Dihydroartemisinin-piperaquine (DHA-PQ) effectively prevents malaria during pregnancy; however, HIV-infected women receiving efavirenz-based antiretroviral therapy have low piperaquine concentrations compared with HIV-negative women. Daily low dose DHA-PQ is predicted to maximize protective efficacy and safety of DHA-PQ chemoprevention in this population.
Keywords: intermittent preventive treatment during pregnancy, dihydroartemisinin-piperaquine, HIV infection, drug–drug interaction
Abstract
Background
A monthly treatment course of dihydroartemisinin-piperaquine (DHA-PQ) effectively prevents malaria during pregnancy. However, a drug–drug interaction pharmacokinetic (PK) study found that pregnant human immunodeficiency virus (HIV)–infected women receiving efavirenz-based antiretroviral therapy (ART) had markedly reduced piperaquine (PQ) exposure. This suggests the need for alternative DHA-PQ chemoprevention regimens in this population.
Methods
Eighty-three HIV-infected pregnant women who received monthly DHA-PQ and efavirenz contributed longitudinal PK and corrected QT interval (QTc) (n = 25) data. Population PK and PK-QTc models for PQ were developed to consider the benefits (protective PQ coverage) and risks (QTc prolongation) of alternative DHA-PQ chemoprevention regimens. Protective PQ coverage was defined as maintaining a concentration >10 ng/mL for >95% of the chemoprevention period.
Results
PQ clearance was 4540 L/day. With monthly DHA-PQ (2880 mg PQ), <1% of women achieved defined protective PQ coverage. Weekly (960 mg PQ) or low-dose daily (320 or 160 mg PQ) regimens achieved protective PQ coverage for 34% and >96% of women, respectively. All regimens were safe, with ≤2% of women predicted to have ≥30 msec QTc increase.
Conclusions
For HIV-infected pregnant women receiving efavirenz, low daily DHA-PQ dosing was predicted to improve protection against parasitemia and reduce risk of toxicity compared to monthly dosing.
Clinical Trials Registration
An estimated 28 million pregnancies occurred in malaria-endemic regions of Africa in 2015, placing infants at risk for the complications of placental malaria, including intrauterine growth retardation, preterm birth, low birth weight, and mortality [1]. Human immunodeficiency virus (HIV) infection increases these risks of malaria during pregnancy compared with HIV-uninfected women [2]. To prevent malaria during pregnancy in moderate- to high-transmission areas in Africa, the World Health Organization (WHO) recommends use of insecticide-treated bed nets and intermittent preventive treatment during pregnancy (IPTp) with at least 3 doses of sulfadoxine-pyrimethamine (SP) [3]. HIV-infected women receive malaria chemoprevention with daily trimethoprim-sulfamethoxazole (TMP-SMX), a related antifolate that is also used to prevent other opportunistic infections [3]. Coadministration of SP and TMP-SMX is not recommended due to increased risk for drug toxicity.
Antifolate resistance is now widespread in Africa, threatening the efficacy of SP and TMP-SMX for malaria chemoprevention [4]. A recent placebo-controlled chemoprevention trial among Ugandan children found a protective efficacy of only 7% for SP and 28% for TMP-SMX [5]. The artemisinin-based combination therapy dihydroartemisinin-piperaquine (DHA-PQ) is under active study as an agent for IPTp because DHA rapidly clears circulating parasites and piperaquine (PQ), which has a half-life of several weeks, provides prolonged protection after therapy. Two recent trials of HIV-uninfected pregnant women in Uganda and Kenya, where Plasmodium falciparum predominates, found that administering a treatment course of DHA-PQ every 4 weeks, beginning in the second trimester, was associated with decreased malaria during pregnancy and decreased placental malaria [6, 7]. Population pharmacokinetic /pharmacodynamic (PK/PD) modeling, conducted as part of the Ugandan trial, estimated that venous PQ concentrations of >10.3 ng/mL and >13.9 ng/mL were associated with 95% and 99% protection from parasitemia during pregnancy (Savic et al, unpublished data).
A similar clinical trial evaluated monthly DHA-PQ for IPTp among HIV-infected women receiving efavirenz (EFV)–based antiretroviral therapy (ART) and TMP-SMX prophylaxis in Tororo, Uganda. There was an unusually low malaria burden during the trial, coinciding with district-wide indoor residual spraying of insecticide (IRS), and DHA-PQ did not show benefit [8]. However, an intensive pharmacokinetic (PK) analysis among 26 trial participants revealed that women receiving EFV-based ART experienced a 38% reduction in PQ exposure as measured by the area under the curve to 21 days (AUC0-21d), as compared to pregnant women who did not take EFV [9]. These findings suggest that coadministration of monthly DHA-PQ IPTp and EFV-based ART would result in low protective efficacy in the setting of high malaria transmission. Therefore, our goal was to identify alternative DHA-PQ regimens for HIV-infected pregnant women receiving EFV-based ART that would be predicted to provide optimal protective efficacy and safety. Toward this goal, we used available PK data, which included monthly measurements throughout chemoprevention, paired corrected QT interval (QTc) measurements, and population PK/PD modeling and simulations to predict the impacts of different dosing regimens on plasma levels of PQ.
