Summary
Decreasing HPV seroprevalence at older ages in women in the United States largely reflects increasing cumulative number of sex partners in post-1945 birth cohorts, with limited evidence for significant loss of antibody due to waning immunity with age.
Keywords: human papillomavirus, cancer, sexual revolution, cohort effect
Abstract
Background
The United States has experienced an increase in the incidence of human papillomavirus (HPV)–related cancers that are not screen-detectable. It has been hypothesized, but not directly demonstrated, that this is due to increasing HPV prevalence in the unvaccinated population.
Methods
Female self-reported numbers of lifetime sex partners and HPV serology from the National Health and Nutrition Examination Survey (NHANES) were used to develop mathematical models of sexual partner acquisition and antibody dynamics. Modeled trends in sexual behaviors were compared to incidence data for cervical adenocarcinoma, oropharyngeal cancer, and anal cancer.
Results
The age-specific HPV seroprevalence data were best explained by a partner acquisition model that explicitly accounted for cohort-dependent changes in sexual behavior. Estimates of the mean time to loss of natural antibodies varied by model, ranging from 49 to 145 years. Inferred trends in sexual behavior over the past decades paralleled the increasing incidence of HPV-related cancers in the United States.
Conclusions
The findings suggest that lower HPV seroprevalence in older US women primarily reflects cohort-specific differences in sexual behaviors, and is only marginally attributable to immune waning with age. Our results emphasize the importance of continuing surveillance of sexual behaviors, alongside vaccine status, to predict future disease burden.
Sexually transmitted human papillomavirus (HPV) is the causal agent of various cancers and benign warts in the anogenital tract and oropharynx [1]. While the incidence of squamous cell carcinomas of the cervix has decreased substantially after the introduction of cytology screening in the 1940s [2] the incidence of adenocarcinomas of the cervix, which are poorly detectable through routine screening, has increased [3]. There has been a similar increasing trend in the incidence of HPV-related cancers of the oropharynx [4] and the anus [5], both of which are not routinely screened for in the US population.
HPV prevalence and cancer risk at oral and anogenital sites are consistently associated with markers of sexual activity, especially high number of lifetime sex partners (LTSPs) [1]. It has therefore been suggested that the increasing incidence of non-screen-detected HPV-related cancers in the United States is attributable to significant changes in sexual behaviors over the past decades, including earlier age at sexual debut, increasing number of LTSPs [6], later age at marriage, and more permissive attitudes about premarital sex [7].
It is presumed that these well-documented changes in sexual behavior have led to a population-level increase in HPV exposure, in turn driving the observed cancer incidence trends. However, US population-level surveillance for female genital HPV DNA prevalence was only initiated in 2003 [8], followed by surveillance for oral male and female HPV DNA prevalence in 2008 [9], and male genital HPV DNA prevalence in 2013–2014 [10]. While essential for ongoing impact evaluation of HPV vaccination, these data are insufficient to capture past trends in HPV exposure. In view of this limitation, birth cohort–specific differences in serum antibodies to HPV represent a reasonable biomarker of changing HPV exposure over longer time periods. Approximately 60% of women infected with HPV seroconvert [11], and type-specific serum antibodies provide potential long-term markers for cumulative HPV exposure. Cross-sectional seroprevalence data for 9 high- and low-risk HPV genotypes have been reported from the 2005–2006 round of the National Health and Nutrition Examination Survey (NHANES) [12], showing a decline in seroprevalence with age, consistent with the reported cohort-specific differences in sexual behavior [6]. Direct inference from this age-specific cross-sectional analysis is difficult, however, because declining prevalence in older age groups could be due to cohort effects or waning immunity, or both [12–14]. Simple mathematical models have previously been applied to interpret possible cohort effects in HPV seroprevalence data in the United Kingdom [15], using catalytic models with age at sexual debut as a proxy for cohort-specific risk of infection. Here, we extend this approach by developing a nonlinear age-cohort model of new partner acquisition based on actual self-reported number of LTSPs in the United States, and couple the sexual exposure model to a dynamic in-host model of type-specific antibody status. Integrating the models with US cross-sectional data for 9 different high- and low-risk HPV types (HPV-6, -11, -16, -18, -31, -33, -45, -52, and -58), we perform model selection and evaluate the relationship between changing sexual behaviors and HPV exposure against competing models of age-associated waning immunity.
