Over 48 weeks, maraviroc partnered with a dual nucleoside/nucleotide reverse transcriptase inhibitor backbone was a safe and effective switch for a ritonavir-boosted protease inhibitor (PI/r) in HIV-1–infected patients with R5-tropic virus, virologically suppressed on a PI/r-based regimen.
Keywords: maraviroc, switch, HIV-1, antiretroviral, comorbidity
Abstract
Background. Alternative combination antiretroviral therapies in virologically suppressed human immunodeficiency virus (HIV)–infected patients experiencing side effects and/or at ongoing risk of important comorbidities from current therapy are needed. Maraviroc (MVC), a chemokine receptor 5 antagonist, is a potential alternative component of therapy in those with R5-tropic virus.
Methods. The Maraviroc Switch Study is a randomized, multicenter, 96-week, open-label switch study in HIV type 1–infected adults with R5-tropic virus, virologically suppressed on a ritonavir-boosted protease inhibitor (PI/r) plus double nucleoside/nucleotide reverse transcriptase inhibitor (2 N(t)RTI) backbone. Participants were randomized 1:2:2 to current combination antiretroviral therapy (control), or replacing the protease inhibitor (MVC + 2 N(t)RTI arm) or the nucleoside reverse transcriptase inhibitor backbone (MVC + PI/r arm) with twice-daily MVC. The primary endpoint was the difference (switch minus control) in proportion with plasma viral load (VL) <200 copies/mL at 48 weeks. The switch arms were judged noninferior if the lower limit of the 95% confidence interval (CI) for the difference in the primary endpoint was < −12% in the intention-to-treat (ITT) population.
Results. The ITT population comprised 395 participants (control, n = 82; MVC + 2 N(t)RTI, n = 156; MVC + PI/r, n = 157). Baseline characteristics were well matched. At week 48, noninferior rates of virological suppression were observed in those switching away from a PI/r (93.6% [95% CI, −9.0% to 2.2%] and 91.7% [95% CI, −9.6% to 3.8%] with VL <200 and <50 copies/mL, respectively) compared to the control arm (97.6% and 95.1% with VL <200 and <50 copies/mL, respectively). In contrast, MVC + PI/r did not meet noninferiority bounds and was significantly inferior (84.1% [95% CI, −19.8% to −5.8%] and 77.7% [95% CI, −24.9% to −8.4%] with VL <200 and <50 copies/mL, respectively) to the control arm in the ITT analysis.
Conclusions. These data support MVC as a switch option for ritonavir-boosted PIs when partnered with a 2-N(t)RTI backbone, but not as part of N(t)RTI-sparing regimens comprising MVC with PI/r.
Clinical Trials Registration. NCT01384682.
Widespread use of combination antiretroviral therapy (cART) has transformed human immunodeficiency virus type 1 (HIV-1) infection into a chronic condition, with near-normal life expectancy [1]. Recommended regimens are currently 3-drug combinations including at least 2 of the 4 classes of ART (https://aidsinfo.nih.gov/guidelines). While treatment-limiting toxicities have been reduced considerably in recent years, even low-grade side effects can affect adherence and patient satisfaction [2]. Several switch studies have explored novel antiretroviral combinations. Early findings from some very small nucleos(t)ide reverse transcriptase inhibitor (N(t)RTI)–sparing switch studies [3–5] including maraviroc (MVC) [6, 7] have been encouraging, but none are definitive.
MVC, the first licensed [8] chemokine receptor 5 (CCR5)-receptor antagonist, targets a host co-receptor critical for HIV entry into CD4+ T-cells and monocyte/macrophages. MVC is safe and well tolerated, with favorable renal and lipid profiles (http://www.selzentry.com). These factors are likely to be of increasing importance as the HIV-infected population ages with greater risks of increased cardiovascular disease (CVD) and declining renal function. MVC may also have additional anti-inflammatory activity that could further reduce risks of specific end-organ disease [9].
A key barrier to using MVC in routine care settings is the need for characterization of virus co-receptor tropism prior to use. Assay systems can now determine HIV co-receptor tropism based not only on HIV RNA but also on HIV DNA [10, 11]. The latter means tropism for the CCR5 co-receptor can be determined in virologically suppressed patients, thus allowing MVC to be used as a switch option. This testing platform is tentatively endorsed by the German/Austrian (http://www.daignet.de/site-content/hiv-therapie/leitlinien-1) and European guidelines [12], but with a weak evidence base.
The Maraviroc Switch (MARCH) study aimed to define whether, in HIV-1–infected adults on a stable 2-N(t)RTI + ritonavir-boosted protease inhibitor (PI/r) cART regimen and with R5 virus as determined by proviral DNA tropism testing, replacing either the PI/r or 2 N(t)RTIs with MVC provided noninferior virological efficacy and improvements in safety and tolerability relative to remaining on a 2-N(t)RTI + PI/r regimen.
METHODS
Study Design
MARCH is an international, multicenter, randomized (1:2:2), open-label, 96-week noninferiority switch study of MVC 300 mg twice daily with a 2-N(t)RTI backbone vs MVC 150 mg twice daily (recommended dosing in the presence of a pharmacokinetic enhancer [http://www.selzentry.com]) with a PI/r vs continuing the current 2-N(t)RTI + PI/r (control) regimen.
Study Population
Participants were included if they were HIV-1–infected adults aged ≥18 years, with plasma HIV RNA (viral load [VL]) <200 copies/mL on a stable (>24 weeks) 2-N(t)RTI + PI/r regimen. Participants were excluded if they were pregnant or breastfeeding, had known genotypic resistance and/or prior virological failure/rebound, had an anticipated need to modify current cART for toxicity in the next 6 months, or had active hepatitis B coinfection, or if specific hematological/biochemical parameters were outside protocol-specified ranges. Other exclusions included use of medications contraindicated with MVC, acute therapy for serious infection/medical illness, use of immunomodulators ≤30 days prior to enrollment, current alcohol/illicit substance use that would conflict with study conduct, or compulsorily detained. The protocol and patient information statement and consent form were approved by the ethics committee/institutional review board at all participating sites. Written informed consent was obtained from all participants.
Proviral DNA Tropism Testing for Eligibility
For population tropism genotyping, several in‐house protocols have been optimized and cover most subtypes and circulating recombinant forms [13–15]. Overall, the interpretation of the V3 sequence is via algorithms that predict the likelihood of co‐receptor usage—for example, geno2pheno (http://www.geno2pheno.org). For MARCH study eligibility, participants were deemed to have R5 virus if each of the 3 sequences of the relevant portion of the V3 loop had a false-positive rate of ≥10% using the standard geno2pheno algorithm or if 2 of 3 sequences were R5 and 1 sequence failed to amplify. A single repeat sample could be drawn in the case of test failure. In this circumstance, the patient's virus was declared R5 if there were ≥3 R5 sequences and no X4 from both samples. Tropism testing was conducted at 14 certified laboratories [16], each using its own laboratory procedures.
Assessments
A computer-generated randomization sequence with a blocking factor of 5, stratified by site, was created by the study statistician. Eligible participants were randomly assigned through an electronic case report form. At the time of sample size reduction (see below), arm allocation was changed to using a minimization strategy (www.sghms.ac.uk/depts/phs/guide/randser.htm) to reduce the risk of imbalance between arms.
