Summary
Respiratory mucosal immunization of humanized mice with an adenoviral-vectored tuberculosis vaccine induces antigen-specific human T-cell responses in the lung and, compared with parenteral immunization, superior protection against pulmonary Mycobacterium tuberculosis infection in a human T-cell–dependent manner.
Keywords: Tuberculosis, humanized mice, viral-vectored vaccines, respiratory mucosal immunization.
Abstract
Background.
The translation of preclinically promising novel tuberculosis vaccines to ultimate human applications has been challenged by the lack of animal models with an immune system equivalent to the human immune system in its genetic diversity and level of susceptibility to tuberculosis.
Methods.
We have developed a humanized mice (Hu-mice) tuberculosis model system to investigate the clinical relevance of a novel virus-vectored (VV) tuberculosis vaccine administered via respiratory mucosal or parenteral route.
Results.
We find that VV vaccine activates T cells in Hu-mice as it does in human vaccinees. The respiratory mucosal route for delivery of VV vaccine in Hu-mice, but not the parenteral route, significantly reduces the humanlike lung tuberculosis outcomes in a human T-cell–dependent manner.
Conclusions.
Our results suggest that the Hu-mouse can be used to predict the protective efficacy of novel tuberculosis vaccines/strategies before they proceed to large, expensive human trials. This new vaccine testing system will facilitate the global pace of clinical tuberculosis vaccine development.
Tuberculosis is the leading infectious cause of mortality worldwide [1]. The BCG vaccine, the only licensed tuberculosis vaccine, has been used globally for >6 decades but is suboptimal against pulmonary tuberculosis [2]. Thus, there is a need to develop novel tuberculosis vaccines and vaccination strategies that can be used to replace the BCG vaccine or boost BCG-induced protective immunity in the lung [3, 4].
Preclinical vaccine evaluation in conventional animal models has helped screen for promising novel tuberculosis vaccines and vaccination strategies, thus representing a critical step toward clinical development [4–6]. However, humans are the sole natural host of Mycobacterium tuberculosis, and their genetic makeup, immune system, and anti–M. tuberculosis responses are much different from those in other animal species [7]. As a result, whether or not a promising tuberculosis vaccine may improve tuberculosis protection in humans remains unknown until the large costly efficacy trials are completed [8, 9].
The most advanced novel tuberculosis vaccine, MVA85A, which has undergone the canonical preclinical and clinical assessment process for >10 years, has been shown to provide no enhanced protection in a human efficacy trial [7–10]. This represents a long, resource-consuming journey of vaccine assessment and has been one of the major bottlenecks in clinical tuberculosis vaccine development. This situation emphasizes the development and application of novel and affordable preclinical tuberculosis animal models. The humanized mouse (Hu-mouse) model that closely recapitulates human immune system has emerged as an attractive surrogate model system for human infectious disease research [11–14]. Hu-mice are highly susceptible to M. tuberculosis infection and generate humanlike T-cell responses as well as immunopathologic lung tissue findings seen in human tuberculosis [12, 13, 15]. However, their value for evaluating novel tuberculosis vaccines and/or vaccination strategies has remained unexplored.
In the current study, we show the model of M. tuberculosis–infected Hu-mice to be a suitable human surrogate for interrogating the potency of novel tuberculosis vaccines/concepts. Using this model, we find that a novel virus-vectored (VV) tuberculosis vaccine, developed in our laboratory and with recently completed phase I human studies, activates T cells in Hu-mice as it does in human vaccinees [16, 17]. The respiratory mucosal (RM) route for delivery of VV vaccine in Hu-mice, but not the parenteral route, significantly reduces humanlike lung tuberculosis outcomes in a human T-cell–dependent manner. This new vaccine testing system will facilitate the global pace of clinical tuberculosis vaccine development.
MATERIALS AND METHODS
Detailed methods are provided in the online Supplement.
Generation of Hu-Mice
Humanized NOD-Rag1tm1MomIl2rgtm1Wjl (NRG) mice were generated as described elsewhere [18] (Supplementary Figure S1).
Immunization of Hu-Mice
Hu-mice were immunized intranasally or intramuscularly with AdHu5Ag85A [17, 19]. The BCG vaccine was prepared as described elsewhere [20] and delivered subcutaneously.
Pulmonary Infection With M. tuberculosis
M. tuberculosis was prepared as described elsewhere [21]. Hu-mice were infected with M. tuberculosis via the respiratory route.
Depletion of Human CD4+ and CD8+ T Cells
OKT-4 (100 μg) and OKT-8 (50 μg) monoclonal antibodies were injected intraperitoneally to deplete human CD4+ and CD8+ T cells [13].
Isolation of Mononuclear Cells
Peripheral blood mononuclear cells and bronchoalveolar lavage, lung, and spleen specimens were obtained as described elsewhere [19, 20].
Intracellular Cytokine Staining and Flow Cytometry
Intracellular cytokine staining was performed with T cells after ex vivo stimulation with live BCG. Stained cells were acquired on a LSR II cytometer, and data were analyzed using FlowJo software version 10 (TreeStar, Ashland, OR, USA).
Measurement of Tuberculosis Disease Outcome Indices
Illness score, lung and spleen bacterial load, and lung acid-fast bacilli, gross pathology, and histopathology scores were determined 4 weeks after M. tuberculosis infection [13, 22].
Immunohistochemical Visualization of Human CD4+, CD8+, and CD68+ Cells in Lungs
Immunohistochemical staining of human CD4, CD8, and CD68 was performed on deparaffinized sections by using anti–human CD4, CD8, and CD68 monoclonal antibodies.
Statistical Analysis
Two-tailed Student t tests for comparison between 2 groups and 1-way analysis of variance followed by post-test Tukey analysis for multiple-group comparison were performed using GraphPad Prism software. Results were considered significant for P values ≤.05, and approaching significance for P values ≤.10 but >.05.
RESULTS
Reconstitution of Human Immune Cells in Both Circulation and RM Tissue of Hu-Mice
To generate Hu-mice for the current study, human cord blood CD34-enriched hematopoietic stem cells were injected intrahepatically into sublethally irradiated newborn NRG mice (Supplementary Figure S1). At 90–120 days after hematopoietic stem cell injection, frequencies of human immune cells in the peripheral blood and the lung were analyzed using flow cytometry. Individual Hu-mice had varying frequencies of circulating human CD45+ leukocytes, human CD3+ T cells, and human CD14+ monocytes/macrophages in peripheral blood mononuclear cells (Supplementary Figure S2A). Their lung mucosal tissues were also well populated by human-origin leukocytes with >50% of human CD45+ mononuclear cells being CD3+ and 10% being CD14+ (Supplementary Figure S2B and S2C). Immunohistochemistry of lung sections revealed abundant human CD4+ and CD8+ T cells and human CD68+ macrophages throughout the entire lung (Supplementary Figure S2D). Thus, the Hu-mice used in this study demonstrate reconstitution of human immune cells not only in the circulation but also in the respiratory mucosa.