METHODS
Data Sources
NHANES
NHANES is a series of ongoing cross-sectional surveys conducted by the Centers for Disease Control and Prevention. Over 2-year cycles, NHANES performs physical examinations and interviews of a representative sample of the civilian, noninstitutionalized population of the United States. Self-reported number of LTSPs among females aged 20–59 were analyzed for eight 2-year survey cycles, from 1999–2000 to 2013–2014. For the cycles 2009–2010 and later, self-reported LTSPs among women aged 18 and 19 years were available and included in the analysis. For the survey cycle 2005–2006, serum from women aged 18–59 was analyzed for presence of immunoglobulin G antibodies against 9 HPV types (6, 11, 16, 18, 31, 33, 45, 52, and 58) using a competitive Luminex Assay (cLIA).
Surveillance, Epidemiology, and End Results Database
The National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database records cancer cases in approximately 25% of the US population. Data from 9 SEER registries (1973–2013) were queried for cervical adenocarcinoma (CAC) in women, and oropharyngeal cancer (OPC) and anal cancer (AC) in both men and women. Analyses were restricted to subsites commonly associated with HPV-positive tumors in individuals of age 15 years and older (see Supplementary Data 1 for details). Incidence rates were computed for 5-year intervals between 1975–1979 and 2005–2009, and a 4-year interval between 2010 and 2013.
Statistical Analyses
NHANES
Descriptive analyses were performed using the “survey” package (version 3.31) in the statistical software R, to account for the complex survey design. The 2-year interview weights were used for the sexual behavior analyses, and the 2-year mobile examination center weights were used for the serum antibody analyses. For the seroprevalence and sexual behavior data used in the mathematical modeling analyses, age at examination was generally categorized into 5-year groups. For the seroprevalence analyses based on stratification by self-reported number of LTSPs, age at examination was categorized into 10-year age groups, and the LTSPs were stratified into groups of 1, 2–3, 4–6, 7–9, 10–19, and 20–50 LTSPs. The choice of cut-points and exclusion of individuals with >50 LTSPs were motivated by the underlying distribution of LTSPs (Supplementary Figure 1). Differences in mean number of serotypes were evaluated based on a 2-tailed Z-test at the 5% significance level.
SEER
Data were analyzed using SEER*Stat software (November 2015 submission). Age-adjusted incidence rates and 95% confidence intervals were computed using the 2000 US Standard Population (19 age groups, Census P25-1130).
Mathematical Models
For the analysis of the population-based female LTSPs and seroprevalence data, we developed a series of mathematical models. Because the data used to inform the models were collected from a pre-HPV vaccine survey, the impact of HPV vaccination was not modeled. Details about model development and parameter inference are provided in Supplementary Data 2. In short, we first used LTSP data to parameterize a Bayesian model of age- and cohort-dependent sexual partner acquisition in US females. Thereby, we assumed that a woman’s probabilistic rate of partner acquisition varied over age, and was further adjusted to account for cohort effects. Fitting the model to the LTSP data, we then inferred the age- and cohort-specific contributions to the rate of new sexual partner acquisition. The acquisition rate of a specific HPV type was modeled as the product of the partner acquisition rate and the per-partnership acquisition probability, which accounts for both the partner’s HPV prevalence and the per-partnership transmission probability. Next, we combined the age- and cohort-dependent model of HPV acquisition with an in-host model of HPV dynamics that accounts for possible seroconversion upon infection with a new type, and describes waning of antibody-conferred protection against reinfection from new sexual partners. We used likelihood-based methods to parameterize a family of candidate models based on cross-sectional seroprevalence data from NHANES. The simplest model (M1) assumes an age-dependent partner acquisition rate, a constant per-partnership probability of acquiring any given HPV type, and allows for immune waning (Table 1). The second model (M2) accounts for both age- and cohort-dependent partner acquisition, but posits complete absence of immune waning. Model 3 (M3) extends M2 to account for immune waning. Model 4 (M4) extends M3 to account for a linear age-dependent variation in the per-partnership acquisition probability. Model 5 (M5) is identical to M4 except that the HPV acquisition probability is modeled to depend on the birth cohort instead of age. In model 6 (M6) we further generalize M4 and M5 and assumed the acquisition probability to depend linearly on both age and cohort. Finally, in model 7 (M7), as an extension to the linear model M6, we assumed the acquisition probability to be proportional to the nonlinear partner acquisition rate. Finally, goodness of fit for the different models M1–M7 was ascertained with the Akaike information criterion (AIC) and the coefficient of determination (COD).
