New approach to determine the inorganic carbon permeability across the chloroplast envelope, and to measure the role of aquaporin Pip1,2 on CO2 permeability.
Keywords: CCM, chloroplast bicarbonate permeability, chloroplast CO2 permeability, MIMS, photosynthesis
Abstract
To support photosynthetic CO2 fixation by Rubisco, the chloroplast must be fed with inorganic carbon in the form of CO2 or bicarbonate. However, the mechanisms allowing the rapid passage of this gas and this charged molecule through the bounding membranes of the chloroplast envelope are not yet completely elucidated. We describe here a method allowing us to measure the permeability of these two molecules through the chloroplast envelope using a membrane inlet mass spectrometer and 18O-labelled inorganic carbon. We established that the internal stromal carbonic anhydrase activity is not limiting for this technique, and precisely measured the chloroplast surface area and permeability values for CO2 and bicarbonate. This was performed on chloroplasts from several plant species, with values ranging from 2.3 × 10–4 m s–1 to 8 × 10–4 m s–1 permeability for CO2 and 1 × 10–8 m s–1 for bicarbonate. We were able to apply our method to chloroplasts from an Arabidopsis aquaporin mutant, and this showed that CO2 permeability was reduced 50% in the mutant compared with the wild-type reference.
Introduction
The permeability of biological membranes to the gases CO2 and O2 is an extremely important property of cells of all microbial and multicellular organisms where respiration and photosynthesis are the most important energetic processes sustaining life. This is the reason why being able to measure the permeability of membranes through which these gases diffuse and to understand how sufficient permeability is achieved and modulated are of considerable interest (Endeward et al., 2014; Kai and Kaldenhoff, 2014; Raven and Beardall, 2016). In this respect, this study is focused on measuring and understanding CO2 (and ) permeability of plant chloroplasts where photosynthesis is the major process occurring and CO2 is being consumed and O2 is evolved in equimolar amounts.
Our understanding of CO2 permeability has evolved over the past 100 years. Initially it was proposed that lipid-based biological membranes were very permeable to CO2, based on the lipid/water partitioning of the gas, and that they posed little diffusion resistance to its movement (Missner et al., 2008; Endeward et al., 2014). This view was supported by measurements of artificial lipid membrane systems (Missner et al., 2008). However, over the past 10 years, it has became increasingly apparent that this diffusion can be greatly slowed by the inclusion of various components which are found in functional biological membranes such as sterols (cholesterol) and protein complexes, to create the possible reality that CO2 permeability may be restricted to an extent which requires the introduction of specialized CO2 transfer protein complexes to speed up CO2 entry. These transfer complexes are proposed to be members of the aquaporin family of water channel proteins found in most biological membranes (Boron et al., 2011; Itel et al., 2012; Endeward et al., 2014; Kai and Kaldenhoff, 2014).
Our understanding of the CO2 permeability of biological membranes has been heavily dependent on the measurement techniques employed to study CO2 transfer in artificial lipid membranes and liposomes as well as intact biological cells and organelles (Endeward et al., 2014). These methods have demonstrated that the various measurement techniques have intrinsic limitations and advantages with regard to their applicability and inherent capability to measure quantitatively accurate CO2 permeability values. Stopped flow spectrophotometry techniques tend to give comparatively low permeability values, being limited by the time resolution of the measurement techniques; mass spectrometric 18O exchange techniques give comparatively higher values, making use of the relatively slow kinetics of isotopic equilibrium and increased time resolution; and pH microelectrode techniques appear to give the highest values but are limited to use with relatively large surface area artificial membrane systems or large fixed position cells (Missner et al., 2008). Based on these factors, we have focused on the use of MS 18O exchange techniques, which offer the ability to work with cells and chloroplasts containing carbonic anhydrase (CA) with a fast time resolution.
Our understanding of the transfer of CO2 into plant chloroplasts and its ultimate fixation by Rubisco into sugars depends on whether plants possess a CO2-concentrating mechanism (CCM) to enhance CO2 around Rubisco relative to the external ambient concentration. For C3 plants such a tobacco and wheat, it is assumed that they can be modelled as a simple passive diffusion of CO2 down a concentration gradient from the external air to the sites of Rubisco in the chloroplast. The diffusion pathway to CO2 is a series of resistances including the stomata, the cell wall and plasma membrane, the cytosolic pathway, and the chloroplast envelope. The non-stomatal resistances are responsible for determining the total mesophyll conductance, of which the chloroplast component is proposed to contribute ~30–40% (Tholen and Zhu, 2011). There is considerable interest in understanding what contributes to mesophyll conductance, and how it may differ, as variation in these resistances can contribute to photosynthetic efficiency when leaves are CO2 limited (Tholen et al., 2012, 2014; Flexas et al., 2013). For C4 plants such as maize and sorghum, the situation is quite different, with Rubisco being contained in the chloroplasts of the bundle sheath cells and CO2 being supplied by decarboxylation of a C4 acid. The primary passive supply of CO2 occurs in the cytosol of the mesophyll cells where CO2 is converted to by CA for primary fixation by phosphenolpyruvate (PEP) carboxylase (Hatch, 1987).
This study focuses on exploring how a mass spectrometric 18O exchange technique can be applied to isolated C3 plant chloroplasts to understand their CO2 and envelope conductance properties. The findings of the study demonstrate that the approaches taken with chloroplast isolation, membrane inlet mass spectrometry (MIMS), and theoretical modelling can be applied to obtain reasonable estimates of chloroplast CO2 permeability for a range of species, can also be extended to infer permeability values, and can demonstrate the differences in CO2 conductance caused by the presence of chloroplast membrane aquaporins.
Materials and methods
Plant material and growing conditions
Spinach (Spinacea oleracae) leaves were obtained fresh from local markets. Arabidopsis thaliana ecotype Col-0 and atpip1;2-1 (Heckwolf et al. 2011) seeds, and Nicotiana benthamiana seeds were sown directly on to Debco seed raising potting mix (Debco Pty Ltd, Australia). After 2 weeks, 10 plants were transferred and grown in pots with a mix of Debco Plugger starter plus and Seed Raising (3:1 v:v) supplied with Scotts osmocote exact mini (1 g kg–1) (Scotts International, The Netherlands). Pisum sativum seeds were directly planted into a pot with the same potting mix. Plants were grown for ~45 d in a growth chamber under controlled conditions (16:8 h photoperiod, light at 250 µmol quanta m–2 s–1, 25 °C:20 °C day:night temperature, and relative humidity at 60%, watered every 2 d, for N. benthamiana and P. sativa); (8:16 h photoperiod, light at 250 µmol quanta m–2 s–1, 22 °C:22 °C day:night temperature and relative humidity at 60%, watered every 2 d, for A. thaliana).
Yeast (Saccharomyces cerevisiae) INVSc1: MATa his3D1 leu2 trp1-289 ura3-52 MAT his3D1 leu2 trp1-289 ura3-52::human CA were grown on YPD broth (–leucine,–uracil) at 29 °C overnight under continuous shaking.