Significantly Enhanced Protection Against Pulmonary Tuberculosis Disease Outcomes by Parenteral BCG Immunization in Hu-Mice
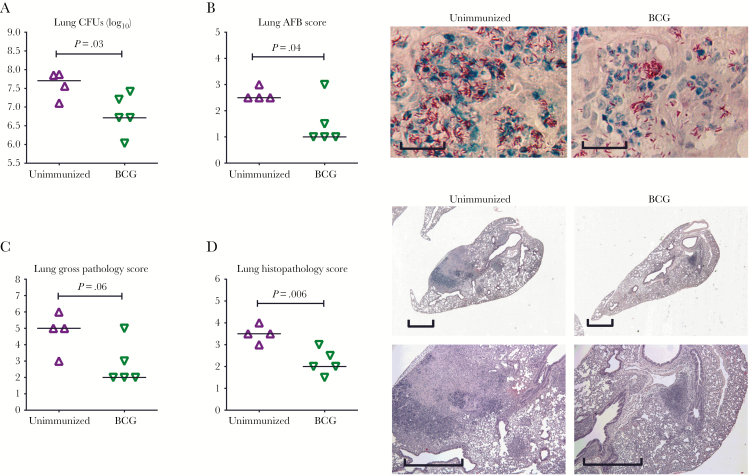
With major immune cell composition characterized in Hu-mice, as the first step we determined the potential value of this model for testing the efficacy of novel tuberculosis vaccines and concepts by parenteral delivery of BCG vaccine, the only approved tuberculosis vaccine with well-evidenced but suboptimal lung protection [23]. Hu-mice were immunized subcutaneously with BCG vaccine for 4 weeks and subsequently infected with M. tuberculosis. Compared with unimmunized animals, BCG-immunized animals were significantly better protected, as exemplified by improved illness scores (Supplementary Figure S3), and significantly reduced M. tuberculosis burden (Figure 1A and 1B), gross pathologic changes (Figure 1C), and microscopic granulomatous lesions (Figure 1D) in the lung. These data suggest that BCG vaccine, as a human vaccine and when tested in Hu-mice, provides a degree of lung protection as observed in humans, lending strong support to the value of this humanized model for testing novel tuberculosis vaccines and vaccination strategies.
Figure 1.
Pulmonary tuberculosis disease outcomes in humanized mice (Hu-mice) immunized parenterally with BCG vaccine. Animals were infected with Mycobacterium tuberculosis at 1 × 104 colony-forming units (CFUs) per animal via the pulmonary route at 4 weeks after BCG immunization and euthanized for assessment of tuberculosis disease outcome indices 4 weeks after infection. A, Scatterplot comparing M. tuberculosis burden in the lung, assessed by CFU assay. B, Scatterplot (left) comparing M. tuberculosis burden by scoring the density of acid-fast–stained M. tuberculosis bacilli and representative micrographs of acid-fast–stained (red) M. tuberculosis bacilli in lung tissue sections. Scale bar indicates 50 µm. C, Scatterplots comparing gross lung pathology scores. D, Scatterplots of scores for microscopic histopathologic changes in the lung and representative micrographs of lung sections stained with hematoxylin-eosin, comparing the extent of granulomatous lesions and necrosis. Scale bar represents 1 mm. Horizontal lines in all scatterplots represent median values. Data are representatives of 2 independent experiments, with 4 animals per unimmunized group and 5 animals per BCG vaccine group.
Markedly Increased M. tuberculosis–Specific Human CD4+ T-Cell Responses in Hu-Mice Lungs After RM Immunization
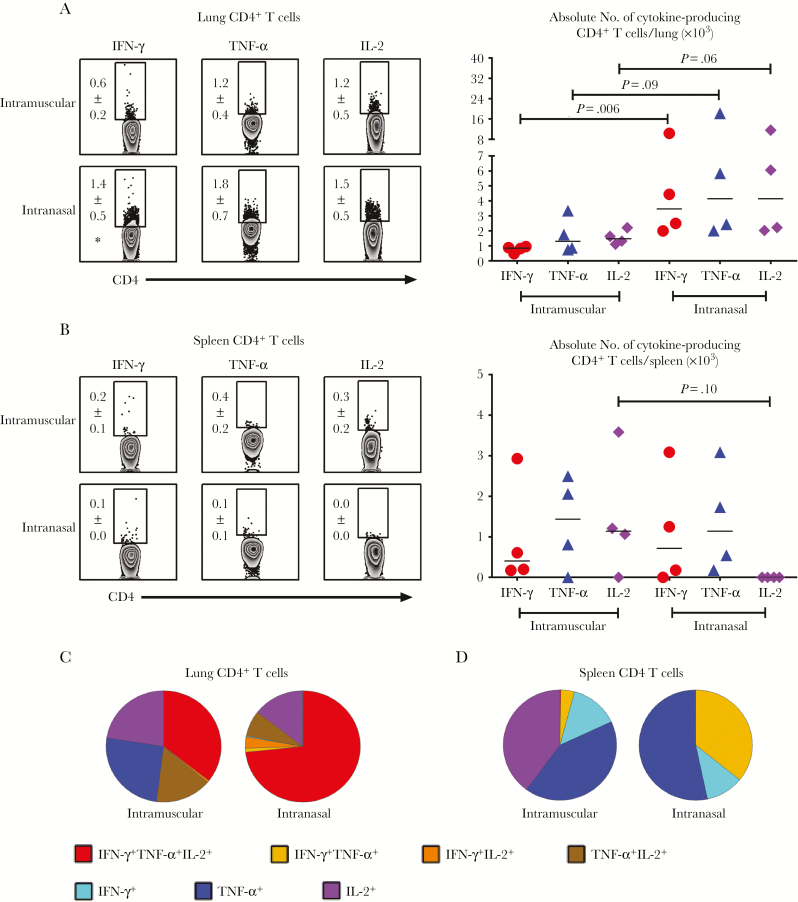
To begin evaluating novel tuberculosis vaccines/strategies in Hu-mice, we examined human T-cell responses after parenteral (intramuscular) or RM (intranasal) immunization with a novel adenoviral-vectored (VV) vaccine expressing an immunodominant M. tuberculosis antigen (Ag), Ag85A. Although early-phase human studies have been completed for this promising VV vaccine [16, 17], whether it enhances protection in humans remains completely unknown. Thus, 4 weeks after parenteral and RM immunization, quantities of Ag-specific cytokine-producing CD4+ and CD8+ T cells were analyzed in bronchoalveolar lavage, lung, and spleen specimens. We found that animals immunized via both routes lacked T cells in the airway (Supplementary Figure S4A). Total T-cell numbers in the lung and spleen did not differ significantly between parenterally and RM-immunized animals (Supplementary Figure S4B and S4C).
