We apply inference methods based on the previously defined relative risk statistic to suggest the prominent relative roles of children aged ≤10 years (particularly those aged 3–6 years) in propagating respiratory syncytial virus epidemics in the United States during 2001–2012.
Keywords: RSV, age groups, relative risk, epidemic drivers, transmission modeling
Abstract
Background
While circulation of respiratory syncytial virus (RSV) results in high rates of hospitalization, particularly among young children and elderly individuals, little is known about the role of different age groups in propagating annual RSV epidemics.
Methods
We evaluate the roles played by individuals in different age groups during RSV epidemics in the United States between 2001 and 2012, using the previously defined relative risk (RR) statistic estimated from the hospitalization data from the Healthcare Cost and Utilization Project. Transmission modeling was used to examine the robustness of our inference method.
Results
Children aged 3–4 years and 5–6 years each had the highest RR estimate for 5 of 11 seasons included in this study, with RSV hospitalization rates in infants being generally higher during seasons when children aged 5–6 years had the highest RR estimate. Children aged 2 years had the highest RR estimate during one season. RR estimates in infants and individuals aged ≥11 years were mostly lower than in children aged 1–10 years. Highest RR values aligned with groups for which vaccination had the largest impact on epidemic dynamics in most model simulations.
Conclusions
Our estimates suggest the prominent relative roles of children aged ≤10 years (particularly among those aged 3–6 years) in propagating RSV epidemics. These results, combined with further modeling work, should help inform RSV vaccination policies.
Annual epidemics of respiratory syncytial virus (RSV) take place in the United States, exerting a heavy toll in severe outcomes in infants and young children [1–5], as well as older adults [6–8], with rates of associated bronchiolitis hospitalizations in infants being particularly high [9, 10]. Rates of hospitalization with RSV infection present in the diagnosis drop off dramatically with age (Table 1 in the article by Zhou et al [6] and Table 1 in this article), but those represent only a fraction of all hospitalizations associated with RSV infections. Various strategies have been used to estimate the rates of RSV-associated hospitalization in different age groups, including inpatient surveillance in the community [3], prospective cohort studies [8], regression-based statistical analyses [6, 7], and procedures for correcting missing or misclassified diagnoses by attributing certain proportions of hospitalizations with certain diagnoses to different etiologies [5]. Still, our understanding of the hospitalization burden stemming from RSV circulation is incomplete [11], particularly for the adult populations [12].
Table 1.
Seasonal Counts and Rates of Hospitalization With Respiratory Syncytial Virus Mentioned in the Diagnosis, by Age Group
| Age / Season | 2001–2002 | 2002–2003 | 2003–2004 | 2004–2005 | 2005–2006 | 2006–2007 | 2007–2008 | 2008–2009 | 2009–2010 | 2010–2011 | 2011–2012 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| <1 y | |||||||||||