METHODS
Study Setting and Participants
The clinical trial that provided samples for our analyses was conducted in Tororo, Uganda, from December 2014 through October 2015. The region has a history of high malaria transmission intensity, but a district-wide program of IRS was implemented in December 2014, with a subsequent dramatic decrease in malaria burden [10, 11]. Eligible women were ≥16 years of age; infected with HIV type 1 (HIV-1); lived within 30 km of the study site; and had a pregnancy between 12 and 28 weeks’ gestation [8]. Written informed consent was obtained from all study participants. The study protocol was approved by the Makerere University School of Biomedical Sciences Research and Ethics Committee, the Uganda National Council for Science and Technology, and the University of California, San Francisco Committee on Human Research. The clinical trial registration number is NCT02282293.
Study Design and Randomization
All women were prescribed ART with standard dosing of EFV/tenofovir/lamivudine and a once-daily dose of TMP-SMX (160 mg TMP/800 mg SMX). Study subjects were randomized to receive either a treatment course of DHA-PQ (360 mg DHA/2880 mg PQ-phosphate divided into 3 consecutive daily oral doses, Holley-Cotec Pharmaceuticals) every 4 weeks or placebo. Administration of the first dose of DHA-PQ was observed in clinic, and the following doses were taken at home. DHA-PQ chemoprevention was started at 16, 20, 24, or 28 weeks’ gestational age and continued every 4 weeks through birth or 40 weeks’ gestational age.
Clinical Procedures
At enrollment, study participants received a long-lasting insecticide-treated bed net, underwent a physical examination, and had blood collected. All women participated in routine visits at 4-week intervals and returned to the clinic for all of their medical needs. At routine visits, participants underwent a capillary blood draw for blood smear, plasma sample, and dried blood spot collection. At select visits, venous samples were obtained for similar studies.
Pharmacokinetic Sampling
Intensive PK Data
Details of the intensive PK study have been reported [9]. In brief, 28 consenting women from the trial were enrolled into an intensive PK substudy prior to their 28-week study visit [9]. Venous samples were collected on the day of the third daily dose of DHA-PQ at gestational week 28, and included samples predose (0 hours) and at 0.5, 1, 2, 3, 4, 6, 8, and 24 hours postdose. Capillary plasma samples were collected at 24 hours and at 4, 7, 14, and 21 days postdose.
Sparse PK Data
A convenience sample of 82 women provided capillary plasma samples permitting trough PQ concentration measurements at routine prenatal visits occurring every 4 weeks, starting 4 weeks after the first DHA-PQ administration. Capillary samples were obtained by fingerprick prior to administration of DHA-PQ using SAFE-T-FILL ethylenediaminetetraacetic acid–containing capillary blood collection tubes. Plasma was obtained by centrifugation of PK samples within 60 minutes of collection at 2000g for 10 minutes, and then stored at –80°C. PQ base concentrations were determined using high-performance liquid chromatography–tandem mass spectrometry, as previously described [12]. Modification and partial validation of the original method for PQ quantitation was performed, to cover a concentration range of 0.50–1000 ng/mL. The lower limit of quantification (LLOQ) was 0.50 ng/mL and the coefficient of variation was <10% for quality control samples [12].
Protective Piperaquine Concentration
Due to lower than anticipated malaria burden observed in the clinical trial, PQ pharmacodynamic (PD) endpoints could not be determined [8]. Therefore, protective PQ concentrations used for this study were estimated based on a randomized placebo-controlled trial conducted before institution of IRS in Tororo. This trial studied HIV-uninfected pregnant women who received DHA-PQ for IPTp [7]. A simultaneous PK/PD model from the trial defined the relationship between PQ concentration and the probability of parasitemia measured by loop-mediated isothermal amplification (LAMP). Using a conservative measure at the upper bounds of uncertainty from the model, PQ concentrations >10.3 ng/mL and >13.9 ng/mL provided 95% and 99% probability, respectively, of being parasitemia free during pregnancy (Savic et al, unpublished data). For the analysis in the current report, 10 ng/mL and 14 ng/mL were used as the protective PQ concentrations. The time above these protective PQ concentrations was used as the PD endpoint. Protective PQ coverage was defined as maintaining a PQ concentration of >10 ng/mL for 95% of the chemoprevention period (ie, from initiation of chemoprevention at 20 weeks’ gestation through delivery).
Unlike the earlier trial of HIV-uninfected pregnant women from which the protective PQ concentrations were determined, all participants in this trial received TMP-SMX daily as part of standard management of their HIV infections. To account for potential additive protection against malaria with the combination of TMP-SMX and DHA-PQ, a protective PQ concentration of 5 ng/mL was also tested.
Population PK/PD Modeling and Simulation
A nonlinear mixed effects approach was used for the PQ population PK model development. The first-order conditional estimation method was employed during the analysis. In the first step, a separate model for intensive venous samples was established. A joint model was then developed including both venous and capillary samples. PQ concentrations below the LLOQ were excluded, as they represented a small proportion of the PK data (2.2% of specimens).
Different structural models, including 1-, 2-, and 3-compartment models, with first-order absorption and with/without lag time were considered to fit the PQ data. The individual PK parameters were assumed to be log-normally distributed, and residual error variance was described by the proportional model. The relationship between capillary and venous samples was modeled using a linear relationship with estimated slope and fixed intercept of 0. The model appropriateness was assessed by the likelihood ratio test, inspection of the diagnostic plots, and internal model validation techniques, including visual and numerical predictive checks.