Chloroplast isolation
An 8–10 g aliquot of fresh leaves was ground for 2 s with a Polytron mechanical homogenizer (Kinematica Gmbh, Germany) in 20 ml of isolation buffer (sorbitol 330 mmol l–1, MOPS 30 mmol l–1 adjusted at pH 7.8, EDTA 2 mmol l–1, BSA 1.5 g l–1). After filtration through eight layers of miracloth (Wattman, USA), the extract was centrifuged at 1500 g for 90 s (Sorvall rotor SS34). The pellet was delicately resuspended in 4 ml of isolation buffer. Starch and nuclei were spun down by a centrifugation at 120 g for 45 s. Crude chloroplasts were concentrated to 500 μl by centrifugation (1500 g, 90 s) and then pipetted onto the top of a linear Percoll gradient (50% Percoll, sorbitol 330 mmol l–1, MOPS 30 mmol l–1 adjusted at pH 7.8, EDTA 2 mmol l–1, BSA 1.5 g l–1) previously autoformed by centrifugation for 1 h at 20 000 g. Pure chloroplasts were collected from the bottom fraction after centrifugation at 5000 g for 5 min and kept in the dark and on ice for <1 h before the permeability assay. Chloroplast integrity as assayed by reaction with ferricyanide (Mourioux and Douce, 1981) was found to be 80–85%. We routinely used phase-contrast microscopy (Leica DM5500 B, Germany) to monitor the integrity of the chloroplast preparation.
Determination of size and number
Pure chloroplasts were examined under a fluorescence microscope (Leica DM5500 B, Germany) and then quantified using flow cytometry. Samples of diluted chloroplasts were analysed using Fortessa and LSRII cytometers (BD Biosciences, USA). Size was determined using forward scatter (FSC) intensity after calibration against size reference beads (1, 2, 4, 6, 10, and 15 µm; Flow cytometry size calibration kit, Molecular Probes, ThermoFisher Scientific, USA). Absolute chloroplast number was determined by mixing the sample with a calibrated suspension of microspheres that have specific fluorescence emissions (CountBright absolute counting beads; Molecular Probes, ThermoFisher Scientific). Data were processed using FlowJo software (Flow Jo LLC, USA) by plotting side scatter (SSC) against FSC or fluorescence against FSC. In parallel to this, the Chl a concentration was determined spectrophotometrically after pigment extraction in 100% methanol (MacKinney, 1941). Surface area was calculated for chloroplast particles, assuming they were spherical, and summed up to obtain the total surface value. This value was normalized by the absolute chloroplast count to give an average surface area per chloroplast and surface area injected per assay. The same has been done for the yeast suspension.
Permeability assay
For low enrichment assays, 2.5 × 10–3 mol l–1 (final concentration) of low 18O-enriched NaH13CO3 (equilibrated against 1.2% [18O]water) was added to the reaction buffer (EPPS 100 mmol l–1 at pH 7.8, sorbitol 330 mmol l–1) in the mass inlet mass spectrometer (MIMS) cuvette (600 μl total volume, see Supplementary Fig. S1 at JXB online). Dextran-bound acetazolamide (Ramidus AB, Sweden) was also added (1.2 µg ml–1 to get an equivalent effect of 1.2 µmol ml–1 acetazolamide on bovine CA) to eliminate external CA activity. After chemical equilibration was reached (from 200 s to 300 s), chloroplast solution was added (<1/60th cuvette volume) in the dark, and the concentration of 13C18O16O (m/z=47) species was monitored over time by MIMS (Isoprime100, Isoprime, UK). In high enrichment assays, 13C18O18O (m/z=49) and 13C16O16O (m/z=45) were also monitored, and highly enriched [18O]bicarbonate for these assays was equilibrated against 99% [18O]water. 18O-enriched inorganic carbon (Ci) was prepared by incubating 0000.5 mol l–1 NaH13CO3 with either 1.2% (1% added labelled water plus 0.2% natural abundance in the unenriched water) or 99% H218O in a sealed vial at room temperature for at least 24 h. In low enriched experiments, the added inorganic carbon was assumed to be in the form of with an enrichment of 3.5%, which was predicted from a model of labelling in 1.2% [18O]water. In-house Python scripts were used to record and process data from the mass spectrometer.
Mathematical modelling procedures
Modelled time courses and curve-fitting of data shown herein were done with a biochemical network simulation program, COPASI, available on the internet (copasi.org) and described in detail by Hoops et al. (2006). We used COPASI to simulate time courses of reaction intermediate changes in a two-compartment model (external and chloroplast stroma) where reactants are linked in a biochemical network described in Fig. 1. We used the deterministic approach based on solving a set of differential equations (ODEs) shown in Supplementary Figs S3 and S4, and the modelling parameters described in Supplementary Tables S1 and S2.
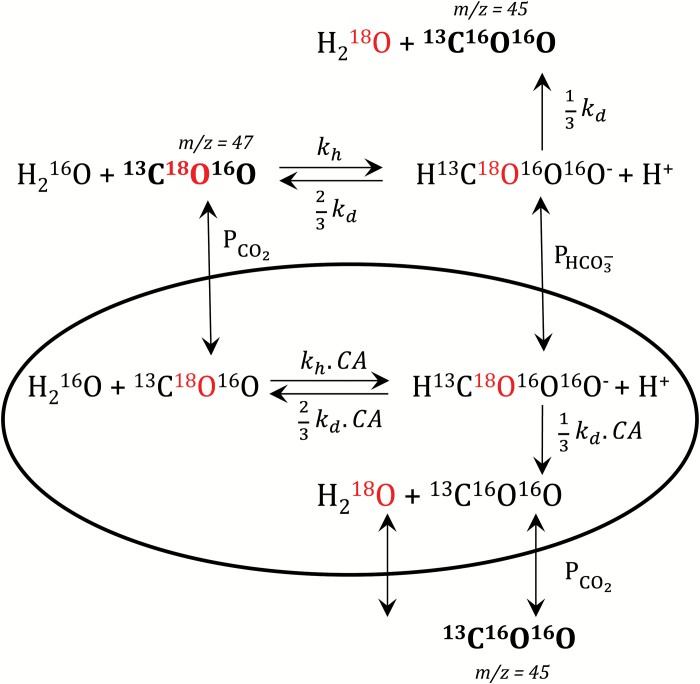
Fig. 1.
A diagram of the assumed exchange of inorganic carbon isotopes with water used in the mathematical simulations and modelling described herein. , permeability of chloroplast; , CO2 permeability of chloroplast; kh, CO2 hydration rate constant; kd, dehydration rate constant; CA, scaling factor of carbonic anhydrase activity. As the loss of 18O from Ci species is the key component of measurements, 18O is labelled in red. Ci species measurable in the mass spectrometer are in bold, and mass over charge value (m/z) specified. 13C-labelled inorganic carbon is used for all assays.
We used the LSoda deterministic solving routine with a time step of 0.1 s and ran the model for 600 s. Initial concentrations of labelled CO2 and species were set as listed in Supplementary Table S1 and the injection of chloroplasts was simulated by changing the chloroplast envelope area from 0 to 30 m2 m–3 at 200 s or 300 s.