| Count | 44 855 | 38 518 | 40 855 | 34 133 | 38 272 | 38 346 | 39 289 | 34 462 | 37 810 | 37 126 | 28 692 |
| Rate, cases/100 000 | 2049 | 1766 | 1856 | 1546 | 1725 | 1697 | 1721 | 1542 | 1734 | 1715 | 1334 |
| 1 y | |||||||||||
| Count | 8677 | 7245 | 8213 | 7040 | 7436 | 7372 | 7986 | 7328 | 8921 | 8775 | 6757 |
| Rate, cases/100 000 | 400.9 | 331.4 | 377.9 | 321.5 | 338.5 | 334.4 | 356.2 | 324.2 | 402.9 | 403.3 | 311 |
| 2 y | |||||||||||
| Count | 2825 | 2267 | 2713 | 2343 | 2637 | 2485 | 2869 | 2690 | 3601 | 3645 | 2801 |
| Rate, cases/100 000 | 134 | 104.8 | 124.2 | 108 | 120.4 | 113.1 | 130.2 | 120.1 | 159.3 | 164.3 | 128.4 |
| 3 y | |||||||||||
| Count | 1059 | 908 | 1128 | 930 | 1069 | 1038 | 1112 | 1220 | 1727 | 1764 | 1364 |
| Rate, cases/100 000 | 50.6 | 43 | 52.1 | 42.6 | 49.1 | 47.3 | 50.4 | 55.1 | 76.9 | 78 | 61.4 |
| 4 y | |||||||||||
| Count | 437 | 351 | 526 | 476 | 465 | 419 | 490 | 489 | 790 | 829 | 661 |
| Rate, cases/100 000 | 20.8 | 16.7 | 24.8 | 21.9 | 21.1 | 19.1 | 22.1 | 22 | 35.3 | 36.7 | 29.2 |
| 5 y | |||||||||||
| Count | 209 | 161 | 226 | 207 | 233 | 197 | 237 | 274 | 399 | 452 | 320 |
| Rate, cases/100 000 | 9.8 | 7.6 | 10.7 | 9.7 | 10.7 | 8.9 | 10.7 | 12.3 | 17.8 | 20.1 | 14.1 |
| 6 y | |||||||||||
| Count | 118 | 88 | 116 | 102 | 117 | 109 | 133 | 146 | 209 | 257 | 200 |
| Rate, cases/100 000 | 5.4 | 4.1 | 5.5 | 4.9 | 5.5 | 5 | 6 | 6.6 | 9.4 | 11.5 | 8.9 |
| 7 y | |||||||||||
| Count | 66 | 56 | 58 | 66 | 61 | 57 | 67 | 96 | 124 | 160 | 88 |
| Rate, cases/100 000 | 3 | 2.6 | 2.7 | 3.1 | 2.9 | 2.7 | 3.1 | 4.3 | 5.6 | 7.2 | 3.9 |
| 8 y | |||||||||||
| Count | 48 | 44 | 36 | 34 | 42 | 45 | 45 | 51 | 73 | 88 | 76 |
| Rate, cases/100 000 | 2.2 | 2 | 1.6 | 1.6 | 2 | 2.1 | 2.1 | 2.3 | 3.3 | 4 | 3.4 |
| 9 y | |||||||||||
| Count | 40 | 24 | 20 | 29 | 36 | 36 | 44 | 49 | 85 | 55 | 34 |
| Rate, cases/100 000 | 1.8 | 1.1 | 0.9 | 1.3 | 1.7 | 1.7 | 2.1 | 2.3 | 3.8 | 2.5 | 1.5 |
| 10 y | |||||||||||
| Count | 31 | 28 | 36 | 24 | 35 | 32 | 37 | 43 | 55 | 57 | 48 |
| Rate, cases/100 000 | 1.3 | 1.2 | 1.6 | 1.1 | 1.5 | 1.4 | 1.6 | 1.9 | 2.4 | 2.5 | 2.1 |
| 11 y | |||||||||||
| Count | 33 | 27 | 19 | 25 | 31 | 19 | 19 | 38 | 48 | 60 | 40 |
| Rate, cases/100 000 | 1.4 | 1.2 | 0.8 | 1.1 | 1.4 | 0.8 | 0.8 | 1.7 | 2.1 | 2.6 | 1.7 |
| 12–17 y | |||||||||||
| Count | 106 | 84 | 106 | 81 | 109 | 116 | 142 | 151 | 194 | 218 | 149 |
| Rate, cases/100 000 | 0.8 | 0.6 | 0.8 | 0.6 | 0.8 | 0.8 | 1 | 1.1 | 1.4 | 1.6 | 1.1 |
| 18–49 y | |||||||||||
| Count | 191 | 132 | 177 | 150 | 238 | 235 | 318 | 373 | 493 | 568 | 465 |
| Rate, cases/100 000 | 0.3 | 0.2 | 0.2 | 0.2 | 0.3 | 0.3 | 0.4 | 0.5 | 0.7 | 0.8 | 0.6 |
| 50–64 y | |||||||||||
| Count | 156 | 120 | 144 | 162 | 267 | 219 | 320 | 328 | 616 | 723 | 564 |
| Rate, cases/100 000 | 0.7 | 0.5 | 0.6 | 0.6 | 1 | 0.8 | 1.1 | 1.1 | 2 | 2.2 | 1.7 |
| ≥65 y | |||||||||||
| Count | 297 | 190 | 252 | 190 | 406 | 327 | 573 | 663 | 950 | 1331 | 1174 |
| Rate, cases/100 000 | 1.6 | 1 | 1.3 | 1 | 2.1 | 1.7 | 2.8 | 3.2 | 4.5 | 6.2 | 5.3 |
Data are from the State Inpatient Databases of the Healthcare Cost and Utilization Project [18].