Eligible participants were randomized ≤60 days of screening and seen subsequently at weeks 4, 12, and every 12 weeks thereafter until week 96. At each study visit vital signs, targeted physical examination, changes in randomly assigned therapy and concomitant medications, adverse events (AEs), HIV-1 RNA with a lower limit of detection of at least <200 copies/mL at the local laboratories, T-cell enumeration, and safety laboratory tests (biochemistry/hematology/pregnancy) were collected. Fasting (≥8 hours) lipid and glycemic parameters were performed at all visits (except week 36) in year 1 and annually thereafter. Annual assessments in all patients included anthropometric measurements, bone mineral density (BMD), and peripheral and central fat using dual-energy X-ray absorptiometry (DXA) scanning and quality of life (QoL) using the 12-Item Short-Form Health Survey (SF-12) instrument. ART adherence was assessed at weeks 4, 48, and 96 using a validated self-reporting 7-day recall adherence tool [17]. Plasma and sera were collected at each study visit; plasma and a buffy coat were taken for central genotypic resistance testing (protease and reverse transcriptase sequencing) and genotypic tropism testing in those with confirmed virological failure (defined below).
Endpoints
The primary endpoint was the comparison of proportions of participants with HIV RNA <200 copies/mL 48 weeks after randomization between control and each switch arm. Secondary endpoints included difference between control and switch arms over 48 weeks as follows. Virologic: proportion with plasma HIV RNA <50 copies/mL; time to virological failure defined as plasma HIV RNA ≥200 copies/mL on randomized therapy, on 2 occasions ≥7 days apart; time to loss of virological response defined by virological failure, permanent discontinuation of randomized treatment, new AIDS-defining illness, death, or withdrawal from the study; change in plasma HIV RNA log10 copies/mL; frequency of plasma virus “blips” (nonsustained VL >200 copies/mL); genotypic resistance at virological failure. Immunologic: changes from baseline in CD4+ T-cell count (absolute and percentage). Clinical: rates of opportunistic disease, serious non-AIDS-defining illness, and non-AIDS-related mortality. Metabolic and body composition: changes from baseline in fasting lipids (total cholesterol, low-density lipoprotein cholesterol [LDL-c], high-density lipoprotein cholesterol, triglycerides), fasting glucose, and insulin; absolute 10-year CVD risk assessment using the Framingham risk score (http://cvdrisk.nhlbi.nih.gov); rates of initiation/changes in existing lipid-lowering therapies; changes from baseline in body fat and BMD derived from DXA; changes in 10-year fracture risk (FRAX algorithm) and bone turnover markers. Safety: changes from baseline in selected serum biochemical parameters, including estimated glomerular filtration rate (GFR); proportions experiencing and types of serious adverse events (SAEs); proportions experiencing AEs, and types and severity of AEs. Adherence: self-reported at weeks 4 and 48. QoL: change from baseline health status scores using the SF-12 health status.
Statistical Analysis
In May 2013, the sample size was reduced to 380 (from the original 560) participants randomized 1:2:2 (76:152:152) to control; MVC + 2 N(t)RTIs; MVC + PI/r. This was in response to the slower than expected enrollment. The sample size reduction resulted in a loss of power from 90% to 80% to demonstrate virological noninferiority of the switch arms vs control.
Treatment was to continue until the last randomized participant completed 96 weeks of follow-up or had permanently withdrawn from follow-up. The criteria by which the experimental switch regimens were judged noninferior to the control regimen was if the lower limit of the 95% confidence interval (CI) for the difference in virological response between each experimental arm and the control arm did not extend below −12%. Tests for noninferiority were primarily performed in the intention-to-treat (ITT) study population. A supportive analysis of the per-protocol (PP) population was also pre-planned. For secondary and exploratory efficacy endpoints, the ITT population was primary, followed by the PP population. Safety data were analyzed by randomized arm on available data. All comparisons were pairwise between an experimental arm and the control arm. All differences were experimental arm minus control arm. The χ2 test, or where cell sizes were less than Fisher exact test, were used to compare proportions–tests for means and Cox proportional hazards for incidence rates. All statistical tests were 2-sided and considered significant at α < .05. Statistical analyses were performed using SAS version 9.4 and Stata 13 software.
RESULTS
Study Status
The first and last randomizations were 19 January 2012 and 12 February 2014, respectively. All participants completed 48 weeks of follow-up by 14 January 2015. The data for this analysis were extracted on 6 February 2015. Participant follow-up is ongoing.
Participant Disposition
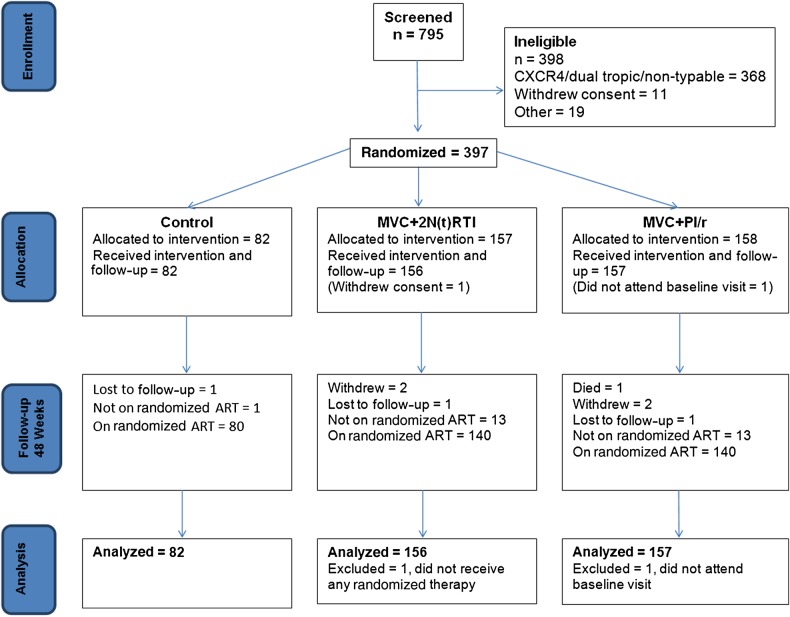
Seven hundred ninety-five participants were screened from sites in 13 countries (Argentina, Australia, Canada, Chile, France, Germany, Ireland, Japan, Mexico, Poland, Spain, Thailand, United Kingdom). Disposition and analysis populations are described in greater detail in Figure 1. The ITT population comprised 395 participants who commenced randomized therapy, attended baseline, and had ≥1 study visit.
Figure 1.
Maraviroc Switch Study recruitment and participant disposition. Abbreviations: ART, antiretroviral therapy; MVC, maraviroc; N(t)RTI, nucleoside/nucleotide reverse transcriptase inhibitor; PI/r, ritonavir-boosted protease inhibitor.
Baseline Characteristics
Baseline characteristics were well balanced across the arms (Table 1). Twenty-three percent of participants were women; the mean age was 43 years; 56% and 32% were of white and Hispanic/Latino ethnicity, respectively. Sixty percent had category A disease; 96% had plasma VL <50 copies/mL; the mean CD4+ T-cell count was 617 cells/μL, and the mean ART duration was 6.1 years. Sixty-eight percent were on tenofovir-based N(t)RTI backbones; 35%, 28%, and 17% were on ritonavir-boosted atazanavir, lopinavir (LPV/r), or darunavir, respectively (Table 2). Overall, the types of N(t)RTI backbone were similar across arms; however, abacavir/lamivudine was used in 22% of the control arm vs 12% and 13% of the MVC + 2 N(t)RTI and MVC + PI/r arms, respectively. The most common PI/r was ritonavir-boosted atazanavir, then LPV/r in all 3 arms. LPV/r was the PI/r used in 21% of the MVC + 2 N(t)RTI arm, compared with 35% and 32% in the control and MVC + PI/r arms, respectively. Mean Framingham 10-year CVD risk (available in 377 participants) was 6.64% (standard deviation [SD], 6.16%): 6.87% (SD, 7.21%) in the control (n = 78), 6.3% (SD, 5.56%) in the MVC + 2 N(t)RTI (n = 144), and 6.84% (SD, 6.15%) in the MVC + PI/r (n = 155) arms. The mean T-score at the lumbar spine site only (Table 1) showed mild osteopenia in all 3 arms.