Table 1.
Model Selection
| Model Structure | Model Fitting | |||||
|---|---|---|---|---|---|---|
| Model | Partner Acquisition | HPV Acquisition Probabilitya | Immune Waning | AIC (IQR) | COD, % (IQR) | Mean Time, y, to Loss of Immunity (IQR) |
| M1 | Age | Constant | Yes | –8.0 (–8.8 to –7.2) | 58.5 (56.7–60.1) | 68.5 (64.9–74.6) |
| M2 | Age & cohort | Constant | No | –12.2 (–13.8 to –10.4) | 65.8 (61.1–70.0) | … |
| M3 | Age & cohort | Constant | Yes | –13.3 (–14.7 to –11.3) | 74.3 (70.0–78.0) | 144.9 (108.7–192.3) |
| M4 | Age & cohort | Linear in age | Yes | –11.6 (–13.2 to –9.2) | 74.6 (69.2–79.0) | 64.1 (40.7–161.3) |
| M5 | Age & cohort | Linear in cohort | Yes | –12.0 (–13.4 to –10.5) | 75.8 (72.1–79.4) | 51.0 (36.2–82) |
| M6 | Age & cohort | Linear in age, cohort | Yes | –10.6 (–12.0 to –8.8) | 76.9 (72.6–80.6) | 49.0 (33.0–79.4) |
| M7 | Age & cohort | Proportional to female partner acquisition rate | Yes | 24.2 (15.4–46.2) | –25.2 (–46.9 to –1.9) | >5000 |
Abbreviations: AIC, Akaike information criteria; COD, coefficient of determination; HPV, human papillomavirus; IQR, interquartile range.
aPer new partner.
RESULTS
Parameter Inference and Model Selection
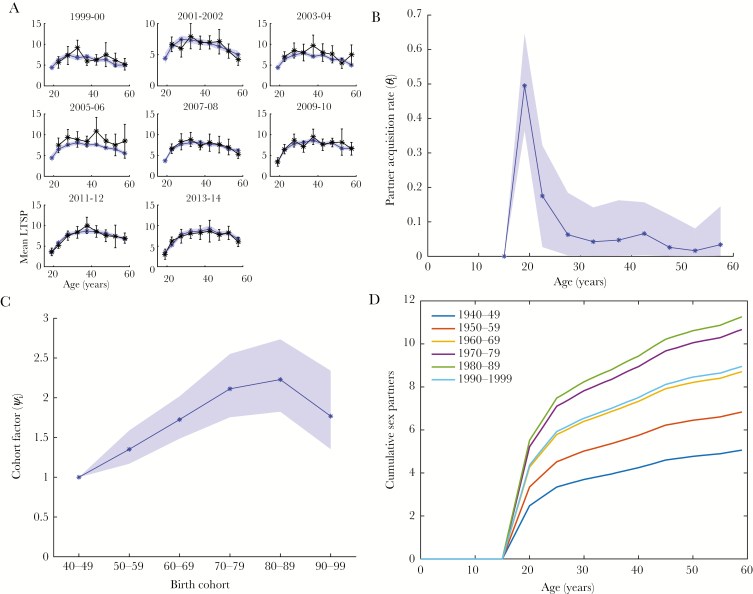
First, we parameterized the sexual partner acquisition model based on female self-reported LTSP data from NHANES (Figure 1A). The age-specific acquisition rates for the reference birth cohort (1940–1949) are shown in Figure 1B. The median acquisition rate reached its peak during the first 5 years after sexual debut at 0.50 (95% credible interval [CI], .36–.65) new partners per year, then decreased to 0.18 (95% CI, .03–.32) between ages 20 and 25, fluctuated between .04 and .07 until age 45, and further decreased to .02–.03 thereafter. The partner acquisition rate increased approximately linearly until the 1970–1979 cohort, where it was 2.1-fold higher (95% CI, 1.82–2.73) than in the reference cohort (Figure 1C). For women born in 1980–1989, the cohort factor plateaued at 2.4 (95% CI, 1.8–2.6), and for the 1990–1999 birth cohort, it decreased to 1.8 (95% CI, 1.4–2.3). Of note, the data available to inform the latter birth cohort are scarce. Figure 1D shows the median cohort-stratified cumulative LTSPs between ages 15 and 59 as predicted by the model. Finally, neglecting cohort effects in the partner acquisition model yielded substantially worse fits to the NHANES seroprevalence data (Supplementary Figure 2).