Given the high burden of severe outcomes associated with RSV, it would be useful to understand the roles of different population groups in propagating RSV epidemics in the community. Such information should contribute to our overall understanding of RSV epidemiology and help inform mitigation efforts particularly vaccination, with a variety of RSV vaccine candidates for different populations being in different stages of development [13, 14].
Elsewhere, we developed a method for assessing the roles of different subpopulations in transmission during epidemics of infectious diseases [15–17]. The idea of that method is that subpopulations that play a disproportionate role during the outbreak’s ascent due to increased susceptibility and/or contact rates can be related to the relative risk (RR) statistic that evaluates the change in the subpopulation’s proportion among all cases in the population before versus after the epidemic’s peak (see Methods). Previously, we estimated the RR statistics in select age groups for several influenza epidemics in the United States, using data on hospitalized cases for the inference [15]. An important aspect of the method we proposed is that the results do not depend on case-hospitalization rates in different age groups [15]. Moreover, we used simulations in the context of influenza and pertussis epidemics [15, 16] to show an association between a higher value for an RR statistic in a given age group and a higher impact of vaccinating an individual in that age group on the reduction in the epidemic’s initial reproductive number/growth rate.
In this article, we estimate the RR statistic in different age groups for 11 RSV epidemics in the United States, using data on RSV hospitalizations in a collection of US states between 2001 and 2012 [18]. We consider a fine age stratification for young children to examine how their role during RSV epidemics changes with age progression. We compare the RR estimates in different age groups across 11 RSV epidemics and use simulations of transmission dynamics to investigate the relation between the values of the RR statistic in different age groups and the relative roles that those groups play in propagating RSV epidemics. We then discuss the relevance of our findings to the potential impact of vaccination.
METHODS
Data
We used weekly data on hospitalizations with an RSV diagnosis (both primary and contributing; International Classification of Diseases, Ninth Revision, codes 079.6, 466.11, and 480.1) from the State Inpatient Databases of the Healthcare Cost and Utilization Project (HCUP), maintained by the Agency for Healthcare Research and Quality through an active collaboration [18]. This database contains hospital discharges from community hospitals in participating states. We compiled data from the 2001–2002 RSV season through the 2011–2012 RSV season for 10 different age groups (see the “Before and After the Peak Counts” subsection) and 26 participating states that reported continuously to HCUP between week 27 in 2001 and week 26 in 2012. Those states (that represented about 54.3% of the US population between 2001 and 2012) are Arizona, California, Colorado, Connecticut, Georgia, Iowa, Illinois, Kentucky, Maryland, Minnesota, Missouri, North Carolina, Nebraska, New Jersey, Nevada, Ohio, Oregon, Rhode Island, South Carolina, South Dakota, Tennessee, Utah, Vermont, Washington, Wisconsin, and West Virginia. Each RSV season was defined as a period between calendar week 27 of one year through calendar week 26 of the next year. One year (2006) had 53 calendar weeks; the corresponding season was defined as the period from week 27 of 2006 through week 25 of 2007. Aggregate hospitalization data were used in our analyses, with no informed consent from participants sought.