Immunization induced a predominantly M. tuberculosis Ag–specific CD4+ T-cell response in the lung (Figure 2A) and spleen (Figure 2B), compared with negligible CD8+ T-cell responses (Supplementary Figure S5A and S5B). In contrast, unimmunized Hu-mice lacked Ag-specific T cells (Supplementary Figure S6). Compared with parenteral immunization, RM immunization induced significantly greater Ag-specific CD4+ T-cell responses in the lung parenchyma, particularly interferon (IFN) γ+ CD4+ T-cell responses (Figure 2A). On the other hand, parenteral immunization seemed to induce more Ag-specific interleukin 2 (IL-2)+ CD4+ T cells in the spleen (Figure 2B).
Figure 2.
Characterization of Mycobacterium tuberculosis antigen (Ag)–specific polyfunctional human CD4+ T-cell responses after viral-vectored (VV) immunization. A, Representative dot plots showing frequencies (means with standard errors of the mean) and scatterplot showing absolute numbers of M. tuberculosis Ag–specific interferon (IFN) γ+, tumor necrosis factor (TNF) α+, and interleukin 2 (IL-2)+ CD4+ T cells in the lungs of animals immunized intramuscularly or intranasally. *P < .05 (compared with intramuscular immunization). B, Representative dot plots showing frequencies (means with standard errors of the mean) and scatterplot showing absolute numbers of M. tuberculosis Ag–specific IFN-γ+, TNF-α+, and IL-2+ CD4+ T cells in the spleens of animals immunized intramuscularly or intranasally. Mononuclear cells isolated from these sites 4 weeks after VV immunization were stimulated ex vivo with mycobacterial Ags, stained intracellularly for cytokines, and analyzed with flow cytometry. C, D, Pie charts showing average proportions of M. tuberculosis Ag–specific CD4+ T cells expressing a single or a combination of 2 or 3 cytokines in the lung (C) and spleen (D) of animals immunized intramuscularly or intranasally. Data are representative of 2 independent experiments, with 4 animals per group.
Because T cells that coexpress multiple cytokines have been implicated in protection, we examined the polyfunctionality of Ag-specific CD4+ T cells. The majority of RM immunization–induced CD4+ T cells in the lung were polyfunctional, coexpressing IFN-γ, tumor necrosis factor (TNF) α, and IL-2 (red) (Figure 2C) or IFN-γ and TNF-α in the spleen (yellow) (Figure 2D), in contrast to predominantly monofunctional counterparts with parenteral immunization (Figure 2C and 2D). These data indicate that although VV tuberculosis immunization can engage human cells in Hu-mice to induce Ag-specific T-cell responses, RM immunization induces much stronger polyfunctional T-cell responses in the lung than parenteral immunization. These data show inducible antituberculosis T cells caused by VV immunization in Hu-mice before M. tuberculosis exposure.
Markedly Increased Secondary M. tuberculosis–Specific Human T-Cell Responses to Pulmonary M. tuberculosis Infection in RM-Immunized Hu-Mice
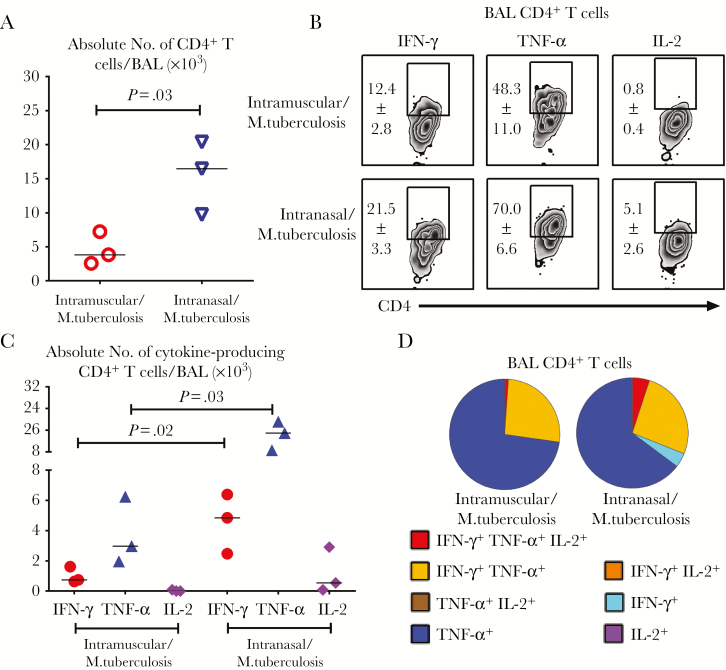
We next investigated to what extent such Ag-experienced human T cells could respond to pulmonary M. tuberculosis infection, because the secondary T-cell responses in the lung are key to tuberculosis protection. Contrary to the lack of airway T cells before M. tuberculosis infection (Supplementary Figure S4A), total CD4+ T cells were recruited to the airways of animals immunized either parenterally (intramuscularly) or via the RM (intranasally) within 6 days after M. tuberculosis infection (Figure 3A). However, compared with those immunized parenterally, infected animals immunized via the RM route had significantly more total CD4+ T cells in the airways (Figure 3A) and, importantly, greater frequencies (Figure 3B) and numbers (Figure 3C) of M. tuberculosis Ag–specific IFN-γ+, TNF-α+, or IL-2+ CD4+ T cells in the airways. Polyfunctional profiles of airway M. tuberculosis Ag–specific CD4+ T cells were similar for the 2 immunization routes (Figure 3D).
Figure 3.
Secondary Mycobacterium tuberculosis antigen (Ag)–specific human T-cell responses in the airway to pulmonary M. tuberculosis infection. A, Scatterplot showing numbers of total CD4+ T cells recruited to the airways (bronchoalveolar lavage [BAL] specimen) at 6 days after pulmonary M. tuberculosis infection (5 × 105 colony-forming units [CFUs] per animal) in humanized mice (Hu-mice) immunized parenterally (intramuscularly) or through the respiratory mucosa (intranasally). B, Representative dot plots showing frequencies (means with standard errors of the mean) of M. tuberculosis Ag–specific interferon (IFN) γ+, tumor necrosis factor (TNF) α+, and interleukin 2 (IL-2)+ CD4+ T cells among total CD4+ T cells in the airways of animals immunized intramuscularly or intranasally after pulmonary M. tuberculosis infection. C, Scatterplot showing numbers of M. tuberculosis Ag–specific cytokine-producing CD4+ T cells in the airways of animals immunized intramuscularly or intranasally after M. tuberculosis infection. D, Pie charts comparing average proportions of M. tuberculosis Ag–specific CD4+ T cells expressing a single cytokine or a combination of 2 or 3 cytokines in the airways (BAL specimen) of animals immunized intramuscularly or intranasally. Horizontal lines in A and C represent median values. Data are from a single experiment with 3 animals per group.