The final population PK model of PQ was used for the simulation of new dosing scenarios. One hundred groups of 100 subjects were simulated for each dosing regimen, and comparisons of the percentage of women who achieved protective PQ coverage were made using the mean values of the groups. Dosing strategies were selected to maximize protective efficacy and safety. Simulated regimens included monthly dosing (2880 mg PQ-phosphate and 360 mg DHA divided into 3 consecutive daily oral doses once per month), weekly dosing (960 mg PQ-phosphate and 120 mg DHA every 7 days), and 2 daily dosing options (320 mg PQ-phosphate with 40 mg DHA daily and 160 mg PQ-phosphate with 20 mg DHA daily). The weekly and daily regimens were also simulated with a DHA-PQ loading dose at the start of chemoprevention (1 DHA-PQ treatment course at gestational week 20, followed by the weekly regimen starting on day 7 or day 28 of chemoprevention or daily regimens starting on day 4 of chemoprevention).
Time above the protective PQ concentration, protective PQ coverage, and peak PQ concentration during pregnancy were predicted based on the PK model. A comparison between the PQ PK profiles of HIV-infected and HIV-uninfected pregnant women was estimated using previously determined PK parameters and the model from HIV-uninfected women (Savic et al, unpublished data).
A linear model was developed to determine the relationship between PK and QTc using simultaneous PK-toxicity modeling, with estimated baseline and slope as functions of drug concentration at the time of QTc measurement. Analyses were performed using NONMEM (version 7.3), Stata (version 14.2), and R (version 3.3.2) software.
RESULTS
Summary of Clinical Results
In the clinical trial, 200 pregnant women with HIV were randomized to receive either malaria chemoprevention with DHA-PQ every 4 weeks or placebo [8]. Eighty-three women who received IPTp with DHA-PQ and EFV-based ART contributed PK data (Table 1). There were no significant differences in enrollment characteristics (age, weight, gestational age, CD4 cell count, LAMP positivity) between the women who provided PK samples and those who did not. Women who were not receiving ART prior to enrollment began EFV-based ART at least 4 weeks prior to all PK studies. All participants were followed through delivery. There were no episodes of malaria among participants included in this analysis, and parasite prevalence was 1.6% as detected by LAMP (Table 1). For the analyses described in this report, only participants from the DHA-PQ arm were included.
Table 1.
Characteristics of Study Participants Receiving Monthly Dihydroartemisinin-Piperaquine
| Characteristic (N = 83a) | No. (%) |
|---|---|
| Age, y, mean (SD) | 29.8 (7.0) |
| CD4 count, cells/µL, mean (SD) | 525 (249) |
| Receiving ART at enrollmentb | 67 (81) |
| Malaria incidence during study period, events per person-year | 0 |
| Parasitemia detected during study periodc | 5/312 (1.6) |
| Placental malaria by histopathology | 5/81 (6.1) |
| Gestational age at first DHA-PQ dose | |
| 16 wk | 19 (23) |
| 20 wk | 25 (30) |
| 24 wk | 20 (24) |
| 28 wk | 19 (23) |
| No. of trough PQ capillary concentrations | 127 |
| 24 wk | 42 (33) |
| 28 wk | 1 (0.8) |
| 32 wk | 69 (54) |
| 36 wk | 1 (0.8) |
| 40 wk | 14 (11) |
| No. of intensive PQ concentrations, (venous/capillary) | 378 (246/132) |
| No. of PQ concentrations below the limit of quantitation (sparse/intensive) | 11 (10/1) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: ART, antiretroviral therapy; DHA-PQ, dihydroartemisinin-piperaquine; PQ, piperaquine; SD, standard deviation.
aTwenty-eight subjects contributed intensive and 82 subjects contributed sparse pharmacokinetic data.
bEfavirenz started ≥4 weeks prior to pharmacokinetic measurements.
cBy loop-mediated isothermal amplification from a dried blood spot.
Population Pharmacokinetic Model
Raw PQ Data
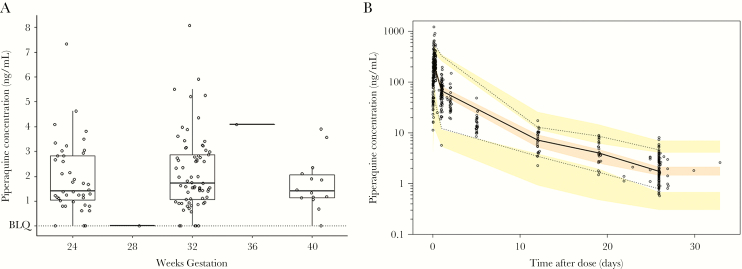
There were 505 PQ concentrations available: 378 were from 28 individuals enrolled in the intensive PK substudy at gestational week 28, and 127 were monthly trough concentrations from 82 individuals (1–4 samples per subject) taken between 20 gestational weeks and delivery (Figure 1). Ten sparse PK specimens (9.2%) and 1 intensive specimen (0.2%) had concentrations below the LLOQ and were excluded from analysis. Among the 117 detectable PQ trough measurements, the median PQ concentration was 1.75 ng/mL (interquartile range, 1.16–2.93 ng/mL [range, 0.58–8.08 ng/mL]; Figure 1A).
Figure 1.
A, Distribution of trough piperaquine (PQ) concentrations by gestational week. Dots show measured concentrations. Box plots represent the median and interquartile range. Concentrations below the limit of quantification (BLQ) were excluded from the analysis. B, Visual predictive check of final pharmacokinetic model. Dots represent measured PQ concentrations, the solid line is the observed median, and the dashed lines are the 5th and 95th percentiles of the observed data. Shaded areas represent simulated 5th, 50th, and 95th percentiles with uncertainty. Model fit is adequate if shaded areas from simulated data encompass solid and dashed lines representing the observed data.
