Acute respiratory virus infections often have a prolonged duration in cystic fibrosis patients and predispose the cystic fibrosis lung to bacterial colonization. An experimental enterovirus infection model couples the ΔF508-mutation in CFTR to delayed virus clearance and defective antiviral immunity.
Keywords: antibody response, antiviral defense, Coxsackievirus, cystic fibrosis, immunity
Abstract
Acute respiratory virus infections predispose the cystic fibrosis (CF) lung to chronic bacterial colonization, which contributes to high mortality. For reasons unknown, respiratory virus infections have a prolonged duration in CF. Here, we demonstrate that mice carrying the most frequent cystic fibrosis transmembrane conductance regulator (CFTR) mutation in humans, ΔF508, show increased morbidity and mortality following infection with a common human enterovirus. ΔF508 mice demonstrated impaired viral clearance, a slower type I interferon response and delayed production of virus-neutralizing antibodies. While the ΔF508 mice had a normal immune cell repertoire, unchanged serum immunoglobulin concentrations and an intact immune response to a T-cell–independent antigen, their response to a T-cell–dependent antigen was significantly delayed. Our studies reveal a novel function for CFTR in antiviral immunity and demonstrate that the ΔF508 mutation in cftr is coupled to an impaired adaptive immune response. This important insight could open up new approaches for patient care and treatment.
Cystic fibrosis (CF) is caused by mutations in a chloride channel encoded by the cystic fibrosis transmembrane conductance regulator (CFTR) gene. It is one of the most common monogenic diseases in the Caucasian population. CFTR is expressed on the apical membrane of most epithelial cells and defective CFTR leads to disturbances in the electrolyte balance in secretory epithelia. This negatively affects the function of epithelial organs, including the lungs, intestine, and pancreas [1].
Although improved treatments have increased survival rates from a few years to over 40 years for most CF patients, recurrent and chronic bacterial lung infections resulting in respiratory failure remain a major cause of early mortality. At present it is not understood why individuals with CF suffer from such life-threatening infections. Current observations suggest that viral infections (eg, enteroviruses including rhinovirus, respiratory syncytial virus [RSV], etc.), may be important contributing factors, as they predispose the lower airways to bacterial colonization [2, 3].
For reasons unknown, individuals with CF suffer from virus infections with a prolonged duration [4, 5]. Considering the aforementioned clinical importance of virus infections in CF [2, 3], knowledge regarding how mutations in CFTR affect the antiviral immune response is warranted. An impaired innate antimicrobial defense in epithelial cells, as demonstrated in cells from the lung [6], may contribute to excessive virus replication. Several studies have shown that CFTR is expressed by immune cells, including mononuclear monocytes and lymphocytes [7–11], but little is known about the functional role of CFTR in immune cells [9, 12] and how mutations in CFTR affect the immune responses to infections.
Enteroviruses such as rhinoviruses and Coxsackie B viruses (CVBs) are a common cause of respiratory tract infections (eg, “the common cold”) both in healthy individuals and patients with CF [2, 5, 13]. Rhinoviruses infect and replicate in the upper airways whereas CVBs infect via both the fecal–oral route and the respiratory tract, and have a broader tissue tropism [14]. Studies examining the immune response to rhinoviruses were long hampered by a lack of suitable small animal models, while CVB-infected mice develop diseases similar to those seen in humans [15] and have been used extensively to study the host immune responses to infection (eg, [15–17]).
The role for CFTR in the host immune response to CVB infection has not been investigated. In contrast, interferons (IFNs) and several IFN-inducible genes (ISGs) have been implicated in the host response to such infections. Studies in animal models have shown that type I (IFN-α and IFN-β), but not type II (IFN-γ) interferons, are essential for the early immune response to, and survival following, CVB infections [18]. A more recently described group of IFNs, type III IFNs (IFN-λs), have so far not been studied under in vivo conditions, but have an antiviral effect against CVBs in vitro [19, 20]. ISGs shown to be important in the defense against CVBs include genes coding for proteins involved in the recognition of viruses (eg, TLR3 and MDA5) and in antiviral defense (eg, iNOS, PKR, OAS) (eg, [16, 17, 21, 22]). Type I IFNs are also known to be of general importance for the activation of the adaptive antiviral immune response [23–25]. The production of neutralizing antibodies after infection that prevent the virus from spreading and that also assist in clearing infectious viral particles from the body, is particularly important during enterovirus infections both in mouse and humans [26–28].
The goal of this study was to increase our knowledge and understanding of the defective antiviral immune response linked with mutations in CFTR. We hypothesized that delayed virus clearance in hosts with a mutated CFTR is due to a defect in extrapulmonary antiviral immunity. To address this hypothesis, we made use of the Cftrtm1EUR mouse model (here denoted ΔF508) carrying the cftr mutation most commonly found in humans, F508del [29, 30]. The normal survival rates of the ΔF508 mouse model and the broad tropism of the enterovirus Coxsackievirus B3 (CVB3) enabled us to study both the systemic and the tissue-specific immune responses to enterovirus infection in a host with a defective CFTR. We demonstrate that the ΔF508 mutation causes delayed virus clearance, which culminates in a high mortality rate. Importantly, we link delayed virus clearance to a defective adaptive immune response and we demonstrate the therapeutic potential of passive immunization.
METHODS
The present study made use of the Cftrtm1EUR mouse model (homozygous ΔF508 mice and wild-type (wt) littermate controls) on a C57BL/6J background [29, 30] and coxsackievirus B3 Nancy (CVB3). A detailed material and methods description is given in the Supplementary Material Methods.
Ethics Statement
The mice were housed and experiments performed according to local and national regulations, and the study was approved by the Stockholm South Animal Ethics Board.