Table 1.
Maraviroc Switch Study Baseline Characteristics
| Characteristic | Control (n = 82)a | MVC + 2 N(t)RTIs (n = 156)a | MVC + PI/r (n = 157)a | Total (N = 395)a |
|---|---|---|---|---|
| Female, No. (%) | 20 (24.4) | 35 (22.4) | 35 (22.3) | 90 (22.8) |
| Age, y, mean (SD) | 43.6 (10.5) | 43.7 (10.5) | 42.7 (9.6) | 43.3 (10.1) |
| Ethnicity, No. (%) | ||||
| African heritage | 0 (0.0) | 6 (3.9) | 4 (2.6) | 10 (2.5) |
| Asian | 9 (11.0) | 10 (6.4) | 15 (9.6) | 34 (8.6) |
| Australian Aboriginal | 0 (0.0) | 0 (0.0) | 1 (0.6) | 1 (0.3) |
| White | 42 (51.2) | 99 (63.5) | 80 (51.0) | 221 (56.0) |
| Hispanic or Latino | 31 (37.8) | 40 (25.6) | 56 (35.7) | 127 (32.2) |
| Other | 0 (0.0) | 1 (0.6) | 1 (0.6) | 2 (0.5) |
| CDC category C, No. (%) | 21 (25.6) | 36 (23.1) | 30 (19.1) | 87 (22.0) |
| Baseline HIV RNA <50 copies/mL | 96.3% | 96.8% | 95.5% | 96.2% |
| CD4+ T cells/µL, mean (SD) | 634.8 (244.5) | 596.3 (253.3) | 637.6 (252.7) | 617.0 (251.2) |
| Nadir CD4+ T cells/µL, mean (SD) | 258 (169) | 206 (156) | 238 (143) | 213 (162) |
| ART duration, y, mean (SD) | 6.3 (4.5) | 5.7 (3.8) | 6.4 (4.4) | 6.1 (4.2) |
| GFR, mL/min, mean (SD) | 99.39 (20.59) | 106.61 (71.77) (n = 155) | 100.73 (22.37) | 102.77 (48.11) (n = 394) |
| Total cholesterol, mmol/L, mean (SD) | 4.87 (1.1) (n = 79) | 4.89 (1.04) (n = 146) | 4.89 (1.01) (n = 155) | 4.89 (1.04) (n = 380) |
| LDL-c, mmol/L, mean (SD) | 2.82 (0.96) (n = 79) | 2.87 (0.86) (n = 142) | 2.86 (0.94) (n = 150) | 2.86 (0.91) (n = 371) |
| HDL-c, mmol/L, mean (SD) | 1.21 (0.29) (n = 79) | 1.26 (0.39) (n = 146) | 1.26 (0.41) (n = 155) | 1.25 (0.38) (n = 380) |
| TGs, mmol/L, mean (SD) | 1.91 (0.99) (n = 79) | 1.77 (0.93) (n = 144) | 1.98 (1.4) (n = 152) | 1.88 (1.16) (n = 375) |
| Right hip T-score, mean (SD) | −0.3 (1.28) (n = 76) | −0.71 (1.1) (n = 131) | −0.6 (1.04) (n = 143) | −0.58 (1.13) (n = 350) |
| Lumbar (T2–T4) T-score, mean (SD) | −0.75 (1.23) (n = 77) | −1.24 (1.35) (n = 134) | −1.18 (1.27) (n = 143) | −1.11 (1.3) (n = 354) |
Abbreviations: ART, antiretroviral therapy; CDC, Centers for Disease Control and Prevention; GFR, glomerular filtration rate; HDL-c, high-density lipoprotein cholesterol; HIV, human immunodeficiency virus; LDL-c, low-density lipoprotein cholesterol; MVC, maraviroc; N(t)RTI, nucleoside/nucleotide reverse transcriptase inhibitor; PI/r, ritonavir-boosted protease inhibitor; SD, standard deviation; TGs, triglycerides;
a If data are available for fewer participants, this will be indicated in brackets against each baseline characteristic.
Table 2.
Maraviroc Switch Study Baseline Antiretroviral Therapy
| Characteristic | Control (n = 82) | MVC + 2 N(t)RTIs (n = 156) | MVC + PI/r (n = 157) | Total (N = 395) |
|---|---|---|---|---|
| N(t)RTI | ||||
| TDF/FTC | 38 (46.3) | 82 (52.6) | 83 (52.9) | 203 (51.4) |
| TDF/3TC | 13 (15.9) | 26 (16.7) | 25 (15.9) | 64 (16.2) |
| ABC/3TC | 18 (22.0) | 19 (12.2) | 20 (12.7) | 57 (14.4) |
| ZDV/3TC | 6 (7.3) | 20 (12.8) | 17 (10.8) | 43 (10.9) |
| Other | 7 (8.5) | 9 (5.6) | 12 (7.6) | 28 (7.1) |
| PI/r | ||||
| ATV/r | 27 (32.9) | 58 (37.2) | 55 (35.0) | 140 (35.4) |
| LPV/r | 29 (35.4) | 33 (21.2) | 50 (31.9) | 112 (28.4) |
| DRV/r | 13 (15.9) | 32 (20.5) | 20 (12.7) | 65 (16.5) |
| SQV/r | 9 (11.0) | 24 (15.4) | 22 (14.0) | 55 (13.92) |
| FPV/r | 4 (4.9) | 8 (5.1) | 10 (6.4) | 22 (5.6) |
| IDV/r | 0 (0.0) | 1 (0.6) | 0 (0.00) | 1 (0.3) |
Data are presented as No. (%).
Abbreviations: 3TC, lamivudine; ABC, abacavir; ATV/r, ritonavir-boosted atazanavir; DRV/r, ritonavir-boosted darunavir; FPV/r, ritonavir-boosted fosamprenavir; FTC, emtricitabine; IDV/r, ritonavir-boosted indinavir; LPV/r, ritonavir-boosted lopinavir; MVC, maraviroc; N(t)RTI, nucleoside/nucleotide reverse transcriptase inhibitor; PI/r, ritonavir-boosted protease inhibitor; SQV/r, ritonavir-boosted saquinavir; TDF, tenofovir disoproxil fumarate; ZDV, zidovudine.