Before and After the Peak Counts
Data on the cumulative number/rate per 100000 of RSV hospitalizations in different age groups during each season (presented in Table 1) were part of the motivation for the following age splitting (in years) for our analysis: <1, 1, 2, 3–4, 5–6, 7–10, 11–17, 18–49, 50–64, and ≥65. For each season and each state s, we defined the peak week P(s) for that state as the calendar week with the highest total number of RSV hospitalizations in the state (with results of the analyses utilizing a 3-week moving average presented in the Supporting Information, section S2). Each case in the state s during each given season was classified as before-the-peak case if it occurred in or prior to week ; after-the-peak case if it occurred in or after week . We exclude cases occurring between weeks and from the analysis because of the misclassification of counts as before or after-the-peak stemming from the noise in the count data for the hospitalized cases, differences in case-hospitalization rates (proportion of RSV infections that result in hospitalizations with an RSV diagnosis) for the various age groups, and other factors that may result in different peak times for the counts of the hospitalized cases vs. cases of infection in the community. For each age group g, before-and-after the peak seasonal counts in that age group in different states were combined into national before-and-after the peak counts B(g) and A(g) correspondingly.
Statistical Inference
The point estimate for the seasonal relative risk RR(g) in an age group g is
| (1) |
The estimates and confidence bounds for relative risks in each group were obtained in a Bayesian framework following the methodology in [15]. Briefly, as the number of cases of infection in each age group is large and case-hospitalization rates (see the previous subsection) are low, we assume that the numbers of reported cases in each age group before an after the peak are Poisson distributed. Posterior samples of size 100 000 for the Poisson parameters (with a flat prior) corresponding to the counts are generated; for each i = 1,..,100000, the corresponding parameters are plugged into eq. 1 to generate an estimate for the relative risk. The mean and the credible interval for the sample (i = 1,..,100000) are then extracted.
Simulations
We simulated RSV epidemics in an age-stratified population (based on the 2016 US population), modeling the transmission dynamics using a stratified mass action SIR framework (see Supporting Information for full details). Importantly, we allowed for variability in the distribution of susceptibility to infection within each age group for each fixed average susceptibility to infection within that age group. We have simulated 250000 epidemics by varying the distribution of susceptibility to infection in different age groups within the ranges described in the Supporting Information, keeping other transmission parameters fixed. For each simulated epidemic, we have calculated, for each age group, its relative risk RR (defined according to eq. 1), as well as the impact of giving 1 dose of a perfect vaccine to each of 1 million members of that age group (equivalent to removing 1 million members of that age group from the transmission process) on the epidemic’s initial effective reproductive number/growth rate in the whole population. We then estimated the proportion of epidemics for which the group with the highest RR is also the group for which 1000000 doses of a perfect vaccine would yield the largest reduction in the epidemic’s initial effective reproductive number . Additionally, we compared the set of epidemics for which there was a discordance between the group with the leading RR and the group with the highest impact of administering 1 million vaccine doses on reducing (discordant epidemics) with the whole collection of 250000 simulated epidemics in terms of the distributions of susceptibility to infection in different age groups.