RESULTS
Decreased Survival and Increased Viral Loads in Organs of ΔF508 Mice Challenged With CVB3
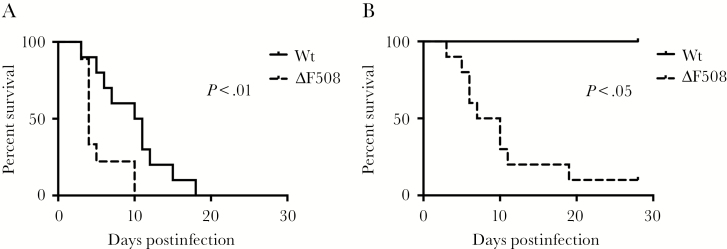
To evaluate if the common ΔF508 mutation in the cftr gene affects susceptibility to enterovirus infection, we infected ΔF508 mice and wild-type (wt) littermate controls with two different doses of CVB3 via the intraperitoneal route. While both wt and ΔF508 mice eventually succumbed to infection with the higher virus dose (Figure 1A), the median survival time was longer in wt animals (10 days) compared to ΔF508 mice (4 days). Even with a virus dose that was not lethal to the wt mice, the majority of ΔF508 mice died after infection (Figure 1B). These results showed that ΔF508 mice have an impaired ability to survive a CVB3 infection.
Figure 1.
ΔF508 mice demonstrate an increased mortality rate after infection with Coxsackievirus. ΔF508 mice (dotted lines) and wild-type (wt) littermate controls (solid lines) were infected with Coxsackievirus B3 (CVB3) and monitored for 28 days. A, Wt (n = 10) and ΔF508 (n = 9) mice infected with 104 plaque-forming units (PFU) CVB3/mouse. B, wt (n = 3) and ΔF508 (n = 10) infected with 102 PFU CVB3/mouse. Log-rank (Mantel–Cox) test.
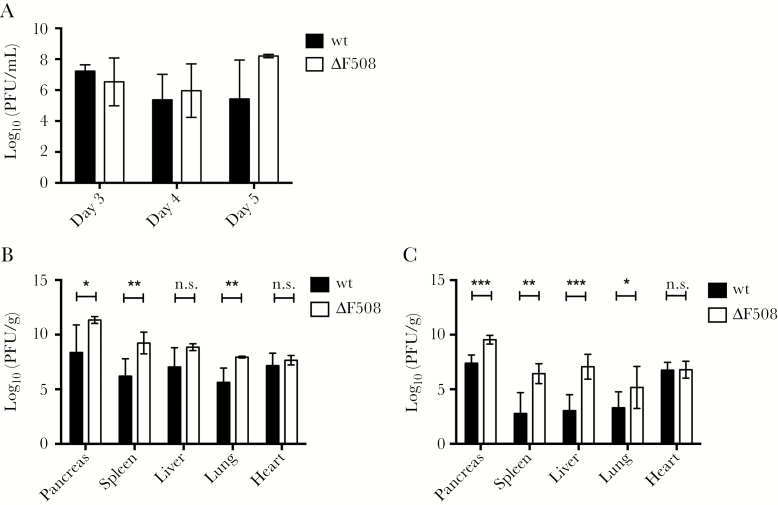
Viremia measurements revealed no differences between the infected wt and ΔF508 mice (Figure 2A), suggesting that the ΔF508 mutation did not affect early viral replication and dissemination. CVB3, however, has a broad tissue tropism and virus titers in organs from systemically infected animals normally peak between days 3 and 5 postinfection [15, 21]. By measuring infectious virus particles in organs collected on day 5 postinfection we detected significant differences in the titers of replicating virus between the wt and ΔF508 mice (Figure 2B). At the same time point, substantial histopathological signs of infection in certain organs from the infected mice, including extensive damage to the exocrine pancreas, mild hepatitis, and some disruption of the splenic structure were observed. No major differences were observed, however, between the wt and ΔF508 mice (Supplementary Figure 1).
Figure 2.
Sustained high level viral replication in ΔF508 mice after infection with Coxsackievirus. Wild-type (wt; black bars) and littermate ΔF508 mice (white bars) were infected with Coxsackievirus B3 (102 plaque-forming units [PFU]/mouse). A, Blood was harvested on days 3 (n = 3–5 animals per genotype), 4 (n = 9–12 animals per genotype), and 5 (n = 3–4 animals per genotype) postinfection for viremia measurements using plaque assay. B, Titers of replicating virus particles in organs harvested on day 5 postinfection were measured by plaque assay (n = 4–7 animals per genotype). C, Titers of replicating virus particles in organs harvested on day 7 postinfection were measured by plaque assay (n = 6–8 animals per genotype). A–C, Plaque assay results are presented as log10(PFU/g wet tissue or mL blood) and represent means ± standard deviation *P < .05, **P < .01, ***P < .001 and n.s, nonsignificant, Mann–Whitney test.
By day 7 postinfection there were even more striking differences between wt and ΔF508 mice in terms of virus replication (Figure 2C). Thus, CVB3 continued to replicate at a high rate in ΔF508 at a time point when virus titers declined in wt mice.
A Delayed Induction of IFN-α Production by ΔF508 Mice in Response to Poly(I:C)
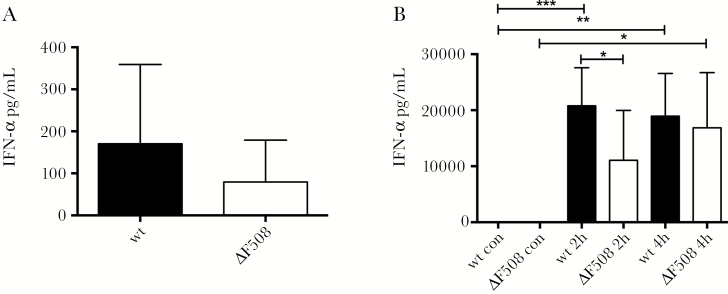
The importance of type I IFNs for survival after CVB infection [18] led us to assess IFN-α levels in the CVB3-infected mice. Based on previous studies [21], serum IFN-α levels were measured at 48 hours post CVB3 infection and we discovered a trend towards a lower production of IFN-α in ΔF508 mice compared to wt mice (Figure 3A). To make sure each animal was exposed to the same amount of viral stimuli, we employed an alternative method to determine the IFN-α response to infection. Polyinosinic:polycytidylic acid (poly(I:C)) is a commonly used mimic of viral replication intermediates that induces IFN production. It is recognized by the same pattern recognition receptors (PRRs) as enteroviruses (TLR3 and MDA5 [15, 21]) and is known to elicit an IFN response rapidly after administration to mice [31, 32].