Primary Endpoint and Other Major Secondary Outcomes
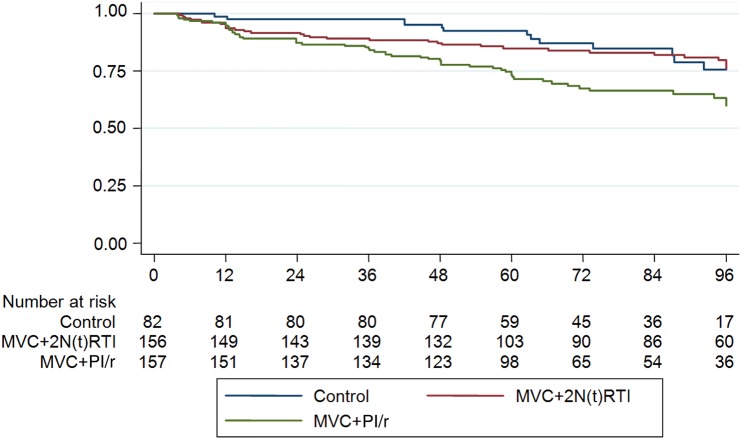
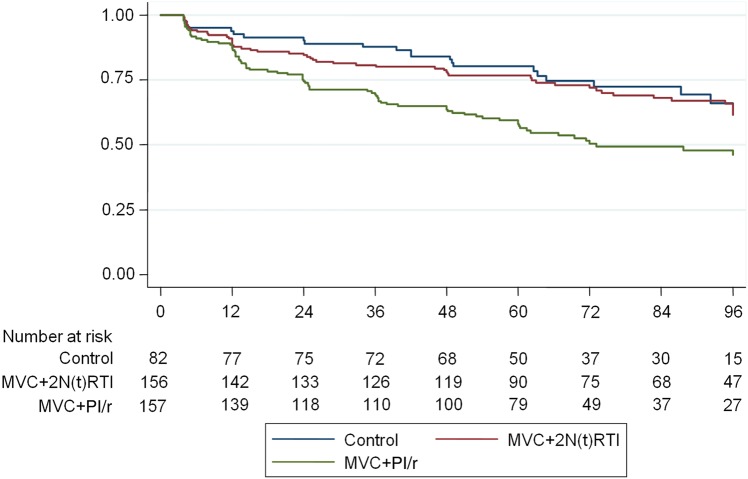
The N(t)RTI-sparing regimen of MVC + PI/r did not meet noninferiority criteria and was significantly inferior to the control arm in the ITT analysis (Table 3). In contrast, high rates of virological suppression (<200 copies/mL and <50 copies/mL) were maintained in those on the control arm and those switching to MVC + 2 N(t)RTIs. Time to loss of virological suppression to <200 copies/mL and <50 copies/mL is shown in Figures 2 and 3, respectively. Loss of virological suppression began early (within the first 24 weeks) after the randomized switch in those on the N(t)RTI-sparing regimen. The hazard ratio for loss of virological response <200 copies/mL and <50 copies/mL over 48 weeks, respectively, was 2.41 (95% CI, 1.31–4.43; P = .005) and 2.16 (95% CI, 1.34–3.48; P = .001), respectively, for the MVC + PI/r arm vs control arm. No formal analysis of patient-reported adherence at weeks 4 and 48 was performed, as only 1.3% reported taking approximately half or less of their ART. Week 4 and week 48 seven-day recall adherence was available for 97% and 96% of the ITT population, respectively; of these, 88% (week 4) and 90% (week 48) reported taking all of their ART, and 10% (week 4) and 8% (week 48) reported taking most of their ART. There was no difference in 7-day recall between the switch arms. Eleven participants ceased randomized therapy for high VL; of these 0, 5, and 6 were in the control, MVC + 2 N(t)RTI, and MVC + PI/r arms, respectively. Up to week 48, there were 18 confirmed VL “blips” in 17 individuals, 13 of which (72%) occurred in the MVC + PI/r arm.
Table 3.
Maraviroc Switch Study Virological Outcomes: 48-Week Data
| Analysis | Arm | Below Threshold, % | Difference, % | (95% CI) |
|---|---|---|---|---|
| Intention to treat | ||||
| <50 copies/mL | Control | 95.1 | Reference | |
| MVC + 2 N(t)RTIs | 91.7 | −3.5 | (−9.6 to 3.8) | |
| MVC + PI/r | 77.7 | −17.4 | (−24.9 to −8.4) | |
| <200 copies/mL | Control | 97.6 | Reference | |
| MVC + 2 N(t)RTIs | 93.6 | −4.0 | (−9.0 to 2.2) | |
| MVC + PI/r | 84.1 | −13.5 | (−19.8 to −5.8) | |
| Noncompletion = failure | ||||
| <50 copies/mL | Control | 93.9 | Reference | |
| MVC + 2 N(t)RTIs | 87.2 | −6.7 | (−13.8 to 1.5) | |
| MVC + PI/r | 74.5 | −19.4 | (−27.4 to −9.9) | |
| <200 copies/mL | Control | 96.3 | Reference | |
| MVC + 2 N(t)RTIs | 88.5 | −7.9 | (−14.1 to −.4) | |
| MVC + PI/r | 80.3 | −16.1 | (−23.1 to −7.6) | |
| Per-protocol | ||||
| <50 copies/mL | Control | 96.3 | Reference | |
| MVC + 2 N(t)RTIs | 97.1 | 0.9 | (−4.2 to 6.9) | |
| MVC + PI/r | 83.6 | −12.7 | (−19.8 to −4.3) | |
| <200 copies/mL | Control | 98.8 | Reference | |
| MVC + 2 N(t)RTIs | 98.6 | −0.2 | (−3.8 to 4.4) | |
| MVC + PI/r | 90 | −8.8 | (−14.2 to −2.1) | |
Abbreviations: CI, confidence interval; MVC, maraviroc; N(t)RTI, nucleoside/nucleotide reverse transcriptase inhibitor; PI/r, ritonavir-boosted protease inhibitor.
Figure 2.
Proportion of participants with virologic response (<200 copies/mL), by week. Abbreviations: MVC, maraviroc; N(t)RTI, nucleoside/nucleotide reverse transcriptase inhibitor; PI/r, ritonavir-boosted protease inhibitor.
Figure 3.
Proportion of participants with virologic response (<50 copies/mL), by week. Abbreviations: MVC, maraviroc; N(t)RTI, nucleoside/nucleotide reverse transcriptase inhibitor; PI/r, ritonavir-boosted protease inhibitor.
Changes in Immunological and Metabolic Parameters and Quality of Life Over 48 Weeks
CD4+ T cells increased 40, 39, and 29 cells/μL in the control, MVC + 2 N(t)RTI, and MVC + PI/r arms, respectively, from baseline. There was a significant decrease in both the mean total (−0.45 mmol/L; P < .0001) and LDL-c (−0.27 mmol/L; P ≤ .0002) in patients switching to the MVC + 2 N(t)RTI arm compared with control (Table 4), but these declines did not translate into a significant change in Framingham 10-year CVD risk score. Of the available BMD change data (70, 123, and 127 in the control, MVC + 2 N(t)RTI, and MVC + PI/r arms, respectively), the T-score at the lumbar spine declined a mean of −0.05 (95% CI, −.12 to .02) in the control arm vs a gain of 0.08 (95% CI, −.00 to .16) in the MVC + 2 N(t)RTI arm (P = .03 vs control) and a gain of 0.10 (95% CI, .04–.17) in the MVC + PI/r arm (P = .0028 vs control). At the right hip, T-score changes in control vs the MVC + 2 N(t)RTI arm were nonsignificant: −0.12 (95% CI, −.12 to .02) and −0.12 (95% CI, −.25 to .01), respectively (P = .069). Participants in the N(t)RTI-sparing arm had a mean positive T-score gain at the right hip of 0.07 (95% CI, −.01 to .16; P = .01) vs the control arm. In comparing the control to each switch arm, there were no significant percentage changes in physical or mental QoL domains.