RESULTS
Estimates of RR in Different Age Groups During Eleven RSV Epidemics in the United States (2001–2012)
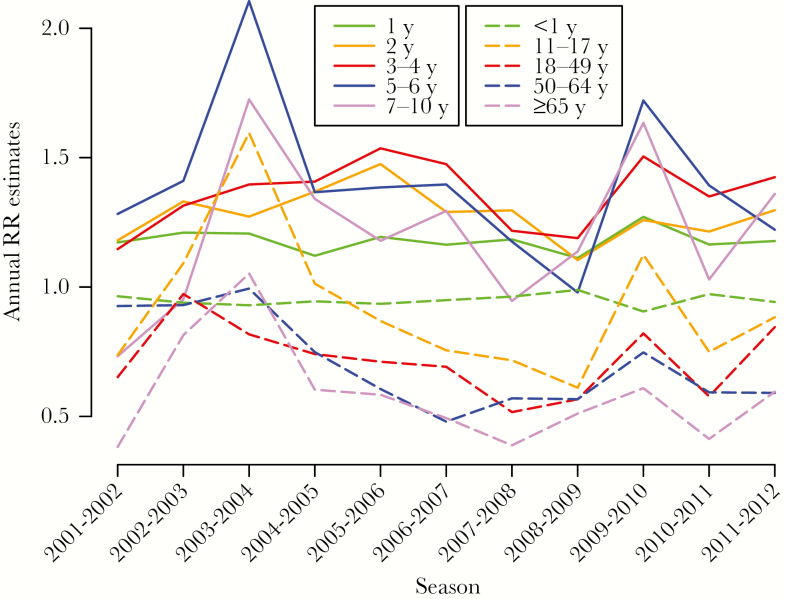
Table 1 presents the cumulative number and the rate per 100000 of RSV hospitalizations during each season in select age groups between 2001–2012 for the 26 states included in our analyses (see Methods). There is an apparent upward trend in the annual hospitalization counts/rates in all age groups over 3 years of age (being most pronounced in adults), likely indicative of temporal changes in RSV detection/diagnostic practices. We note that differences in the counts/rates of hospitalization with an RSV diagnosis by age need not correspond to differences in the rates of RSV-associated hospitalization, or infection by age – see also the 1st paragraph of the Introduction. Figure 1 presents the seasonal estimates of the relative risks RR (eq. 1) in select age groups (with numerical estimates/confidence bounds presented in Tables S4/S5 in the Supporting Information). Children aged 3–4 years and 5–6 years each had the highest RR estimate for 5/11 seasons in the data, with RSV hospitalization rates in infants being generally higher during seasons when children aged 5–6 years had the highest RR estimates (Table 1 and Figure 1). Children aged 2 years had the highest RR estimate during one season, with RR estimates in children aged 2 years being higher than the ones in children aged 1 year during 9/11 seasons. RR estimates in infants and individuals aged 11 years and older (dashed lines) were mostly lower than in children aged 1–10 years (solid lines), with the biggest exception being the RR estimate for children aged 11–17 years during the 2003–04 season. Additionally, RR estimates in children aged under 5 years were more stable than the RR estimates in school-age children, with the latter estimates spiking during the 2003–04 and the 2009–10 seasons (which also represent the two largest influenza epidemics during the study period).
Figure 1.
Annual relative risk (RR) estimates for the 10 age groups used in the analysis.
Simulated Epidemics
For the 250000 simulated epidemics, the highest RR estimate belonged to children aged 3–4 years and 5–6 years in 47.6% of simulations each, to children aged 7–12 years in 2.8% of simulations, to adolescents aged 13–19 in 1.9% of simulations, and to adults aged 20–39 years in 0.05% (133/250000) of simulations. Overall, 73.5% of simulated epidemics were concordant, meaning that the age group with the highest RR was also the group for which vaccination of 1 million individuals yielded the biggest reduction in the epidemic’s initial effective reproductive number/growth rate. This proportion conconcordant varied from 86% among simulated epidemics for which children aged 5–6 years had the highest RR estimate, to 68.3% among simulated epidemics for which children aged 3–4 years had the highest RR estimate, to 0.1% among simulated epidemics for which children aged 13–19 years had the highest RR estimate, to 0% (none) among simulated epidemics for which either children aged 7–12 years or adults aged 20–39 years had the highest RR estimate. Comparing the features of concordant to discordant epidemics, for which the greatest impact of vaccination was not seen in the group with the highest RR, we found that for each age group, discordant epidemics with the highest RR belonging to that age group had, on average, a lower fraction of susceptible individuals in that age group, but a higher susceptibility to infection per susceptible individual in that age group (see Table S3 in the Supporting Information).