Figure 1.
Modeling of new partner acquisition. The age-cohort model for the rate of acquisition of new sex partners is parameterized in a Bayesian framework. A. Visualization of model fit (blue: median and 95% credible interval) to mean number of lifetime sex partners (LTSPs) from 8 survey cycles in the National Health and Nutrition Examination Survey (black: point estimate and 95% confidence interval). B, Median and 95% credible interval for age-dependent partner acquisition rates in the reference birth cohort. C, Median and 95% credible interval for the cohort scaling factors of the partner-acquisition rates (relative to the reference birth cohort). Note that only limited data support the estimates for later birth cohorts, especially the 1980–1989 and 1990–1999 cohorts. D, Model-predicted age-specific median LTSPs, stratified by birth cohort.
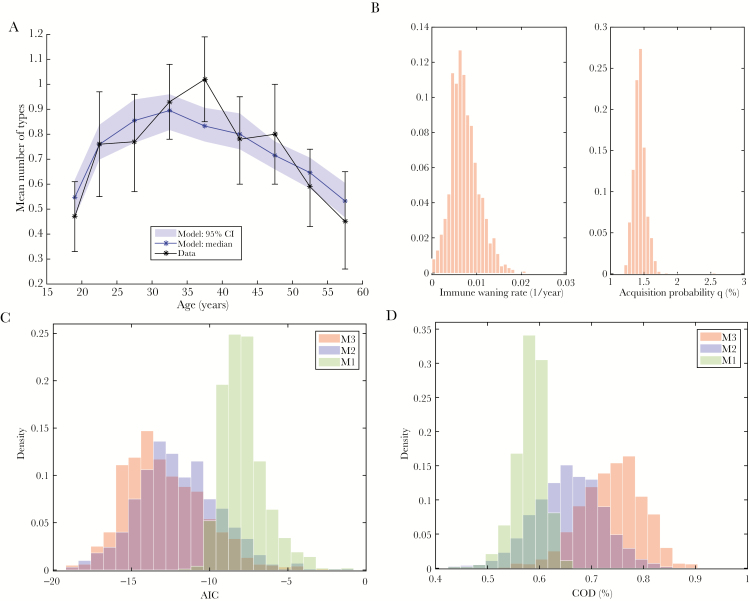
For model selection (Table 1), we first focused on models with a constant per partnership probability of HPV acquisition (models M1–M3). Model M3 with age and cohort dependent partner acquisition rates, a constant per-partnership HPV acquisition probability, and positive immune waning provided the best fit with a median AIC of –13.3 (interquartile range [IQR], –14.7 to –11.3) and a median COD of 74.3% (IQR, 70.0%–78.0%) (Figure 2A). The estimated median time to loss of antibody detection was 145 years (IQR, 109–192 years) (Figure 2B). Consistent with this estimate of a very small rate of immune waning estimate, model M2 with zero immune waning provided an only slightly inferior fit with a median AIC of –12.2 (IQR, –13.8 to –10.4) and median COD of 65.8% (IQR, 61.1%–70.0%). When neglecting the cohort dependence of partner acquisition (M1), the fit was poor, with a median AIC of –8.0 (IQR, –8.8 to –7.2) and a median COD of 58.5% (IQR, 56.7%–60.1%). AIC- and COD-based comparisons between the 3 model variants are shown in Figures 2C and 2D, respectively.
Figure 2.
Modeling of seroprevalence. A, Model M3 (blue: median and 95% credible interval [CI] of the quasi-posterior) is fit to the mean number of serotypes in the 2005–2006 National Health and Nutrition Examination Survey (black: point estimate and 95% confidence interval). B, Histograms approximating the quasi-posterior densities of the immune waning rate and the per-partnership human papillomavirus acquisition probability for model M3. C, Comparison of the quasi-posterior density of Akaike information criteria (AIC) in the best-fitting model (M3; red) to the corresponding densities for 2 alternative models, namely the model without immune waning (M1; blue), and the model without cohort effects in the partner acquisition rate (M2). D, Comparison of the coefficient of determination (COD) between models M1, M2, and M3 (as in C).