Table 4.
Changes in Lipid Parameters
| Characteristic | Arm | No. | Mean | (95% CI) | P Value |
|---|---|---|---|---|---|
| Total cholesterol (mmol/L) | Control | 77 | 0.06 | (−.15 to .28) | |
| MVC + 2 N(t)RTIs | 134 | −0.45 | (−.59 to −.31) | <.0001 | |
| Difference | 0.51 | (.26–.76) | |||
| MVC + PI/r | 150 | 0.34 | (.19–.48) | ||
| Difference | −0.27 | (−.53 to −.02) | .0345 | ||
| HDL-c (mmol/L) | Control | 77 | 0.05 | (.00–.11) | |
| MVC + 2 N(t)RTIs | 134 | 0.04 | (−.01 to .08) | ||
| Difference | 0.02 | (−.06 to .09) | .628 | ||
| MVC + PI/r | 150 | 0.10 | (.04–.16) | ||
| Difference | −0.04 | (−.14 to .05) | .3591 | ||
| LDL-c (mmol/L) | Control | 77 | 0.10 | (−.08 to .28) | |
| MVC + 2 N(t)RTIs | 132 | −0.27 | (−.38 to −.16) | ||
| Difference | 0.37 | (.17–.57) | .0002 | ||
| MVC + PI/r | 143 | 0.18 | (.05–.30) | ||
| Difference | −0.07 | (−.29 to .14) | .4871 | ||
| TGs (mmol/L) | Control | 77 | −0.0795 | (−.2816 to .1227) | |
| MVC + 2 N(t)RTIs | 134 | −0.4016 | (−.5418 to −.2615) | ||
| Difference | 0.3222 | (.0835–.5608) | .0084 | ||
| MVC + PI/r | 147 | 0.1146 | (−.1857 to .4148) | ||
| Difference | −0.194 | (−.6326 to .2445) | .3842 |
Abbreviations: CI, confidence interval; HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein cholesterol; MVC, maraviroc; N(t)RTI, nucleoside/nucleotide reverse transcriptase inhibitor; PI/r, ritonavir-boosted protease inhibitor; TGs, triglycerides.
Safety Findings at 48 Weeks
Eight hundred eighty-four AEs were reported; 86% were determined as not related or probably not related to study drugs. There was no hepatic safety signal. Mean changes in GFR (mL/minute) were nonsignificant, with mean changes of −1.96 (95% CI, −6.11 to 2.19), −9.54 (95% CI, −20.89 to 1.80), and −0.69 (95% CI, −3.61 to 2.24) in the control, MVC + 2 N(t)RTI, and MVC + PI/r arms, respectively. Up to week 48, 35 participants (2 in the control, 16 in the MVC + 2 N(t)RTI, and 17 in the MVC + PI/r arms) ceased randomized therapy; in 6 participants (1, 4, and 1 in the control, MVC + 2 N(t)RTI, and MVC + PI/r arms, respectively), an AE was the reason given for stopping randomized therapy. Thirty-seven SAEs occurred; 8 (9.76% of the control arm), 15 (9.62% of the MVC + 2 N(t)RTI arm), and 14 (8.92% MVC + PI/r arm) events. During the 48 weeks of follow-up, 1 patient died, 1 patient in the MVC + PI/r arm developed an AIDS-defining illness (multidermatomal herpes zoster), and 9 had serious non-AIDS-defining events (2 and 7 in the control and MVC arms, respectively). One myocardial infarction event was reported as a safety alert in a patient on MVC. Of note, this patient's lifestyle and cardiac congenital malformation placed them at increased CVD risk.
Resistance
Twenty-five participants (1, 6, and 18 in the control, MVC + 2 N(t)RTI, and MVC + PI/r arms, respectively) had confirmed virological failure during 48 weeks of follow-up. As shown in Table 5, sequencing was successful in 23 of 25 (median VL, 2280 copies/mL); repeat tropism testing (phenotypic testing as a DNA sample was not available in all) [18] was successful in 18 of the 25 participants. The majority of patients had virological failure without any emergent major mutations in the protease or reverse transcriptase (15 of 23 [65%]); 15 of 18 (83%) samples with a repeat tropism remained R5-tropic. Major protease mutations were found in 2 participants, L90M in one and I50V in the other. Surprisingly, 4 participants (1, 2, and 1 in the control, MVC + 2 N(t)RTI, and MVC + PI/r arms, respectively) had emergent major nonnucleoside reverse transcriptase inhibitor (NNRTI) mutations; in 3 of these, repeat tropism confirmed R5-tropic virus.
Table 5.
Emergent Resistance in Maraviroc Switch Study Participants With Confirmed Virological Failure Over 48 Weeks
| Study Arm | Confirmed Virological Failure | Viral Load at Viral Failure, Copies/mL | Country of Enrollment | PI and RT Sequencing Results | Tropism (Phenotypic Assessment) at Viral Failure |
|---|---|---|---|---|---|
| Control | Week 36 | 2280.0 | Argentina | K103KN | CCR5 |
| MVC + 2 N(t)RTIs | Week 12 | 7248.0 | Argentina | M41L, T215E | CCR5 |
| MVC + 2 N(t)RTIs | Week 12 | 55 808.0 | Mexico | M184V, K101E, Y181C, G190A | CCR5 |
| MVC + 2 N(t)RTIs | Week 12 | 2810.0 | Germany | L10I, K65R; V106I | Test failed |
| MVC + 2 N(t)RTIs | Week 36 | 4812.0 | Spain | L10I, A71V, M184V | CCR5 |
| MVC + 2 N(t)RTIs | Week 12 | 1133.0 | Poland | L90M, L10I, A71V, M184MV | CCR5 |
| MVC + PI/r | Week 48 | 891.0 | Argentina | None | CCR5 |
| MVC + PI/r | Week 48 | 730.0 | Argentina | L10I | Test failed |
| MVC + PI/r | Week 24 | 60 800.0 | Argentina | I50V, L10I, L33FL | CXCR4 |
| MVC + PI/r | Week 4 | 30 600.0 | Argentina | None | CCR5 |
| MVC + PI/r | Week 36 | 111 941.0 | Chile | None | CXCR4 |
| MVC + PI/r | Week 12 | 1157.0 | Mexico | None | CCR5 |
| MVC + PI/r | Week 36 | 5565.0 | Mexico | A62V, T215S | CCR5 |
| MVC + PI/r | Week 48 | 63 140.0 | Mexico | None | CXCR4 |
| MVC + PI/r | Week 48 | 5935.0 | Canada | None | CCR5 |
| MVC + PI/r | Week 36 | 1200.0 | Germany | K20I | Test failed |
| MVC + PI/r | Week 12 | 2208.0 | Germany | None | CCR5 |
| MVC + PI/r | Week 12 | 45 600.0 | Germany | None | CCR5 |
| MVC + PI/r | Week 12 | 2275.0 | Germany | E138A | CCR5 |
| MVC + PI/r | Week 12 | 545.0 | Poland | V32AV | Test failed |
| MVC + PI/r | Week 12 | 1924.0 | Poland | None | Test failed |
| MVC + PI/r | Week 36 | 2264.0 | Poland | None | CCR5 |
| MVC + PI/r | Week 48 | 821.0 | Poland | L10I | CCR5 |
Mutations highlighted in bold are major mutations of PI and RT (nucleoside reverse transcriptase inhibitor and nonnucleoside reverse transcriptase inhibitor).