DISCUSSION
Annual RSV epidemics in the United States exert a heavy burden of severe disease in young children, particularly infants [1–5], and among the elderly [6–8, 12], but the role that the different age groups play in the dynamics of RSV infections in the community remains unclear. In this study, we examine the role of individuals in different age groups in propagating the annual RSV epidemics in the United States by applying the RR statistic introduced in our earlier work [15–17] to the US hospitalization data between 2001–2012 [18]. Additionally, we study the robustness of our inference method by relating the values for the RR statistic in various age groups to the impact of vaccination of individuals in those age groups on the epidemic’s initial growth rate using simulations. Our results indicate that the highest estimates for the RR statistic belonged to either children aged 3–4 years or 5–6 years for most RSV epidemics in the US between 2001–2012. Moreover, rates of hospitalization in infants with RSV present in the diagnosis were generally higher during those seasons when children aged 5–6 years had the highest estimate for the RR statistic. This suggests the significant relative role of children in an age subgroup above 4 years of age during RSV epidemics, a finding that has received limited illustration in the literature (compare to [19, 20]). Additionally, our estimates for the RR statistic for infants and individuals over the age of 11 years were generally lower than the ones for children aged 1–10 years.
While several vaccine candidates for the infant and the pediatric populations are currently in different stages of development [13, 14], the schedule for RSV vaccine administration in non-infant populations is unclear. Such a schedule should account for both the direct protection imparted by vaccines, as well as the impact of vaccination on RSV transmission in the community. We hope that our study makes a contribution towards examining those issues in suggesting the prominence of children aged ≤10 years (particularly pre-school and younger school-age children) relative to children aged over 10 years and adults in propagating RSV epidemics.
There is some uncertainty in the relation between the value of the RR statistic in a given age group and the role played by an average individual in that age group in transmission dynamics. Building on our earlier work ([15, 16]), we have attempted to address this issue through simulations of transmission dynamics in the context of RSV epidemics. Similarly to [15, 16], we have found an association between having the highest value for an RR statistic in an age group and the highest impact of vaccination of an individual in that age group on the epidemic’s initial growth rate/reproductive number. At the same time, this association was violated for certain combinations of contacts rates and distributions of susceptibility to infection in different age groups, particularly when the distribution of susceptibility to infection in some age groups was significantly more heterogenous than in others (see Supporting Information, section S1). Given that the estimates of the RR statistic for the different age subgroups of children between the ages of 1 to 10 years (which vary a good deal in terms of their contact rates and distribution of susceptibility to infection) were mostly higher than the RR estimates in different subgroups of individuals aged over 10 years, this suggests that the relative role of an individual aged 11 years and older in propagating RSV epidemics was generally lower than the relative role of a child aged 1–10 years.
The interpretation of our RR estimates with regard to the relative role of different age subgroups of children aged ≤10 years during RSV epidemics may be biased by certain factors. For infants aged <1 year, the immunological status of the younger infants changes through the course of RSV epidemics due to the waning immunity rendered by maternal antibodies [21–23]. Furthermore, the population of children under 1 year of age changes during the course of an outbreak through new births and aging, with major differences in susceptibility to infection between the newborn children and those close to the age of 1 year. Both phenomena should distribute infections among infants more uniformly during the different epidemic periods and bias the estimates of the RR statistic in infants toward the null value of 1. Additionally, infants under the age of 6 months often do not seroconvert following RSV infections [24, 25], and they may be prone to additional RSV infections during the same season. Moreover, those subsequent infections are more likely to occur during the epidemic’s descent compared to the initial infections, which would bias the RR estimate for infants downward. For children aged 1 year, the RR estimates in that age group are generally lower than the RR estimates for children aged 2–6 years. At the same time, the results of our simulations suggest that the role of children aged 1 year could be underestimated by the RR statistic compared to older children if those older children have higher contact rates but a less uniform distribution of susceptibility to infection, with a lower fraction of susceptible individuals compared to children aged 1 year. A reverse bias in the interpretation of RR estimates is also possible, and it could be relevant for children aged 7–10 years compared to children aged 3–6 years. Further work on the dynamics of RSV infection in young (including school-age) children is needed to evaluate the roles of different subgroups of those children during RSV epidemics.