The COPASI software is able to fit the model to data from time course experiments. The curve fitting and parameter estimation is described by Hoops et al. (2006) and uses a weighted sum of squares minimization approach using the Levenberg–Marquardt algorithm provided by the program, and the root mean square was checked to be <5%. We used this capability to derive the actual experimentally observed hydration and dehydration rate constants in the assay medium (kh and kd) from pre-experiments with highly enriched NaH13CO3. Those values are then used in the low enriched NaH13CO3 experiment with chloroplasts for the estimation of and values for the assay.
Results
The 18O exchange technique
CO2 and permeability across biological membranes into an internal compartment containing CA can be studied using MIMS and 18O-labelled inorganic carbon. Techniques based on the kinetics of exchange of 18O from labelled Ci species with [16O]water have been used by two groups employing somewhat different approaches. Silverman and colleagues starting in 1974 (Silverman, 1974; Silverman et al., 1976) studied CO2 and permeability in red blood cells using highly 18O-enriched inorganic carbon (equilibrated with highly 18O-enriched water). They demonstrated permeabilities to both CO2 and bicarbonate and bicarbonate exchange which were facilitated by Cl–/ exchange activity of the band III protein anion exchanger. They developed mathematical analysis techniques to derive CO2 and permeability values from time courses, which followed the changes in CO2 isotopes after the sequential addition of labelled Ci and then erythrocytes over a period of ≥10 min.
Subsequently, Itada and Forster (1977) explored the application of a similar technique, using low enriched [18O]Ci species (equilibrated with 1.2% 18O-enriched water, see the Materials and methods) to develop a simplified mathematical approach (as fewer labelled species are involved) to study the same phenomena (Itada and Forster, 1977). This approach has been subsequently developed further using numerical curve-fitting procedures to describe the operation and limitations of this system in greater detail (Wunder and Gros, 1998; Wunder et al., 1998). We have used a similar approach with a reduced enrichment procedure to determine CO2 and permeability of isolated chloroplasts, and have tested some hypotheses with highly 18O-enriched Ci (details of our procedures are described in the Materials and methods). Figure 1 shows the chemical and diffusion reactions, which we have assumed to occur when creating the mathematical model which we have used to simulate and analyse the results obtained from our time course experiments shown in Fig. 6.
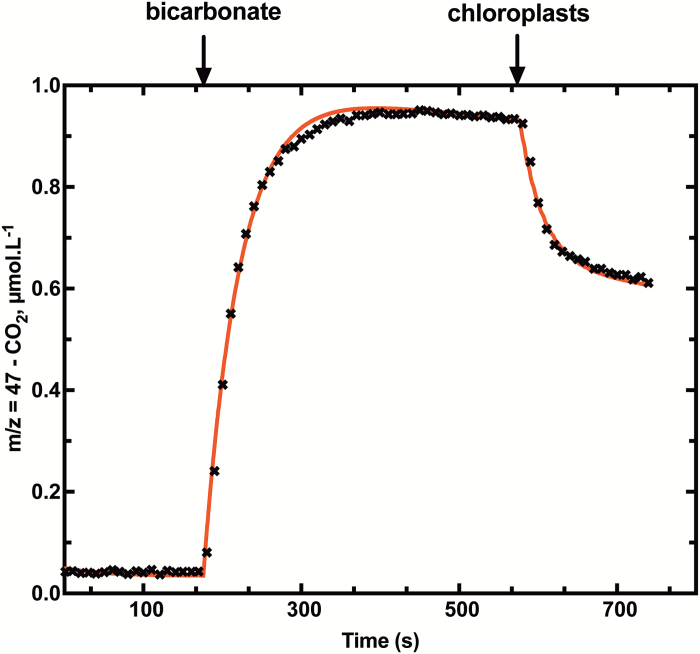
Fig. 6.
Time course (black crosses) of 13C18O16O (m/z=47) and curve fitting (red line) using COPASI software as described in the Materials and methods. Low 18O-enriched Ci (2.5 mM) was injected at t=170 s, and chloroplasts at t=577 s after chemical equilibrium had been reached.
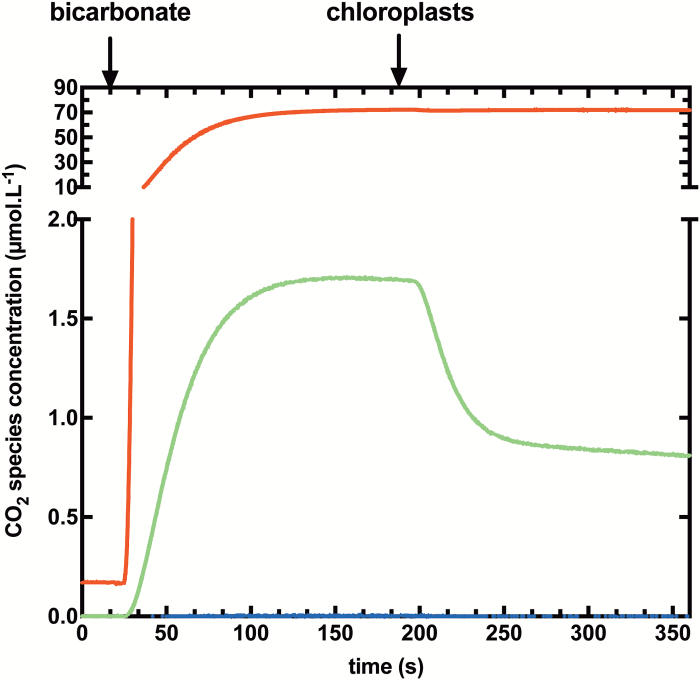
We have produced time courses for experiments conducted with chloroplasts using both low (Fig. 2) and highly (Fig. 3) enriched Ci species. After reaching a chemical equilibrium between CO2 and , chloroplasts are added in the dark, initiating a rapid drop of 18O-labelled CO2 species (m/z=49 and m/z=47), followed by a new isotopic equilibrium. Permeabilities are derived from the study of these kinetics. The primary difference between the two experimental systems is that in the highly enriched system, all three masses of labelled CO2 isotopes (m/z=49, m/z=47, and m/z=45) are significantly present and are changing, whereas singly labelled CO2 (m/z=47) is the dominant 18O-labelled CO2 species which changes over time in the low enriched system. In this condition, 13C18O16O (m/z=47) represent ~1–2% of total CO2 species in the solution (Fig. 2).
Fig. 2.
Typical time course for a low 18O-enriched permeability assay. Changes in the concentrations of 13C18O18O (m/z=49, blue), 13C18O16O (m/z=47, green), and 13C16O16O (m/z=45, red) species are shown. After injection of low 18O-enriched Ci (equilibrated against 1.2% [18O]water), chemical equilibrium is reached before chloroplast injection at 200 s.