Next, we considered the effects of allowing for a nonconstant probability of acquiring a given HPV type from a new sex partner (models M4–M7). The linear models (M4–M6) lead to similar model fits that were all inferior to the best-fitting model M3 (Table 1). The corresponding estimates of the time to immune waning were found to be 2- to 3-fold lower compared to model M3, with a mean time to loss of antibody detection of 51 years (IQR, 36–82 years) for the best-fitting model M4. In models M5 and M6 we found a decreasing trend for the acquisition probability by cohort, corresponding to a smaller risk of acquiring HPV from a new partner in a younger birth cohort (Supplementary Table 1). Finally, in model M7 where the HPV prevalence in partners mirrored the partner acquisition rate, the model fit was poor (Table 1; Supplementary Figure 2).
LTSP-Stratified Seroprevalence
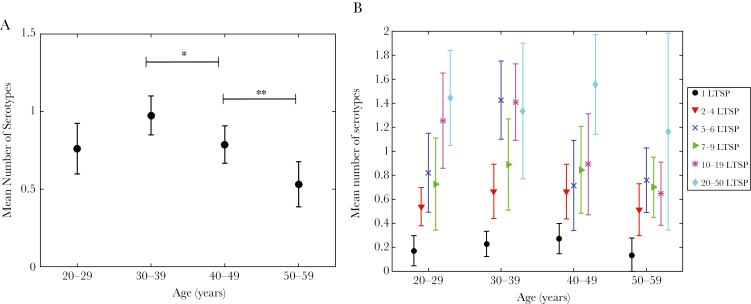
The models predicted relatively small rates of immune waning, with a mean time to loss of detectable antibodies on the order of 50–150 years (Table 1). In consequence, we hypothesized that seroprevalence would be relatively constant across age among individuals with similar exposure history. To test this hypothesis, we analyzed the mean number of serotypes stratified by age group and number of LTSPs. In the unstratified data, there was a significant decrease in the mean number of serotypes between the age groups of 30–39 and 40–49 years, and the age groups of 40–49 and 50–59 years, respectively (Figure 3A). In the stratified analysis, however, the majority of LTSP strata did not exhibit such a decrease in number of serotypes (Figure 3B). In the strata with 1, 2–4, and 7–9 LTSPs, the slight decrease in older age groups was consistent with presence of small but nonnegligible immune waning over time. The same observation was made in the strata with 20–50 LTSPs, but wide confidence intervals precluded definite conclusions. In the 5–6 and 10–19 LTSP strata, we found a significant decrease in the mean number of serotypes between the age groups of 30–39 and 40–49 years, but no further decrease between the age groups of 40–49 and 50–59 years.
Figure 3.
Cross-sectional seroprevalence in the US population. A, Age-stratified, cross-sectional number of detectable serotypes among US females (mean and 95% confidence interval). There was a significant decrease in mean number of serotypes between the age groups of 30–39 and 40–49 years (*P < .05), and the age groups of 40–49 and 50–59 years (**P < .01), respectively. B, The cross-sectional measure from (A) is stratified by cumulative sexual exposure as measured by self-reported number of lifetime sex partners (LTSPs) up to 50 total partners. For the strata “LTSP = 5–6” and “LTSP = 10–19,” there was a significant decrease in mean number of serotypes between the age groups of 30–39 and 40–49 years (P < .01 and P < .05, respectively), but no difference between the age groups of 40–49 and 50–59 years. In the remaining LTSP strata, no decrease in mean number of serotypes was observed after age 30–39 years.
Cancer Incidence Comparisons
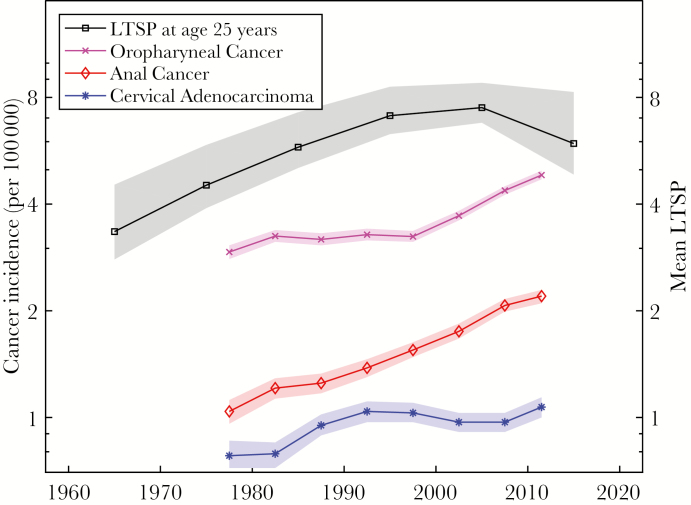
The overlay of age-adjusted incidence of CAC, OPC, and AC from 1975–2013 with the modeled mean number of LTSPs at age 25 in Figure 4 shows the similar pattern of both increasing sexual exposure via increasing LTSPs and increasing incidence of non-screen-detected HPV-associated cancers over time at the population level. The LTSP time series starts in 1965 when individuals born in 1940 (the oldest modeled cohort) reached the age of 25 and continues until 2015.