PK Model Estimations
The final PK parameter estimates based on the structural 2-compartment model with lag time are presented in Table 2. Typical oral clearance (CL/F) of PQ in pregnant HIV-infected women receiving EFV was estimated to be 4540 L/day with interindividual variability of 26%. Correlation between venous and capillary concentrations revealed a linear relationship, with a venous/capillary ratio of 0.776. The visual predictive check, shown in Figure 1B, demonstrates good PK model predictability and ability of the model to accurately simulate anticipated variability in population.
Table 2.
Estimated Piperaquine Pharmacokinetic Parametersa
| Parameter | Value (Relative SE) | Interindividual Variability (Relative SE) |
|---|---|---|
| No. (subjects/observations) | 83/494 | |
| Clearance, L/d | 4540 (7%) | 26% (13%) |
| Volume of central compartment, L | 4310 (17%) | 31% (38%) |
| Intercompartmental clearance, L/d | 2280 (14%) | … |
| Volume of peripheral compartment, L | 15300 (11%) | … |
| Rate constant of absorption, 1/d | 29.3 (32%) | 101% (14%) |
| Lag time, d | 0.0381 (2%) | … |
| Ratio, venous/capillary | 0.776 (17%) | … |
| Proportional error | 46% (9%) | … |
Abbreviation: SE, standard error.
aDose administered as piperaquine phosphate and concentration measured as piperaquine base.
Using the PQ population PK model, with monthly DHA-PQ, no study participants achieved protective PQ coverage, defined as maintaining a PQ concentration >10 ng/mL for >95% of the time on DHA-PQ. When the lower PQ concentration of 5 ng/mL was evaluated, 3.6% of the study participants achieved protective PQ coverage. The median cumulative PQ AUC during pregnancy was 2848 ng × hour/mL (standard deviation, 1145 ng × hour/mL [range, 1014–8322 ng × hour/mL]).
Simulations
Achieving Adequate PQ Protection
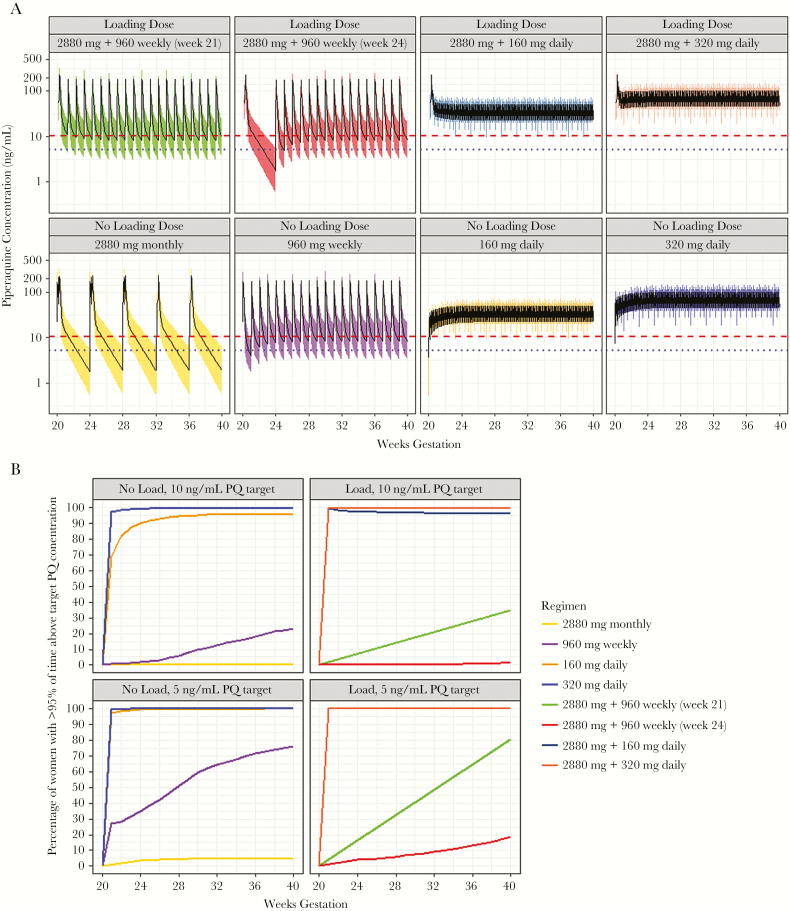
The predicted time above the protective PQ concentrations is presented in Table 3 and visualized for each DHA-PQ regimen in Figure 2. Less than 1% of women receiving monthly DHA-PQ and less than a quarter of women receiving weekly DHA-PQ achieved protective PQ coverage (Table 3 and Figure 2). In contrast, daily dosing achieved significantly improved coverage compared to monthly (P < .001) or weekly regimens (P < .001); 96.0% of women receiving the lower tested dose and 99.9% of those receiving the higher tested dose achieved protective PQ coverage. Adding a loading dose at the initiation of chemoprevention significantly improved the percentage of women who achieved protective PQ coverage for weekly regimens (P < .001), but did not for daily regimens (Table 3).
Table 3.