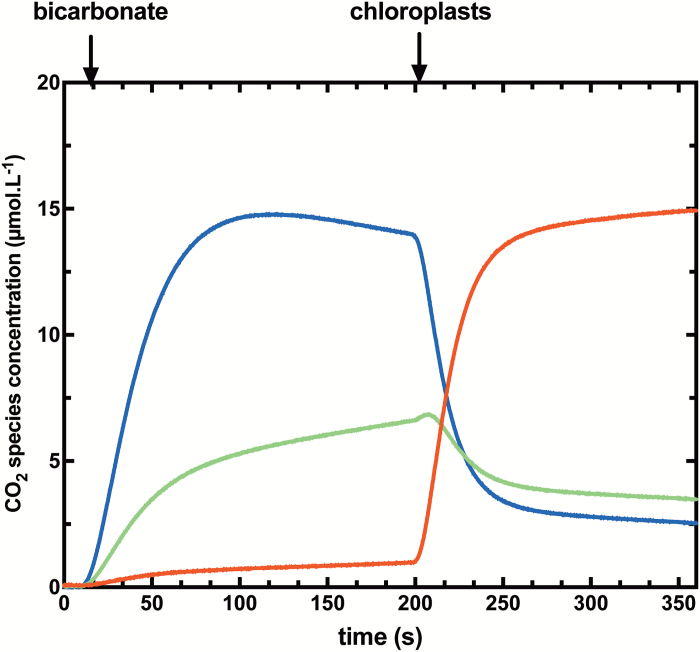
Fig. 3.
Typical time course for a highly 18O-enriched assay. Changes in concentrations of 13C18O18O (m/z=49, blue), 13C18O16O (m/z=47, green), and 13C16O16O (m/z=45, red) species are shown. Conditions were the same as Fig. 2 but using highly enriched [18O]Ci equilibrated against 99% [18O]water, instead of low 18O-enriched Ci.
Assumption about internal CA activity
One important assumption of this technique and modelling is that there is sufficient CA within the internal compartment (stroma of the chloroplast) to equilibrate the 18O isotope rapidly between the Ci species and the water. Thus, it is important to test this assumption. In this regard, although the singly labelled species experiment is simpler to analyse, it conveys less information on the extent of the isotopic equilibrium catalysed by CA within the chloroplasts due to the lack of an intermediate CO2 species (m/z=47 in Fig. 3), which can convey information about incomplete isotopic exchange.
As it is not possible to measure the stromal (internal) CA activity of our chloroplast preparations, we simulated the isotopic exchange between labelled Ci species and water (in a scenario of highly enriched [18O]bicarbonate) assuming a range of CA activities (103- to 106-fold increased exchange rates compared with the uncatalysed reaction) (Supplementary Fig. S2). A ≥106-fold increase in exchange rate is necessary to reach complete exchange with stromal water. Below this activity, production of singly 18O-labelled CO2 (m/z=47) occurs after the chloroplast injection. In our experience, we have observed consumption of singly 18O-labelled CO2 (m/z=47) when we inject the chloroplasts (Fig. 3), indicating that CA activity inside the chloroplast appears sufficient to achieve at least a 106-fold increase in Ci/water exchange rates. It should be noted that there are differences in the overall kinetics of the modelling and experimental results. In particular, mass 47 (singly labelled CO2) continues to rise in the modelling after the initial drop caused by injection of chloroplasts, and this does not happen in the actual experiments with chloroplasts (Fig. 3; Supplementary Fig S7). This may indicate a deficiency in the modelling.
Measuring chloroplast dimensions
A key parameter of our mathematical model and all permeability calculations is A, the area of chloroplast envelope per volume of assay (Supplementary Fig. S3; Supplementary Table S1). Surprisingly, the size and shape of isolated chloroplasts are not well described in the literature. An estimation of size and number on a small subpopulation by microscopy gives low precision data (for a review on cell counting, see Guillard and Sieracki, 2005). Indeed haemocytometer counts have low precision (within ±20% of the true count) due to random error and the relatively small numbers of cells counted, even when many replicate counts are made (Pringle and Mor, 1975; reviewed by Guillard et al., 2005). In addition, counts are frequently inaccurate due to systematic errors (Berkson et al., 1940; Pringle and Mor, 1975; Guillard and Sieracki, 2005).
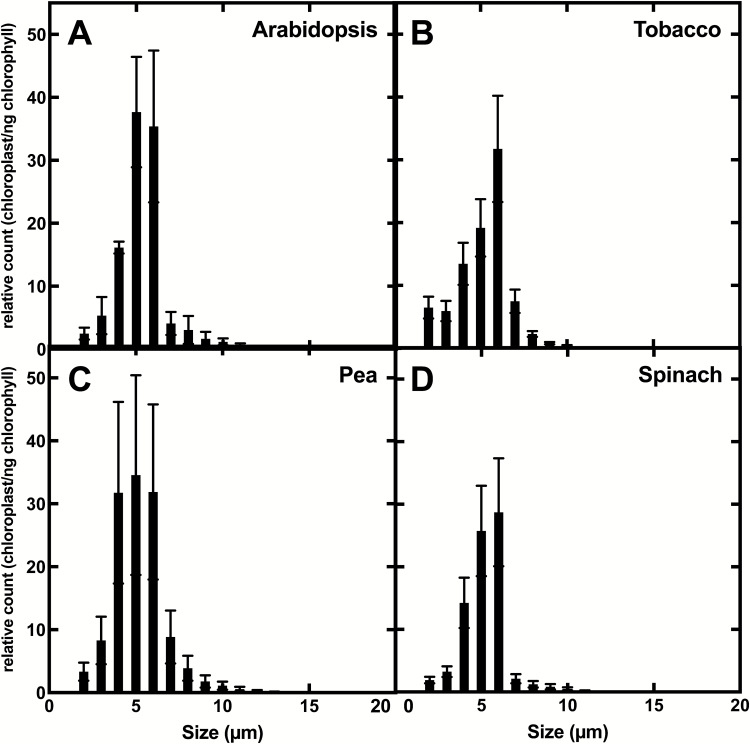
We chose to use flow cytometry to determine the diameter and the number of chloroplasts in our samples because a large number of individual chloroplasts can be counted, providing a high intrinsic precision to the counts. In addition, the technique describes the diversity of size and shapes present in the sample. Raw data from flow cytometry (the ratio between SSC and FSC) did not show a strong ellipsoid shape of the chloroplasts (data not shown), and consequently we assumed chloroplasts to be spherical for surface area calculations. Regardless of species origin, the majority of purified chloroplasts we obtained have a diameter between 4 µm and 6 µm (Fig. 4). As explained in the Materials and methods, we are calculating the surface area of each chloroplast for ~100 000–200 000 counts, and obtain the absolute concentration of chloroplasts and their surface area in our preparations. Surface area per chloroplast is given in Table 1.
Fig. 4.
Chloroplasts size distribution for four C3 plant species, Arabidopsis (Arabidopsis thaliana) (A), tobacco (Nicotiana benthamiana) (B), pea (Pisum sativa) (C), and spinach (Spinacea oleracea) (D). Size and number were determined by flow cytometry, and are normalized by Chl a concentration.
Table 1.
