tract sites despite robust immune responses. Although the primary rationale for selection of antigenic and phase variants is believed to be the evasion of host antibodies resulting in persistence, other possibilities exist, such as differential adherence to host cells or regulation of host cell invasion and egress.
Additional studies are needed to define other factors that facilitate survival in reproductive tract tissues. For example, the life cycle of M. genitalium within reproductive tract tissues is essentially unknown, as are the mechanisms of invasion and the importance of intraepithelial cell residence for persistence and immune evasion. In addition, characterization of the surface-exposed antigenic epitopes important for antibody-mediated killing should be a priority for the future development of effective vaccines. Finally, the use of well- defined animal models, the continued exploration of enzymes required for recombination, and the regulation of recombination and other critical processes in this unique bacterium are critical to our understanding of the pathobiology of this emerging pathogen and its associated disease processes.
Notes
Acknowledgments. We acknowledge Vsevolod Popov, PhD, ScD (University of Texas Medical Branch, Galveston) for providing electron micrographs of M. genitalium, Gwen Wood, PhD (University of Washington, Seattle) for critical reading of the manuscript and assistance in designing the figures and Raul Burgos, PhD, for Fig. 2.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial support. This work is an outcome of an experts technical consultation on M. genitalium, supported by the Division of Microbiology and Infectious Diseases of the National Institute of Allergy and Infectious Diseases (contract HHSN272201300012I), with the University of Alabama at Birmingham Sexually Transmitted Infections Clinical Trials Group. This project was also supported in part by the National Institute of General Medical Sciences, National Institutes of Health, which funds the Louisiana Clinical and Translational Science Center (grant U54 GM104940) and the National Institute of Allergy and Infectious Diseases (grants R21 AI109332 and R21 AI107402).
Supplement sponsorship. This work is part of a supplement sponsored by the University of Alabama at Birmingham Sexually Transmitted Infections Clinical Trials Unit and the National Institute of Allergy and Infectious Diseases.
Potential conflicts of interest. Both authors: No reported conflicts of interest. Both authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Tully JG, Taylor-Robinson D, Cole RM, Rose DL. A newly discovered Mycoplasma in the human urogenital tract. Lancet 1981; 1:1288–91. [DOI] [PubMed] [Google Scholar]
- 2. Hatchel JM, Balish MF. Attachment organelle ultrastructure correlates with phylogeny, not gliding motility properties, in Mycoplasma pneumoniae relatives. Microbiology 2008; 154:286–95. [DOI] [PubMed] [Google Scholar]
- 3. Henderson GP, Jensen GJ. Three-dimensional structure of Mycoplasma pneumoniae’s attachment organelle and a model for its role in gliding motility. Mol Microbiol 2006; 60:376–85. [DOI] [PubMed] [Google Scholar]
- 4. Tully JC, Taylor-Robinson D, Rose DL, Cole RM, Bove JM. Mycoplasma genitalium, a new species from the human urogenital tract. Int J Syst Bacteriol 1983; 33:387–96. [Google Scholar]
- 5. Dallo SF, Baseman JB. Adhesin gene of Mycoplasma genitalium exists as multiple copies. Microb Pathog 1991; 10:475–80. [DOI] [PubMed] [Google Scholar]
- 6. Jensen JS, Uldum SA, Søndergård-Andersen J, Vuust J, Lind K. Polymerase chain reaction for detection of Mycoplasma genitalium in clinical samples. J Clin Microbiol 1991; 29:46–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Palmer HM, Gilroy CB, Claydon EJ, Taylor-Robinson D. Detection of Mycoplasma genitalium in the genitourinary tract of women by the polymerase chain reaction. Int J STD AIDS 1991; 2:261–3. [DOI] [PubMed] [Google Scholar]
- 8. Jensen JS, Orsum R, Dohn B, Uldum S, Worm AM, Lind K. Mycoplasma genitalium: a cause of male urethritis? Genitourin Med 1993; 69:265–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Horner PJ, Gilroy CB, Thomas BJ, Naidoo RO, Taylor-Robinson D. Association of Mycoplasma genitalium with acute non-gonococcal urethritis. Lancet 1993; 342:582–5. [DOI] [PubMed] [Google Scholar]
- 10. Jensen JS, Hansen HT, Lind K. Isolation of Mycoplasma genitalium strains from the male urethra. J Clin Microbiol 1996; 34:286–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jensen JS, Fernandes P, Unemo M. In vitro activity of the new fluoroketolide solithromycin (CEM-101) against macrolide-resistant and -susceptible Mycoplasma genitalium strains. Antimicrob Agents Chemother 2014; 58:3151–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jensen JS, Bradshaw CS, Tabrizi SN, Fairley CK, Hamasuna R. Azithromycin treatment failure in Mycoplasma genitalium-positive patients with nongonococcal urethritis is associated with induced macrolide resistance. Clin Infect Dis 2008; 47:1546–53. [DOI] [PubMed] [Google Scholar]