Figure 4.
Trends in sexual behaviors and cancer incidence. Age-adjusted incidence trends for adenocarcinomas of the cervix, and carcinomas of the oropharynx and anus (1975–2013, 5-year intervals; point estimate and 95% confidence interval) are compared to the modeled mean number of lifetime sex partners (LTSPs) among females of age 25 years (1965–2015, 10-year intervals; median and 95% credible interval). The y-axis for cancer incidence is on the left (logarithmic scale), and the y-axis for mean LTSPs is on the right (linear scale logarithmic scale).
DISCUSSION
We developed mathematical models to investigate the relationship between changing sexual behaviors and cumulative HPV exposure as measured by cross-sectional seroprevalence from a nationally representative dataset. We found that lower HPV seroprevalence at older ages in the United States can be largely explained by cohort-specific changes in sexual behaviors. While models that allow for immune waning in addition to cohort effects in partner acquisition yield marginally better fits to the seroprevalence data, waning immunity appears to have a limited impact on age-specific seroprevalence patterns in US women.
As a marker of cumulative exposure to a sexually transmitted viral infection in the population, HPV seroprevalence is expected to increase rapidly around the average age of sexual debut, and then to plateau as individuals enter longer-term, stable relationships. In the 2005–2006 NHANES survey [12], however, HPV seroprevalence peaked in women in their 30s and declined thereafter, especially in women aged 50–59 years. The authors hypothesized that this pattern could represent an apparent decline in antibody detectability concomitant with the immunosenescence of aging (ie, waning immunity) or could represent a cohort effect. With respect to the latter possibility, we noted that the women comprising the 50- to 59-year age group in 2005–2006 represented the 1946–1956, or early to mid–Baby Boomer, birth cohorts. These women would have reached sexual debut during the so-called Sexual Revolution of the late 1960s and early 1970s, a period marked by changing female roles, contraceptive choices, and more permissive attitudes toward premarital sex [7, 16, 17]. Our models confirmed the presence of a cohort effect, whereby Baby Boomers had 2.5-fold fewer LTSPs compared to the first cohort of Millennials (born 1980–1989). The Sexual Revolution cohort effect is also reflected in several sexual attitude and behavior surveys in the United States [6, 7].
Despite a clear sexual behavior difference between the older and younger birth cohorts, it remained unclear whether this LTSP difference was sufficient to explain the 60% lower HPV seroprevalence at ages 50–59 years relative to the peak in the mid-30s. Because the magnitude of immune waning after natural seroconversion is largely inestimable given currently available data [12–14], we approached the evaluation of the relative contribution of cohort effects and immune waning by comparing competing mechanistic mathematical models with varying assumptions regarding immune waning in the presence and absence of cohort effects. Based on these analyses, immune waning was estimated to be small, with inferred mean time to loss of natural antibodies of 50 years or more. Thus, our findings suggest that the pronounced age-dependence of cross-sectional seroprevalence among US women (Figure 3A) may primarily be driven by cohort-specific differences in sexual behaviors rather than waning immunity. Consistent with these conclusions, the cross-sectional seroprevalence patterns were substantially attenuated upon stratification by cumulative sexual exposure (Figure 3B). Indeed, most LTSP strata exhibited largely age-independent seroprevalence profiles, with a slight decay in older age groups that is consistent with small immune waning.
Few studies have evaluated seropersistence of antibodies following natural infection over time, precluding direct validation of our model-derived estimates of immune waning. The Finnish Maternity Cohort reported high rates of seropersistence up to 5 years in young pregnant women [16]. While a study in Costa Rica reported lower overall rates of seropersistence, the rates of seropersistence were similar across age groups, with a slightly lower seroprevalence only in the oldest age group (>65 years) [18], which is consistent with our modeled estimates. The reason for the lower rates of seropersistence in the Costa Rica compared with the Finnish Maternity Cohort are unclear, but may be related to the lack of standardization of the seroassays used in the studies. Additional support of our conclusions comes from a modeling study of HPV seroprevalence in women in the United Kingdom, which similarly concluded that cohort effects provided a better explanation of age-specific declines in HPV seroprevalence, with minimal contribution from waning immunity with age [15]. Because immune waning plays a critical role in health economic projection models [17–19], further research on seropersistence to confirm our estimates is warranted.