Percentage of Women Predicted to Achieve Protective Piperaquine Coveragea and Expected Degree of Corrected QT Interval Prolongation With Proposed Dosing Regimens
| Piperaquine Dose | No. of DHA-PQ Tabletsb | Mean Time >10 ng/mL (95% CI)c | Mean Percentage of Women Above the Protective PQ Target >95% of the Time, % (95% CI)c | Mean Percentage of Women With >30 msec Delta QTc, % (95% CI)d | ||
|---|---|---|---|---|---|---|
| 5 ng/mL | 10 ng/mL | 14 ng/mL | ||||
| 2880 mg monthlye | 9 tablets once monthly | 38.0 (35.7–40.7.3) | 5.4 (1.4–9.5) | .4 (0–2.0) | .1 (0–1.0) | 1.8 (0–6.0) |
| 960 mg weekly | 3 tablets once weekly | 64.8 (60.4–69.1) | 76.5 (69.5–83.5) | 24.0 (16.0–32.5) | 7.2 (3.0–12.5) | .8 (0–2.0) |
| 160 mg daily | Half-tablet daily | 98.9 (98.0–99.6) | 99.9 (99.0–100) | 96.0 (92.0–99.0) | 86.8 (80.0–93.0) | 0 |
| 320 mg daily | 1 tablet daily | 99.9 (99.7–100) | 100 (100–100) | 99.9 (99.0–100) | 99.4 (97.0–100) | 0 |
| 2880 mg + 960 mg weekly beginning at 21 wk | 9 tablets, then 3 tablets weekly beginning at 21 wk | 69.7 (65.1–73.9) | 80.7 (74.0–87.0) | 33.6 (26.0–41.0) | 14.6 (9.0–20.0) | 1.4 (0–4.0) |
| 2880 mg + 960 mg weekly beginning at 24 wk | 9 tablets, then 3 tablets weekly beginning at 24 wk | 59.5 (54.7–63.7) | 18.6 (11.0–27.0) | 1.6 (0–4.5) | .3 (0–1.5) | 1.7 (0–5.0) |
| 2880 mg + 160 mg daily | 9 tablets, then half-tablet daily | 99.2 (99.1–99.9) | 99.8 (99.5–100) | 96.4 (92.5–99.0) | 88.5 (82.5–95.0) | 1.6 (0–4.0) |
| 2880 mg + 320 mg daily | 9 tablets, then 1 tablet daily | 99.9 (99.8–100) | 100 (100–100) | 99.8 (99.0–100) | 99.4 (98.0–100) | 1.4 (0–4.0) |
Abbreviations: CI, confidence interval; DHA-PQ, dihydroartemisinin-piperaquine; PQ, piperaquine; QTc, corrected QT interval.
aMaintaining the protective PQ concentration >95% of the time from gestational week 20 to delivery.
bRefers to adult tablets (40 mg dihydroartemisinin/320 mg PQ-phosphate).
cAll differences within columns are statistically significant with P < .001 except for: mean time >10 ng/mL 160 mg daily vs 2880 mg + 160 mg daily (P = .91), 320 mg daily vs 2880 mg + 160 mg daily (P = .09), 320 mg daily vs 2880 mg + 320 mg daily (P = 1.0), 2880 mg + 160 mg daily vs 2880 mg + 320 mg daily (P = .07); 5 ng/mL target, all regimens containing 160 mg daily or 320 mg daily had P = 1.0; 10 ng/mL target, 160 mg daily vs 2880 mg + 160 mg daily (P = .94), 320 mg daily vs 2880 mg + 320 mg daily (P = 1.0), 2880 mg monthly vs 2880 mg + 960 mg weekly at 24 weeks (P = .01); 14 ng/mL target, 2880 mg monthly vs 2880 mg + 960 mg weekly at 24 weeks (P = 1.0), 320 mg daily vs 2880 mg + 320 mg daily (P = 1.0).
d P < .001 for all comparisons between regimens containing 2880 mg vs those that did not, and when comparing 960 mg weekly vs either daily dose, otherwise P > .05.
eFor monthly regimens, milligrams of PQ are divided into equal doses over 3 consecutive days.
Figure 2.
Results of simulations. A, Predicted piperaquine (PQ) concentrations from different dihydroartemisinin-piperaquine (DHA-PQ) chemoprevention regimens. All simulations assumed chemoprevention started at 20 weeks and continued through delivery (40 weeks). The horizontal dashed line represents 10 ng/mL, which has been associated with 95% protection from parasitemia during pregnancy. The blue dotted line indicates a PQ concentration of 5 ng/mL. B, Percentage of women receiving the indicated regimens of DHA-PQ who achieve protective PQ coverage by gestational week. Protective PQ coverage is defined as maintaining a PQ concentration of >10 ng/mL or >5 ng/mL for 95% of the time starting at gestational week 20.
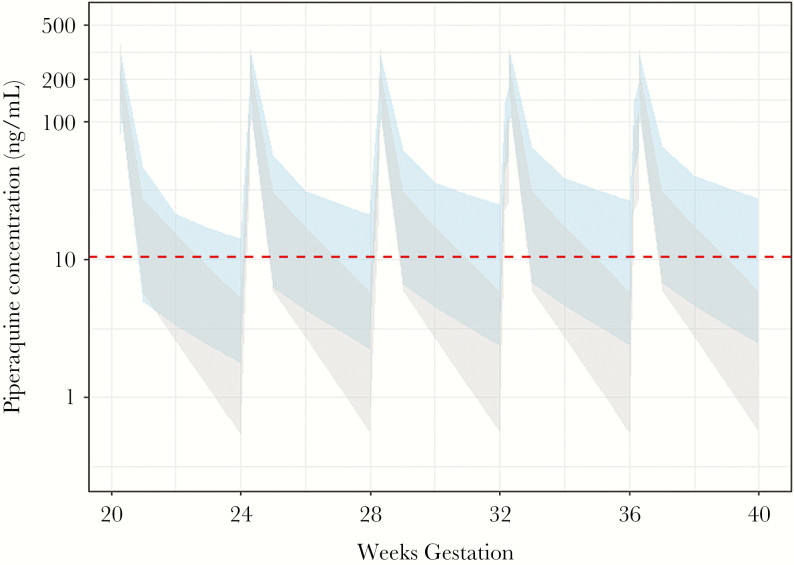
Protective PQ coverage with monthly DHA-PQ was compared between HIV-uninfected women and HIV-infected women (all receiving EFV) using the previously determined parameters (Savic et al, unpublished data) (Figure 3). As expected, the percentage of women who achieved protective PQ coverage was predicted to be significantly lower for HIV-infected women compared with HIV-uninfected women (0.4% vs 24.3%; P < .001).