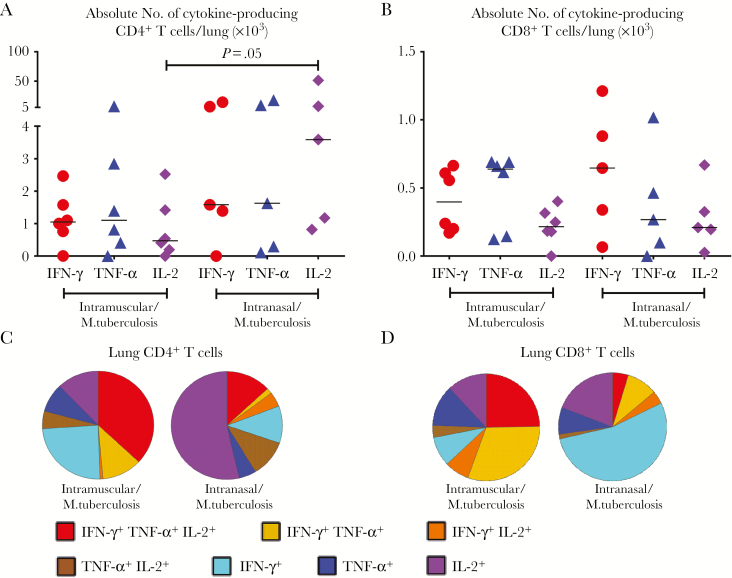
As in the airways, RM-immunized animals also had significantly more M. tuberculosis Ag–specific, particularly IL-2+, CD4+ T cells in their lung parenchyma, with more animals responding highly to infection, compared with parenterally immunized animals (Figure 4A). Contrary to the lack of CD8+ T-cell responses before infection (Supplementary Figure S5A), M. tuberculosis Ag–specific CD8+ T-cell responses in the lung were increased in both parenterally and RM-immunized animals (Figure 4B). In contrast to those in the airway, CD4+ T cells in the lung parenchyma of RM-immunized animals became monofunctional after infection (Figure 4C), after being polyfunctional before infection (Figure 2C). Lung CD8+ T cells in these animals were also mostly monofunctional (Figure 4D). These data suggest that VV vaccine–activated human T cells can respond well to pulmonary M. tuberculosis exposure. RM-immunized animals have much greater and qualitatively different T-cell responses compared with their parenterally immunized counterparts.
Figure 4.
Secondary respiratory mucosal Mycobacterium tuberculosis antigen (Ag)–specific human T-cell responses in the lung parenchyma to pulmonary M. tuberculosis infection. A, B, Scatterplots showing numbers of M. tuberculosis Ag–specific cytokine-producing CD4+ (A) and CD8+ (B) T cells in the lung parenchyma of animals immunized intramuscularly or intranasally 6 days after pulmonary M. tuberculosis infection (5 × 105 colony-forming units per animal). C, D, Pie charts comparing average proportions of M. tuberculosis Ag–specific CD4+ T (C) or CD8+ (D) T cells expressing a single cytokine or a combination of 2 or 3 cytokines in the lung parenchyma of animals immunized intramuscularly or intranasally. Horizontal lines in A and B represent the median values. Data are representative of 2 independent experiments, with 6 animals in the intramuscular and 5 in the intranasal immunization group.
Robust Secondary Human T-Cell Responses to Pulmonary M. tuberculosis Infection in RM-Immunized but Not Unimmunized Hu-Mice
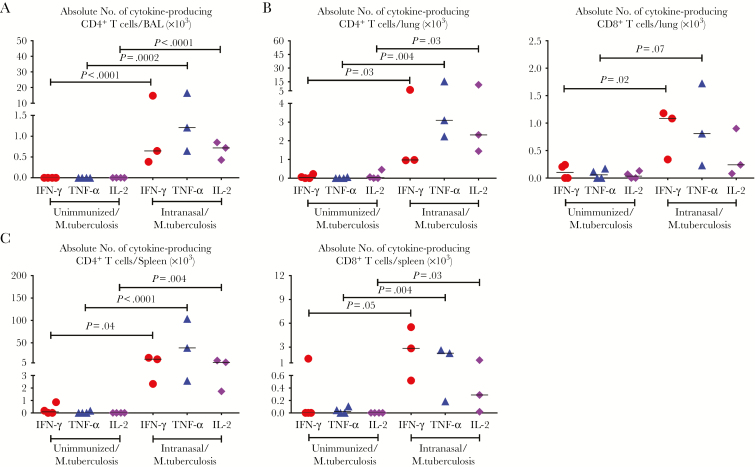
Thus far, we have demonstrated the superiority of RM immunization, compared with parenteral immunization, in human T-cell responses before and after M. tuberculosis exposure. To further examine the potency and establish a key role of RM immunization in the observed secondary T-cell responses in Hu-mice, we set up independent experiments to compare secondary T-cell responses in the lungs of RM-immunized Hu-mice with those in their unimmunized counterparts after pulmonary M. tuberculosis exposure. M. tuberculosis infection alone in unimmunized Hu-mice failed to induce any Ag-specific cytokine-producing CD4+ or CD8+ T-cell responses in either the airways (Figure 5A) or the lungs (Figure 5B) 6 days after M. tuberculosis exposure. The same held true in the spleen (Figure 5C).
Figure 5.
Antigen (Ag)–specific T-cell responses to pulmonary Mycobacterium tuberculosis infection in immunized humanized mice (Hu-mice) compared with unimmunized animals. Scatterplots compare total numbers of M. tuberculosis Ag–specific cytokine-producing CD4+ T cells in the airways (A), CD4+ (B, left) and CD8+ (B, right) T cells in the lung parenchyma, and spleen (C) 6 days after pulmonary M. tuberculosis infection (5 × 105 colony-forming units per animal). Horizontal lines represent median values. Data presented are from 1 experiment, with 3 animals per group. These are exact 4-digit P values with those ≥.0010 rounded to the first non-zero digit.
In stark contrast, RM-immunized animals generated robust, rapid secondary M. tuberculosis Ag–specific CD4+ and CD8+ T-cell responses, with significantly increased IFN-γ+, TNF-α+, or IL-2+ CD4+ and CD8+ T cells seen not only in the airways and lung of RM-immunized animals, but also in a distal tissue site, the spleen (Figure 5A–5C). These data lend further evidence that VV-based RM immunization robustly activates human T cells capable of rapid secondary responses to pulmonary M. tuberculosis infection in Hu-mice.
