Abstract
Cryptosporidium viatorum is a globally distributed pathogenic species of Cryptosporidium that has only ever been recorded from humans, until now. For the first time, we molecularly characterised a novel subtype of C. viatorum (subtype XVbA2G1) from the endemic Australian swamp rat (Rattus lutreolus) using the small subunit of nuclear ribosomal RNA (SSU) gene and then subtyped it using the 60-kilodalton glycoprotein (gp60) gene. In total, faecal samples from 21 swamp rats (three were positive for C. viatorum), three broad toothed rats (Mastacomys fuscus) and two bush rats (Rattus fuscipes) were tested for Cryptosporidium. The long-term, isolated nature of the swamp rat population in Melbourne's drinking water catchment system (where public access is prohibited), the lack of C. viatorum from other mammals and birds living within the vicinity of this system and its genetic distinctiveness in both the SSU and gp60 gene sequences from other species of Cryptosporidium collectively suggest that C. viatorum might be endemic to native rats in Australia. The current state of knowledge of epidemiological surveys of Cryptosporidium of rats and the zoonotic potential are further discussed in light of the finding of C. viatorum. Long-term studies, with the capacity to repetitively sample a variety of hosts in multiple localities, in different seasons and years, will allow for greater insight into the epidemiological patterns and zoonotic potential of rare Cryptosporidium species such as C. viatorum.
Keywords: Cryptosporidium viatorum, Rattus lutreolus, Molecular identification, 60-Kilodalton glycoprotein (gp60), Small subunit of nuclear ribosomal RNA (SSU), Zoonotic potential
Graphical abstract
Highlights
-
•
First detection of Cryptosporidium viatorum (XVbA2G1) in a non-human host.
-
•
Found in native Australian swamp rat Rattus leutreolus.
-
•
Appraisal of current state of knowledge of Cryptosporidium in rats.
-
•
Cognisance of epidemiology and zoonotic potential through long-term monitoring.
1. Introduction
Cryptosporidium is a genus of protozoan parasites that are recognised as a leading cause of diarrhoea and malnutrition, particularly in developing regions around the world (Sow et al., 2016, Kotloff, 2017, Squire and Ryan, 2017). At least 34 species and more than 40 genotypes are recognised to infect humans and other animals (Zahedi et al., 2016), 21 of which are reported to have zoonotic potential (Xiao and Fayer, 2008, Ryan et al., 2014, Zahedi et al., 2016, Xiao and Feng, 2017). Cryptosporidium viatorum was first described in 2012 from travellers returning to the United Kingdom from the Indian subcontinent (Elwin et al., 2012). Thus far, C. viatorum has been found in people endemic to or returning from the following countries: Bangladesh, Barbados, Colombia, Ethiopia, Guatemala, India, Kenya, Nepal, Nigeria and Pakistan (Elwin et al., 2012, Insulander et al., 2013, Lebbad et al., 2013, Adamu et al., 2014, Ayinmode et al., 2014, Stensvold et al., 2015, de Lucio et al., 2016, Sanchez et al., 2017, Ukwah et al., 2017).
Clinical symptoms associated with cryptosporidiosis linked to C. viatorum from Swedish and British-based travellers to Bangladesh, Guatemala, India, Kenya, Nepal and Pakistan have included diarrhoea, abdominal pain, nausea, fever, headache, vomiting and marked weight loss, with illness lasting from 9 to 30 days (Elwin et al., 2012, Lebbad et al., 2013). Other studies reporting C. viatorum infection were in HIV-positive patients or children; the symptoms could either not be distinguished or were not recorded (Adamu et al., 2014, Ayinmode et al., 2014, de Lucio et al., 2016, Sanchez et al., 2017, Ukwah et al., 2017).
Because C. viatorum is currently the only species of Cryptosporidium found exclusively in humans, there has been speculation as to whether C. viatorum occurs in a domestic or sylvatic animal reservoir host (Elwin et al., 2012, Lebbad et al., 2013, Stensvold et al., 2015, Sanchez et al., 2017). However, to date, there has been no report of C. viatorum in an animal species other than human.
The Melbourne Water project (Nolan et al., 2013, Koehler et al., 2016b) is an ongoing survey of eukaryotic microbes including Cryptosporodium and Giardia (since June of 2009) in faecal deposits from feral and endemic wildlife within the closed water catchments supplying the city of Melbourne, Australia, with drinking water. Of the faecal samples collected and tested to date, the majority has been from eastern grey kangaroos, European rabbits, Sambar deer, swamp wallabies and common wombats. Interest in pathogens of lesser studied species of native wildlife led to the collection of faeces from three of Australia's native rat species, the Australian swamp rat, Rattus lutreolus, the bush rat, Rattus fuscipes and the broad-toothed rat, Mastacomys fuscus.
Here, we molecularly characterise a novel subtype of Cryptosporidium from a native rat and compare it to existing subtypes of C. viatorum using markers from the small subunit of nuclear ribosomal RNA (SSU) gene and the 60 kilodalton glycoprotein (gp60) gene which allowed for the subtyping of C. viatorum (Stensvold et al., 2015). We also appraise the survey history of Cryptosporidium from all known species of rats.
2. Materials and methods
2.1. Sample collection and DNA extraction
Since June of 2009, the flood plain where the Yarra River feeds the Upper Yarra Reservoir (∼100 km east of Melbourne [latitude: −37.673563; longitude: 145.89612]) was surveyed 17 times as part of our ongoing Melbourne Water project which monitors wildlife faecal samples from multiple catchments on a monthly basis for potential waterborne pathogens (Nolan et al., 2013, Koehler et al., 2016b). A total of 1394 faecal samples from foxes, cats, deer, kangaroos, rabbits, rats, wombats and waterbirds (mostly Australian wood ducks) have been collected from within the ‘closed’ catchment called Upper Yarra. A closed catchment refers to the land surrounding a reservoir where public access is prohibited and human activity is restricted to employees of the water management corporation. The Upper Yarra catchment (33,670 hectares) was established in 1888, in accordance with the Closed Catchment Policy that set aside the land for drinking water collection (Parks Victoria, 2002). Cryptosporidium species and genotypes that have been discovered in the Upper Yarra catchment include: C. cuniculus (from rabbits), C. hominis, C. parvum, C. ryanae, C. ubiquitum (from deer) and Cryptosporidium sp. duck-like genotype (from waterbirds) (Nolan et al., 2013, Koehler et al., 2016b).
In recent years, with the growth of grasses and reeds along the banks of the reservoir, we have noticed well-defined rodent runways (Koehler and Haydon, personal observations). Rat faeces were collected on three occasions: 3 September, 2015 (n = 12 samples) (Spring), 27 July, 2016 (n = 1) (Winter) and 26 April, 2017 (n = 13) (Autumn). Faecal samples were identified in the field as belonging to rodents based on the identification of rodent runways and faecal morphology (Triggs, 2004). Samples were taken in a ‘haphazard’ collection manner (Manly and Navarro Alberto, 2015), while trying to avoid collecting faeces from the same runway to minimise duplication of collection from the same host. DNAs were extracted from faecal samples using the MoBio (Carlsbad, CA, USA) described previously (Koehler et al., 2016b).
2.2. Host identification
The molecular identification of the rodent hosts using faecal DNA was achieved by PCR-based amplification of a 421 bp region employing universal vertebrate cytochrome b (cytb) primers mcb398 5′-TAC CAT GAG GAC AAA TAT CAT TCT G-3′ and mcb869 5′-CCT CCT AGT TTG TTA GGG ATT GAT CG-3’ (Verma and Singh, 2003) and analysis of amplicons.