Figure 3.
Comparison of predicted piperaquine (PQ) concentrations in pregnant women receiving malaria chemoprevention with a standard monthly regimen of dihydroartemisinin-PQ and also receiving (gray or light gray) or not receiving (blue or dark gray) efavirenz-based antiretroviral therapy. The dotted line indicates a PQ concentration of 10 ng/mL, which has been associated with 95% protection from parasitemia during pregnancy.
Peak Piperaquine Concentration and QTc Analysis
Risk of drug toxicity is related primarily to peak drug concentrations. In the case of PQ, there is concern for cardiotoxicity, with demonstrated prolongation of the QTc interval [13]. Twenty-five trial participants contributed intensive PQ PK data and had their QTc measured after their third daily dose of DHA-PQ. The PK model was used to predict the PQ concentration at the time of QTc determination. A linear model with the equation QTc = modeled baseline QTc + [PQ] × 0.777 / 10000 best described the association between PQ PK and QTc.
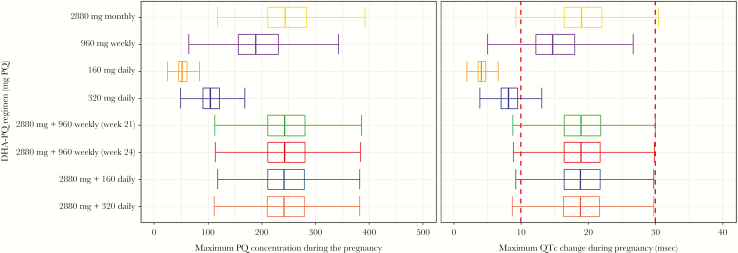
Figure 4 summarizes the predicted peak PQ concentrations during pregnancy and QTc changes for each DHA-PQ regimen. Peak PQ concentrations were highest among regimens that included at least 1 monthly (2880 mg PQ over 3 days) DHA-PQ dose. The mean maximum PQ concentration with a monthly regimen was 250 ng/mL (95% confidence interval [CI], 238–262 ng/mL), with a maximum concentration of 549 ng/mL. Daily regimens had the lowest predicted PQ peak concentrations, with a mean of 53.3 ng/mL (95% CI, 51.1–55.7 ng/mL) for 160 mg PQ daily, and 107 ng/mL (95% CI, 101–112 ng/mL) for 320 mg PQ daily. Greater than 30 msec of QTc prolongation at some point during the pregnancy was predicted for <2% of women taking at least 1 monthly dose of DHA-PQ (including a loading dose), for 0.8% of women receiving a weekly regimen without a loading dose, and for none of the women receiving daily regimens. Indeed, the model predicted that none of the women receiving a 160 mg daily DHA-PQ regimen would experience >10 msec QTc prolongation.
Figure 4.
The predicted range of maximum piperaquine (PQ) concentrations and predicted maximum change in corrected QT interval (QTc) during pregnancy with the different dihydroartemisinin-piperaquine (DHA-PQ) chemoprevention regimens.
Peak PQ concentrations with monthly DHA-PQ were predicted to be significantly higher for HIV-infected women compared with HIV-uninfected women (mean, 250 vs 190 ng/mL; P < .001).
DISCUSSION
IPTp with DHA-PQ has great promise to reduce the burden of malaria during pregnancy in African women, but its benefits may be lost in HIV-infected women receiving EFV-based ART due to EFV-mediated cytochrome P450 (CYP) 3A4 induction, which leads to increased PQ clearance and reduced plasma exposure [9]. Considering other drugs received by study participants, tenofovir, lamivudine, and TMP-SMX are not known to change CYP3A4 or CYP2C19 activity, making them less likely to contribute to these findings [14–16].
Less than 1% of women who received EFV and monthly DHA-PQ were predicted to achieve defined ideal protective PQ coverage. Although the protective efficacy of monthly DHA-PQ among trial participants could not be quantified in the setting of low malaria burden, the PQ PK model strongly suggests that, in the presence of high malaria transmission intensity, monthly DHA-PQ will not adequately prevent parasitemia. To identify regimens with improved PQ exposure, we simulated alternative DHA-PQ dosing schemes including weekly and daily schedules, with and without a treatment course of DHA-PQ at the beginning of chemoprevention. Time above the protective PQ concentration increased with more frequent, lower-dose DHA-PQ administration, with daily DHA-PQ predicted to have the optimal protective PQ coverage and lowest risk of cardiotoxicity.
Prior to the institution of IRS in Tororo, HIV-infected women who received daily TMP-SMX experienced a high burden of malaria during pregnancy, and one-third had placental malaria [17]. Therefore, in settings of high malaria burden and antifolate resistance, HIV-infected women who take daily TMP-SMX will likely require an additional agent for effective malaria chemoprevention. PQ models in nonpregnant adult males and Ugandan children have predicted that a weekly dose of 960 mg PQ should be considered to improve the protective efficacy of DHA-PQ [18, 19]. We also found improved PQ coverage with weekly regimens, with up to 34% of women predicted to achieve protective PQ coverage.