Markedly Improved T-Cell–Dependent Antituberculosis Immune Protection by RM Immuni‑zation in Hu-Mice
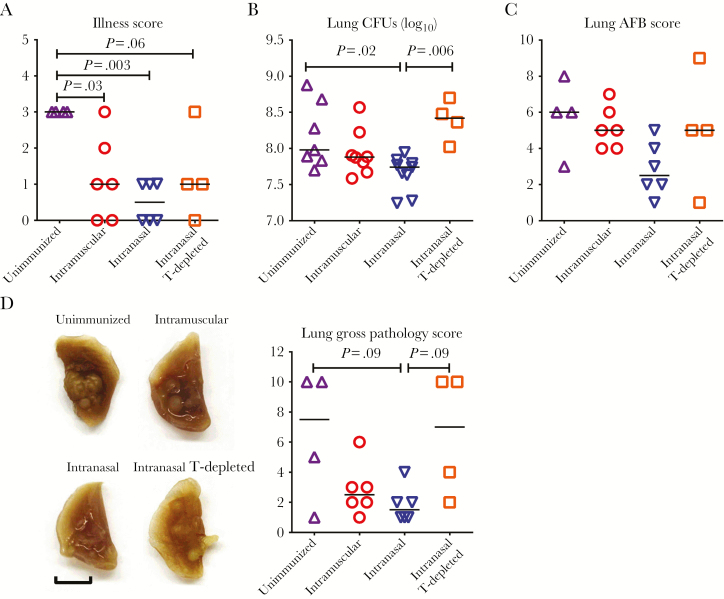
Next, to investigate whether VV vaccine–activated antituberculosis human T cells could improve protection against pulmonary M. tuberculosis infection, we assessed a set of tuberculosis disease outcomes, including illness scores, lung M. tuberculosis bacillary burden, and lung macroscopic and microscopic pathologic changes. Compared with findings in unimmunized animals, both RM (intranasal) and parenteral (intramuscular) immunization routes reduced illness scores after M. tuberculosis infection, RM immunization the most significantly (Figure 6A). In keeping with this finding, RM-immunized animals had significantly reduced M. tuberculosis burden in the lung, as assessed by M. tuberculosis colony-forming unit (CFU) assay (Figure 6B), accompanied by decreased acid-fast-stained bacilli in the lung (Figure 6C and Supplementary Figure S7).
Figure 6.
Pulmonary tuberculosis disease outcomes in immunized humanized mice (Hu-mice) and the requirement of vaccine-induced immunity for protection. A, Scatterplot comparing illness scores of unimmunized animals, animals immunized parenterally (intramuscularly) or via the respiratory mucosa (intranasally), and animal immunized intranasally and depleted of human CD4+ and CD8+ T cells (T-depleted). All animals were infected with Mycobacterium tuberculosis (1 × 104 colony-forming units [CFUs] per animal) via the pulmonary route 4 weeks after viral-vectored immunization and euthanized for assessment of tuberculosis disease outcome indices 4 weeks after infection. Illness scoring was carried out using a rating model in which 0 indicated no symptoms; 1, mild piloerection; 2, marked piloerection and reduced activity; 3, hunched and less active; and 4, lethargic. B, Scatterplot comparing M. tuberculosis burden in the lung, assessed by CFU assay. C, Scatterplot comparing M. tuberculosis burden by scoring the density of acid-fast–stained M. tuberculosis bacilli (AFB). D, Representative lung lobes showing the degree of gross pathologic change, indicated by the size and the number of tuberculosis lesion nodules. Scale bar represents 5 mm. Scatterplot compares gross lung pathology scores. Horizontal lines in all scatterplots represent median values. Data presented in A, C, and D are pooled from 2 independent experiments, with the indicated number of animals per group. Data in B are pooled from 3 independent experiments, with the indicated number of animals per group.
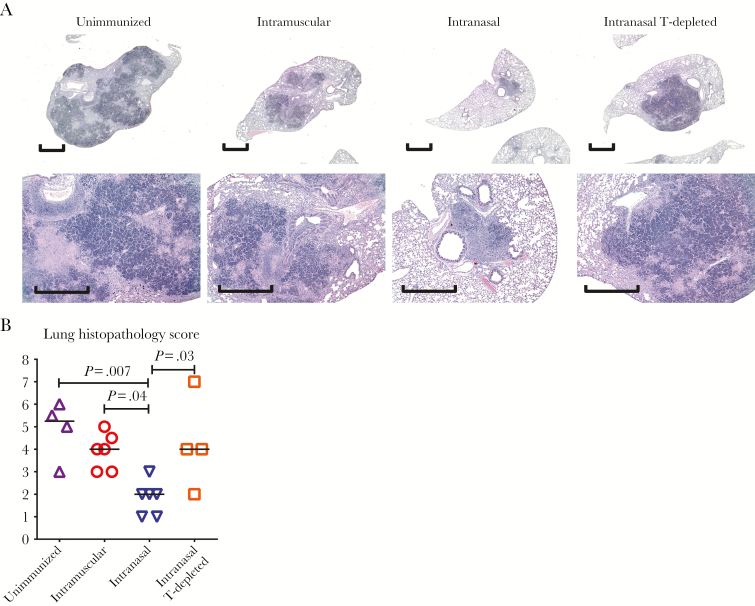
In contrast, the M. tuberculosis burdens in the lungs of parenterally immunized animals were unreduced, being as high as in unimmunized controls, about 8 log CFUs (Figure 6B and 6C and Supplementary Figure S7). Such magnitude of infection in Hu-mice was 2 logs higher than the usual levels of infection in the lung of conventional unimmunized mice, indicating their high sensitivity to M. tuberculosis, as in humans. Macroscopically, compared with large and smaller nodules visible on the lungs of unimmunized and parenterally immunized animals, respectively, the smallest lung nodules were seen in RM-immunized animals (Figure 6D). Microscopically, the lungs of unimmunized animals displayed extensive granulomatous tuberculosis lesions, characterized by solidification, giant mononuclear cell formation, and necrosis (Figure 7A and 7B), resembling many aspects of human tuberculosis. Consistent with much-reduced gross pathologic changes, the lungs of RM-immunized animals had the most significantly reduced granulomatous lesions (Figure 7B). With immunohistochemistry, human CD4+/CD8+ T cells and human CD68+ macrophages were visualized within and at the periphery of small granulomas in RM-immunized animals (Supplementary Figure S8).
Figure 7.
Pulmonary tuberculosis histopathologic findings in immunized humanized mice (Hu-mice). Unimmunized animals, animals immunized parenterally (intramuscularly) or via the respiratory mucosa (intranasally), and animals immunized intranasally and depleted of human CD4+ and CD8+ T cells (T-depleted) were infected with Mycobacterium tuberculosis (1 × 104 colony-forming units per animal) via the pulmonary route 4 weeks after viral-vectored immunization and euthanized 4 weeks after infection. A, Representative micrographs of lung sections stained with hematoxylin–eosin, comparing the extent of granulomatous lesions and necrosis. Scale bar represents 1 mm. B, Scatterplot comparing the scores of microscopic histopathologic changes in the lung. Horizontal lines in B represent the median values, and data in B are pooled from 2 independent experiments with the indicated number of animals per group.