Figure 3.
ΔF508 mice show a delayed interferon-alpha (IFN-α) response after stimulation with poly(I:C). A, Wild-type (wt; n = 6) and ΔF508 (n = 8) mice were infected with Coxsackievirus B3 (104 plaque-forming units [PFU]/mouse) and serum was drawn on day 2 postinfection. B, Wt (n = 8–14 per time point) and ΔF508 (n = 6–8 per time point) mice were stimulated with poly(I:C) (100 μg/mouse, intraperitoneal) and serum was drawn at 2 hours and 4 hours after stimulation. IFN-α levels in serum from wt mice (black bars) and ΔF508 mice (white bars) from infected mice (A) or mice stimulated with Poly(I:C) (B) were measured using enzyme-linked immunosorbent assay. Data is presented as means ± SD *P < .05, **P < .01, and ***P < .001, two-way analysis of variance with Holm–Sidak’s correction.
Before stimulating the animals with poly(I:C) we measured the basal mRNA expression levels of the PRRs TLR3 and MDA5 in different organs of uninfected wt and ΔF508 mice. No differences in their expression levels were observed (Supplementary Figure 2). We then injected mice with poly(I:C) and measured serum IFN-α levels. At 2 hours postinjection, ΔF508 mice produced significantly lower levels of IFN-α compared to wt mice. In contrast, at 4 hours postinjection this difference was no longer observed, suggesting that the F508del mutation caused a delay in the IFN-α response upon poly(I:C) stimulation (Figure 3B).
IFN-α is primarily produced by immune cells (mainly dendritic cells [DC]), while IFN-β and type III IFNs are produced by both immune and parenchymal cells (reviewed in [33]). Hence, we also measured IFN-β and IFN-λ mRNA expression levels in different organs from poly(I:C)-stimulated wt and ΔF508 mice. Both groups responded to the injection but there were no statistically significant differences in the IFN-β (Supplementary Figure 3) and IFN-λ (Supplementary Figure 4) mRNA expression levels between the wt and ΔF508 mice.
Together, these studies revealed that ΔF508 mice have a delayed IFN-α response to viral stimuli.
ΔF508 Mice Have no Major Impairment in the Expression of Antiviral Defense Genes
The basal expression levels of iNOS and OAS mRNA did not differ between the wt and ΔF508 mice (Supplementary Figures 5A and B), whereas the expression of both iNOS and OAS was increased in most organs in both wt and ΔF508 mice following poly(I:C) stimulation and only a few minor differences were observed between the wt and ΔF508 mice (Supplementary Figure 5A and B). These are unlikely to contribute dramatically to the lower survival of ΔF508 mice following virus challenge.
Impaired Production of Virus-neutralizing Antibodies in ΔF508 Mice
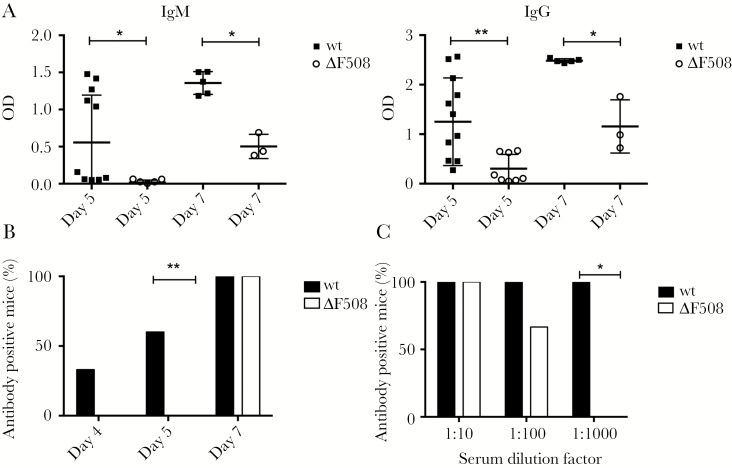
We next investigated if the delay in the innate response to virus was mimicked in an impaired adaptive immune response. Using enzyme-linked immunosorbent assay (ELISA) we measured serum levels of IgM and IgG virus-binding antibodies. At day 5 postinfection around half of the wt mice had detectible IgM and IgG levels, while the levels in ΔF508 mice remained close to the detection limit (Figure 4A). Two days later (day 7 postinfection), all mice had a detectible IgM and IgG responses to the virus. However, the antibody titers in serum from the ΔF508 mice were significantly lower than in wt mice (Figure 4A).
Figure 4.
Mice carrying the ΔF508 mutation have a delayed antibody response to Coxsackievirus B3 (CVB3). A, Wt and ΔF508 mice were infected with 102 PFU CVB3/mouse. Virus-specific IgM and IgG antibodies were measured in serum samples harvested from infected animals on day 5 (n = 8–12 per genotype) and 7 (n = 3–5 per genotype) postinfection using a CVB3 virus like particles-specific enzyme-linked immunosorbent assay. B, C, Serum drawn on days 4 (n = 4–6 animals per genotype), 5 (n = 7–15 animals per genotype), and 7 (n = 3–8 animals per genotype) postinfection were evaluated for the presence of neutralizing antibodies, as described in Supplementary Material Methods. B, The percentage of antibody-positive wt (black bars) and ΔF508 (white bars) mice at the indicated days postinfection is shown. C, The percentage of antibody-positive wt mice (black bars, n = 8) and ΔF508 mice (white bars, n = 3) at indicated serum dilutions on day 7 postinfection. Data in A is presented as mean ± SD *P < .05, **P < .01. Mann–Whitney test (A) and Fisher exact test (B, C). Abbreviation: OD, optical density.