Determination of chloroplast permeabilities (CO2 and ) for different plant species as described in Fig. 6; shown are means ± SDs (n=3 different chloroplast isolation from three different plants)
Also included are values for yeast expressing human CA internally (from three independent cultures). Average surface area for each chloroplast or yeast was determined by flux cytometry
| (m s–1) | (m s–1) | Surface (m2) per unit | |
|---|---|---|---|
| Plant chloroplast | |||
| Spinach | 6.9 × 10–4±1.24 × 10–4 | 9.9 × 10–9±5 × 10–10 | 1.27 × 10–10±2.78 × 10–11 |
| Tobacco | 3.9 × 10–4±2.0 × 10–5 | 1.1 × 10–8±4 × 10–10 | 1.21 × 10–10±4.92 × 10–11 |
| Pea | 8.0 × 10–4±5.5 × 10–5 | 9.8 × 10–9±9 × 10–10 | 1.22 × 10–10±3.79 × 10–11 |
| Arabidopsis | 2.3 × 10–4±6.4 × 10–5 | 1.0 × 10–8±5 × 10–10 | 1.24 × 10–10±4.68 × 10–11 |
| Yeast (S. cerevisiae) | |||
| INVSc1: MATa his3D1 leu2 trp1-289 ura3-52 MAT his3D1 leu2 trp1-289 ura3- 52::human CA | 1.09 × 10–3±1.72 × 10–4 | 9.7 × 10–9±1 × 10–10 | 2.56 × 10–10±5.75 × 10–11 |
Modelling and measuring CO2 and permeabilities
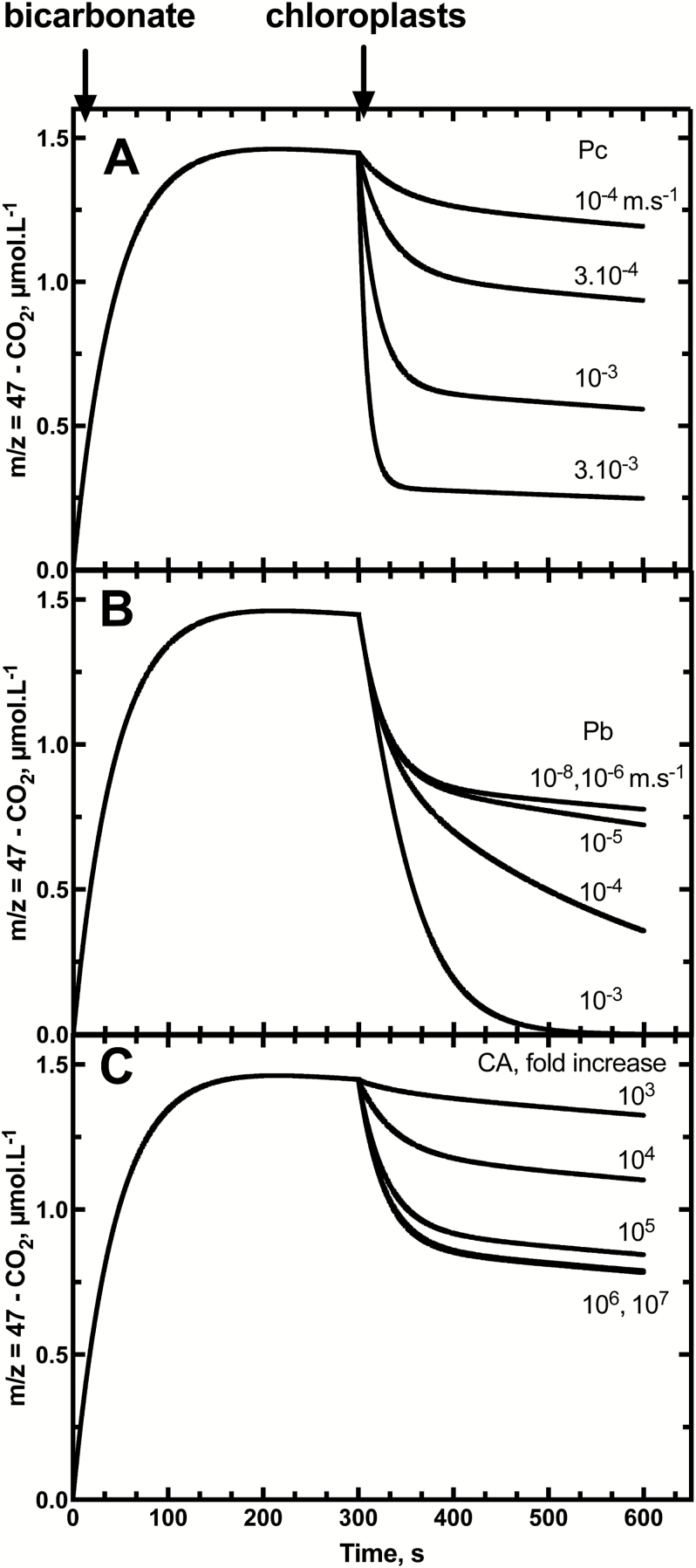
Isotopic exchange between labelled Ci (in the scenario of low 18O-enriched bicarbonate equilibrated against 1% [18O]water) and water was mathematically simulated to predict the effect of variation of CO2 and permeability (Fig. 5A, B), and chloroplast CA activity (Fig. 5C). This modelling used chloroplast dimensions per assay similar to those observed in actual assays (Tables 1, 2; Supplementary Table S2). After chemical equilibration between CO2 and bicarbonate during the first 300 s, the addition of chloroplasts induced an obvious consumption of labelled CO2 (m/z=47). For CO2 permeabilities ranging from 3 × 10–3 m s–1 to 10–4 m s–1, this drop is measurable and significantly different from one condition to the other (Fig. 5A). Significant differences are also present if the bicarbonate permeability varies between 10–6 m s–1 and 10–3 m s–1 (Fig. 5B).
Fig. 5.
The modelled effects of variation in chloroplast envelope CO2 permeability (; A), permeability (; B), and in internal chloroplast CA activity (C) on the decrease in 13C18O16O (m/z=47) species in the assay after the injection of chloroplasts. In (A) was varied from 10–4 m s–1 to 3 × 10–3 m s–1 (was fixed at 10–8 m s–1, CA at 107-fold increase). In (B) was varied from 10–8 m s–1 to 10–3 m s–1 ( was fixed at 5 × 10–4 m s–1, CA at 107-fold increase). In (C), CA activity was modelled as the fold increase in the interconversion between CO2 and within the chloroplast stroma and was varied between a 103 and 107 increase in the hydration and dehydration rate constants (was fixed at 5 × 10–4 m s–1 and at 10–8 m s–1). The modelling procedures are described in the Materials and methods and supplementary information, based on the use of low 18O-enriched bicarbonate equilibrated against 1.2% [18O]water. Labelled bicarbonate is added at time zero (1 mmol l–1 total) and chloroplasts are added after 300 s of equilibration in the assay.
Similar to what is shown in Supplementary Fig. S2, internal CA activity has an influence on the kinetics of isotopic exchange. If the CA activity increases the CO2 hydration rate and bicarbonate dehydration rate by 1000-fold, the drop of labelled CO2 (m/z=47) is too small to be measured (Fig. 5C). With 106- and 107-fold increases in the catalysis rate, the drop and time course is similar, and has reached its maximum indicating, as in Supplementary Fig. S2, that a 106-fold increase is sufficient to achieve complete isotopic exchange of internal Ci species with water.