PCR was carried out in a reaction volume of 50 μl using a standard reaction buffer, 3.0 mM of MgCl2, 200 μM of each dNTP, 50 pmol of each primer, 1 U of GoTaq polymerase (Promega, USA) and 2 μl of genomic DNA (except for the no-template controls, where H2O was added). The PCR conditions were: 94 °C for 5 min (initial denaturation), followed by 35 cycles of 94 °C for 30 s (denaturation), 51 °C for 30 s (annealing) and 72 °C for 30 s (extension), with a final extension of 72 °C for 5 min. A restriction fragment length polymorphism (RFLP) analysis was conducted using the FastDigest TaqI enzyme (Thermo Fisher Scientific, USA) according to the manufacturer's protocol; digests were separated in a 1.5% agarose gel by electrophoresis for 30 min at 90 V. Amplicons representing each unique banding patterns were selected, individually treated with shrimp alkaline phosphatase and exonuclease I (Thermo Fisher Scientific), according to the manufacturer's instructions, and then subjected to bi-directional automated sequencing (BigDye® Terminator v.3.1 chemistry, Applied Biosystems, USA) using the same primers as for PCR amplification. The resultant sequences were compared with sequences in GenBank using the BLASTn algorithm, and levels of identity established. Sequences from this study were deposited in the GenBank database under the accession numbers MG021319 – MG021323.
Host identification was achieved by phylogenetic analyses of the cytb sequence data, conducted by the neighbor joining (NJ) distance method (Saitou and Nei, 1987) in the program MEGA v.7.0.20 (Kumar et al., 2016). Evolutionary distances were computed using the number of differences method (Nei and Kumar, 2000), including transitions and transversions for the nucleotide data. Rates of evolution among sites were considered uniform and gaps were treated using pairwise deletion. A total of 2000 bootstrap replicates were performed and are reported as bootstrap percentages (BP).
2.3. Literature survey of Cryptosporidium in rats
A comprehensive literature search was performed using Google Scholar (google.scholar.com) using search terms “rat”, “Rattus” and “Cryptosporidium” in order to find all known epidemiological surveys of rats for Cryptosporidium. The search results, including host, locality, prevalence and parasite species or genotype, are summarised in Supplementary Table 1.
2.4. Nested PCR assays for Cryptosporidium
Nested PCR assays were carried out for the small subunit of nuclear ribosomal RNA (SSU) (Alves et al., 2003) and 60-kilodalton glycoprotein (gp60) (Stensvold et al., 2015) genes using the same set-up as described in section 2.2, except that for the secondary PCR, 1 μl of template from the primary PCR was carried over to the secondary PCR. No-template (negative) controls were included at all steps, and no-template controls were carried over from the primary to the secondary (nested) PCR. A well-known positive control sample (C. parvum DNA) was included in each PCR run.
An established SSU-nested PCR was conducted as described previously (Alves et al., 2003). In brief, the primary PCR (∼760 bp) was carried out using primers 18SiCF2 (forward: 5′-GAC ATA TCA TTC AAG TTT CTG ACC-3′) and 18SiCR2 (reverse: 5′-CTG AAG GAG TAA GGA ACA ACC-3′), followed by secondary (nested) PCR (∼590 bp) using primers 18SiCF1 (forward: 5′-CCT ATC AGC TTT AGA CGG TAG G-3′) and 18SiCR1 (reverse: 5′-TCT AAG AAT TTC ACC TCT GAC TG-3′). Both the primary and secondary PCRs utilized the following cycling conditions: 94 °C for 5 min (initial denaturation), followed by 45 cycles of 94 °C for 30 s (denaturation), 58 °C for 30 s (annealing) and 72 °C for 30 s (extension), with a final extension of 72 °C for 10 min.
Once it was determined that the taxon of Cryptosporidium identified was related to C. viatorum, the nested PCR protocol for gp60 of C. viatorum was conducted essentially as described by Stensvold et al. (2015). In brief, primary PCR of a partial region (1192 bp) of the gp60 gene from 2 μl of genomic DNA (expect for no-template controls) was conducted using primers CviatF2 (forward 5′-TTC ATT CTG ACC CCT CAT AG-3′) and CviatR5 (reverse: 5′-GTC TCC TGA ATC TCT GCT TAC TC-3′); 1 μl of template from the primary PCR was carried over to the secondary PCR conducted using primers CviatF3 (forward: 5′-GAG ATT GTC ACT CAT CAT CGT AC-3′) and CviatR8 (reverse: 5’ –CTA CAC GTA AAA TAA TTC GCG AC-3′) to produce a product of ∼950 bp. Both primary and secondary PCR conditions were: 94 °C for 5 min (initial denaturation), followed by 35 cycles of 94 °C for 30 s (denaturation), 52 °C for 30 s (annealing) and 72 °C for 1 min (extension), with a final extension of 72 °C for 5 min. No-template (negative) controls were included at all steps, and no-template controls were carried over from the primary to the secondary (nested) PCR. A well-known positive control sample (C. parvum DNA) was included in each PCR run.
2.5. Sequencing of amplicons and phylogenetic analysis of sequence data for Cryptosporidium
Nested PCR amplicons were individually treated with shrimp alkaline phosphatase and exonuclease I (Thermo Scientific, USA), according to the manufacturer's instructions, and then subjected to bi-directional automated sequencing (BigDye® Terminator v.3.1 chemistry, Applied Biosystems, USA) using the same primers as employed in the secondary (nested) PCR. Sequence quality was verified by comparison with corresponding electropherograms using the program Geneious v.10.2.3 (Kearse et al., 2012). Sequences were aligned using the program MAFFT (Katoh et al., 2002) with the option ‘E-INS-i’, which is recommended for alignments that contain multiple conserved domains and long gaps, and alignments were manually adjusted using the program Mesquite v.3.10 (Maddison and Maddison, 2015). Sequences were then compared with those available in the GenBank database using BLASTn (NCBI, USA).
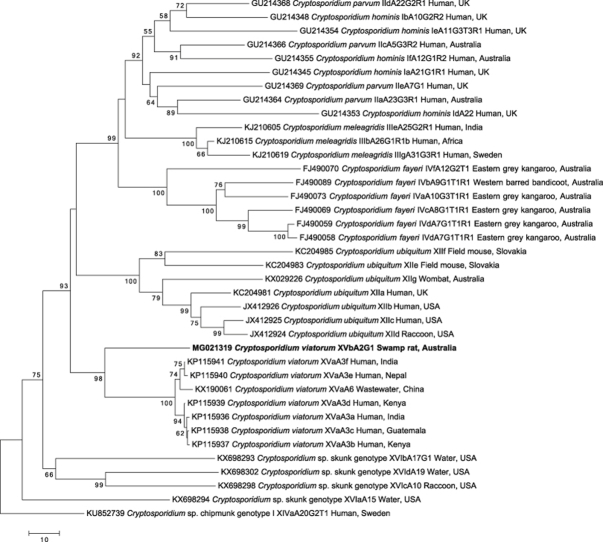
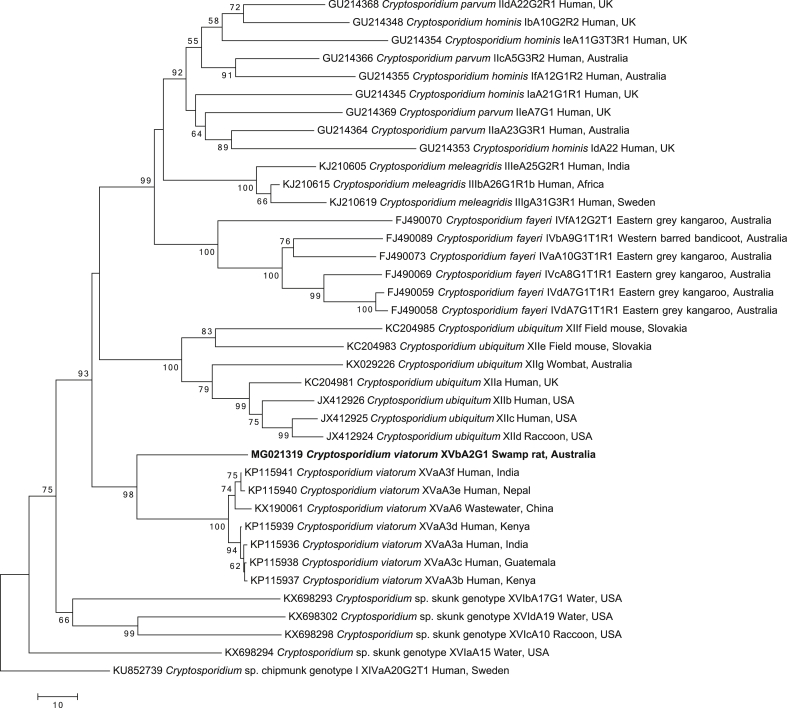
The SSU tree (Fig. 1; Supplementary Table 2) was constructed with the intent of precisely placing the novel genotype within the overall Cryptosporidium tree (cf. Ruecker et al., 2012, Stenger et al., 2015). To do this, we included as many representative sequences of unique Cryptosporidium species and genotypes as possible from the top portion of the Cryptosporidium phylogeny (cf. Ruecker et al., 2012, Stenger et al., 2015), while leaving out species and genotypes from the bottom half of the phylogeny, which would decrease the quality of the alignment. This ‘cleansed database’ approach to building Cryptosporidium phylogenetic trees based on the SSU gene region, where sequences from GenBank are critically evaluated to removed errors and redundancies, is well established (cf. Ruecker et al., 2012). Typically, sequences from environmental samples collected from water have not been included when assessing Cryptosporidium phylogeny, as they are not derived from a particular host species, but they were included here because the initial BLASTn results showed a close relatedness between Cryptosporidium from environmental samples and C. viatorum. Cryptosporidium baileyi (GenBank accession no. L19068) was used to root the tree.