As not all of the antibodies that bind to a virus are capable of preventing infection, we next assessed whether the antibodies produced by the infected mice were able to neutralize CVB replication. In wt mice, serum neutralizing antibodies had already appeared by day 4 postinfection (Figure 4B). In stark contrast, none of the ΔF508 mice had detectible levels of neutralizing antibodies at this time point. On day 5 postinfection over 60% of the wt mice capable to neutralize virus, while no such neutralization was observed in serum harvested from ΔF508 mice. On day 7 postinfection both wt and ΔF508 mice were antibody positive (Figure 4B). However, the titers of neutralizing antibodies were significantly lower in ΔF508 mice than in wt mice (Figure 4C). Taken together, this demonstrated that ΔF508 mice are impaired in their ability to efficiently raise antibodies against CVB3.
Passive Immunization Protects ΔF508 Mice From Infection
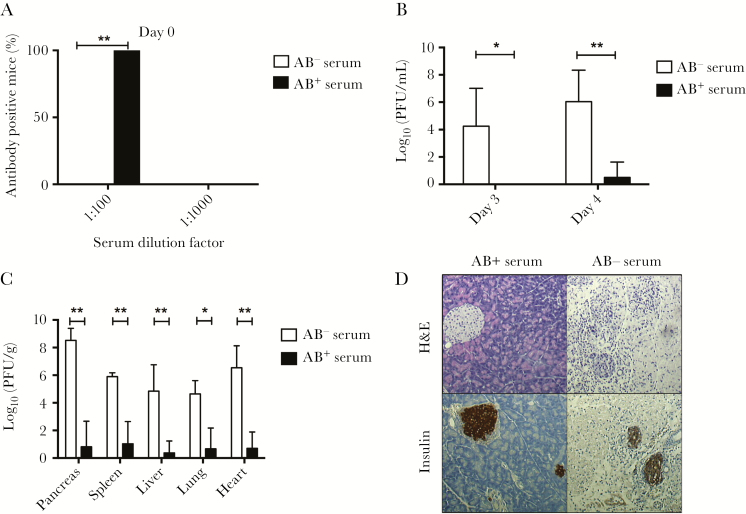
If the lack of a rapid and efficient antibody response to CVB3 was the underlying cause of the increased viral replication and mortality seen in ΔF508 mice challenged with CVB3, it would be expected that the transfer of antibodies from previously infected mice should provide protection against infection. To both test this hypothesis, and to make a preclinical assessment of the therapeutic potential of the passive transfer of antibodies for the protection of ΔF508 mice against viral infection, ΔF508 mice received either antibody positive or naive serum 1 day before challenge with CVB3 and were followed until day 7 postinfection The successful transfer of neutralizing antibodies to the ΔF508 mice receiving immune serum was confirmed by neutralization assay (Figure 5A). Mice that received antibody-positive serum had significantly lower levels of viremia on days 3 and 4 postinfection (Figure 5B). These animals also had lower levels of replicating virus particles in different organs (Figure 5C), as well as less exocrine pancreatic tissue damage (Figure 5D). These results demonstrated that the transfer of neutralizing antibodies prevents CVB3 infection in ΔF508 mice.
Figure 5.
Passive immunization protects ΔF508 mice from Coxsackievirus B3 (CVB3) infection and tissue damage. Antibody-positive (immune) and negative (nonimmune) sera was prepared from previously infected wt mice, as described in the Supplementary Material Methods section. Passive immunizations were performed 24 hours prior to CVB3 challenge by the administration of 400 µl (intraperitoneal) immune or nonimmune sera to the ΔF508 mice. A, The neutralizing capacity of sera drawn at 24 hours after passive immunization was measured at 2 separate dilutions, 1:100 and 1:1000, as described in the Supplementary Material Methods. The percentage of animals that had sera with virus-neutralizing capacity is shown as the percentage of all animals in each respective group. Black and white bars indicate animals receiving immune (AB+; n = 5) and nonimmune (AB−; n = 5) sera, respectively. B, C, Passively immunized ΔF508 mice were infected with CVB3 (102 plaque-forming units [PFU]/mouse). Blood was drawn on days 3 and 4 postinfection for viremia measurements (B). C, On day 7 postinfection, the mice were sacrificed and the indicated organs were retrieved for histological analysis and measurements of replicating virus particles. Note that in the group receiving nonimmune serum, one mouse had to be sacrificed on day 6 postinfection due to CVB3-induced illness. The results are presented as log10(PFU/g wet tissue or mL blood) and represents means ± SD. D, Representative images of pancreas sections from CVB3-infected ΔF508 mice receiving antibody-negative (AB−; n = 5) or antibody-positive (AB+; n = 5) sera, respectively. Upper panels, H&E staining. Lower panels, immunohistochemistry using primary antibody detecting insulin positive cells within the pancreas parenchyma. Original magnification × 10. *P < .05, **P < .01. Fisher exact test (A) and Mann–Whitney test (B, C).
ΔF508 Mice Display no Differences in Major Lymphocyte Populations and Have Normal Levels of IgM and IgG Antibodies at Steady State
The impaired antibody response to CVB3 infection suggested a deficiency in the immune system of ΔF508 mice. To test this hypothesis, we analyzed major lymphocyte populations (B and T cells, invariant natural killer T cells, and natural killer cells) in spleens from ΔF508 and wt littermate controls. We did not detect any statistically significant differences in the proportions of any of the immune cell subsets studied (Supplementary Figure 6A–I).