- 13. Totten P, Jensen NL, Khosopour CM, Gillespie CW, Golden MR, Totten PA. Azithromycin and doxycycline resistant profiles of Mycoplasma genitalium and association with treatment outcomes. Sex Transm Infect 2013; 89(Suppl 1):0.19.1. [Google Scholar]
- 14. Jensen JS, Björnelius E, Dohn B, Lidbrink P. Use of TaqMan 5’ nuclease real-time PCR for quantitative detection of Mycoplasma genitalium DNA in males with and without urethritis who were attendees at a sexually transmitted disease clinic. J Clin Microbiol 2004; 42:683–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hjorth SV, Bjornelius E, Lidbrink P et al. . Sequence-based typing of Mycoplasma genitalium reveals sexual transmission. J Clin Microbiol 2006; 44:2078–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Iverson-Cabral SL, Astete SG, Cohen CR, Rocha EP, Totten PA. Intrastrain heterogeneity of the mgpB gene in Mycoplasma genitalium is extensive in vitro and in vivo and suggests that variation is generated via recombination with repetitive chromosomal sequences. Infect Immun 2006; 74:3715–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Iverson-Cabral SL, Astete SG, Cohen CR, Totten PA. mgpB and mgpC sequence diversity in Mycoplasma genitalium is generated by segmental reciprocal recombination with repetitive chromosomal sequences. Mol Microbiol 2007; 66:55–73. [DOI] [PubMed] [Google Scholar]
- 18. Ma L, Mancuso M, Williams JA et al. . Extensive variation and rapid shift of the MG192 sequence in Mycoplasma genitalium strains from patients with chronic infection. Infect Immun 2014; 82:1326–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kokotovic B, Friis NF, Jensen JS, Ahrens P. Amplified-fragment length polymorphism fingerprinting of Mycoplasma species. J Clin Microbiol 1999; 37:3300–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ma L, Martin DH. Single-nucleotide polymorphisms in the rRNA operon and variable numbers of tandem repeats in the lipoprotein gene among Mycoplasma genitalium strains from clinical specimens. J Clin Microbiol 2004; 42:4876–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. García-Morales L, González-González L, Querol E, Piñol J. A minimized motile machinery for Mycoplasma genitalium. Mol Microbiol 2016; 100:125–38. [DOI] [PubMed] [Google Scholar]
- 22. Fraser CM, Gocayne JD, White O et al. . The minimal gene complement of Mycoplasma genitalium. Science 1995; 270:397–403. [DOI] [PubMed] [Google Scholar]
- 23. Margulies M, Egholm M, Altman WE et al. . Genome sequencing in microfabricated high-density picolitre reactors. Nature 2005; 437:376–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McGowin CL, Ma L, Jensen JS et al. . Draft genome sequences of four axenic Mycoplasma genitalium strains isolated from Denmark, Japan, and Australia. J Bacteriol 2012; 194:6010–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Inamine JM, Ho KC, Loechel S, Hu PC. Evidence that UGA is read as a tryptophan codon rather than as a stop codon by Mycoplasma pneumoniae, Mycoplasma genitalium, and Mycoplasma gallisepticum. J Bacteriol 1990; 172:504–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Himmelreich R, Plagens H, Hilbert H, Reiner B, Herrmann R. Comparative analysis of the genomes of the bacteria Mycoplasma pneumoniae and Mycoplasma genitalium. Nucleic Acids Res 1997; 25:701–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McGowin CL, Ma L, Martin DH, Pyles RB. Mycoplasma genitalium-encoded MG309 activates NF-κB via Toll-like receptors 2 and 6 to elicit proinflammatory cytokine secretion from human genital epithelial cells. Infect Immun 2009; 77:1175–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McGowin CL, Radtke AL, Abraham K, Martin DH, Herbst-Kralovetz M. Mycoplasma genitalium infection activates cellular host defense and inflammation pathways in a 3-dimensional human endocervical epithelial cell model. J Infect Dis 2013; 207:1857–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dehon PM, Hagensee ME, Sutton KJ, Oddo HE, Nelson N, McGowin CL. Histological evidence of chronic Mycoplasma genitalium-induced cervicitis in HIV-infected women: A Retrospective Cohort Study. J Infect Dis 2016; 213:1828–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dehon PM, McGowin CL. Mycoplasma genitalium infection is associated with microscopic signs of cervical inflammation in liquid cytology specimens. J Clin Microbiol 2014; 52:2398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McGowin CL, Popov VL, Pyles RB. Intracellular Mycoplasma genitalium infection of human vaginal and cervical epithelial cells elicits distinct patterns of inflammatory cytokine secretion and provides a possible survival niche against macrophage-mediated killing. BMC Microbiol 2009; 9:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Svenstrup HF, Jensen JS, Gevaert K, Birkelund S, Christiansen G. Identification and characterization of immunogenic proteins of Mycoplasma genitalium. Clin Vaccine Immunol 2006; 13:913–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Iverson-Cabral SL, Manhart LE, Totten PA. Detection of Mycoplasma genitalium-reactive cervicovaginal antibodies among infected women. Clin Vaccine Immunol 2011; 18:1783–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tully JG, Taylor-Robinson D, Rose DL, Furr PM, Graham CE, Barile MF. Urogenital challenge of primate species with Mycoplasma genitalium and characteristics of infection induced in chimpanzees. J Infect Dis 1986; 153:1046–54. [DOI] [PubMed] [Google Scholar]