Fig. 1.
Phylogenetic relationships of small subunit of nuclear ribosomal RNA (SSU) gene nucleotide sequence data (aligned over 563 bp) of selected Cryptosporidium taxa in relation to the novel C. viatorum genotype using the neighbor joining distance method. Individual GenBank accession numbers precede species name, followed by host common name and locality descriptors. Bootstrap support values (based on 2000 iterations) are indicated next to supported branches. Cryptosporidium baileyi was chosen as the outgroup. The novel genotype from this study is in bold-type. Scale bar indicates the number of nucleotide substitutions per site.
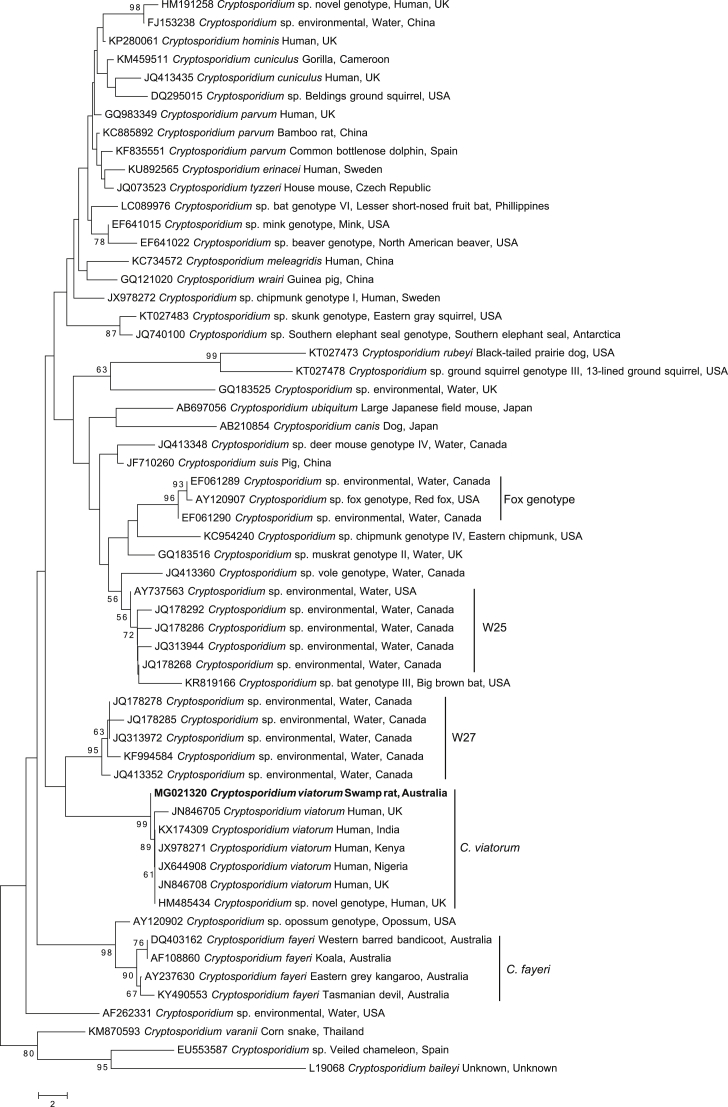
In order to obtain an accurate alignment of homologous characters, an alignment of inferred amino acid sequence data was used to construct the GP60 tree (Fig. 2; Supplementary Table 3). At present, primers for gp60 (the standard gene for subtyping Cryptosporidium; Ryan et al., 2014) have been developed for only a few species of Cryptosporidium [C. cuniculus, C. hominis and C. parvum (see Strong et al., 2000); C. fayeri (see Power et al., 2009); C. ubiquitum (see Li et al., 2014); C. meleagridis (see Stensvold et al., 2014); C. viatorum (see Stensvold et al., 2015); C. sp. skunk genotype (Yan et al., 2017)]. Amino acid sequences representing multiple members of each available gp60 subtype were included in the tree to provide comparative GP60 diversity within taxa (Fig. 2). Phylogenetic analyses of both nucleotide (for SSU) and amino acid (for gp60) sequence data were conducted by the NJ distance method, as detailed in section 2.2. Cryptosporidium sp. chipmunk genotype I (GenBank accession no. KU852739) was used to root the tree.
Fig. 2.
Phylogenetic relationships of amino acid sequences inferred from the 60 kilodalton glycoprotein (gp60) gene (286 amino acids) for selected Cryptosporidium taxa in relation to the novel C. viatorum subtype using the neighbor joining distance method. Individual GenBank accession numbers precede species name, followed by host common name and locality descriptors. Bootstrap support values (based on 2000 iterations) are indicated next to supported branches. Cryptosporidium sp. chipmunk genotype was chosen as the outgroup. The novel subtype from this study is in bold type. Scale bar indicates the number of amino acid changes per site.
2.6. Alignments and pairwise comparisons and prediction of protein sequences
An alignment of the GP60 amino acid sequence of the novel C. viatorum subtype with those of the six known human C. viatorum subtypes was made using MAFFT in Geneious (Fig. 3). A table of pairwise amino acid similarities amongst C. viatorum subtypes was also constructed using Geneious (Table 1). The online servers ProP 1.0 (www.cbs.dtu.dk/services/ProP/) (Duckert et al., 2004), NetNGlyc 1.0 (www.cbs.dtu.dk/services/NetNGlyc/) (Blom et al., 2004) and NetOGlyc 4.0 (www.cbs.dtu.dk/services/NetOGlyc/) (Steentoft et al., 2013) were used to predict furin cleavage sites, N-glycosylation sites and O-glycosylation sites, respectively. The sites predicted for the novel subtype were compared with those predicted by Stensvold et al. (2015).
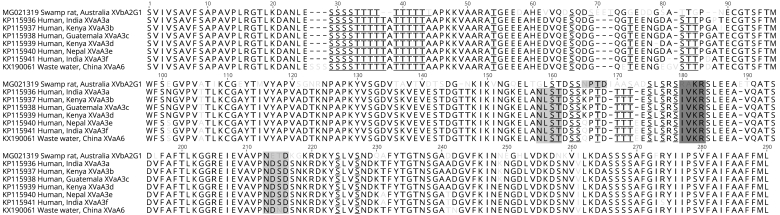
Fig. 3.