Using ELISA, we measured total serum concentrations of IgM and IgG in naive ΔF508 and littermate control mice (Supplementary Figure 6J). This analysis failed to reveal any differences between ΔF508 and wt mice. Collectively, these analyses suggested that the ΔF508 polymorphism confers no generalized alterations in splenic lymphocyte development or changes in systemic immunoglobulin concentrations.
ΔF508 Mice Display a Delayed Response to a T-Cell–Dependent Antigen
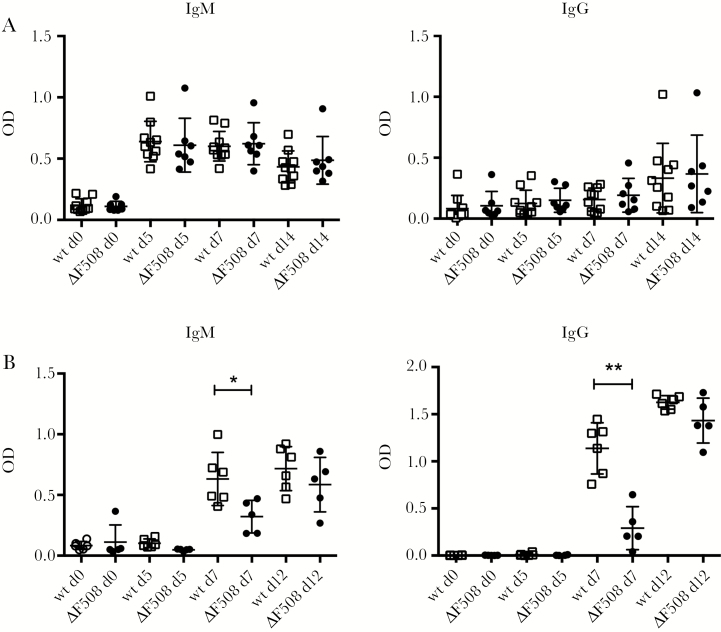
We next performed a more detailed study examining the antibody responses in the ΔF508 mice. B cells can raise antibodies to antigens either in a T-cell–independent (TI) or T-cell–dependent (TD) manner [34]. By injecting mice with 2,4,6-trinitrophenyl (TNP)-Ficoll and measuring nitrophenyl (NP)-specific antibodies in serum, we investigated whether ΔF508 mice have a defective TI antibody response [35]. This analysis revealed no differences in the production of NP-specific IgM or IgG antibodies between ΔF508 and wt mice at any of the time points examined (Figure 6A). This is a strong indication that the ΔF508 mice have intact antibody responses to TI antigen.
Figure 6.
ΔF508 mice have an intact T-cell–independent antibody response but show a delayed response to a T-cell–dependent antigen. A, Wild-type (wt; n = 10; black circles) and ΔF508 (n = 7; open squares) mice were injected with the T-cell–independent antigen 2,4,6-trinitrophenyl (TNP)-Ficoll (10 µg/mouse, intraperitoneal [IP]). B, Wt (n = 6; black circles) and ΔF508 (n = 5; open squares) mice were challenged with the T-cell–dependent antigen recombinant Semliki Forest virus expressing beta-galactosidase (rSFV-βGal; 1 × 105 Infectious units [IU]/mouse, intravenous [IV]). Serum was drawn at the indicated time points (days 0, 5, 7, and 12 postinjection) and levels of nitrophenyl (NP)-specific antibodies in serum (A) and βGal (B) specific IgM and IgG were measured using enzyme-linked immunosorbent assay. Open squares and black circles represents wt and ΔF508 mice, respectively. Data are presented as mean ± standard deviation *P < .05, **P < .01. Mann−Whitney test. Abbreviation: OD, optical density.
To address whether the ΔF508 mice have a normal antibody response to a TD antigen, we made use of recombinant Semliki Forest virus expressing beta-galactosidase (SFV-βGal) [36], which has previously been shown to elicit a strong antibody response in immunized mice [37]. Mice were injected with rSFV-βGal and the levels of βGal-specific antibodies were measured in serum samples drawn on days 0, 5, 7, and 12 postinjection. ΔF508 mice had significantly lower levels of βGal-specific IgM and IgG antibodies on day 7 postimmunization (Figure 6B). However, by day 12, this difference was no longer observed and the antibody levels in F508 mice were similar to those in wt mice (Figure 6B). This showed that the ΔF508 mice have the same ability to raise antibodies as wt mice, but that the time between antigen exposure and fully activated antibody production is longer in the ΔF508 mice, in a similar manner to that seen with CVB3 infection.
DISCUSSION
Much research in CF has focused on the immune functions of the airways. However, several observations [38–40] indicate that the impact of a defective CFTR must be seen in a broader context, taking both the immune system and other organs into consideration. The present study used a well-characterized model for human infections with enteroviruses and demonstrates that mice homozygous for ΔF508, the most common CFTR mutation in man, show increased mortality following a Coxsackievirus infection. A lower survival was accompanied by a reduced ability to attenuate virus replication in several organs, a delayed IFN-α response to viral stimuli, as well as the failure to efficiently raise neutralizing antibodies in response to infection.
A previous study showed that mice completely deficient in CFTR have a delayed clearance of RSV from the lungs, but did not provide a mechanistic explanation for this observation [41]. In the present study, experiments were conducted to identify the underlying cause of the greater mortality seen in ΔF508 mice challenged with CVB3. As judged by viremia measurements, no differences existed between the percentages of wt and ΔF508 mice that became infected with CVB3. This is in keeping with most, but not all, studies, suggesting that human CF is not associated with more frequent viral infections (eg, [4, 5, 42]). In contrast, significantly higher levels of infectious virus particles were measured in numerous organs from ΔF508 mice, suggesting that the ΔF508 mice are impaired in their ability to hinder viral replication and clear the virus. This novel observation deserved further investigation.