- 35. Wood GE, Iverson-Cabral SL, Patton DL, Cummings PK, Cosgrove Sweeney YT, Totten PA. Persistence, immune response, and antigenic variation of Mycoplasma genitalium in an experimentally infected pig-tailed macaque (Macaca nemestrina). Infect Immun 2013; 81:2938–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jurstrand M, Jensen JS, Magnuson A, Kamwendo F, Fredlund H. A serological study of the role of Mycoplasma genitalium in pelvic inflammatory disease and ectopic pregnancy. Sex Transm Infect 2007; 83:319–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wood G, Patton D, Cummings P, Inverson-Cabral S, Totten P. Experimental infection of pig-tailed macaques (Macacca nemestrina) with Mycoplasma genitalium. Infect Immun 2017; 85:e00738-16. doi:10.1128/IAI.00738-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McGowin CL, Spagnuolo RA, Pyles RB. Mycoplasma genitalium rapidly disseminates to the upper reproductive tracts and knees of female mice following vaginal inoculation. Infect Immun 2010; 78:726–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vandepitte J, Weiss HA, Kyakuwa N et al. . Natural history of Mycoplasma genitalium infection in a cohort of female sex workers in Kampala, Uganda. Sex Transm Dis 2013; 40:422–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Oakeshott P, Aghaizu A, Hay P et al. . Is Mycoplasma genitalium in women the “new chlamydia?” a community-based prospective cohort study. Clin Infect Dis 2010; 51:1160–6. [DOI] [PubMed] [Google Scholar]
- 41. Cohen CR, Nosek M, Meier A et al. . Mycoplasma genitalium infection and persistence in a cohort of female sex workers in Nairobi, Kenya. Sex Transm Dis 2007; 34:274–9. [DOI] [PubMed] [Google Scholar]
- 42. McGowin CL, Spagnuolo RA, Pyles RB. Mycoplasma genitalium rapidly disseminates to the upper reproductive tracts and knees of female mice following vaginal inoculation. Infect Immun 2010; 78:726–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Peterson SN, Bailey CC, Jensen JS et al. . Characterization of repetitive DNA in the Mycoplasma genitalium genome: possible role in the generation of antigenic variation. Proc Natl Acad Sci U S A 1995; 92:11829–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ma L, Jensen JS, Myers L et al. . Mycoplasma genitalium: an efficient strategy to generate genetic variation from a minimal genome. Mol Microbiol 2007; 66:220–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ma L, Jensen JS, Mancuso M et al. . Genetic variation in the complete MgPa operon and its repetitive chromosomal elements in clinical strains of Mycoplasma genitalium. PLoS One 2010; 5:e15660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Palmer GH, Bankhead T, Lukehart SA. ‘Nothing is permanent but change’—antigenic variation in persistent bacterial pathogens. Cell Microbiol 2009; 11:1697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Burgos R, Pich OQ, Ferrer-Navarro M, Baseman JB, Querol E, Piñol J. Mycoplasma genitalium P140 and P110 cytadhesins are reciprocally stabilized and required for cell adhesion and terminal-organelle development. J Bacteriol 2006; 188:8627–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lluch-Senar M, Querol E, Piñol J. Cell division in a minimal bacterium in the absence of ftsZ. Mol Microbiol 2010; 78:278–89. [DOI] [PubMed] [Google Scholar]
- 49. Ma L, Jensen JS, Mancuso M, Myers L, Martin DH. Kinetics of genetic variation of the Mycoplasma genitalium MG192 gene in experimentally infected chimpanzees. Infect Immun 2015; 84:747–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Criss AK, Kline KA, Seifert HS. The frequency and rate of pilin antigenic variation in Neisseria gonorrhoeae. Mol Microbiol 2005; 58:510–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Burgos R, Wood GE, Young L, Glass JI, Totten PA. RecA mediates MgpB and MgpC phase and antigenic variation in Mycoplasma genitalium, but plays a minor role in DNA repair. Mol Microbiol 2012; 85:669–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Burgos R, Totten PA. Characterization of the operon encoding the Holliday junction helicase RuvAB from Mycoplasma genitalium and its role in mgpB and mgpC gene variation. J Bacteriol 2014; 196:1608–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Burgos R, Totten PA. MG428 is a novel positive regulator of recombination that triggers mgpB and mgpC gene variation in Mycoplasma genitalium. Mol Microbiol 2014; 94:290–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Torres-Puig S, Broto A, Querol E, Piñol J, Pich OQ. A novel sigma factor reveals a unique regulon controlling cell-specific recombination in Mycoplasma genitalium. Nucleic Acids Res 2015; 43:4923–36. [DOI] [PMC free article] [PubMed] [Google Scholar]