Daily low-dose DHA-PQ was estimated to provide protective PQ coverage for >98% of women. In addition to potentially improved prevention of parasitemia, we suggest that daily low-dose DHA-PQ chemoprevention during pregnancy for HIV-infected women receiving EFV-based ART may also minimize the risk of selection for drug resistance. We estimated that women receiving EFV-based ART and monthly DHA-PQ would spend an average of 62% of the chemoprevention period at risk for parasitemia with potentially detectable PQ concentrations. Plasma PQ concentrations as low as 2.4 ng/mL have been associated with the selection for genetic markers of decreased susceptibility to antimalarials [20]. With daily DHA-PQ chemoprevention, <1% of women would be estimated to sustain PQ concentrations that permit parasitemia as detected by LAMP. Optimizing DHA-PQ dosing for chemoprevention could mitigate the potential risk for drug resistance.
High PQ concentrations have been associated with clinically significant QTc prolongation [13]. Efavirenz has also been associated with increases in QTc [21, 22]. In our analysis, QTc prolongation was linearly associated with PQ concentrations. Estimated peak PQ concentrations during pregnancy with monthly DHA-PQ were somewhat higher for HIV-infected women receiving EFV compared with those in HIV-uninfected women in the simulated data (Savic et al, unpublished data). This was an unanticipated finding, as EFV is not believed to affect PQ absorption. Sensitivity analysis suggested that the estimate of the absorption rate constant, which defines maximum concentration (Cmax), was highly influenced by data from 1 individual. Even with this high absorption constant and presence of EFV, none of the regimens resulted in Cmax values predicted to cause clinically significant QTc prolongation. Most importantly, modeling of low-dose daily DHA-PQ predicted minimal risk of QTc prolongation with excellent protective PQ coverage compared with monthly DHA-PQ with or without EFV.
Our study had some limitations. First, due to the unexpectedly low burden of malaria at the study site, insufficient power was available to assess PD endpoints. Second, the protective efficacy of daily TMP-SMX could not be determined. The protective efficacy of TMP-SMX during pregnancy is unknown, as it is standard of care for HIV-infected pregnant women. Considering other populations, among infants who received placebo or TMP-SMX in Uganda, the protective efficacy of TMP-SMX was 28%–49% [5, 23]. In a randomized trial, HIV-infected adults who continued TMP-SMX experienced a protective efficacy of 70% against malaria compared with those who stopped the drug per WHO recommendations [24]. To explore the DHA-PQ dosing implications with the additive benefit of TMP-SMX, we evaluated the time above a protective PQ concentration that was lowered by 50% (eg, 5 ng/mL) for each regimen. Even with this modified PQ concentration target, the daily DHA-PQ regimens remained optimal. Third, a biomarker that predicts the maximum benefit of IPTp is not available. We used the PD target of nearly eliminating LAMP positivity during pregnancy for >95% of women, as LAMP positivity has been associated with placental malaria, and to limit the potential selection of drug resistance [20, 25]. Fourth, daily or weekly DHA-PQ administration over a 6-month period during pregnancy has not been tested clinically, and side effects or impact on parasite determinants of drug resistance with this regimen have not been assessed.
Efavirenz-based ART is currently the WHO first-line treatment for HIV during pregnancy [26]. We found that, for women receiving EFV-based ART in the absence of highly effective vector control measures such as IRS, low-dose daily DHA-PQ during pregnancy is likely to provide much greater protection against malaria than the monthly regimen that has been most studied. As future clinical trials are designed to evaluate the efficacy and safety of DHA-PQ chemoprevention for HIV-infected women during pregnancy, daily low-dose DHA-PQ appears to be the most promising regimen for chemoprevention. Our findings also support a potential benefit to switching to dolutegravir-based ART during pregnancy, which would avoid the drug–drug interaction responsible for decreased PQ concentrations with concurrent use of EFV [27]. Optimizing the dosing of DHA-PQ for chemoprevention in the setting of drug–drug interactions will be an important advancement in malaria control.
Notes
Acknowledgments. We thank the women who participated in the study, the dedicated study staff, practitioners at the Tororo District Hospital, members of the Infectious Diseases Research Collaboration, and members of the University of California, San Francisco Drug Research Unit.