The curve fitting of our experimental time courses has been performed with a biochemical network simulation program, COPASI (Fig. 6), using the ODEs presented in Supplementary Fig. S3. This approach was used to obtain the values of CO2 permeability and bicarbonate permeability reported in Table 1. However, it has also been possible to estimate CO2 permeability graphically from chloroplast injection time courses as shown in Supplementary Fig. S4. Values obtained with this empirical method have been compared with values assumed in the modelled time course shown in Fig. 5A, and show a very strong linear correlation (Supplementary Fig. S6). We used this technique to determine values rapidly for a number of experiments. Supplementary Fig. S7 shows that our assay methods appear valid for a wide range of chlorophyll concentrations per assay (0.2–3 µg Chl ml–1) but there is a curvilinear relationship when injection volumes are increased (Supplementary Fig. S8) and this is evident above 10 µl per 600 µl assay. Our assay procedures used <10 µl of chloroplast solution per assay.
Measurements from a range of species
We have performed CO2 and bicarbonate permeability assays on chloroplasts from a range of different C3 plants. As described previously, we have determined precisely the surface of the interface between the outside and the inside (stroma and thylakoids of chloroplast). With our isolation conditions, all isolated chloroplasts have a similar size for the different species tested (Fig. 4) and so surface areas per assay are comparable in our experiments. Where bicarbonate permeability is nearly identical for all species at 10–8 m s–1, CO2 permeability varies from 2.3 × 10–4 m s–1 for Arabidopsis to 8.0 × 10–4 m s–1 for pea.
For comparison, we have also tested our method on yeast cells overexpressing human CA internally. We have checked that CA activity was sufficient to carry out our method (Supplementary Fig. S9), and the time course with the highly enriched Ci (in 18O) is similar to the chloroplasts, with an activity estimated as a >106-fold increase. In yeast, bicarbonate permeability was low, similar to chloroplasts, but CO2 permeability is higher at 1 × 10–3 m s–1.
Arabidopsis aquaporin knockouts
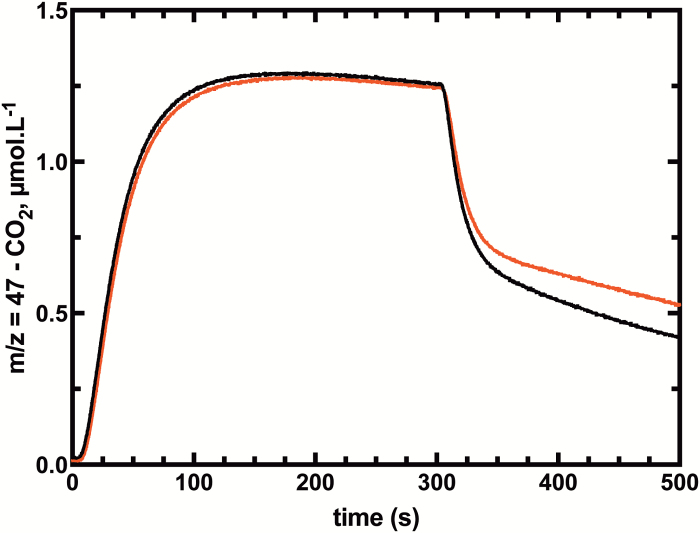
In order to test our method on estimating CO2 permeability, we chose an Arabidopsis mutant that has been described to have a lower CO2 permeability, atpip1;2-1 (Heckwolf et al., 2011). This is a knockout mutant of an aquaporin, orthologous to NtAQP1 which is proposed to facilitate CO2 diffusion across membranes (Uehlein et al., 2008). Despite the lack of absolute evidence of the localization of NtAQP1, different proteomic studies have found it in the chloroplast envelope (Beebo et al., 2013). In our experiments, the decrease in 13C18O16O (m/z=47) species in the assay after the injection of chloroplasts is lower for the mutant atpip1;2-1 than for the wild type (Fig. 7). This difference results in a calculated CO2 permeability of atpip1;2-1 which is half that of the wild type at 10–4 m s–1 (Table 2).
Fig. 7.
Time course of 13C18O16O (m/z=47) species for the wild type and PIP1.2 mutants. Low 18O-enriched Ci (2.5 mmol l–1) was injected at t=5 s, and chloroplasts from the wild type (black) or PIP1,2 (red) were injected at t=300s after chemical equilibrium had been reached. Final chlorophyll concentrations were 1.9 μg ml–1 and 2.0 μg ml–1 for the wild type and mutant, respectively.
Table 2.
Measurements of chloroplast permeability for the Arabidopsis thaliana wild type and atpip1;2-2 mutant
Chloroplast permeabilities were determined using the same principle as described for Table 1; shown are means ± SDs (n=4 different chloroplast isolations from four different plants)
| Chloroplast permeability | Wild type | atpip1;2-1 |
|---|---|---|
| (m s–1) | 2.27 × 10–4±6.6 × 10–5 | 1.02 × 10–4±5.9 × 10–5 |
| (m s–1) | 1.0 × 10–8±5 × 10–10 | 1.0 × 10–8±7 × 10–10 |
Discussion
This study demonstrates that MIMS-18O Ci exchange techniques can be used to measure the CO2 and permeabilities of isolated chloroplasts. For simple measurements of permeability, it is most convenient to use low 18O-enriched Ci species where changes in singly labelled CO2 (m/z=47) are followed, as it allows simpler mathematics for curve fitting and parameter estimation. However, the use of highly 18O-enriched Ci species can be complementary when establishing the validity of assumptions concerning internal CA activity. The measurements with isolated chloroplasts from a range of species give values that are close to those estimated to be necessary to support measured CO2 gas exchange within the chloroplast in the C3 leaf supported by passive CO2 diffusion (Evans et al., 2009; Tholen and Zhu, 2011). However, a range of values was obtained from different species (8 × 10–4–2.3 × 10–4 m s–1) which indicates either real variation between species or other variable limitations of the technique which are discussed below. In addition to measuring CO2 permeability, the technique also established that all chloroplasts had a consistently low permeability to (10–8 m s–1) which has never been directly measured before, although this has been inferred from previous studies of Ci accumulation within the chloroplast following the pH gradient between the inside and outside (Werdan and Heldt, 1972)
Our results with yeast allow us to compare our techniques with others in the literature. It is possible to find different values for CO2 permeability ranging from 5 × 10–5 m s–1 (Heckwolf et al., 2011) to 1.7 × 10–4 m s–1 (Navarro-Ródenas et al., 2012), passing through 10–4 m s–1 (Ding et al., 2013). Those three studies employ the method of stopped flow based on intracellular pH changes caused by CO2 injection. In this study using the MIMS-18O approach, we are measuring a of ~10–3 m s–1 which is 10 times higher than in these previous studies. The particular characteristics and limitations of the various measurement techniques have been previously reviewed (Endeward et al., 2014), and limitations with time resolution reduce the CO2 permeabilities derived from the stopped flow technique. The MIMS-18O technique has fewer limitations with time resolution but there are other inherent sources of error, which need to be considered, and these are discussed below.