Our study has some additional limitations. While we have used state-specific peaks of RSV hospitalizations to characterize the before-vs.-after the peak cases, further asynchrony in RSV epidemics within each state may make this categorization of some cases for the corresponding local outbreaks inaccurate. We note that this would bias all the RR estimates toward the null value of 1, whereas the data allows for a delineation of the groups with the highest RR estimates. For the 2003–04 and 2009–10 seasons (that had the two largest influenza epidemics during the study period), values of the RR statistic for RSV epidemics for school-age children were the highest during the study period. There are several possible explanations for this including immunological interference between influenza and RSV, and changes in diagnosis/laboratory testing practices through the course of a season, possibly stemming from changes in perception regarding the potential viral etiologies for the hospitalized cases. The latter might introduce a bias in the RR estimates for a number of RSV seasons, and the scope of that bias is difficult to assess with the available data. We also note that annual rates of RSV hospitalization in different age groups (Table 1) suggest temporal (year-to-year) changes in testing practices; however this should not bias the RR estimates (that are derived separately during each season), unless testing/diagnostic practices change through the course of the season in a manner that is not uniform for all age groups [15, 17].
We believe that despite the above limitations, our study sheds new light on the role of different age groups during RSV epidemics, a subject that had received limited attention in the literature. It suggests the prominence of children under the age of 10 years (particularly those aged 3–6 years) in propagating RSV epidemics, pointing to a potential benefit of RSV vaccine administration in those age groups for mitigating RSV epidemics in the whole community. We hope that our results combined with further modeling work should help inform the potential benefits that different vaccination strategies for RSV will have in the different population groups, including infants and the elderly.
Supplementary Material
Notes
Acknowledgments. We would like to thank the HCUP Partner states, which voluntarily provide their data to the project and without which this research would not be possible (data available at: https://www.hcup-us.ahrq.gov/partners.jsp); and Zeynal Karaca, PhD, and Jenny Schnaier, MA, who helped us with this project on behalf of the AHRQ.
Disclaimer. This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial support. This work was supported by the National Institute of General Medical Sciences (award U54GM088558), support for M. L., E. G., and H. H. N.), the Bill and Melinda Gates Foundation (support for C. J. W.), and the Multinational Influenza Seasonal Mortality Study, led by the Division of International Epidemiology and Population Studies, the Fogarty International Center, and the National Institutes of Health (support for C. V.).
Potential conflict of interest. M. L. reports receiving grants from the National Institute of General Medical Sciences, National Institutes of Health, during the conduct of the study; and personal fees from Affinivax, personal fees from Merck, grants and personal fees from Pfizer, grants from PATH Vaccine Solutions, outside the submitted work. All other authors report no potential conflicts.
The author has submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Bont L, Checchia PA, Fauroux B et al. Defining the epidemiology and burden of severe respiratory syncytial virus infection among infants and children in western countries. Infect Dis Ther 2016; 5:271–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. US CDC. Respiratory Syncitial Virus Infection. http://www.cdc.gov/rsv/about/infection.html. Accessed 1 July 2017.