Financial support. This work was supported by the National Institutes of Health (grant numbers R01 AI117001-02 [to P. J. R. and F. A.] and P01 HD059454 [to G. D.]). E. W. was supported by a National Institutes of Health Research Training Grant (grant number T32 GM007546-41).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. World malaria report 2015. Geneva, Switzerland: WHO, 2015. [Google Scholar]
- 2. ter Kuile FO, Parise ME, Verhoeff FH et al. . The burden of co-infection with human immunodeficiency virus type 1 and malaria in pregnant women in sub-Saharan Africa. Am J Trop Med Hyg 2004; 71:41–54. [PubMed] [Google Scholar]
- 3. World Health Organization. Updated WHO policy recommendation: intermittent preventive treatment of malaria in pregnancy using sulfadoxine-pyrimethamine (IPTp-SP). Geneva, Switzerland: WHO, 2012. [Google Scholar]
- 4. Walker PG, Floyd J, Ter Kuile F, Cairns M. Estimated impact on birth weight of scaling up intermittent preventive treatment of malaria in pregnancy given sulphadoxine-pyrimethamine resistance in Africa: a mathematical model. PLoS Med 2017; 14:e1002243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bigira V, Kapisi J, Clark TD et al. . Protective efficacy and safety of three antimalarial regimens for the prevention of malaria in young Ugandan children: a randomized controlled trial. PLoS Med 2014; 11:e1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Desai M, Gutman J, L’lanziva A et al. . Intermittent screening and treatment or intermittent preventive treatment with dihydroartemisinin-piperaquine versus intermittent preventive treatment with sulfadoxine-pyrimethamine for the control of malaria during pregnancy in western Kenya: an open-label, three-group, randomised controlled superiority trial. Lancet 2015; 386:2507–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kakuru A, Jagannathan P, Muhindo MK et al. . Dihydroartemisinin-piperaquine for the prevention of malaria in pregnancy. N Engl J Med 2016; 374:928–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Natureeba P, Kakuru A, Muhindo M et al. . Intermittent preventive treatment with dihydroartemisinin-piperaquine for the prevention of malaria among HIV-infected pregnant women. J Infect Dis 2017; 216:29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kajubi R, Huang L, Jagannathan P et al. . Antiretroviral therapy with efavirenz accentuates pregnancy-associated reduction of dihydroartemisinin-piperaquine exposure during malaria chemoprevention. Clin Pharmacol Ther 2017; 102:520–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kamya MR, Arinaitwe E, Wanzira H et al. . Malaria transmission, infection, and disease at three sites with varied transmission intensity in Uganda: implications for malaria control. Am J Trop Med Hyg 2015; 92:903–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Katureebe A, Zinszer K, Arinaitwe E et al. . Measures of malaria burden after long-lasting insecticidal net distribution and indoor residual spraying at three sites in Uganda: a prospective observational study. PLoS Med 2016; 13:e1002167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kjellin LL, Dorsey G, Rosenthal PJ, Aweeka F, Huang L. Determination of the antimalarial drug piperaquine in small volume pediatric plasma samples by LC-MS/MS. Bioanalysis 2014; 6:3081–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Manning J, Vanachayangkul P, Lon C et al. . Randomized, double-blind, placebo-controlled clinical trial of a two-day regimen of dihydroartemisinin-piperaquine for malaria prevention halted for concern over prolonged corrected QT interval. Antimicrob Agents Chemother 2014; 58:6056–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wen X, Wang JS, Backman JT, Laitila J, Neuvonen PJ. Trimethoprim and sulfamethoxazole are selective inhibitors of CYP2C8 and CYP2C9, respectively. Drug Metab Dispos 2002; 30:631–5. [DOI] [PubMed] [Google Scholar]
- 15. Nekvindová J, Masek V, Veinlichová A et al. . Inhibition of human liver microsomal cytochrome P450 activities by adefovir and tenofovir. Xenobiotica 2006; 36:1165–77. [DOI] [PubMed] [Google Scholar]
- 16. Barry M, Mulcahy F, Merry C, Gibbons S, Back D. Pharmacokinetics and potential interactions amongst antiretroviral agents used to treat patients with HIV infection. Clin Pharmacokinet 1999; 36:289–304. [DOI] [PubMed] [Google Scholar]
- 17. Natureeba P, Ades V, Luwedde F et al. . Lopinavir/ritonavir-based antiretroviral treatment (ART) versus efavirenz-based ART for the prevention of malaria among HIV-infected pregnant women. J Infect Dis 2014; 210:1938–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Permala J, Tarning J, Nosten F, White NJ, Karlsson MO, Bergstrand M. Prediction of improved antimalarial chemoprevention with weekly dosing of dihydroartemisinin-piperaquine. Antimicrob Agents Chemother 2017; 61:e02491–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sambol NC, Tappero JW, Arinaitwe E, Parikh S. Rethinking dosing regimen selection of piperaquine for malaria chemoprevention: a simulation study. PLoS One 2016; 11:e0154623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Conrad MD, Mota D, Foster M et al. . Impact of intermittent preventive treatment during pregnancy on Plasmodium falciparum drug resistance-mediating polymorphisms in Uganda. J Infect Dis 2017; 216:1008–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Abdelhady AM, Shugg T, Thong N et al. . Efavirenz inhibits the human ether-a-go-go related current (hERG) and induces QT interval prolongation in CYP2B6*6*6 allele carriers. J Cardiovasc Electrophysiol 2016; 27:1206–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shimabukuro-Vornhagen A, Rybniker J, Zoghi S et al. . Acquired long QT syndrome and torsade de pointes associated with HIV infection. Case Rep Med 2010; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kamya MR, Kapisi J, Bigira V et al. . Efficacy and safety of three regimens for the prevention of malaria in young HIV-exposed Ugandan children: a randomized controlled trial. AIDS 2014; 28:2701–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kasirye RP, Baisley K, Munderi P et al. . Incidence of malaria by cotrimoxazole use in HIV-infected Ugandan adults on antiretroviral therapy: a randomised, placebo-controlled study. AIDS 2016; 30:635–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kapisi J, Kakuru A, Jagannathan P et al. . Relationships between infection with Plasmodium falciparum during pregnancy, measures of placental malaria, and adverse birth outcomes. Malar J 2017; 16:400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Geneva, Switzerland: WHO, 2016. [PubMed] [Google Scholar]
- 27. Lewis JM, Railton E, Riordan A, Khoo S, Chaponda M. Early experience of dolutegravir pharmacokinetics in pregnancy: high maternal levels and significant foetal exposure with twice-daily dosing. AIDS 2016; 30:1313–5. [DOI] [PMC free article] [PubMed] [Google Scholar]