While we discovered a clear difference in the levels of IFN-α in the serum between wt and ΔF508 mice stimulated with poly(I:C), no or only small differences were observed in the mRNA expression of PRRs and ISGs. Thus, the decreased antiviral immunity in ΔF508 mice appeared to result from alterations in immune cells rather than defects within nonlymphoid organs.
Variation in the levels of replicating virus particles in organs from wt and ΔF508 mice was apparent on day 5 postinfection, a time point after which the virus titers typically begin to decline in wt mice [16, 27]. Antibodies are particularly crucial in the clearance of enteroviruses in both mice and humans [26–28], and we noted that production of virus-binding antibodies and antibodies with virus-neutralizing capacity was delayed in the ΔF508 mice. These observations, coupled with the impaired clearance of replicating virus particles from organs and the protective effect seen with the transfer of antibody-positive sera, clearly suggested that a defective antibody response contributed to the uncontrolled virus replication and heightened mortality seen in ΔF508 mice. Indeed, it is very likely that the uncontrolled virus replication led to multiorgan failure and thereby the fatal outcome.
In analogy with human CF (eg, [43]), our analysis of relevant immune cell subsets in wt and ΔF508 mice did not reveal any significant differences between the groups. Similarly, the levels of total IgM and IgG did not differ between the animals. Collectively, this suggest that the delayed antibody response in ΔF508 mice may be explained by a functional defect within the immune cell compartment or a loss or altered frequency of specific immune cell subtypes. The ΔF508 mice had a normal response to a TI antigen, suggesting that B-cell activation, antibody secretion, and isotype class switching are normal in these mice. Similar conclusions have been drawn in studies examining B cells in human CF (eg, [43]). Indeed, although individuals develop hypergammaglobulinemia in parallel with progression to more severe disease, most individuals with CF are hypogammaglobulinemic or have normal immunoglobulin levels early in life [43, 44].
Here, we discovered that the ΔF508 mice had a delayed response to a TD antigen. This observation shifts the focus to other cell types that are important in a TD antibody response, namely T cells and antigen presenting cells (eg, DC and macrophages). Altered helper and cytotoxic T-cell functions have been reported in human CF [43, 45]. Defective DC functions have also been suggested to occur in human CF and the ΔF508 mouse model, including altered cell numbers and altered antigen presenting capacity [11, 30]. The observations of the DCs are interesting considering the difference seen in our studies in the production of IFN-α between wt and ΔF508 mice. Indeed, plasmacytoid DCs (pDCs) are the major producers of IFN-α in response to poly(I:C) [46] and some virus infections [47]. Although the present study did not examine the cellular source of the IFN-α, it provides an indication that pDC numbers may be lower or that DC function is impaired. IFNs are important for promoting a T-cell response and the maturation of B cells into antibody-secreting plasma cells [23–25]. Thus, it is tempting to speculate that the delayed IFN-α secretion may have contributed to the slower production of neutralizing antibodies in the ΔF508 mice. Future studies should address whether there may be a defect in pDC or other DCs and T cells in the ΔF508 mouse which might explain the delayed antibody response.
Infections with enteroviruses such as rhinoviruses are common in humans and show a prolonged duration in individuals with CF [4, 5], thereby contributing to CF lung exacerbations [5, 13, 48–50]. Here, we showed that passive immunization prevented aggressive infection and enterovirus-induced tissue damage in the ΔF508 mice. This indicates that intravenous immunoglobulin (IVIG) treatment could be useful in humans for quenching excessive virus particles while awaiting the activation of the adaptive immune response.
Another important implication of our findings is that individuals carrying the ΔF508 mutation may not only have altered antibody responses to viruses. Indeed, kinetics of the antibody responses to bacteria and vaccines may also be affected and additional studies should be performed to assess the antibody response of the ΔF508 mice to these.
The life expectancy of patients with CF has increased dramatically over the last decades, mainly as a result of improved treatment and care. However, respiratory bacterial infections still remain a major risk factor for morbidity and mortality in CF. Respiratory viral infections increase the risk of chronic bacterial colonization of the CF airways [48–50]. To our knowledge, our study is the first to demonstrate that a homozygote defect in cftr impairs the host immune response to a type of virus known to cause respiratory tract infections in CF patients [5, 13]. Our observations showing inefficient viral clearance in the ΔF508 mice are in accord with the prolonged duration of respiratory virus infections observed in human CF [4, 5]. By providing a novel biological explanation of the delayed viral clearance, our study contributes to an increased understanding of both the role of CFTR in host antiviral defense and the impact of mutations in cftr on immune functions, which could open up new approaches for patient care and treatment.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgements. Dr V. Hytönen Tampere University, Finland, is gratefully acknowledged for sharing CVB3 VLPs. The authors also thank Dr G. Karlsson-Hedestam, Karolinska Institutet, and members of the Flodström-Tullberg group for fruitful discussions.
Previous presentation. Part of this work was presented at the 17th European Study Group on the Molecular Biology of Picornaviruses (EUROPIC), San Raphael, 2012, France; Keystone meeting on Innate Immunity: Sensing the Microbes and Damage Signals (Q7), Keystone, USA, 2012; and the 37th European CF Conference of the European Cystic Fibrosis Society, Gothenburg, 2014.