Abbreviations: MVC, maraviroc; N(t)RTI, nucleoside/nucleotide reverse transcriptase inhibitor; PI, protease inhibitor; PI/r, ritonavir-boosted protease inhibitor; RT, reverse transcriptase.
DISCUSSION
This is the largest randomized study using genotypic assessment of virus tropism using HIV DNA to determine the likelihood of MVC activity in a switch setting. The findings strengthen the evidence base for this testing platform at the cutoffs used. Just under 50% of those screened were ineligible because virus tropism was determined as X4/dual tropic or nonclassifiable. This international study had a novel design for a switch study, separating the “traditional” components of a PI/r-based regimen to test the contribution of each component to virological control, metabolic changes, and side effects. MVC represented an attractive switch choice as it is potent and well tolerated, with neutral impacts on lipids and renal function. Somewhat surprisingly, the N(t)RTI-sparing arm was significantly inferior in regard to virological control compared with the control arm over 48 weeks of follow-up. This excess loss of virological control was not explained by reduced adherence as measured in the study. In contrast, those randomized to MVC in lieu of their PI/r and remaining on their original 2-N(t)RTI backbone retained high rates of virological suppression similar to those remaining on their original regimen of PI/r + 2 N(t)RTIs. These data suggest that a PI/r is not an effective “backbone” for MVC in the switch/simplification setting. These unexpected findings highlight the importance of randomized trials of this nature. The findings are in line with findings from some other N(t)RTI-sparing studies [19], including one that combined MVC with raltegravir [20], and suggest that specific inhibition of reverse transcriptase may be more important than previously thought.
One of the rationales for performing switch studies is to test other benefits including side effects, tolerability, renal safety, metabolic changes, and immunological benefits. In this switch study, MVC was confirmed as safe and well tolerated. The study confirms the neutral impact of MVC on renal function. There were potentially beneficial changes in lipids in those switching away from PI/r, but these changes, while important, did not translate into a significant change in the 10-year cardiovascular risk using the Framingham equation over the relatively short period of follow-up. In the longer term, these lipid changes may have a positive impact on reducing cardiovascular risk. There were also significant, albeit small improvements in lumbar spine BMD in both MVC switch arms and at the right hip in the MVC + PI/r switch arm.
All 3 arms showed CD4+ T-cell gains over 48 weeks of follow-up, this despite the patients already being on cART for a median of just over 6 years. However, in those switching to MVC, CD4+ T-cell gain was not enhanced compared to the control arm. CD8+ T cells increased significantly over the 48 weeks (data not shown) in the MVC arms. The clinical significance of this is unknown, but confirms previous findings and is thought to be an effect on T-cell trafficking via CCR5 blockade [21].
Although rates of both confirmed virological failure and virological blipping were significantly higher in the N(t)RTI-sparing arm, in those who could be sequenced, only 2 patients had an emergent major PI mutation, suggesting that virological failure in this setting would not result in loss of future treatment options with a PI/r-based regimen for the majority of patients. The relatively high rates of emergent NNRTI resistance suggest that patients with transmitted NNRTI resistance or prior NNRTI failure were enrolled in the study, something we tried to avoid when designing the exclusions for study participation. Importantly, most participants appeared to retain R5-tropic virus, so the reasons for virological failure could not be ascribed to emergent X4-tropic virus, at least using the clinical cutoffs we applied. In addition, 30% of participants with virological failure had no emergent protease or reverse transcriptase mutations and their virus remained R5-tropic, suggesting that poor adherence—not detected through the 7-day recall at weeks 4 and 48—might have contributed to viral failure.
In summary, this large international randomized study demonstrates that MVC with a 2-N(t)RTI backbone, in those with R5-tropic virus determined by genotypic tropism testing, is a switch/simplification option for patients virologically suppressed on PI/r–N(t)RTI regimens. MVC was safe and well tolerated, with favorable impact on lipids and neutral effects on renal function over 48 weeks.
Notes
Acknowledgments. We extend our grateful thanks to all the volunteers who have been participating in this study.
Financial support. The study was funded from the following sources: an independent academic grant from ViiV Healthcare and Pfizer Ltd to the University of New South Wales, Australia; the Australian Government Department of Health and Ageing; and the University of New South Wales. The Kirby Institute is affiliated with the Faculty of Medicine, University of New South Wales, Australia.
Potential conflicts of interest. S. E. reports grants from ViiV Healthcare/Pfizer. J. K. R. reports grants from Gilead and personal fees from Abbott, AbbVie, Bionor, Bristol-Myers Squibb (BMS), Cipla, Gilead, Janssen, Merck, and ViiV. W. S. became an employee of GlaxoSmithKline (GSK) K.K., Tokyo, Japan, on 1 April 2015, and at this point relinquished his role as principal investigator of the Nagoya Medical Center and membership of the Maraviroc Switch PSC. A. C. is an employee of ViiV Healthcare. R. K. reports grants from ViiV and personal fees from ViiV, Merck Sharp & Dohme (MSD), Janssen, Gilead, Siemens, and Roche. A. C. reports travel expenses from Gilead Sciences, Janssen, and BMS. R. H. has received grants from, served as an ad hoc advisor to, or has spoken at various events sponsored by Pfizer, GSK, Abbott, Merck, Tobira Therapeutics, Virco, and Quest Diagnostics; has served as a consultant for ViiV Healthcare, Tobira Therapeutics, Selah Genomics, and Quest Diagnostics; and holds stock in Merck, Illumina, Gilead, Zizowist Diagnostics, and Northern Lipids. In addition, he is supported by the Canadian Institutes of Health Research/GSK Research Chair in Clinical Virology. M. L. has received research grant support from Abbott, Merck Research Laboratories, and Pfizer. Jose Gatell has received research funding, consultancy fees, or lecture sponsorships, or has served on advisory boards for Abbott, Boehringer Ingelheim, BMS, Gilead Sciences, GSK, MSD, Pfizer, Theratechnologies, and Tibotec. I. W. has received research