Because T cells are critical to antituberculosis immunity in humans [24–26] and RM VV vaccine–activated Ag-specific T cells (Figure 2) robustly responded to M. tuberculosis exposure in the lung (Figures 3–5), we determined the role of vaccine-induced human T cells in markedly improved protection by RM VV immunization. To this end, CD4+ and CD8+ T cells were depleted in RM-immunized animals 1 week before M. tuberculosis infection, with human CD4+/CD8+ T-cell–depleting monoclonal antibodies administered twice (100 and 50 μg per animal for anti-CD4 and anti-CD8, respectively), 2 days apart. Compared with T-cell–competent RM-immunized animals, T-cell depletion significantly worsened the initially RM VV vaccine–improved tuberculosis disease outcomes to levels close to those seen either in unimmunized or parenterally (intramuscularly) immunized animals, including illness, lung CFU and acid-fast-stained bacilli scores, and macroscopic and microscopic granulomatous lesions (Figure 6 and 7 and Supplementary Figure S7).
Together, the above data indicate that the Hu-mouse is a highly susceptible humanlike model for stringent tuberculosis vaccine testing. The novel RM VV immunization strategy, but not parenteral immunization, can significantly improve lung tuberculosis protection in a human T-cell–dependent manner.
DISCUSSION
A major challenge the tuberculosis vaccine field faces is that the knowledge from conventional animal models is of little value in predicting the efficacy in humans. s a result, the protective efficacy of a promising vaccine in humans remains unknown until costly clinical efficacy trials are completed [8–10]. Indeed, although extensive preclinical studies have demonstrated the efficacy of a novel tuberculosis vaccine, MVA85A, it has been shown to be ineffective in infants or adults with human immunodeficiency virus infection [7–9, 27, 28]. It is now recognized that the gap between humans and conventional animal models in genetics, M. tuberculosis susceptibility, tuberculosis disease outcomes, and immune responses to vaccination severely diminishes the value of such animal models for predicting vaccine efficacy in humans [7, 8, 28]. Therefore, besides efforts to improve tuberculosis vaccines, there is a need to develop novel, affordable animal models amenable to efficacy prediction in humans (http://pipelinereport.org/2016/tb-prevention).
The current study provides evidence that Hu-mice represent a viable solution for this challenge. We have used Hu-mice as a surrogate human M. tuberculosis challenge model to evaluate the efficacy of a novel VV tuberculosis vaccine, AdHu5Ag85A, and a new RM vaccination paradigm. To date, this vaccine has completed a range of preclinical evaluation [19, 29–32] and early-phase human studies [16, 17], but its protective efficacy is still unknown. Although the RM tuberculosis vaccination concept has been shown to be promising in humans [33], its human efficacy is still unknown, despite a study by Jeyanathan et al [29] showing its superiority over parenteral route in a more clinically relevant nonhuman primate (NHP) model.
In the current study, we showed that this model system recapitulates not only the high susceptibility to M. tuberculosis and tuberculosis disease outcomes in humans but also the characteristics of human T-cell responses to AdHu5Ag85A vaccine in humans, as reported in a phase I trial by Smaill et al [17]. With the efficacy of BCG vaccine validated in Hu-mice as demonstrated in humans [23], we tested the efficacy of AdHu5Ag85A in this model and showed RM immunization to be superior to parenteral route in markedly improving tuberculosis disease outcomes. We also demonstrate that improved protection by RM immunization in Hu-mice is mediated by human T cells. Our conclusion is in basic agreement with earlier findings in the NHP model [29].
The strength of our study is the provision of a small animal system with much lessened ethical and financial constraints that is still equipped with the major human immune cells, for more reliable prediction of protective efficacy by a novel tuberculosis vaccine and/or vaccination concept in humans [6, 11]. We believe this to be an imperative step forward in tuberculosis vaccine development. Presently, it takes 10–15 years and enormous resources to complete all preclinical and clinical studies before the human efficacy of a vaccine is known [9, 34]. With such a small animal–based humanized model as the one currently available, the global pace of clinical tuberculosis vaccine development may be expedited. Thus, further study could be discouraged for any promising tuberculosis vaccines/immunization concepts that failed to activate human T cells and significantly enhance lung protection (http://pipelinereport.org/2016/tb-prevention).
M. tuberculosis is a highly human-adapted pathogen and fails to elicit the same type of infection, pathologic, and immune responses in many conventional animal models as it does in humans [6, 19, 32]. As a result, the mycobacterial load (typically reduced by >0.5 log CFUs) is used as the main readout for protective efficacy, rather than disease prevention, the ultimate protective readout in humans [9]. A handful of studies have reported the validity of Hu-mice to model humanlike tuberculosis disease outcomes [12, 13, 15, 35]. However, the value of Hu-mice for evaluations of novel tuberculosis vaccines has remained unexplored.
Detailed evaluation of vaccine-activated human T-cell subsets before and after M. tuberculosis challenge reveals a human T-cell activation profile in our model to be similar to that in humans, as reported in the phase I trial of this vaccine [17], a profile poorly reflected in murine models [19]. VV parenteral immunization in trial participants led to a predominant CD4+ T-cell response. Similarly, immunization in Hu-mice also led to predominantly antimycobacterial CD4+ T-cell responses. Furthermore, the polyfunctional property of CD4+ T cells was also comparable between Hu-mice and the trial participants. In comparison, antimycobacterial CD4+ T cells were hardly detectable in AdHu5Ag85A-immunized conventional mice [19].
The T-cell response to pulmonary M. tuberculosis infection is known to be much delayed compared with that to other respiratory pathogens in conventional animal models [24–26, 36, 37]. Although there are limited data from human studies, much-delayed tuberculin skin test conversion in M. tuberculosis–exposed humans suggests that M. tuberculosis–specific T-cell responses are delayed in humans as well [38]. Indeed, in our current study, the unimmunized Hu-mice failed to produce human T-cell responses in the lung and spleen for at least 6 days after M. tuberculosis exposure. Because the early events leading to delayed T-cell responses in humans remain to be understood, the Hu-mouse model can be used to dissect the mechanisms.
In contrast to unimmunized or parenterally immunized Hu-mice, potent Ag-specific human CD4+ T-cell responses in the lung were present and rapidly increased in RM-immunized Hu-mice before and after M. tuberculosis exposure, respectively. Such accelerated T-cell responses were correlated with improved tuberculosis disease outcomes in RM-immunized Hu-mice. Parenteral immunization reduced illness scores but not bacterial burden or disease in the lung. These findings suggest that parenteral immunization strategy might induce tissue tolerance but not resistance to M. tuberculosis, further supporting our conclusion that RM immunization provides superior protection compared with a parenteral route.