Financial support. This work was supported by Karolinska Institutet, the Swedish Foundation for Strategic Research, the Swedish Research Council, Riksförbundet Cystisk Fibros (Sweden), and Erica Lederhausens Minnesstiftelse (Sweden).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Elborn JS. Cystic fibrosis. Lancet 2016; 388:2519–31. [DOI] [PubMed] [Google Scholar]
- 2. Billard L, Le Berre R, Pilorge L, Payan C, Hery-Arnaud G, Vallet S. Viruses in cystic fibrosis patients’ airways. Crit Rev Microbiol 2017; 43:1–19. [DOI] [PubMed] [Google Scholar]
- 3. Flight W, Jones A. The diagnosis and management of respiratory viral infections in cystic fibrosis. Expert Rev Respir Med 2017; 11:221–7. [DOI] [PubMed] [Google Scholar]
- 4. Dijkema JS, van Ewijk BE, Wilbrink B, Wolfs TF, Kimpen JL, van der Ent CK. Frequency and duration of rhinovirus infections in children with cystic fibrosis and healthy controls: a longitudinal cohort study. Pediatr Infect Dis J 2016; 35:379–83. [DOI] [PubMed] [Google Scholar]
- 5. van Ewijk BE, van der Zalm MM, Wolfs TF et al. . Prevalence and impact of respiratory viral infections in young children with cystic fibrosis: prospective cohort study. Pediatrics 2008; 122:1171–6. [DOI] [PubMed] [Google Scholar]
- 6. Zheng S, De BP, Choudhary S et al. . Impaired innate host defense causes susceptibility to respiratory virus infections in cystic fibrosis. Immunity 2003; 18:619–30. [DOI] [PubMed] [Google Scholar]
- 7. Bubien JK, Kirk KL, Rado TA, Frizzell RA. Cell cycle dependence of chloride permeability in normal and cystic fibrosis lymphocytes. Science 1990; 248:1416–9. [DOI] [PubMed] [Google Scholar]
- 8. Johansson J, Vezzalini M, Verzè G et al. . Detection of CFTR protein in human leukocytes by flow cytometry. Cytometry A 2014; 85:611–20. [DOI] [PubMed] [Google Scholar]
- 9. McDonald TV, Nghiem PT, Gardner P, Martens CL. Human lymphocytes transcribe the cystic fibrosis transmembrane conductance regulator gene and exhibit CF-defective cAMP-regulated chloride current. J Biol Chem 1992; 267:3242–8. [PubMed] [Google Scholar]
- 10. Sorio C, Buffelli M, Angiari C et al. . Defective CFTR expression and function are detectable in blood monocytes: development of a new blood test for cystic fibrosis. PLoS One 2011; 6:e22212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xu Y, Krause A, Limberis M, Worgall TS, Worgall S. Low sphingosine-1-phosphate impairs lung dendritic cells in cystic fibrosis. Am J Respir Cell Mol Biol 2013; 48:250–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mueller C, Braag SA, Keeler A, Hodges C, Drumm M, Flotte TR. Lack of cystic fibrosis transmembrane conductance regulator in CD3+ lymphocytes leads to aberrant cytokine secretion and hyperinflammatory adaptive immune responses. Am J Respir Cell Mol Biol 2011; 44:922–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Esther CR Jr, Lin FC, Kerr A, Miller MB, Gilligan PH. Respiratory viruses are associated with common respiratory pathogens in cystic fibrosis. Pediatr Pulmonol 2014; 49:926–31. [DOI] [PubMed] [Google Scholar]
- 14. Palansch M, Oberste MS, Whitton JL. Enteroviruses: polioviruses, coxsackieviruses, echoviruses, and newer enteroviruses. In: Knipe DM and Howley PM, eds. Fields Virology, 6th ed. Vol. 1 Lippincott Williams & Wilkins, 2013:490–530. [Google Scholar]
- 15. Mena I, Fischer C, Gebhard JR, Perry CM, Harkins S, Whitton JL. Coxsackievirus infection of the pancreas: evaluation of receptor expression, pathogenesis, and immunopathology. Virology 2000; 271:276–88. [DOI] [PubMed] [Google Scholar]
- 16. Flodström M, Horwitz MS, Maday A, Balakrishna D, Rodriguez E, Sarvetnick N. A critical role for inducible nitric oxide synthase in host survival following coxsackievirus B4 infection. Virology 2001; 281:205–15. [DOI] [PubMed] [Google Scholar]
- 17. Flodström-Tullberg M, Hultcrantz M, Stotland A et al. . RNase L and double-stranded RNA-dependent protein kinase exert complementary roles in islet cell defense during coxsackievirus infection. J Immunol 2005; 174:1171–7. [DOI] [PubMed] [Google Scholar]
- 18. Wessely R, Klingel K, Knowlton KU, Kandolf R. Cardioselective infection with coxsackievirus B3 requires intact type I interferon signaling: implications for mortality and early viral replication. Circulation 2001; 103:756–61. [DOI] [PubMed] [Google Scholar]
- 19. Lind K, Richardson SJ, Leete P, Morgan NG, Korsgren O, Flodström-Tullberg M. Induction of an antiviral state and attenuated coxsackievirus replication in type III interferon-treated primary human pancreatic islets. J Virol 2013; 87:7646–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lind K, Svedin E, Utorova R, Stone VM, Flodström-Tullberg M. Type III interferons are expressed by Coxsackievirus-infected human primary hepatocytes and regulate hepatocyte permissiveness to infection. Clin Exp Immunol 2014; 177:687–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hühn MH, McCartney SA, Lind K, Svedin E, Colonna M, Flodström-Tullberg M. Melanoma differentiation-associated protein-5 (MDA-5) limits early viral replication but is not essential for the induction of type 1 interferons after Coxsackievirus infection. Virology 2010; 401:42–8. [DOI] [PubMed] [Google Scholar]
- 22. Richer MJ, Lavallée DJ, Shanina I, Horwitz MS. Toll-like receptor 3 signaling on macrophages is required for survival following coxsackievirus B4 infection. PLoS One 2009; 4:e4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med 2005; 202:637–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Le Bon A, Tough DF. Links between innate and adaptive immunity via type I interferon. Curr Opin Immunol 2002; 14:432–6. [DOI] [PubMed] [Google Scholar]