funds from Gilead Sciences and MSD; consulting funds from BMS and Gilead Sciences; chairing fees from Abbott and MSD; and conference support from MSD, ViiV Healthcare, and Abbott. John Gill reports grants from the University of New South Wales and personal fees as an ad hoc member of national HIV advisory boards to Janssen, Merck, Gilead, and Viiv Healthcare. A. K. reports other support from St Vincent's Hospital. T. P. has received consultancy fees from Gilead, BMS, and Abbvie, and has participated in scientific conferences given with the support of ViiV, Gilead, and Abbvie. S. L. P. received support to attend an international conference from MSD and Gilead. P. M. has received support in the form of research grants awarded to the institution, attendance at advisory boards, honoraria, and/or travel to conferences from Janssen-Cilag, Gilead Sciences, ViiV Healthcare, BMS, and MSD. D. C. reports grants and personal fees from ViiV. K. R. received honoraria or consultation fees from MSD, Roche, Janssen-Cilag, Tibotec, Mylan, and GPO (governmental pharmaceutical organization, Thailand) and has participated in speaker's bureaus from Abbott, Gilead, BMS, Merck, Roche, Janssen-Cilag, GSK, and Thai GPO. J. S. M. reports speaker's fees for Pfizer, Stendahl, and Gilead; consultant fees for MSD, Stendahl, and Pfizer; and research support from BMS, MSD, GSK, and Pfizer. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
APPENDIX
Protocol Steering Committee: Janaki Amin, Juan Alberto Arnaiz, Waldo Belloso, Andrew Clark, Amanda Clarke, David Cooper, Sean Emery, Martin Fisher (deceased), Jose Gatell, John Gill, Andrejz Horban, Rolf Kaiser, Tony Kelleher, Marcelo Losso, Juan Sierra Madero, Patrick Mallon, Sarah Pett, Thierry Prazuck, Juergen Rockstroh, Kiat Ruxrungtham, Jurgen Stellbrink, Wataru Sugiura, Marcelo J Wolff, Ian Woolley. Laboratory group: Anthony Kelleher, Kate Merlin, Julie Yeung, Bertha Fsadni, Kat Marks, Kazuo Suzuki, Nick Rismanto, Horacio Salomon, Andrea E. Rubio, Doris Chibo, Chris Birch, Richard Harrigan, Luke Swenson, Dennison Chan, Thomas Berg, Martin Obermeier, Rolf Kaiser, Eugen Schuelter, Saleta Sierra Aragon, Nadine Luebke, Suzie Coughlan, Jonathan Dean, Wataru Sugiura, Yasumasa Iwatani, Gustavo Reyes Teran, Santiago Avila, Kiat Ruxrungtham, Sunee Sirivichayakul, May Naphassanant, Sasiwimol Ubolyam, Steve Kaye, Sally Land. Data safety monitoring board: Sarah Walker, Richard Haubrich, Edwin DeJesus. Sydney coordinating team: Sean Emery, Sarah L. Pett, Elise Tu, David Silk, Nisha Berthon-Jones, Janaki Amin, Natalie Espinosa, Kymme Courtney-Vega, Noorul Absar, Hila Haskelberg, Rose Robson, Anna Donaldson. Argentina coordinating team: Marcelo Losso, Waldo Belloso, Daniel Guelman, Luciana Gambardella, Mariana Valdovinos. Spain coordinating team: Jose Gatell, Juan Arnaiz, Helena Beleta, Nuria Ramos, Marta Targa. Germany coordinating team: Jurgen Rockstroh, Brigitta Späth, Christoph Boesecke, Angelika Engelhardt. UK coordinating team: Martin Fisher, Nicky Perry, Amanda Clarke. Canada coordinating team: John Gill, Brenda Beckthold. ViiV Healthcare; Pfizer: Andrew Clark, Fraser Drummond, Eric Lefevre, Sharon Corr, Carol Grant.
Sites:
Argentina: CAICI, Rosario: Dr Sergio Lupo, Luciana Peroni; Hospital Italiano, Buenos Aires: Dr Marisa Sanchez, Mariana De Paz Sierra; Hospital Ramos Mejia, Buenos Aires: Dr Marcelo Losso, Guillermo Viloria, Angel Parlante; FUNCEI, Buenos Aires: Dr Emiliano Bissio, Pablo Luchetti, Valeria Confalonieri; Hospital Paroissien: Dr Eduardo Warley, Ines Vieni; Fundacion IDEAA, Buenos Aires: Norma Porteiro, Cecilia Vilas; Hospital Privado, Cordoba: Dr Abel Zarate, Gabriela Mayer.
Australia: Alfred Hospital, Melbourne: Dr Julian Elliot, Michelle Hagenauer; Brisbane Sexual Health and HIV Service, Brisbane: Dr Mark Kelly, Dr Diane Rowling, Abby Gibson, Ngaire Latch, Chantal Tabrett, Elizabeth Warzywoda; St Vincent's Hospital, Sydney: Prof David Cooper, Dr Sarah Pett, Karen MacRae, Brett Sinclair, Kate Sinn; Holdsworth House Medical Practice, Sydney: Dr Mark Bloch, Teo Franic, Trina Vincent, Natasha Stewart, Avindra Jayewardene; Westmead Hospital, Sydney: Dr Dominic Dwyer, Dr Jennifer Kok, Delene Assam, Janette Taylor, Patricia King; Gladstone Road General Practice, Brisbane: Dr David Orth, David Youds; Sexual health/HIV service, Nambour: Dr David Sowden, Colleen Johnston, Suzanne Murray, Jennifer Hehir, Samantha Wadham; O'Brien Street Practice, Adelaide: Dr William Donohue, Jill Thompson; Royal Prince Alfred Hospital, Sydney: Dr Roger Garsia, Geoffrey Turnham, Tracey Madden, June Nvene; Monash Medical Centre, Melbourne: Dr Ian Woolley, Ainsley Gillies, Mellissa Bryant.
Canada: Southern Alberta Clinic, Calgary: Dr John Gill, Brenda Beckthold; University Health Network–Toronto General Hospital, Toronto: Dr Sharon Walmsley, Warmond Chan; Clinique OPUS, Montreal: Dr Roger LeBlanc, Francois Lanteigne, Rima Mouawad, Ines Rahal, Sergio Guber, Sefika Ozturk; Maple Leaf Research, Toronto: Dr Graham Smith, Roberta Halpenny, Tatjana Reko, Jennifer Robinette Hills.
Chile: Fundacion Arriaran, Santiago: Dr Marcelo Wolff, Gladys Allendes.
France: Orleans hospital (CHR Orleans La Source), Orleans: Dr Thierry Prazuck, Francois Laurent Hocqueloux, Barbara de Dieuleveult.
Germany: Johann Wolfgang Goethe-University Hospital, HIVCENTER, Frankfurt: Dr Christoph Stephan, Franziska Ebeling; University of Bonn, Bonn: Prof Juergen Rockstroh, Dr Christoph Boesecke, Brigitta Spath, Angelika Engelhardt; Universitätsklinikum Düsseldorf, Klinik für Gastroenterologie, Dusseldorf: Dr Bjorn-Erik Ole Jensen, Cecilie Feind; Klinik für Immunologie und Rheumatologie, Hannover: Dr Dirk Meyer-Olson, Prof Matthias Stoll, Kirsten Hoeper, Renata Beider; Klinikum der Universitat Zu Koln, Cologne: Prof Gerd Faetkenheur, Ellen Thomas; Baumgarten, MIB Medical Center for Infectious Diseases, Berlin: Dr Axel Baumgarten, Dr Patrick Ingiliz, Andreas Wienbreyer, Daniela Behrendt, Tanja Nienkarken; Gemeinschaftspraxis Jessen Jessen Stein, Berlin: Dr Heiko Jessen, Carmen Zedlack.
Ireland: Mater Misericordiae University Hospital, Dublin: Dr Paddy Mallon, Sibongile Simelane, Jennifer Assmann, Bijan Ghavami-Kia.
Japan: Nagoya Medical Center, Nagoya: Dr Wataru Sugiura, Dr Mayumi Imahashi, Kazue Tanabe, Dr Yoshiyuki Yokomaku, Dr Junji Imamura.
Mexico: Hospital Civil de Guadalajara, Guadalajara: Dr Jaime Andrade-Villanueva, Melva Montes de Oca, Lucero Gonzalez, David Ponce, Andrea Mendoza; Instituto Nacional de Ciencias Medicas y Nutriciòn Salvador Zubiran, Mexico City: Dr Juan Sierra-Madero, Jesus Eduardo Sanchez Hernandez, Eduardo Jaime Ruiz Ballesteros, Sergio del Moral Ponce; Hospital General Regional De Leon i Capasits Leon, Leon: Dr Luis Mosqueda, Monica Lopez.