Mounting evidence has highlighted the critical dependence of vaccine-induced T-cell responses at the lung mucosa for protection [4, 19, 20, 33, 39–41], and the requirement of T cells for host defense against tuberculosis in humans has been well established [24, 26]. The protective role of human T cells is also demonstrated for VV in RM-immunized Hu-mice, as evidenced in the current study by abrogation of otherwise protective immunity on human T-cell depletion. Because the lung is the entry site for M. tuberculosis [42], our findings strongly support RM immunization as a new strategy for tuberculosis prevention in humans. In NHPs and humans, tuberculosis is characterized by lung granuloma formation [43]. However, the conventional mouse models do not reflect the key features of tuberculosis granuloma in NHP or human lungs [6, 19].
We show in the current study that lung tuberculosis granuloma in infected Hu-mice recapitulates that in NHPs and humans, as evidenced by necrotic granulomas that are resided by macrophages and surrounded by CD4+ T cells, and to a lesser extent by CD8+ T cells. Moreover, acid-fast staining of M. tuberculosis bacilli occurred nearly exclusively within granulomas. However, because murine radioresistant immune cells and lung stromal cells are present in Hu-mice, further studies are required to determine potential limitations of Hu-mice model in reflecting human lung tuberculosis granulomas.
Currently, the protective correlates still remain elusive [5–7, 36]. Our data suggest that M. tuberculosis Ag–specific CD4+ T cells in the lung before and after M. tuberculosis infection might be a reliable protective correlate that deserves further investigation in humans. Rapidly emerging Ag-specific human CD4+ T cells in the airways of RM-immunized Hu-mice after infection were characterized by TNF-α monofunctionality, and to a lesser extent TNF-α and IFN-γ bifunctionality with minimal IL-2 production, resembling effector or effector memory CD4+ T cells [44]. In contrast to the airway findings, there was a marked increase in monofunctional IL-2–producing Ag-specific CD4+ T cells in the lungs of RM-immunized Hu-mice after M. tuberculosis infection. These cells thus resembled long-lasting memory CD4+ T cells with proliferative capacity indicating establishment of central memory Ag-specific CD4+ T cells by RM immunization [45, 46]. Facilitated by our Hu-mouse model with easy accessibility to various organs, further “-omic” characterization of T cells will allow biological correlates of protection to be identified.
In conclusion, we show for the first time that the Hu-mouse model can be used as a surrogate human model to evaluate the efficacy of novel tuberculosis vaccines/concepts before they proceed to large human trials. This new vaccine testing system will facilitate the global pace of clinical tuberculosis vaccine development and immune protective correlate identification.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. We are grateful for technical assistance from Philip Nguyen, Xueya Feng, Mary Jo Smith, and animal care staff at the biosafety level 3 facility.
Financial support. This study was supported by the Canadian Institutes of Health Research, the Natural Sciences and Engineering Research Council of Canada, and the Canadian Foundation for Innovation.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Global tuberculosis report 2016. Geneva, Switzerland: WHO; 2017. [Google Scholar]
- 2. Brennan MJ, Thole J. Tuberculosis vaccines: a strategic blueprint for the next decade. Tuberculosis (Edinb) 2012; 92(suppl 1):S6–13. [DOI] [PubMed] [Google Scholar]
- 3. Andersen P, Kaufmann SH. Novel vaccination strategies against tuberculosis. Cold Spring Harb Perspect Med 2014; 4:pii: a018523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xing Z, Jeyanathan M, Smaill F. New approaches to TB vaccination. Chest 2014; 146:804–12. [DOI] [PubMed] [Google Scholar]
- 5. McShane H, Williams A. A review of preclinical animal models utilised for TB vaccine evaluation in the context of recent human efficacy data. Tuberculosis (Edinb) 2014; 94:105–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Myllymäki H, Niskanen M, Oksanen KE, Rämet M. Animal models in tuberculosis research—where is the beef? Expert Opin Drug Discov 2015; 10:871–83. [DOI] [PubMed] [Google Scholar]
- 7. O’Shea MK, McShane H. A review of clinical models for the evaluation of human TB vaccines. Hum Vaccin Immunother 2016; 12:1177–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tameris M, McShane H, McClain JB et al. Lessons learnt from the first efficacy trial of a new infant tuberculosis vaccine since BCG. Tuberculosis (Edinb) 2013; 93:143–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tameris MD, Hatherill M, Landry BS et al. ; MVA85A 020 Trial Study Team Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet 2013; 381:1021–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McShane H, Pathan AA, Sander CR et al. Recombinant modified vaccinia virus Ankara expressing antigen 85A boosts BCG-primed and naturally acquired antimycobacterial immunity in humans. Nat Med 2004; 10:1240–4. [DOI] [PubMed] [Google Scholar]
- 11. Brehm MA, Jouvet N, Greiner DL, Shultz LD. Humanized mice for the study of infectious diseases. Curr Opin Immunol 2013; 25:428–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Calderon VE, Valbuena G, Goez Y et al. A humanized mouse model of tuberculosis. PLoS One 2013; 8:e63331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Heuts F, Gavier-Widén D, Carow B, Juarez J, Wigzell H, Rottenberg ME. CD4+ cell-dependent granuloma formation in humanized mice infected with mycobacteria. Proc Natl Acad Sci U S A 2013; 110:6482–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Leung C, Chijioke O, Gujer C et al. Infectious diseases in humanized mice. Eur J Immunol 2013; 43:2246–54. [DOI] [PubMed] [Google Scholar]
- 15. Nusbaum RJ, Calderon VE, Huante MB et al. Pulmonary tuberculosis in humanized mice infected with HIV-1. Sci Rep 2016; 6:21522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jeyanathan M, Damjanovic D, Yao Y, Bramson J, Smaill F, Xing Z. Induction of an immune-protective T-cell repertoire with diverse genetic coverage by a novel viral-vectored tuberculosis vaccine in humans. J Infect Dis 2016; 214:1996–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Smaill F, Jeyanathan M, Smieja M et al. A human type 5 adenovirus-based tuberculosis vaccine induces robust T cell responses in humans despite preexisting anti-adenovirus immunity. Sci Transl Med 2013; 5:205ra134. [DOI] [PubMed] [Google Scholar]