Our model to calculate CO2 and permeabilities of isolated chloroplasts is based on a number of important assumptions. A major assumption concerns the chloroplast CA activity, which needs to be sufficient for complete isotopic equilibration of Ci species and water within the chloroplast. To validate this hypothesis, we used highly enriched [18O]bicarbonate in our experiments and modelling of the impact of CA activity variation on predicted assay time courses. Modelling shows that a >105-fold increase in the Ci–water interconversion rate over the uncatalysed rate is needed to achieve this both with low and highly enriched Ci techniques (Figs 2, 3; Supplementary Fig. S2). Experiments with tobacco chloroplasts indicate that there is sufficient CA within an isolated C3 chloroplast to achieve this (Fig. 3) and is consistent with calculations which indicate in tobacco that CA activity in the chloroplast is sufficient to speed up interconversion by >4 × 105-fold (Price et al., 1994). If, however, stromal CA was to be limiting, Fig. 5 shows that this would result in underestimating the true value. There have been examples where the in vivo limiting CA activity level has been estimated by model fitting procedures and corrected values obtained (Endeward and Gros, 2005). However, if CA activity levels are in fact significantly limiting, then this approach is likely to introduce its own errors. We have avoided doing this and prefer to verify the existence of sufficient CA levels where this correction does not need to be made.
Another important assumption is that we can precisely determine chloroplast surface area separating the external compartment from the stroma, as this is the interface at which transport and diffusion of CO2 and occur and is a significant calculation input. To achieve this, we chose a flow cytometry approach to obtain an average chloroplast size measurement on a large sampling of the isolated chloroplast preparation. However, in addition to chloroplast counts, it is also important to approximate the geometric shape of the chloroplasts for surface area calculations. This approximation can introduce a bias in our permeability value. In our set of data, the choice of a spherical model instead of an elliptical model would decrease the permeability value by ~4%. However, we chose the spherical model with regard to the images from optical microscopy and indications from the ratio (~1) between the side scatter and the forward scatter in flow cytometry.
In reality, a leaf is composed of different cell layers and types, which contain chloroplasts of different shape and/or size. As measurements shown here are derived from chloroplasts isolated using a particular technique, this may introduce biases. Chloroplasts are being isolated from all leaf cell types, so we have a heterogeneous population of chloroplasts in our preparation as shown in the size distribution (Fig. 4). Our permeability values are an average of all the chloroplasts in the cuvette so there will be an attenuation of any differences in chloroplast permeability between different tissues. In addition, the isolation procedure itself could favour the isolation of a subpopulation of chloroplasts which are more resistant to the isolation protocol, and so obtain the permeability of one specific class of chloroplast. Differences in between the species in Table 1 may in part be due to some of the isolation biases and their differences between species.
We have ignored the influence of unstirred layers both inside and outside the chloroplast which have been identified as factors influencing the MIMS assay (Endeward and Gros, 2009), which have the potential to lead to underestimation of permeabilities of red blood cells by ~30%. However, we have considered their influence to be minor in our assays. In addition, we ignored any influence of H218O accumulation within the chloroplast and the presence of 0.2% natural abundance in the reaction water. We have calculated the latter assumption to reduce PCO2 values by ~20% (data not shown).
Given the observed time courses of the permeability assays and the predicted modelling, the MIMS-18O technique shows good sensitivity for the measurement of values. Given the dimensions of chloroplasts and chlorophyll concentrations of 0.2–3 μg Chl ml–1, we roughly estimate that the technique provides the ability to resolve values in the range of 10–2–10–5 m s–1, which is well within the calculated values for the isolated chloroplasts. The situation for resolving potential variation in permeabilities is perhaps more limited. The technique certainly establishes that the chloroplast is relatively impermeable to , but modelling would appear to show that it is difficult to resolve differences for below 10–6 m s–1.
The measurement technique used here, as is also the case for other methods, obviously requires that assays be conducted in the dark, as uptake of CO2 species by Rubisco in the light would make the technique unworkable. This raises the question of whether the envelope interface properties of the chloroplasts are affected by light. For example, if there was light activation of a CO2-conducting aquaporin then this would not be easily detected and could lead to the underestimation of values. Similar arguments could be made for a light-stimulated pathway for entry if this existed.
Despite these potential errors, the observed values are approaching the range which has been predicted from C3 leaf gas exchange measurements and modelling to be necessary to explain photosynthetic CO2 flux rates based on passive CO2 diffusion assumptions and models (Evans et al., 2009; Tholen and Zhu, 2011). Previous measurements of values for C3 chloroplasts using stopped flow techniques have reported values which are 1–2 orders of magnitude lower than those necessary for photosynthetic CO2 fluxes (Uehlein et al., 2008). This indicates that the MIMS-18O technique has the ability to reveal chloroplast permeability values which are more realistic despite the limitations discussed above. In this context, we can readily detect differences in between Arabidopsis chloroplasts isolated from the wild type and atpip1,2 mutants (Table 2).
Supplementary data
Supplementary data are available at JXB online.
Fig. S1. The MIMS assay cuvette design.
Fig. S2. The modelled effects of variation in internal chloroplast carbonic anhydrase activity on assays using highly enriched [18O]bicarbonate equilibrated against 99% [18O]water.
Fig. S3. Differential equations used for numerical modelling of time courses for changes in singly labelled CO2 species.
Fig. S4. Differential equations used for numerical modelling of time courses for changes in highly enriched [18O]bicarbonate equilibrated against 99% [18O]water in Fig. S2.
Fig. S5. Estimation of from chloroplast injection time courses as shown in Fig. 2.
Fig. S6. Empirical verification of the graphical estimation procedure for the method and equation shown in Fig. S3.
Fig. S7. Correlation between chloroplast number (chlorophyll concentration) and determination of permeability values before correction by chloroplast number.
Fig. S8. Correlation between volume of chloroplasts injected in the MIMS cuvette and the drop in 13C18O16O (m/z=47) at the injection.
Fig. S9. Typical time course for a highly 18O-enriched assay with yeast injection.
Table S1. Parameters and their units and values for the model equations used in Fig. S3.
Table S2. Parameters and their units and values for the model equations used in Fig. S4.
Data deposition
COPASI files of numerical modelling used for the generation of time courses for changes in labeled CO2 species. Dryad Digital Repository. http://dx.doi:10.5061/dryad.2r05d.
Supplementary Material
Acknowledgements
We would like to thank Hannah L. Osborn for providing the yeast strain, and Dr Harpreet Vohra, from the Imaging & Cytometry Facility of The John Curtin School of Medical Research, for her expertise on and teaching of flow cytometry. This work was supported by the University of Illinois as part of the Bill and Melinda Gates Foundation-funded Realizing Increased Photosynthetic Efficiency (RIPE) consortium, and the Australian Research Council’s Centre of Excellence for Translational Photosynthesis. The authors declare no conflict of interest
Glossary
Abbreviations:
- CA
carbonic anhydrase
- CCM
CO2-concentrating mechanism
- Chl
chlorophyll
- Ci
inorganic carbon
- FSC
forward scatter
- MIMS
membrane inlet mass spectrometry
- SSC
side scatter.