Multiple alignment of 286 amino acids inferred from the 60 kilodalton glycoprotein (gp60) gene for representatives of all known subtypes of Cryptosporidium viatorum. GenBank accession number precedes the host common name, followed by country and subtype. A dashes indicates a gap, and light grey text indicates a variable site. Inferred furin cleavage sites are enclosed by a dark grey box. N-glycosylation sites are enclosed by a light grey box. Predicted O-glycosylation sites are underlined.
Table 1.
Pairwise differences (percentage) among Cryptosporidium viatorum for amino acid sequences (below the diagonal) and nucleotides (above the diagonal).
| MG021319 Swamp rat Australia XVbA2G1 | KP115936 Human India XVaA3a | KP115937 Human Kenya XVaA3b | KP115938 Human Guatemala XVaA3c | KP115939 Human Kenya XVaA3d | KP115940 Human Nepal XVaA3e | KP115941 Human India XVaA3f | KX190061 Wastewater China XVaA6 | |
|---|---|---|---|---|---|---|---|---|
| MG021319 Swamp rat, Australia XVbA2G1 | – | 16.3 | 16.4 | 16.4 | 16.7 | 16.4 | 16.9 | 17.3 |
| KP115936 Human, India XVaA3a | 23.1 | – | 0.2 | 0.1 | 1.0 | 1.3 | 2.1 | 3.3 |
| KP115937 Human, Kenya XVaA3b | 23.1 | 0.7 | – | 0.1 | 1.0 | 1.3 | 2.1 | 3.3 |
| KP115938 Human, Guatemala XVaA3c | 23.1 | 0.4 | 0.4 | – | 0.8 | 1.2 | 2.0 | 3.2 |
| KP115939 Human, Kenya XVaA3d | 23.1 | 1.4 | 1.4 | 1.1 | – | 1.5 | 2.4 | 3.7 |
| KP115940 Human, Nepal XVaA3e | 22.0 | 3.2 | 3.2 | 2.8 | 2.5 | – | 0.8 | 2.5 |
| KP115941 Human, India XVaA3f | 22.7 | 4.3 | 4.3 | 3.9 | 3.6 | 1.1 | – | 2.9 |
| KX190061 Wastewater, China XVaA6 | 24.8 | 6.0 | 6.0 | 5.6 | 6.0 | 4.2 | 4.2 | – |
3. Results
3.1. Identification of rat hosts
The results of the PCR-based RFLP (four unique banding patterns) and sequencing of cytb amplicons showed that 21 of the 26 faecal samples tested were from R. lutreolus, 3 were from M. fuscus, 2 were from R. fuscipes. Sequences representing two of the RFLP banding patterns matched R. lutreolus GenBank accession no. GU570661 (448 of 467 bp; 96% identity) and sequences from the other banding patterns matched R. fuscipes GenBank accession no. GU570664 (419 of 447 bp; 96% identity) and M. fuscus GenBank accession no. KY754024 (457 of 467 bp; 98% identity). Although sequence matches using the cytb gene were not 100%, the phylogenetic tree indicated that each of the sequences from the rodent faecal samples grouped closely with R. lutreolus, R. fuscipes and M. fuscus (GenBank accession nos., respectively: MG021321 – MG021323) (Supplementary Fig. 1).
3.2. Literature survey results for Cryptosporidium of rats
Rats have been surveyed for Cryptosporidium (both microscopically and molecularly) in a total of 23 studies from countries including Australia, Brazil, China, Egypt, Japan, Indonesia, Iran, Kenya, Korea, New Zealand, Nigeria, Philippines, Spain and United Kingdom (Supplementary Table 1). There are 66 species of ‘true rats’ in the genus Rattus (Wilson and Reeder, 2005). Prior to the present study, only 6 species had been surveyed for Cryptosporidium. The commonest species of rat studied are the brown or Norway rat (Rattus norvegicus) (n = 1518), the Asian house rat (R. tanezumi) (n = 442) and the black rat (Rattus rattus) (n = 475) (Supplementary Table 1). The majority of the studies (n = 14) undertook molecular characterisation, but 9 relied only on microscopy such that Cryptosporidium species could not be assigned beyond the genotypic level. The most frequently observed Cryptosporidium species and genotypes seen in rats are C. muris (n = 82), C. parvum (n = 53) and Cryptosporidium sp. rat genotype III (n = 37) (Supplementary Table 1).
3.3. Molecular identification and classification as C. viatorum
Of the 26 rat faecal samples examined, three from R. lutreolus (laboratory nos. UY7513, UY7521 and UY7523) (but none from R. fuscipes or M. fuscus) were PCR test-positive for Cryptosporidium by SSU. The SSU sequences determined (represented by accession no. MG021320) were all identical across all 568 nts; a comparison of this novel sequence with the sequence representing C. viatorum (accession no. JN846708) from GenBank revealed 99% identity (four deletion/insertion events). Only one of the gp60 amplicons derived from rat faecal DNAs (UY7513) returned a clean gp60 sequence for Cryptosporidium of 911 bp in length. This sequence (accession no. MG021319) had 84% identity (767 of 911 bp) with C. viatorum (accession no. KP115936) present in GenBank at the time.
The SSU phylogenetic tree (Fig. 1) suggests that the Cryptosporidium taxon in the faecal sample from R. lutreolus is most closely related (537 of 541 bp or 99.3% identity) to the majority of C. viatorum sequences. The sequences of C. viatorum grouped into a highly-supported monophyletic clade (BP = 99%). Five of the previously defined C. viatorum sequences (accession nos. KX174309, JX978271, JX644908, JN846708 and HM485434) are identical, while one (JN846705) has a single nucleotide difference to the other five sequences. The clades that are closest to the C. viatorum clade in the tree are a well-supported clade (BP = 95%) containing multiple sequences representing Cryptosporidium sp. environmental water samples from Canada (Ruecker et al., 2012, Ruecker et al., 2013, Prystajecky et al., 2014) and the strongly-supported (BP = 98%) marsupial clade representing sequences of C. fayeri and Cryptosporidium sp. opossum genotype (Morgan et al., 1999, Xiao et al., 2002, Power et al., 2004, Wait et al., 2017).
The gp60 tree (Fig. 2) also reveals a strongly supported monophyletic C. viatorum clade (BP = 98%). Six members of the previously recognised subtypes XVaA3a to XVaA3f (Stensvold et al., 2015) as well as XVaA6 from wastewater (Huang et al., 2017) appear to cluster tightly in a well-supported clade (BP = 100%) with relatively short branch lengths. The difference in branch length between the novel C. viatorum sequence and the other C. viatorum sequences (accession nos. KP115936 to KP115941) leads to the designation of a new subtype XVb. All of the other Cryptosporidium species and genotypes group into well supported clades, with the exception of C. parvum and C. hominis which remain together within their own clade. Of note is the extent of the maximum level of intraspecific variation recorded to date within C. meleagridis (16.3%), C. fayeri (37.8%), C. ubiquitum (54.3%) and Cryptosporidium sp. skunk genotype (45.6%), compared to that within C. viatorum (26.3%).
3.4. Comparison of gp60 and sequence data
The nucleotide alignment of gp60 (not shown) indicates that the descriptive serine repeat region contains two TCA serine repeats followed by a third TCG serine repeat which, in accordance C. viatorum subtype naming guidelines (Stensvold et al., 2015), designates the name of the novel C. viatorum subtype as XVbA2G1. The pairwise nucleotide sequence difference of the novel subtype to the other C. viatorum subtypes ranged from 16.3% to 17.3% (Table 1). The intra-subtype variation for the known subtypes ranges from 0.1 to 3.7% difference.