Our approach has a number of limitations. First, we evaluated seroprevalence as measured by cLIA, an assay targeting a single immunodominant epitope for each HPV type. We assume that our results would have been similar using enzyme-linked immunosorbent assay (ELISA) serology, which measures a higher number of both neutralizing and nonneutralizing HPV antibodies, based on previous reports of high correlation between cLIA and ELISA in nonvaccinated individuals [20]. Second, because we modeled population-level trends, the model is not designed to capture behavioral and biologic heterogeneity in the population. For example, cohort-specific changes in sexual behaviors were not uniform across all races/ethnicities [6], and antibody stability may differ by HPV type. For example, we note that 2 LTSP strata [5, 6, 10–19] exhibited prevalence patterns similar to those in the nonstratified population (Figure 3A), which may be partially explained by other cohort-dependent sources of heterogeneity and possible reporting biases (see Supplementary Figure 1). Third, due to a lack of HPV DNA prevalence data and uncertainty about per-partnership transmission of HPV [21], the probability of acquiring HPV from a partner is largely unknown. Balancing goodness of fit against complexity, model selection favored the model with a constant partner HPV prevalence across all ages and cohorts. The validity of this model is supported by a recent NHANES survey of male penile HPV prevalence in the United States, showing peak prevalence by age 30 years, which remained stable through age 59 (the oldest age evaluated) [10]. Fourth, the developed age-cohort model of partner acquisition does not account for possible period effects on sexual behaviors. While we cannot exclude that such effects may play a role, we note that, in contrast to premarital sex, attitude towards extramarital sex did not become more permissive in more recent generations [7]. Consequently, it appears that the Sexual Revolution affected younger, unmarried women more than older, married women, which in turn points toward a cohort rather than a period effect.
Our results suggest temporal increases in HPV exposure since the 1960s, a trend that has been frequently reported as the cause of increasing incidence of oropharyngeal and anal cancers, as well as adenocarcinomas of the cervix. While we are unable to directly evaluate whether increased HPV exposure is the primary explanation for the observed trends in HPV-related cancer incidence, Figure 4 indicates a plausible temporal association between the two. However, we acknowledge that we cannot rule out contributions from temporal changes in co-factor exposure as alternative explanations for the observed increase in HPV-related cancer incidence.
In summary, our findings suggest that the age-specific HPV seroprevalence patterns in unvaccinated US women are largely explained by temporal changes in sexual behaviors during and after the Sexual Revolution. The resulting increase in HPV exposure may have precipitated the observed increase in incidence of HPV-related cancers that are not screen detectable. As the highly exposed late Baby Boomer and post–Baby Boomer birth cohorts are reaching the age of peak HPV-associated cancer incidence, observed rates of HPV-related disease may continue to rise until the trend in sexual behaviors is reversed, or the first postvaccine generation enters the age of peak cancer incidence. Taken together, these findings emphasize the need for continued surveillance of sexual behaviors to ensure optimal prediction and control of future disease burden. Finally, it is noteworthy that most health economic models of HPV and cervical cancer prevention do not account for cohort-specific differences in new partner acquisition. In light of the magnitude of these differences and their impact on cumulative HPV exposure in successive generations, inclusion of cohort effects into the models may be necessary for accurate predictions of future disease burden.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Financial support. M. D. R. was partially supported by the Swiss National Science Foundation (P300P2-154583), the National Institutes of Health (K99 CA207872), and the National Science Foundation (DMS-1614838). P. E. G. was partially supported by the National Institutes of Health (R01 CA190366). A. F. R. was partially supported by the Cigarette Restitution Fund Research Grant to the Johns Hopkins Medical Institutions (FY16).