- 18. Dykstra C, Lee AJ, Lusty EJ et al. Reconstitution of immune cell in liver and lymph node of adult- and newborn-engrafted humanized mice. BMC Immunol 2016; 17:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang J, Thorson L, Stokes RW et al. Single mucosal, but not parenteral, immunization with recombinant adenoviral-based vaccine provides potent protection from pulmonary tuberculosis. J Immunol 2004; 173:6357–65. [DOI] [PubMed] [Google Scholar]
- 20. Santosuosso M, McCormick S, Zhang X, Zganiacz A, Xing Z. Intranasal boosting with an adenovirus-vectored vaccine markedly enhances protection by parenteral Mycobacterium bovis BCG immunization against pulmonary tuberculosis. Infect Immun 2006; 74:4634–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Santosuosso M, Zhang X, McCormick S, Wang J, Hitt M, Xing Z. Mechanisms of mucosal and parenteral tuberculosis vaccinations: adenoviral-based mucosal immunization preferentially elicits sustained accumulation of immune protective CD4 and CD8 T cells within the airway lumen. J Immunol 2005; 174:7986–94. [DOI] [PubMed] [Google Scholar]
- 22. Ferreira DM, Moreno AT, Cianciarullo AM, Ho PL, Oliveira ML, Miyaji EN. Comparison of the pulmonary response against lethal and non-lethal intranasal challenges with two different pneumococcal strains. Microb Pathog 2009; 47:157–63. [DOI] [PubMed] [Google Scholar]
- 23. Colditz GA, Brewer TF, Berkey CS et al. Efficacy of BCG vaccine in the prevention of tuberculosis: meta-analysis of the published literature. JAMA 1994; 271:698–702. [PubMed] [Google Scholar]
- 24. Cooper AM. T cells in mycobacterial infection and disease. Curr Opin Immunol 2009; 21:378–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shaler CR, Horvath C, Lai R, Xing Z. Understanding delayed T-cell priming, lung recruitment, and airway luminal T-cell responses in host defense against pulmonary tuberculosis. Clin Dev Immunol 2012; 2012:628293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Urdahl KB, Shafiani S, Ernst JD. Initiation and regulation of T-cell responses in tuberculosis. Mucosal Immunol 2011; 4:288–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ndiaye BP, Thienemann F, Ota M et al. ; MVA85A 030 trial investigators Safety, immunogenicity, and efficacy of the candidate tuberculosis vaccine MVA85A in healthy adults infected with HIV-1: a randomised, placebo-controlled, phase 2 trial. Lancet Respir Med 2015; 3:190–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shay T, Jojic V, Zuk O et al. ; ImmGen Consortium Conservation and divergence in the transcriptional programs of the human and mouse immune systems. Proc Natl Acad Sci U S A 2013; 110:2946–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jeyanathan M, Shao Z, Yu X et al. AdHu5Ag85A respiratory mucosal boost immunization enhances protection against pulmonary tuberculosis in BCG-primed non-human primates. PLoS One 2015; 10:e0135009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Perez de Val B, Villarreal-Ramos B, Nofrarias M et al. Goats primed with Mycobacterium bovis BCG and boosted with a recombinant adenovirus expressing Ag85A show enhanced protection against tuberculosis. Clin Vaccine Immunol 2012; 19:1339–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vordermeier HM, Huygen K, Singh M, Hewinson RG, Xing Z. Immune responses induced in cattle by vaccination with a recombinant adenovirus expressing Mycobacterial antigen 85A and Mycobacterium bovis BCG. Infect Immun 2006; 74:1416–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xing Z, McFarland CT, Sallenave JM, Izzo A, Wang J, McMurray DN. Intranasal mucosal boosting with an adenovirus-vectored vaccine markedly enhances the protection of BCG-primed guinea pigs against pulmonary tuberculosis. PLoS One 2009; 4:e5856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Satti I, Meyer J, Harris SA et al. Safety and immunogenicity of a candidate tuberculosis vaccine MVA85A delivered by aerosol in BCG-vaccinated healthy adults: a phase 1, double-blind, randomised controlled trial. Lancet Infect Dis 2014; 14:939–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kaufmann SH, McElrath MJ, Lewis DJ, Del Giudice G. Challenges and responses in human vaccine development. Curr Opin Immunol 2014; 28:18–26. [DOI] [PubMed] [Google Scholar]
- 35. Lee J, Brehm MA, Greiner D, Shultz LD, Kornfeld H. Engrafted human cells generate adaptive immune responses to Mycobacterium bovis BCG infection in humanized mice. BMC Immunol 2013; 14:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bhatt K, Verma S, Ellner JJ, Salgame P. Quest for correlates of protection against tuberculosis. Clin Vaccine Immunol 2015; 22:258–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dean GS, Clifford D, Whelan AO et al. Protection induced by simultaneous subcutaneous and endobronchial vaccination with BCG/BCG and BCG/adenovirus expressing antigen 85A against Mycobacterium bovis in cattle. PLoS One 2015; 10:e0142270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Huebner RE, Schein MF, Bass JB Jr. The tuberculin skin test. Clin Infect Dis 1993; 17:968–75. [DOI] [PubMed] [Google Scholar]
- 39. Beverley PC, Sridhar S, Lalvani A, Tchilian EZ. Harnessing local and systemic immunity for vaccines against tuberculosis. Mucosal Immunol 2014; 7:20–6. [DOI] [PubMed] [Google Scholar]
- 40. Jeyanathan M, Heriazon A, Xing Z. Airway luminal T cells: a newcomer on the stage of TB vaccination strategies. Trends Immunol 2010; 31:247–52. [DOI] [PubMed] [Google Scholar]
- 41. Kaushal D, Foreman TW, Gautam US et al. Mucosal vaccination with attenuated Mycobacterium tuberculosis induces strong central memory responses and protects against tuberculosis. Nat Commun 2015; 6:8533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Getahun H, Matteelli A, Chaisson RE, Raviglione M. Latent Mycobacterium tuberculosis infection. N Engl J Med 2015; 372:2127–35. [DOI] [PubMed] [Google Scholar]
- 43. Shaler CR, Horvath CN, Jeyanathan M, Xing Z. Within the Enemy’s Camp: contribution of the granuloma to the dissemination, persistence and transmission of Mycobacterium tuberculosis. Front Immunol 2013; 4:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Prezzemolo T, Guggino G, La Manna MP, Di Liberto D, Dieli F, Caccamo N. Functional signatures of human CD4 and CD8 T cell responses to Mycobacterium tuberculosis. Front Immunol 2014; 5:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lindenstrøm T, Knudsen NP, Agger EM, Andersen P. Control of chronic Mycobacterium tuberculosis infection by CD4 KLRG1- IL-2-secreting central memory cells. J Immunol 2013; 190:6311–9. [DOI] [PubMed] [Google Scholar]
- 46. Woodworth JS, Aagaard CS, Hansen PR, Cassidy JP, Agger EM, Andersen P. Protective CD4 T cells targeting cryptic epitopes of Mycobacterium tuberculosis resist infection-driven terminal differentiation. J Immunol 2014; 192:3247–58. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.