Pairwise comparisons showed 8 amino acid deletions, 6 insertions and 53 non-synonymous substitutions when the C. viatorum subtype XVbA2G1 was compared with other C. viatorum subtypes (Fig. 3), with variation ranging from 22.0% to 24.8% across the consensus length of 286 amino acids. Variation among six other known subtypes ranged from 0.4% to 6.0% at the amino acid level.
The “diagnostic” serine repeat region (Fig. 3: amino acid postions 26–32) precedes a threonine repeat region, which includes 10 repeats of threonine with an alanine in the middle of the C. viatorum subtypes from humans but is lacking from subtype XVbA2G1 from the swamp rat (Fig. 3). The furin cleavage site (“RAKR”) in the sequence of subtype XVbA2G1 was at the same position as in C. viatorum subtypes from humans or from wastewater (“IVKR”), but there were two amino acid differences (Fig. 3). In addition, subtype XVbA2G1 differs at two of the recognised N-glycosylation sites from the other subtypes: the first N-glycosylation site is 7 amino acids further downstream, and the second site is at the same position but its sequence is “NETD” rather than “NDSD” or “NDND”. Of the 35 O-glycosylation sites predicted for subtype XVbA2G1 and all other known C. viatorum subtypes, 23 amino acid positions (66%) were conserved among subtypes.
4. Discussion
Since the initial description of C. viatorum from travellers returning to the United Kingdom from the Indian subcontinent, there has been a question as to whether animal reservoirs exist for C. viatorum (Elwin et al., 2012). This question has at least partially been resolved with the discovery of C. viatorum subtype XVbA2G1 from native Australian swamp rats living in an isolated and protected water catchment free from human intervention and in which other wildlife have been continuously surveyed for Cryptosporidium since 2009 (Nolan et al., 2013, Koehler et al., 2016b). Although this subtype is not identical to those found in humans (Stensvold et al., 2015), the molecular and phylogenetic evidence provided here indicates they it belong to the same clade as other subtypes and thus likely represents the same species of Cryptosporidium.
In the following, we (i) provide support for the molecular identification of C. viatorum from R. lutreolus and discuss its relationship to currently known C. viatorum subtypes from humans; (ii) give an historical account of rats in Australia and their role or potential role as hosts for Cryptosporidium; and (iii) discuss the epidemiological context for C. viatorum in Australia and its zoonotic potential.
4.1. Molecular identification of C. viatorum
The sequence differences between the novel subtype (XVbA2G1) and the human C. viatorum subtypes for the SSU gene is only four nucleotides (out of 541) or 0.7% difference (Fig. 1), which borders on the acceptable limits of intraspecific variation for Cryptosporidium (Ruecker et al., 2012, Ryan et al., 2014, Koehler et al., 2016a). Convincingly, subtype XVbA2G1clearly clusters together in a well-supported monophyletic clade with the other C. viatorum sequences from humans (Fig. 1). The inclusion of the majority of unique Cryptosporidium sequences, from the top half of the Cryptosporidium phylogeny (cf. Ruecker et al., 2012), provides further evidence that there is no sequence currently publicly available on GenBank to which the novel subtype matches closer than to the known C. viatorum subtypes from humans (Fig. 1). The closest clades to the C. viatorum clade are the Canadian environmental sequences (collectively referred to as W27 (Ruecker et al., 2012)), taken from water samples, and the marsupial clade, which includes the Australian C. fayeri and the North American opossum genotype (Fig. 1). Unfortunately, the host(s) from which the Canadian environmental water samples originated is unknown (Ruecker et al., 2012). The common locality of Australia (between the marsupial clade and the novel subtype) is interesting, but there is currently not enough information available to lend significant support towards any conjectures about the evolutionary relationship between C. viatorum and the other clades.
The gp60 gene provides clear evidence that XVbA2G1 is a subtype of C. viatorum. The gp60 tree (Fig. 2) suggests a strongly supported monophyletic clade for C. viatorum when compared to the other Cryptosporidium species for which there are gp60 sequences in GenBank (Strong et al., 2000, Power et al., 2009, Li et al., 2014, Stensvold et al., 2014, Stensvold et al., 2015, Huang et al., 2017, Yan et al., 2017). The intraspecific variability of all the other major clades of Cryptosporidium species included within the gp60 tree (Fig. 2) is comparable to that seen within the C. viatorum clade. Indeed, sequence variation among representatives within species (clades) are usually higher than for C. viatorum, except for C. meleagridis (Fig. 2).
The tight grouping of C. viatorum subtypes XVaA3a - XVaA3f could be suggestive of a recent spread of C. viatorum from a source population, which is now being disseminated via global human travel, as there does not appear to be a geographic partitioning according to country (Fig. 2). A more extensive study would be necessary to support this hypothesis, although a population study of gp60 from C. parvum in humans reported that the reduced level of variation within C. parvum could represent a recent adaptation to the human host or a selective sweep (Abal-Fabeiro et al., 2013).
As mentioned previously, the subtype XVbA2G1 is not identical to other C. viatorum subtypes from humans or wastewater, but it is considered similar enough to represent C. viatorum. The genetic difference at the gp60 locus between subtypes is significant enough to suggest geographic isolation between C. viatorum from Australia and C. viatorum from other locations as a possible explanation. Isolation of the Australian subtype coupled with a relatively recent global spread of the human subtypes could also account for these differences; however, many more samples would need to be tested before a definitive conclusion could be made regarding population structure for C. viatorum.
The alignment of the inferred GP60 amino acid sequence data of C. viatorum subtypes further supports the conjecture that subtype XVbA2G1 belongs to C. viatorum based on the conserved the furin cleavage site and the partial conservation of the N and O-glycosylation sites (Fig. 3) (cf. Stensvold et al., 2015). Additionally, the unique serine repeat region, which has been the cornerstone of Cryptosporidium genotyping (Strong et al., 2000, Sulaiman et al., 2005), is followed by a threonine repeat region (Fig. 3). Thus far, C. viatorum is the only species of Cryptosporidium that has a threonine repeat region rather than a truncated serine repeat (Stensvold et al., 2015). In the future, this region of threonine repeats might be useful to assess the classification of novel subtypes of C. viatorum, in addition to the considerably shorter serine repeat region.
4.2. Rats and their role as hosts of Cryptosporidium
The Australian swamp rat (R. lutreolus) and the bush rat (R. fuscipes), are two of seven ‘new endemic’ rats found in Australia (Robins et al., 2010). New endemics are thought to have arrived in the second wave of Australian rodent colonization approximately 1 million years ago Australia whereas ‘old endemic’ rodents (like the broad-toothed rat, M. fuscus) colonized Australia around 10 million years ago (Simpson, 1961, Rowe et al., 2008, Robins et al., 2010). Other rats commonly surveyed for Cryptosporidium have been the invasive Norway or brown rat (R. norvegicus) which originated in China and spread along with human global colonization the black, roof or ship rat (R. rattus) which originated either in Malaysia or India and then colonised the globe along trade routes (Kosoy et al., 2015) and the Asian black rat or house rat (R. tanezumi) which is closely related to and morphologically indistinguishable from R. rattus (Kosoy et al., 2015).
If C. viatorum is endemic to rats, as is suggested in the present study, the current survey effort for Cryptosporidium in rats could factor in to why C. viatorum has not been identified until now. If only the ‘true rats’ (or members of the genus Rattus) are considered, the diversity of rats sampled is very low with only 9% (6 of 66) of the species screened for Cryptosporidium (Wilson and Reeder, 2005; Supplementary Table 1). Of those rats, the invasive R. norvegicus, R. rattus and R. tanezumi account for 95% of those sampled for Cryptosporidium (Supplementary Table 1). Of the 2585 rats examined, 1221 were tested by microscopy only (leaving them identified as Cryptosporidium sp.), and 1364 have been tested by molecular methods. The majority (56%) of the sampling has been done in Japan (n = 516) the United Kingdom (n = 511) and China (n = 417) (Supplementary Table 1). There have been no recorded studies from North America and, of the countries that have been reported as sources of human subtypes of C. viatorum, only Nigeria has had rats surveyed (134 R. norvegicus) for Cryptosporidium (Ayinmode et al., 2014). Including C. viatorum, there have been 9 species and 5 genotypes of Cryptosporidium reported from rats (Supplementary Table 1) the most common being C. muris (n = 82), C. parvum (n = 53) and C. sp. rat genotype III (n = 37). Continued and increased global surveillance of both native and introduced rats for pathogens such as Cryptosporidium would help us gain a clearer understanding of the role these important reservoirs play in the phylogeography and epidemiology of Cryptosporidium.