- 25. Marrack P, Kappler J, Mitchell T. Type I interferons keep activated T cells alive. J Exp Med 1999; 189:521–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McKinney RE Jr, Katz SL, Wilfert CM. Chronic enteroviral meningoencephalitis in agammaglobulinemic patients. Rev Infect Dis 1987; 9:334–56. [DOI] [PubMed] [Google Scholar]
- 27. Mena I, Perry CM, Harkins S, Rodriguez F, Gebhard J, Whitton JL. The role of B lymphocytes in coxsackievirus B3 infection. Am J Pathol 1999; 155:1205–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Misbah SA, Spickett GP, Ryba PC et al. . Chronic enteroviral meningoencephalitis in agammaglobulinemia: case report and literature review. J Clin Immunol 1992; 12:266–70. [DOI] [PubMed] [Google Scholar]
- 29. van Doorninck JH, French PJ, Verbeek E et al. . A mouse model for the cystic fibrosis delta F508 mutation. EMBO J 1995; 14:4403–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Veltman M, Stolarczyk M, Radzioch D et al. . Correction of lung inflammation in a F508del CFTR murine cystic fibrosis model by the sphingosine-1-phosphate lyase inhibitor LX2931. Am J Physiol Lung Cell Mol Physiol 2016; 311:L1000–14. [DOI] [PubMed] [Google Scholar]
- 31. Gitlin L, Barchet W, Gilfillan S et al. . Essential role of mda-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc Natl Acad Sci U S A 2006; 103:8459–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kato H, Takeuchi O, Sato S et al. . Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 2006; 441:101–5. [DOI] [PubMed] [Google Scholar]
- 33. Lazear HM, Nice TJ, Diamond MS. Interferon-λ: immune functions at barrier surfaces and beyond. Immunity 2015; 43:15–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mond JJ, Vos Q, Lees A, Snapper CM. T cell independent antigens. Curr Opin Immunol 1995; 7:349–54. [DOI] [PubMed] [Google Scholar]
- 35. Mond JJ, Lees A, Snapper CM. T cell-independent antigens type 2. Annu Rev Immunol 1995; 13:655–92. [DOI] [PubMed] [Google Scholar]
- 36. Smerdou C, Liljeström P. Two-helper RNA system for production of recombinant Semliki forest virus particles. J Virol 1999; 73:1092–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hidmark AS, Nordström EK, Dosenovic P, Forsell MN, Liljeström P, Karlsson Hedestam GB. Humoral responses against coimmunized protein antigen but not against alphavirus-encoded antigens require alpha/beta interferon signaling. J Virol 2006; 80:7100–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bonvillain RW, Valentine VG, Lombard G, LaPlace S, Dhillon G, Wang G. Post-operative infections in cystic fibrosis and non-cystic fibrosis patients after lung transplantation. J Heart Lung Transplant 2007; 26:890–7. [DOI] [PubMed] [Google Scholar]
- 39. Dimagno MJ, Lee SH, Hao Y, Zhou SY, McKenna BJ, Owyang C. A proinflammatory, antiapoptotic phenotype underlies the susceptibility to acute pancreatitis in cystic fibrosis transmembrane regulator (-/-) mice. Gastroenterology 2005; 129:665–81. [DOI] [PubMed] [Google Scholar]
- 40. Smyth RL, Croft NM, O’Hea U, Marshall TG, Ferguson A. Intestinal inflammation in cystic fibrosis. Arch Dis Child 2000; 82:394–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Colasurdo GN, Fullmer JJ, Elidemir O, Atkins C, Khan AM, Stark JM. Respiratory syncytial virus infection in a murine model of cystic fibrosis. J Med Virol 2006; 78:651–8. [DOI] [PubMed] [Google Scholar]
- 42. Ramsey BW, Gore EJ, Smith AL, Cooney MK, Redding GJ, Foy H. The effect of respiratory viral infections on patients with cystic fibrosis. Am J Dis Child 1989; 143:662–8. [DOI] [PubMed] [Google Scholar]
- 43. Lahat N, Rivlin J, Iancu TC. Functional immunoregulatory T-cell abnormalities in cystic fibrosis patients. J Clin Immunol 1989; 9:287–95. [DOI] [PubMed] [Google Scholar]
- 44. Matthews WJ Jr, Williams M, Oliphint B, Geha R, Colten HR. Hypogammaglobulinemia in patients with cystic fibrosis. N Engl J Med 1980; 302:245–9. [DOI] [PubMed] [Google Scholar]
- 45. Knutsen AP, Slavin RG, Roodman ST, Mueller KR, Marino NL. Decreased T helper cell function in patients with cystic fibrosis. Int Arch Allergy Appl Immunol 1988; 85:208–12. [DOI] [PubMed] [Google Scholar]
- 46. Asselin-Paturel C, Boonstra A, Dalod M et al. . Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat Immunol 2001; 2:1144–50. [DOI] [PubMed] [Google Scholar]
- 47. Deal EM, Lahl K, Narváez CF, Butcher EC, Greenberg HB. Plasmacytoid dendritic cells promote rotavirus-induced human and murine B cell responses. J Clin Invest 2013; 123:2464–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. de Vrankrijker AMM, Wolfs TFW, Ciofu O et al. . Respiratory syncytial virus infection facilitates acute colonization of Pseudomonas aeruginosa in mice. J Med Virol 2009; 81:2096–103. [DOI] [PubMed] [Google Scholar]
- 49. Johansen HK, Høiby N. Seasonal onset of initial colonisation and chronic infection with Pseudomonas aeruginosa in patients with cystic fibrosis in Denmark. Thorax 1992; 47:109–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Petersen NT, Høiby N, Mordhorst CH, Lind K, Flensborg EW, Bruun B. Respiratory infections in cystic fibrosis patients caused by virus, chlamydia and mycoplasma–possible synergism with Pseudomonas aeruginosa. Acta Paediatr Scand 1981; 70:623–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.