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Bosch FX, Broker TR, Forman D et al. . Comprehensive control of human papillomavirus infections and related diseases. Vaccine 2013; 31(suppl 8):I1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yang DX, Soulos PR, Davis B, Gross CP, Yu JB. Impact of widespread cervical cancer screening: number of cancers prevented and changes in race-specific incidence. Am J Clin Oncol 2016. doi:10.1097/COC.0000000000000264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang SS, Sherman ME, Hildesheim A, Lacey JV Jr., Devesa S. Cervical adenocarcinoma and squamous cell carcinoma incidence trends among white women and black women in the United States for 1976–2000. Cancer 2004; 100:1035–44. [DOI] [PubMed] [Google Scholar]
- 4. Chaturvedi AK, Engels EA, Pfeiffer RM et al. . Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol 2011; 29:4294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Joseph DA, Miller JW, Wu X et al. . Understanding the burden of human papillomavirus-associated anal cancers in the US. Cancer 2008; 113:2892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu G, Hariri S, Bradley H, Gottlieb SL, Leichliter JS, Markowitz LE. Trends and patterns of sexual behaviors among adolescents and adults aged 14 to 59 years, United States. Sex Transm Dis 2015; 42:20–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Twenge JM, Sherman RA, Wells BE. Changes in American adults’ sexual behavior and attitudes, 1972–2012. Arch Sex Behav 2015; 44:2273–85. [DOI] [PubMed] [Google Scholar]
- 8. Dunne EF, Unger ER, Sternberg M et al. . Prevalence of HPV infection among females in the United States. JAMA 2007; 297:813–9. [DOI] [PubMed] [Google Scholar]
- 9. Gillison ML, Broutian T, Pickard RK et al. . Prevalence of oral HPV infection in the United States, 2009–2010. JAMA 2012; 307:693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gargano JW, Unger ER, Liu G et al. . Prevalence of genital human papillomavirus in males, United States, 2013–2014. J Infect Dis 2017; 215:1070–79. [DOI] [PubMed] [Google Scholar]
- 11. Dillner J. The serological response to papillomaviruses. Semin Cancer Biol 1999; 9:423–30. [DOI] [PubMed] [Google Scholar]
- 12. Liu G, Markowitz LE, Hariri S, Panicker G, Unger ER. Seroprevalence of 9 human papillomavirus types in the United States, 2005–2006. J Infect Dis 2016; 213:191–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Markowitz LE, Sternberg M, Dunne EF, McQuillan G, Unger ER. Seroprevalence of human papillomavirus types 6, 11, 16, and 18 in the United States: National Health and Nutrition Examination Survey 2003–2004. J Infect Dis 2009; 200:1059–67. [DOI] [PubMed] [Google Scholar]
- 14. Newall AT, Brotherton JM, Quinn HE et al. . Population seroprevalence of human papillomavirus types 6, 11, 16, and 18 in men, women, and children in Australia. Clin Infect Dis 2008; 46:1647–55. [DOI] [PubMed] [Google Scholar]
- 15. Desai S, Chapman R, Jit M et al. . Prevalence of human papillomavirus antibodies in males and females in England. Sex Transm Dis 2011; 38:622–9. [DOI] [PubMed] [Google Scholar]
- 16. af Geijersstam V, Kibur M, Wang Z et al. . Stability over time of serum antibody levels to human papillomavirus type 16. J Infect Dis 1998; 177:1710–4. [DOI] [PubMed] [Google Scholar]
- 17. Garnett GP, Kim JJ, French K, Goldie SJ. Chapter 21: modelling the impact of HPV vaccines on cervical cancer and screening programmes. Vaccine 2006; 24(suppl 3):S3/178–86. [DOI] [PubMed] [Google Scholar]
- 18. Jit M, Choi YH, Laprise JF, Boily MC, Drolet M, Brisson M. Two-dose strategies for human papillomavirus vaccination: how well do they need to protect? Vaccine 2014; 32:3237–42. [DOI] [PubMed] [Google Scholar]
- 19. Laprise JF, Markowitz LE, Chesson HW, Drolet M, Brisson M. Comparison of 2-dose and 3-dose 9-valent human papillomavirus vaccine schedules in the United States: a cost-effectiveness analysis. J Infect Dis 2016; 214:685–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lin SW, Ghosh A, Porras C et al. . HPV16 seropositivity and subsequent HPV16 infection risk in a naturally infected population: comparison of serological assays. PLoS One. 2013; 8(1):e53067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bogaards JA, Xiridou M, Coupé VM, Meijer CJ, Wallinga J, Berkhof J. Model-based estimation of viral transmissibility and infection-induced resistance from the age-dependent prevalence of infection for 14 high-risk types of human papillomavirus. Am J Epidemiol 2010; 171:817–25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.