4.3. Epidemiology and zoonotic potential
Within the ‘closed’ Upper Yarra water catchment, more than 1394 faecal samples have from red fox, feral cats, Sambar deer, Eastern grey kangaroos, European rabbits, rats, common wombats and waterbirds (mostly Australian wood ducks) been examined for Cryptosporidium (Nolan et al., 2013, Koehler et al., 2016b; Koehler unpublished data). In that 9-year time period, Cryptosporidium DNA has been detected in faecal samples from the following hosts: C. cuniculus (from rabbits); C. hominis, C. parvum, C. ryanae and C. ubiquitum (from deer); and Cryptosporidium sp. duck-like genotype (from waterbirds) (Nolan et al., 2013, Koehler et al., 2016b). Despite a strong survey effort over this period, including approximately 10 potential hosts, C. viatorum has only been detected from R. lutreolus in April 2017. Seasonality has been recorded as a contributing factor of Cryptosporidium abundance (summarised in Lapen et al., 2016) with the majority of studies citing Summer and Autumn as the peak seasons, but also particular countries, such as New Zealand, Scotland and Ireland, reported Spring as the peak season. Due to the small sample size collected from the rats in this study (n = 26), very little can be concluded about seasonality as a contributing factor to the presence or absence of C. viatorum. However, no C. viatorum was detected in the first round of sampling (n = 12) in the early Spring of 2015, but it was detected 19 months later in Autumn of 2017. More intensive sampling of the rodent populations should be conducted in the Upper Yarra and surrounding water catchments in order to establish the extent of C. viatorum in the region.
There is potential for C. viatorum to spread to other rats, such as the introduced R. rattus and R. norvegicus, which are found throughout the range of M. fucus, R. lutreolus and R. fuscipes (Seebeck and Menkhorst, 2000). Rats are notorious for spreading zoonotic viruses, bacteria and parasites to humans for much of recorded history (reviewed by Begon, 2003, Banks and Hughes, 2012, Kosoy et al., 2015, Morand et al., 2015). In general, rats' high reproductive potential, omnivorous feeding behaviour and ability to adapt and thrive in close contact with humans all contribute to the zoonotic risk (Banks and Hughes, 2012, Kosoy et al., 2015). If animals such as rats are spread beyond their naturally occurring habitat (i.e. biological invasion) the detrimental impacts on the local ecology could be tremendous on many levels (reviewed by Morand et al., 2015). On the one hand, parasites might be lost during the invasion causing an advantage to the host (enemy release hypothesis) (Torchin et al., 2003). On the other hand, parasites brought along may infect other hosts thereby reducing competition for the invasive host (novel weapon hypothesis) (Callaway and Ridenour, 2004) or the arrival of rats might provide opportunities for parasite spillover (spread of parasites into local reservoirs) (Daszak et al., 2000) or parasite “spillback” (amplification of local pathogens in the invading hosts) (Daszak et al., 2000). As noted by Begon (2003), “One of the great challenges in the study of rodent infections, of rodent-reservoir zoonoses, and of infectious diseases generally, is to understand the evolutionary and the pathogenic basis of variations in virulence from species to species”. The question of whether or not C. viatorum can spread or has spread from rats to humans is best resolved by continued monitoring and surveys of both human and wildlife populations. Furthermore, until more data become available, it is not possible to ascertain whether subtype XVaA6, found in a Chinese wastewater sample (Huang et al., 2017), originates from humans or from rats. However, the grouping with related subtypes (Fig. 2) suggests that it is human-derived.
4.4. Conclusion
For the first time, C. viatorum has been found in a non-human host. The long-term, isolated nature of the swamp rat population in the Upper Yarra water catchment in Victoria, Australia, the lack of C. viatorum from other mammals and birds living within the vicinity and the genetic distinctiveness in both the SSU and gp60 genes, collectively suggest that C. viatorum is endemic to native rats in Australia. The role of rodents and rats, in particular, as prospective reservoirs for C. viatorum should continue to be studied in the future, as the zoonotic potential for pathogen dispersal by rats is high (Kosoy et al., 2015). An increase in the number of rats and diversity of rat species surveyed for Cryptosporidium may help give a clearer understanding of how C. viatorum is transmitted and has been transmitted in the past. Longitudinal studies, like the Melbourne Water project, which repetitively samples a variety of hosts in multiple localities, in different seasons and years, might serve as excellent opportunities to gain greater insight into rare pathogen species and their transmission patterns.
Acknowledgements
Research funding from the Melbourne Water Corporation and Australian Research Council (LP160101299) (RBG, AVK and SRH) is gratefully acknowledged. We thank Kathy Cinque for help in collecting samples.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ijppaw.2018.01.004.
Contributor Information
Anson V. Koehler, Email: anson.koehler@unimelb.edu.au.
Robin B. Gasser, Email: robinbg@unimelb.edu.au.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- Abal-Fabeiro J., Maside X., Bello X., Llovo J., Bartolome C. Multilocus patterns of genetic variation across Cryptosporidium species suggest balancing selection at the gp60 locus. Mol. Ecol. 2013;22:4723–4732. doi: 10.1111/mec.12425. [DOI] [PubMed] [Google Scholar]
- Adamu H., Petros B., Zhang G., Kassa H., Amer S., Ye J., Feng Y., Xiao L. Distribution and clinical manifestations of Cryptosporidium species and subtypes in HIV/AIDS patients in Ethiopia. PLoS Neglected Trop. Dis. 2014;8:e2831. doi: 10.1371/journal.pntd.0002831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves M., Xiao L., Sulaiman I., Lal A.A., Matos O., Antunes F. Subgenotype analysis of Cryptosporidium isolates from humans, cattle, and zoo ruminants in Portugal. J. Clin. Microbiol. 2003;41:2744–2747. doi: 10.1128/JCM.41.6.2744-2747.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayinmode A.B., Zhang H., Dada-Adegbola H.O., Xiao L. Cryptosporidium hominis subtypes and Enterocytozoon bieneusi genotypes in HIV-infected persons in Ibadan, Nigeria. Zoonoses Public Health. 2014;61:297–303. doi: 10.1111/zph.12072. [DOI] [PubMed] [Google Scholar]
- Banks P.B., Hughes N.K. A review of the evidence for potential impacts of black rats (Rattus rattus) on wildlife and humans in Australia. Wildl. Res. 2012;39:78–88. [Google Scholar]
- Begon M. Disease: health effects on humans, population effects on rodents. ACIAR Monogr. Ser. 2003;96:13–19. [Google Scholar]
- Blom N., Sicheritz-Pontén T., Gupta R., Gammeltoft S., Brunak S. Prediction of post-translational glycosylation and phosphorylation of proteins from the amino acid sequence. Proteomics. 2004;4:1633–1649. doi: 10.1002/pmic.200300771. [DOI] [PubMed] [Google Scholar]