Poland: Wojewodzki Szpital Zakazny Centrum Diagnostyki i Terapii AIDS, Warsaw: Dr Andrzej Horban, Dr Anna Ignatowska, Dr Elzbieta Bakowska, Dr Piotr Pulik.
Spain: Hospital Principe de Asturias, Madrid: Dr Jose Sanz-Moreno; Hospital Germans Trias i Pujol, Badalona: Dr Roger Paredes, Jordi Puig; Hospital de la Santa Creu i Sant Pau, Barcelona: Dr Pere Domingo, Mar Gutierrez; Hospital Clínic de Barcelona, Barcelona: Prof Jose Gatell, Dr Ana González-Cordón, Pili Callau; Hospital Universitari i Politecnic La Fe, Valencia: Dr Jose Lopez Aldeguer, Sandra Cuellar Tovar; Virgen Del Rocio University Hospital, Sevilla: Dr Manuel Leal Noval, Dr Inmaculada Rivas; Hospital Regional Carlos Haya De Malaga, Malaga: Dr Marcial Delgado-Fernandez; Hospital La Paz, Madrid: Dr Jose Ramon Arribas, Juan Miguel Castro.
Thailand: Chulalongkorn University Hospital-HIVNAT, Bangkok: Prof Kiat Ruxrungtham, Dr Anchalee Avihingsanon, Wirach Maek-a-nantawat, Jintana Intasan, Walairat Charoenporn, Thidarat Cuprasitrut, Pachuen Jaisomkom, Kanchana Pruksakaew.
United Kingdom: St Mary's Hospital, Imperial College, London: Dr Alan Winston, Scott Mullaney; Brighton & Sussex University NHS Trust, Brighton: Prof Martin Fisher, Dr Amanda Clarke, Lisa Barbour, Nicky Perry, Celia Richardson; Guys' and St Thomas' Hospital, London: Dr Julie Fox, Tammy Murray, Dr Al Teague; Western General Hospital, Edinburgh: Dr Clifford Leen, Sheila Morris; Coventry and Warwickshire Partnership Trust, Coventry: Dr Das Satyajit, Rumun Sandhu, James Tucker.
References
- 1. Nakagawa F, Lodwick RK, Smith CJ et al. Projected life expectancy of people with HIV according to timing of diagnosis. AIDS 2012; 26:335–43. [DOI] [PubMed] [Google Scholar]
- 2. Solomon DA, Sax PE. Current state and limitations of daily oral therapy for treatment. Curr Opin HIV AIDS 2015; 10:219–25. [DOI] [PubMed] [Google Scholar]
- 3. Casado JL, Bañón S, Rodriguez MA et al. Efficacy and pharmacokinetics of the combination of etravirine plus raltegravir as novel dual antiretroviral maintenance regimen in HIV-infected patients. Antiviral Res 2015; 113:103–6. [DOI] [PubMed] [Google Scholar]
- 4. Reliquet V, Chirouze C, Allavena C et al. Nevirapine-raltegravir combination, an NRTI and PI/r sparing regimen, as maintenance antiretroviral therapy in virologically suppressed HIV-1-infected patients. Antivir Ther 2014; 19:117–23. [DOI] [PubMed] [Google Scholar]
- 5. Ofotokun I, Sheth AN, Sanford SE et al. A switch in therapy to a reverse transcriptase inhibitor sparing combination of lopinavir/ritonavir and raltegravir in virologically suppressed HIV-infected patients: a pilot randomized trial to assess efficacy and safety profile: the KITE study. AIDS Res Hum Retroviruses 2012; 28:1196–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Macías J, Recio E, Márquez M et al. Efficacy and safety of once-daily maraviroc plus ritonavir-boosted darunavir in pretreated HIV-infected patients in a real-life setting. HIV Med 2014; 15:417–24. [DOI] [PubMed] [Google Scholar]
- 7. Nozza S, Svicher V, Saracino A et al. State of the art of dual therapy in 2015. AIDS Rev 2015; 17:127–34. [PubMed] [Google Scholar]
- 8. Wilkin TJ, Gulick RM. CCR5 antagonism in HIV infection: current concepts and future opportunities. Annu Rev Med 2012; 63:81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Deeks SG, Tracy R, Douek DC. Systemic effects of inflammation on health during chronic HIV infection. Immunity 2013; 39:633–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Poveda E, Alcamí J, Paredes R et al. Genotypic determination of HIV tropism—clinical and methodological recommendations to guide the therapeutic use of CCR5 antagonists. AIDS Rev 2010; 12:135–48. [PubMed] [Google Scholar]
- 11. Symons J, Vandekerckhove L, Paredes R et al. Impact of triplicate testing on HIV genotypic tropism prediction in routine clinical practice. Clin Microbiol Infect 2012; 18:606–12. [DOI] [PubMed] [Google Scholar]
- 12. Vandekerckhove L, Wensing A, Kaiser R et al. European guidelines on the clinical management of HIV-1 tropism testing. Lancet Infect Dis 2011; 11:394–407. [DOI] [PubMed] [Google Scholar]
- 13. Swenson LC, Moores A, Low AJ et al. Improved detection of CXCR4-using HIV by V3 genotyping: application of population-based and “deep” sequencing to plasma RNA and proviral DNA. J Acquir Immune Defic Syndr 2010; 54:506–10. [DOI] [PubMed] [Google Scholar]
- 14. Swenson LC, Mo T, Dong WW et al. Deep sequencing to infer HIV-1 co-receptor usage: application to three clinical trials of maraviroc in treatment-experienced patients. J Infect Dis 2011; 203:237–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Obermeier M, Symons J, Wensing AM. HIV population genotypic tropism testing and its clinical significance. Curr Opin HIV AIDS 2012; 7:470–7. [DOI] [PubMed] [Google Scholar]
- 16. Tu E, Swenson LC, Land S et al. Results of external quality assessment for proviral DNA testing of HIV tropism in the Maraviroc Switch collaborative study. J Clin Microbiol 2013; 51:2063–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mannheimer S, Friedland G, Matts J et al. The consistency of adherence to antiretroviral therapy predicts biologic outcomes for human immunodeficiency virus-infected persons in clinical trials. Clin Infect Dis 2002; 34:1115–21. [DOI] [PubMed] [Google Scholar]
- 18. Heera J, Valluri S, Craig C et al. First prospective comparison of genotypic vs phenotypic tropism assays in predicting virologic responses to maraviroc (MVC) in a phase 3 study: MODERN. J Int AIDS Soc 2014; 17(4 suppl 3):19519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Achhra AC, Boyd MA. Antiretroviral regimens sparing agents from the nucleoside(tide) reverse transcriptase inhibitor class: a review of the recent literature. AIDS Res Ther 2013; 10:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Katlama C, Assoumou L, Valantin MA et al. Maraviroc plus raltegravir failed to maintain virological suppression in HIV-infected patients with lipohypertrophy: results from the ROCnRAL ANRS 157 study. J Antimicrob Chemother 2014; 69:1648–52. [DOI] [PubMed] [Google Scholar]
- 21. Wilkin TJ, Lalama CM, McKinnon J et al. A pilot trial of adding maraviroc to suppressive antiretroviral therapy for suboptimal CD4+ T-cell recovery despite sustained virologic suppression: ACTG A5256. J Infect Dis 2012; 206:534–42. [DOI] [PMC free article] [PubMed] [Google Scholar]