- 3. Hall CB, Weinberg GA, Iwane MK et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med 2009; 360:588–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hall CB, Weinberg GA, Blumkin AK et al. Respiratory syncytial virus-associated hospitalizations among children less than 24 months of age. Pediatrics 2013; 132:e341–8. [DOI] [PubMed] [Google Scholar]
- 5. Stockman LJ, Curns AT, Anderson LJ, Fischer-Langley G. Respiratory syncytial virus-associated hospitalizations among infants and young children in the United States, 1997-2006. Pediatr Infect Dis J 2012; 31:5–9. [DOI] [PubMed] [Google Scholar]
- 6. Zhou H, Thompson WW, Viboud CG et al. Hospitalizations associated with influenza and respiratory syncytial virus in the United States, 1993-2008. Clin Infect Dis 2012; 54:1427–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goldstein E, Greene SK, Olson DR, Hanage WP, Lipsitch M. Estimating the hospitalization burden associated with influenza and respiratory syncytial virus in New York City, 2003-2011. Influenza Other Respir Viruses 2015; 9:225–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med 2005; 352:1749–59. [DOI] [PubMed] [Google Scholar]
- 9. Faber TE, Kimpen JL, Bont LJ. Respiratory syncytial virus bronchiolitis: prevention and treatment. Expert Opin Pharmacother 2008; 9:2451–8. [DOI] [PubMed] [Google Scholar]
- 10. Moreno MA. Advice for patients. Bronchiolitis and respiratory syncytial virus. Arch Pediatr Adolesc Med 2009; 163:1072. [DOI] [PubMed] [Google Scholar]
- 11. Gilca R, De Serres G, Skowronski D, Boivin G, Buckeridge DL. The need for validation of statistical methods for estimating respiratory virus-attributable hospitalization. Am J Epidemiol 2009; 170:925–36. [DOI] [PubMed] [Google Scholar]
- 12. Branche AR, Falsey AR. Respiratory syncytial virus infection in older adults: an under-recognized problem. Drugs Aging 2015; 32:261–9. [DOI] [PubMed] [Google Scholar]
- 13. Broadbent L, Groves H, Shields MD, Power UF. Respiratory syncytial virus, an ongoing medical dilemma: an expert commentary on respiratory syncytial virus prophylactic and therapeutic pharmaceuticals currently in clinical trials. Influenza Other Respir Viruses 2015; 9:169–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. PATH publications. RSV Vaccine and mAb Snapshot. Sep. 2017 http://www.path.org/publications/files/CVIA_rsv_snapshot_final_0917r.pdf. Accessed 1 October 2017.
- 15. Worby CJ, Chaves SS, Wallinga J, Lipsitch M, Finelli L, Goldstein E. On the relative role of different age groups in influenza epidemics. Epidemics 2015; 13:10–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Worby CJ, Kenyon C, Lynfield R, Lipsitch M, Goldstein E. Examining the role of different age groups, and of vaccination during the 2012 Minnesota pertussis outbreak. Sci Rep 2015; 5:13182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goldstein E, Pitzer VE, O’Hagan JJ, Lipsitch M. Temporally varying relative risks for infectious diseases: implications for infectious disease control. Epidemiology 2017; 28:136–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. SID Database Documentation. Healthcare Cost and Utilization Project (HCUP). Rockville, MD: Agency for Healthcare Research and Quality, 2017. https://www.hcup-us.ahrq.gov/db/state/siddbdocumentation.jsp. Accessed 1 July 2017. [Google Scholar]
- 19. Yamin D, Jones FK, DeVincenzo JP et al. Vaccination strategies against respiratory syncytial virus. Proc Natl Acad Sci U S A 2016; 113:13239–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kinyanjui TM, House TA, Kiti MC, Cane PA, Nokes DJ, Medley GF. Vaccine Induced Herd Immunity for Control of Respiratory Syncytial Virus Disease in a Low-Income Country Setting. PLoS One 2015; 10:e0138018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ogilvie MM, Vathenen AS, Radford M, Codd J, Key S. Maternal antibody and respiratory syncytial virus infection in infancy. J Med Virol 1981; 7:263–71. [DOI] [PubMed] [Google Scholar]
- 22. Ochola R, Sande C, Fegan G et al. The level and duration of RSV-specific maternal IgG in infants in Kilifi Kenya. PLoS One 2009; 4:e8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hacimustafaoglu M, Celebi S, Aynaci E et al. The progression of maternal RSV antibodies in the offspring. Arch Dis Child 2004; 89:52–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jounai N, Yoshioka M, Tozuka M et al. Age-specific profiles of antibody responses against respiratory syncytial virus infection. EBioMedicine 2017; 16:124–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sande CJ, Cane PA, Nokes DJ. The association between age and the development of respiratory syncytial virus neutralising antibody responses following natural infection in infants. Vaccine 2014; 32:4726–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.