- Callaway R.M., Ridenour W.M. Novel weapons: invasive success and the evolution of increased competitive ability. Front. Ecol. Environ. 2004;2:436–443. [Google Scholar]
- Daszak P., Cunningham A.A., Hyatt A.D. Emerging infectious diseases of wildlife–threats to biodiversity and human health. Science. 2000;287:443–449. doi: 10.1126/science.287.5452.443. [DOI] [PubMed] [Google Scholar]
- de Lucio A., Amor-Aramendia A., Bailo B., Saugar J.M., Anegagrie M., Arroyo A., Lopez-Quintana B., Zewdie D., Ayehubizu Z., Yizengaw E., Abera B., Yimer M., Mulu W., Hailu T., Herrador Z., Fuentes I., Carmena D. Prevalence and genetic diversity of Giardia duodenalis and Cryptosporidium spp. among school children in a rural area of the Amhara region, North-west Ethiopia. PLoS One. 2016;11:e0159992. doi: 10.1371/journal.pone.0159992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckert P., Brunak S., Blom N. Prediction of proprotein convertase cleavage sites. Protein Eng. Des. Sel. 2004;17:107–112. doi: 10.1093/protein/gzh013. [DOI] [PubMed] [Google Scholar]
- Elwin K., Hadfield S.J., Robinson G., Crouch N.D., Chalmers R.M. Cryptosporidium viatorum n. sp. (Apicomplexa: Cryptosporidiidae) among travellers returning to Great Britain from the Indian subcontinent, 2007–2011. Int. J. Parasitol. 2012;42:675–682. doi: 10.1016/j.ijpara.2012.04.016. [DOI] [PubMed] [Google Scholar]
- Huang C., Hu Y., Wang L., Wang Y., Li N., Guo Y., Feng Y., Xiao L. Environmental transport of emerging human-pathogenic Cryptosporidium species and subtypes through combined sewer overflow and wastewater. Appl. Environ. Microbiol. 2017;83 doi: 10.1128/AEM.00682-17. e00682–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insulander M., Silverlas C., Lebbad M., Karlsson L., Mattsson J.G., Svenungsson B. Molecular epidemiology and clinical manifestations of human cryptosporidiosis in Sweden. Epidemiol. Infect. 2013;141:1009–1020. doi: 10.1017/S0950268812001665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K., Misawa K., Kuma K., Miyata T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002;30:3059–3066. doi: 10.1093/nar/gkf436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearse M., Moir R., Wilson A., Stones-Havas S., Cheung M., Sturrock S., Buxton S., Cooper A., Markowitz S., Duran C., Thierer T., Ashton B., Meintjes P., Drummond A. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler A.V., Haydon S.R., Jex A.R., Gasser R.B. Is Cryptosporidium from the common wombat (Vombatus ursinus) a new species and distinct from Cryptosporidium ubiquitum? Infect. Genet. Evol. 2016;44:28–33. doi: 10.1016/j.meegid.2016.06.028. [DOI] [PubMed] [Google Scholar]
- Koehler A.V., Haydon S.R., Jex A.R., Gasser R.B. Cryptosporidium and Giardia taxa in faecal samples from animals in catchments supplying the city of Melbourne with drinking water (2011 to 2015) Parasites Vectors. 2016;9:315. doi: 10.1186/s13071-016-1607-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosoy M., Khlyap L., Cosson J.-F., Morand S. Aboriginal and invasive rats of genus Rattus as hosts of infectious agents. Vector Borne Zoonotic Dis. 2015;15:3–12. doi: 10.1089/vbz.2014.1629. [DOI] [PubMed] [Google Scholar]
- Kotloff K.L. The burden and etiology of diarrheal illness in developing countries. Pediatr. Clin. 2017;64:799–814. doi: 10.1016/j.pcl.2017.03.006. [DOI] [PubMed] [Google Scholar]
- Kumar S., Stecher G., Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapen D., Schmidt P., Thomas J., Edge T., Flemming C., Keithlin J., Neumann N., Pollari F., Ruecker N., Simhon A. Towards a more accurate quantitative assessment of seasonal Cryptosporidium infection risks in surface waters using species and genotype information. Water Res. 2016;105:625–637. doi: 10.1016/j.watres.2016.08.023. [DOI] [PubMed] [Google Scholar]
- Lebbad M., Beser J., Insulander M., Karlsson L., Mattsson J.G., Svenungsson B., Axen C. Unusual cryptosporidiosis cases in Swedish patients: extended molecular characterization of Cryptosporidium viatorum and Cryptosporidium chipmunk genotype I. Parasitology. 2013;140:1735–1740. doi: 10.1017/S003118201300084X. [DOI] [PubMed] [Google Scholar]
- Li N., Xiao L., Alderisio K., Elwin K., Cebelinski E., Chalmers R., Santin M., Fayer R., Kvac M., Ryan U., Sak B., Stanko M., Guo Y., Wang L., Zhang L., Cai J., Roellig D., Feng Y. Subtyping Cryptosporidium ubiquitum, a zoonotic pathogen emerging in humans. Emerg. Infect. Dis. 2014;20:217–224. doi: 10.3201/eid2002.121797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddison W.P., Maddison D.R. 3.04 ed. 2015. Mesquite; a Modular System for Evolutionary Analysis. [Google Scholar]
- Manly B.F.J., Navarro Alberto J.A. CRC Press Taylor & Francis; New York: 2015. Introduction to Ecological Sampling. [Google Scholar]
- Morand S., Bordes F., Chen H.W., Claude J., Cosson J.F., Galan M., Czirjak G.A., Greenwood A.D., Latinne A., Michaux J., Ribas A. Global parasite and Rattus rodent invasions: the consequences for rodent-borne diseases. Integr. Zool. 2015;10:409–423. doi: 10.1111/1749-4877.12143. [DOI] [PubMed] [Google Scholar]
- Morgan U.M., Monis P.T., Fayer R., Deplazes P., Thompson R.C. Phylogenetic relationships among isolates of Cryptosporidium: evidence for several new species. J. Parasitol. 1999;85:1126–1133. [PubMed] [Google Scholar]
- Nei M., Kumar S. Oxford university press; 2000. Molecular Evolution and Phylogenetics. [Google Scholar]
- Nolan M.J., Jex A.R., Koehler A.V., Haydon S.R., Stevens M.A., Gasser R.B. Molecular-based investigation of Cryptosporidium and Giardia from animals in water catchments in southeastern Australia. Water Res. 2013;47:1726–1740. doi: 10.1016/j.watres.2012.12.027. [DOI] [PubMed] [Google Scholar]
- Parks Victoria . Parks Victoria; Victoria, Melbourne: 2002. Yarra Ranges National Park Draft Management Plan. [Google Scholar]
- Power M.L., Cheung-Kwok-Sang C., Slade M., Williamson S. Cryptosporidium fayeri: diversity within the gp60 locus of isolates from different marsupial hosts. Exp. Parasitol. 2009;121:219–223. doi: 10.1016/j.exppara.2008.10.016. [DOI] [PubMed] [Google Scholar]
- Power M.L., Slade M.B., Sangster N.C., Veal D.A. Genetic characterisation of Cryptosporidium from a wild population of eastern grey kangaroos Macropus giganteus inhabiting a water catchment. Infect. Genet. Evol. 2004;4:59–67. doi: 10.1016/j.meegid.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Prystajecky N., Huck P.M., Schreier H., Isaac-Renton J.L. Assessment of Giardia and Cryptosporidium spp. as a microbial source tracking tool for surface water: application in a mixed-use watershed. Appl. Environ. Microbiol. 2014;80:2328–2336. doi: 10.1128/AEM.02037-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins J.H., McLenachan P.A., Phillips M.J., McComish B.J., Matisoo-Smith E., Ross H.A. Evolutionary relationships and divergence times among the native rats of Australia. BMC Evol. Biol. 2010;10:375. doi: 10.1186/1471-2148-10-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe K.C., Reno M.L., Richmond D.M., Adkins R.M., Steppan S.J. Pliocene colonization and adaptive radiations in Australia and New Guinea (Sahul): multilocus systematics of the old endemic rodents (Muroidea: Murinae) Mol. Phylogenet. Evol. 2008;47:84–101. doi: 10.1016/j.ympev.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Ruecker N.J., Matsune J.C., Lapen D.R., Topp E., Edge T.A., Neumann N.F. The detection of Cryptosporidium and the resolution of mixtures of species and genotypes from water. Infect. Genet. Evol. 2013;15:3–9. doi: 10.1016/j.meegid.2012.09.009. [DOI] [PubMed] [Google Scholar]