References
- Beebo A, Mathai JC, Schoefs B, Spetea C. 2013. Assessment of the requirement for aquaporins in the thylakoid membrane of plant chloroplasts to sustain photosynthetic water oxidation. FEBS Letters 587, 2083–2089. [DOI] [PubMed] [Google Scholar]
- Berkson J, Magath TB, Hurn M. 1940. The error of estimate of the blood cell count as made with the hemocytometer. American Journal of Physiology 128, 309–323. [Google Scholar]
- Boron WF, Endeward V, Gros G, Musa-Aziz R, Pohl P. 2011. Intrinsic CO2 permeability of cell membranes and potential biological relevance of CO2 channels. Chemphyschem 12, 1017–1019. [DOI] [PubMed] [Google Scholar]
- Ding X, Matsumoto T, Gena P, et al. 2013. Water and CO2 permeability of SsAqpZ, the cyanobacterium Synechococcus sp. PCC7942 aquaporin. Biology of the Cell 105, 118–128. [DOI] [PubMed] [Google Scholar]
- Endeward V, Al-Samir S, Itel F, Gros G. 2014. How does carbon dioxide permeate cell membranes? A discussion of concepts, results and methods. Frontiers in Physiology 4, 382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endeward V, Gros G. 2005. Low carbon dioxide permeability of the apical epithelial membrane of guinea-pig colon. Journal of Physiology 567, 253–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endeward V, Gros G. 2009. Extra- and intracellular unstirred layer effects in measurements of CO2 diffusion across membranes—a novel approach applied to the mass spectrometric 18O technique for red blood cells. Journal of Physiology 587, 1153–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JR, Kaldenhoff R, Genty B, Terashima I. 2009. Resistances along the CO2 diffusion pathway inside leaves. Journal of Experimental Botany 60, 2235–2248. [DOI] [PubMed] [Google Scholar]
- Flexas J, Niinemets U, Gallé A, et al. 2013. Diffusional conductances to CO2 as a target for increasing photosynthesis and photosynthetic water-use efficiency. Photosynthesis Research 117, 45–59. [DOI] [PubMed] [Google Scholar]
- Guillard RRL, Sieracki MS. 2005. Counting cells in cultures with the light microscope. In: Andersen R, ed. Algal culturing techniques. New York: Academic Press, 239–267. [Google Scholar]
- Hatch MD. 1987. C-4 photosynthesis—a unique blend of modified biochemistry, anatomy and ultrastructure. Biochimica et Biophysica Acta 895, 81–106. [Google Scholar]
- Heckwolf M, Pater D, Hanson DT, Kaldenhoff R. 2011. The Arabidopsis thaliana aquaporin AtPIP1;2 is a physiologically relevant CO2 transport facilitator. The Plant Journal 67, 795–804. [DOI] [PubMed] [Google Scholar]
- Hoops S, Sahle S, Gauges R, Lee C, Pahle J, Simus N, Singhal M, Xu L, Mendes P, Kummer U. 2006. COPASI—a COmplex PAthway SImulator. Bioinformatics 22, 3067–3074. [DOI] [PubMed] [Google Scholar]
- Itada N, Forster RE. 1977. Carbonic anhydrase activity in intact red blood cells measured with 18O exchange. Journal of Biological Chemistry 252, 3881–3890. [PubMed] [Google Scholar]
- Itel F, Al-Samir S, Öberg F, et al. 2012. CO2 permeability of cell membranes is regulated by membrane cholesterol and protein gas channels. FASEB Journal 26, 5182–5191. [DOI] [PubMed] [Google Scholar]
- Kai L, Kaldenhoff R. 2014. A refined model of water and CO2 membrane diffusion: effects and contribution of sterols and proteins. Scientific Reports 4, 6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinney G. 1941. Absorption of light by chlorophyll solutions. Journal of Biological Chemistry 140, 315–322. [Google Scholar]
- Missner A, Kügler P, Saparov SM, Sommer K, Mathai JC, Zeidel ML, Pohl P. 2008. Carbon dioxide transport through membranes. Journal of Biological Chemistry 283, 25340–25347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourioux G, Douce R. 1981. Slow passive diffusion of orthophosphate between intact isolated chloroplasts and suspending medium. Plant Physiology 67, 470–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro-Ródenas A, Ruíz-Lozano JM, Kaldenhoff R, Morte A. 2012. The aquaporin TcAQP1 of the desert truffle Terfezia claveryi is a membrane pore for water and CO(2) transport. Molecular Plant-Microbe Interactions 25, 259–266. [DOI] [PubMed] [Google Scholar]
- Price G, Von Caemmerer S, Evans J, Yu J, Lloyd J, Oja V, Kell P, Harrison K, Gallagher A, Badger MR. 1994. Specific reduction of chloroplast carbonic anhydrase activity by antisense RNA in transgenic tobacco plants has a minor effect on photosynthetic CO2 assimilation. Planta 193, 331–340. [Google Scholar]
- Pringle JR, Mor JR. 1975. Methods for monitoring the growth of yeast cultures and for dealing with the clumping problem. Methods in Cell Biology 11, 131–168. [DOI] [PubMed] [Google Scholar]
- Raven JA, Beardall J. 2016. The ins and outs of CO2. Journal of Experimental Botany 67, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman DN. 1974. New approach to measuring rate of rapid bicarbonate exchange across membranes. Molecular Pharmacology 10, 820–836. [Google Scholar]
- Silverman DN, Tu C, Wynns GC. 1976. Depletion of 18O from C18O2 in erythrocyte suspensions. The permeability of the erythrocyte membrane to CO2. Journal of Biological Chemistry 251, 4428–4435. [PubMed] [Google Scholar]
- Tholen D, Ethier G, Genty B, 2014. Mesophyll conductance with a twist. Plant, Cell and Environment 37, 2456–2458. [DOI] [PubMed] [Google Scholar]
- Tholen D, Ethier G, Genty B, Pepin S, Zhu XG. 2012. Variable mesophyll conductance revisited: theoretical background and experimental implications. Plant, Cell and Environment 35, 2087–2103. [DOI] [PubMed] [Google Scholar]
- Tholen D, Zhu XG. 2011. The mechanistic basis of internal conductance: a theoretical analysis of mesophyll cell photosynthesis and CO2 diffusion. Plant Physiology 156, 90–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolleter D, Chochois V, Poiré R, Price GD, Badger MR. 2017. Data from: Measuring CO2 and permeabilities of isolated chloroplasts using a MIMS-18O approach. Dryad Digital Repository. http://dx.doi:10.5061/dryad.2r05d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehlein N, Otto B, Hanson DT, Fischer M, McDowell N, Kaldenhoff R. 2008. Function of Nicotiana tabacum aquaporins as chloroplast gas pores challenges the concept of membrane CO2 permeability. The Plant Cell 20, 648–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werdan K, Heldt HW. 1972. Accumulation of bicarbonate in intact chloroplasts following a pH gradient. Biochimica et Biophysica Acta 283, 430–441. [DOI] [PubMed] [Google Scholar]
- Wunder M, Bollert P, Gros G. 1998. Mathematical modelling of the role of intra- and extracellular activity of carbonic anhydrase and membrane permeabilities of , H2O and CO2 in O-18 exchange. Isotopes in Environmental and Health Studies 34, 197–205. [Google Scholar]
- Wunder MA, Gros G. 1998. 18O exchange in suspensions of red blood cells: determination of parameters of mass spectrometer inlet system. Isotopes in Environmental and Health Studies 34, 303–310. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.