- Ruecker N.J., Matsune J.C., Wilkes G., Lapen D.R., Topp E., Edge T.A., Sensen C.W., Xiao L., Neumann N.F. Molecular and phylogenetic approaches for assessing sources of Cryptosporidium contamination in water. Water Res. 2012;46:5135–5150. doi: 10.1016/j.watres.2012.06.045. [DOI] [PubMed] [Google Scholar]
- Ryan U., Fayer R., Xiao L. Cryptosporidium species in humans and animals: current understanding and research needs. Parasitology. 2014;141:1667–1685. doi: 10.1017/S0031182014001085. [DOI] [PubMed] [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Sanchez A., Munoz M., Gomez N., Tabares J., Segura L., Salazar A., Restrepo C., Ruiz M., Reyes P., Qian Y., Xiao L., Lopez M.C., Ramirez J.D. Molecular epidemiology of Giardia, Blastocystis and Cryptosporidium among indigenous children from the Colombian amazon basin. Front. Microbiol. 2017;8:248. doi: 10.3389/fmicb.2017.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seebeck J., Menkhorst P. Status and conservation of the rodents of Victoria. Wildl. Res. 2000;27:357–369. [Google Scholar]
- Simpson G.G. Historical zoogeography of Australian mammals. Evolution. 1961;15:431–446. [Google Scholar]
- Sow S.O., Muhsen K., Nasrin D., Blackwelder W.C., Wu Y., Farag T.H., Panchalingam S., Sur D., Zaidi A.K., Faruque A.S., Saha D., Adegbola R., Alonso P.L., Breiman R.F., Bassat Q., Tamboura B., Sanogo D., Onwuchekwa U., Manna B., Ramamurthy T., Kanungo S., Ahmed S., Qureshi S., Quadri F., Hossain A., Das S.K., Antonio M., Hossain M.J., Mandomando I., Nhampossa T., Acacio S., Omore R., Oundo J.O., Ochieng J.B., Mintz E.D., O'Reilly C.E., Berkeley L.Y., Livio S., Tennant S.M., Sommerfelt H., Nataro J.P., Ziv-Baran T., Robins-Browne R.M., Mishcherkin V., Zhang J., Liu J., Houpt E.R., Kotloff K.L., Levine M.M. The burden of Cryptosporidium diarrheal disease among children < 24 months of age in moderate/high mortality regions of Sub-Saharan Africa and South Asia, utilizing data from the Global Enteric Multicenter Study (GEMS) PLoS Neglected Trop. Dis. 2016;10:e0004729. doi: 10.1371/journal.pntd.0004729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire S.A., Ryan U. Cryptosporidium and Giardia in Africa: current and future challenges. Parasites Vectors. 2017;10:195. doi: 10.1186/s13071-017-2111-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steentoft C., Vakhrushev S.Y., Joshi H.J., Kong Y., Vester-Christensen M.B., Katrine T., Schjoldager B., Lavrsen K., Dabelsteen S., Pedersen N.B. Precision mapping of the human O-GalNAc glycoproteome through SimpleCell technology. EMBO J. 2013;32:1478–1488. doi: 10.1038/emboj.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenger B.L., Clark M.E., Kvac M., Khan E., Giddings C.W., Prediger J., McEvoy J.M. North American tree squirrels and ground squirrels with overlapping ranges host different Cryptosporidium species and genotypes. Infect. Genet. Evol. 2015;36:287–293. doi: 10.1016/j.meegid.2015.10.002. [DOI] [PubMed] [Google Scholar]
- Stensvold C.R., Beser J., Axen C., Lebbad M. High applicability of a novel method for gp60-based subtyping of Cryptosporidium meleagridis. J. Clin. Microbiol. 2014;52:2311–2319. doi: 10.1128/JCM.00598-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stensvold C.R., Elwin K., Winiecka-Krusnell J., Chalmers R.M., Xiao L., Lebbad M. Development and application of a gp60-based typing assay for Cryptosporidium viatorum. J. Clin. Microbiol. 2015;53:1891–1897. doi: 10.1128/JCM.00313-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong W.B., Gut J., Nelson R.G. Cloning and sequence analysis of a highly polymorphic Cryptosporidium parvum gene encoding a 60-kilodalton glycoprotein and characterization of its 15-and 45-kilodalton zoite surface antigen products. Infect. Immun. 2000;68:4117–4134. doi: 10.1128/iai.68.7.4117-4134.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulaiman I.M., Hira P.R., Zhou L., Al-Ali F.M., Al-Shelahi F.A., Shweiki H.M., Iqbal J., Khalid N., Xiao L. Unique endemicity of cryptosporidiosis in children in Kuwait. J. Clin. Microbiol. 2005;43:2805–2809. doi: 10.1128/JCM.43.6.2805-2809.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torchin M.E., Lafferty K.D., Dobson A.P., McKenzie V.J., Kuris A.M. Introduced species and their missing parasites. Nature. 2003;421:628–630. doi: 10.1038/nature01346. [DOI] [PubMed] [Google Scholar]
- Triggs B. Oxford University Press; South Melbourne: 2004. Tracks, Scats and Other Traces: a Field Guide to Australian Mammals. [Google Scholar]
- Ukwah B.N., Ezeonu I.M., Ezeonu C.T., Roellig D., Xiao L. Cryptosporidium species and subtypes in diarrheal children and HIV-infected persons in Ebonyi and Nsukka, Nigeria. J. Infect. Dev. Ctries. 2017;11:173–179. doi: 10.3855/jidc.8034. [DOI] [PubMed] [Google Scholar]
- Verma S., Singh L. Novel universal primers establish identity of an enormous number of animal species for forensic application. Mol. Ecol. Resour. 2003;3:28–31. [Google Scholar]
- Wait L.F., Fox S., Peck S., Power M.L. Molecular characterization of Cryptosporidium and Giardia from the tasmanian devil (Sarcophilus harrisii) PLoS One. 2017;12:e0174994. doi: 10.1371/journal.pone.0174994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D.E., Reeder D.M. JHU Press; 2005. Mammal Species of the World: a Taxonomic and Geographic Reference. [Google Scholar]
- Xiao L., Fayer R. Molecular characterisation of species and genotypes of Cryptosporidium and Giardia and assessment of zoonotic transmission. Int. J. Parasitol. 2008;38:1239–1255. doi: 10.1016/j.ijpara.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Xiao L., Feng Y. Molecular epidemiologic tools for waterborne pathogens Cryptosporidium spp. and Giardia duodenalis. Food Waterborne Parasitol. 2017 doi: 10.1016/j.fawpar.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L., Sulaiman I.M., Ryan U.M., Zhou L., Atwill E.R., Tischler M.L., Zhang X., Fayer R., Lal A.A. Host adaptation and host-parasite co-evolution in Cryptosporidium: implications for taxonomy and public health. Int. J. Parasitol. 2002;32:1773–1785. doi: 10.1016/s0020-7519(02)00197-2. [DOI] [PubMed] [Google Scholar]
- Yan W., Alderisio K., Roellig D.M., Elwin K., Chalmers R.M., Yang F., Wang Y., Feng Y., Xiao L. Subtype analysis of zoonotic pathogen Cryptosporidium skunk genotype. Infect. Genet. Evol. 2017;55:20–25. doi: 10.1016/j.meegid.2017.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahedi A., Paparini A., Jian F., Robertson I., Ryan U. Public health significance of zoonotic Cryptosporidium species in wildlife: critical insights into better drinking water management. Int. J. Parasitol. Parasites Wildl. 2016;5:88–109. doi: 10.1016/j.ijppaw.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.