Summary:
This trial failed to show the equivalence of placebo to amoxicillin in term infants <2 months of age with isolated fast breathing who did not have other clinical signs of illness or hypoxemia and were managed in primary care settings.
Keywords: fast breathing, young infants, amoxicillin, newborn, clinical trial.
Abstract
Background.
Integrated Management of Childhood Illness recommends that young infants with isolated fast breathing be referred to a hospital for antibiotic treatment, which is often impractical in resource-limited settings. Additionally, antibiotics may be unnecessary for physiologic tachypnea in otherwise well newborns. We tested the hypothesis that ambulatory treatment with oral amoxicillin for 7 days was equivalent (similarity margin of 3%) to placebo in young infants with isolated fast breathing in primary care settings where hospital referral is often unfeasible.
Methods.
This randomized equivalence trial was conducted in 4 primary health centers of Karachi, Pakistan. Infants presenting with isolated fast breathing and oxygen saturation ≥90% were randomly assigned to receive either oral amoxicillin or placebo twice daily for 7 days. Enrolled infants were followed on days 1–8, 11, and 14. The primary outcome was treatment failure by day 8, analyzed per protocol. The trial was stopped by the data safety monitoring board due to higher treatment failure rate and the occurrence of 2 deaths in the placebo arm in an interim analysis.
Results.
Four hundred twenty-three infants fulfilled per protocol criteria in the amoxicillin arm and 426 in the placebo arm. Twelve infants (2.8%) had treatment failure in the amoxicillin arm and 25 (5.9%) in the placebo arm (risk difference, 3.1; P value .04). Two infants in the placebo arm died, whereas no deaths occurred in the amoxicillin arm. Other adverse outcomes, as well as the proportions of relapse, were evenly distributed across both study arms.
Conclusions.
This trial failed to show equivalence of placebo to amoxicillin in the management of isolated fast breathing without hypoxemia or other clinical signs of illness in term young infants.
Clinical Trials Registration.
(See the Editorial Commentary by Jehan and Qazi on pages 190–1.)
Infections are a major cause of morbidity and mortality among neonates in lower- and middle-income countries [1]. The World Health Organization (WHO) estimates that globally 9% of child mortality is attributable to neonatal sepsis or pneumonia [2]. WHO recently updated the Integrated Management of Childhood Illness (IMCI) chart booklet on the identification of “very severe disease” in young infants using 7 clinical signs: not feeding well, convulsions, fast breathing (respiratory rate ≥60 breaths/minute), chest indrawing, body temperature ≥37.5°C or ≤35.5°C, and movement only when stimulated or no movement at all [3, 4]. The presence of any one of these clinical signs detected by a health worker is an indicator for a referral to hospital.
Fast breathing alone is a nonspecific sign with high sensitivity but low specificity for serious illness. Fast breathing is also highly prevalent in newborns as it can be due to benign reasons including transient tachypnea of the newborn [5–8] and to overwrapping [9], a common practice in many Pakistani communities. The prevalence of isolated fast breathing ranges from 4.7% to 21.6% [3, 10–12]. In our experience in Karachi, isolated fast breathing as a clinical sign was observed in 16.4% of young infants in a clinical study of newborn infections: 21.6% among infants aged 0–6 days and 11.3% among infants aged 7–59 days. Follow-up of these infants showed no statistical difference in case fatality rate among young infants with isolated fast breathing (0.3%) vs young infants without any clinical sign of illness (0.2%).
The WHO IMCI tools recommend that infants with respiratory rate ≥60 breaths per minute should be referred to hospital. This recommendation is often regarded as impractical in resource-limited settings. Social, cultural, and economic factors prevent parents from accepting or adhering to the advice [13]. A recent trial suggested that antibiotics may not be necessary for children presenting with only fast breathing pneumonia in older children aged 2–59 months [14].
In the light of our observational findings, combined with the high rates of refusal to accept referral advice, a potential increase in antimicrobial resistance due to the overuse of antibiotics, the risk of nosocomial infection after hospitalization, and the strain on already overburdened health facilities, we decided to conduct a randomized double-blind placebo-controlled equivalence trial for the treatment of fast breathing in young infants 0–60 days of age. It was hypothesized that the proportion of treatment failure in each arm is 4% and difference of <3% in the proportions of treatment failure was labeled as equivalent.
METHODS
Study Design and Participants
This trial was conducted at the primary healthcare (PHC) centers of 1 urban and 3 periurban communities in Karachi, Pakistan. The periurban communities were located in coastal areas (Ibrahim Hyderi, Ali Akbar Shah Goth, and Rehri Goth) while the urban community was located in an industrial area (Bhains colony). The total catchment population was approximately 370 000 [15] with a crude birth rate of 33 per 1000 population. Approximately 10 000 pregnancies per year have been actively followed at home by locally recruited and trained community health workers (CHWs) [16]. In 2013, 58% of births were conducted at home with the help of traditional/unskilled birth attendants, and the neonatal mortality rate was found to be 35 per 1000 live births [15].
For the present trial, each PHC center was staffed with 1 physician, 1 lady health visitor (LHV), and 3 CHWs. The LHVs have government sponsored the nursing training of 2 years and supervise the Lady Health Worker Program of Pakistan. The CHWs were local women with high school diplomas who received project specific training in identification of illness in young infants. The PHC center provided free healthcare including medications to children <5 years of age and facilitated referral for those who were in need of hospitalization.
Young infants having any sign(s) of illness, including isolated fast breathing, were referred to the PHC center by CHWs during their regular home visits or were brought by parents/caretakers. Infants with isolated fasting breathing and oxygen saturation ≥90% (assessed by pulse oximetry) were identified for participation in this trial. Infants were excluded if they were not residents of the catchment area, were preterm (born at <37 weeks, assessed through modified Ballard scoring by physician within 1 week of birth), or had any of the following signs: presence of audible murmur, any concurrent signs of severe infection (poor feeding and poor suck, movement only when stimulated, severe chest indrawing, axillary temperature ≥38.0°C or ≤35.5°C, cyanosis, bulging fontanel, unable to feed, unable to cry, apnea, convulsions, unconscious), persistent vomiting, weight 1800 grams at the time of presentation, major congenital malformations or suspected chromosomal abnormalities, hospitalization for illness in the last 2 weeks, or previous enrollment in the trial.
In the PHC center, infants were registered by an LHV and assigned a unique identification (ID) number. The LHV counted the respiratory rate, then weighed and measured the temperature of the infant. Information was recorded on the outpatient department form and the infant was referred to the physician. Very sick young infants were taken directly to the physician without a formal LHV examination to prevent any delay in treatment.
The study physician evaluated the young infant for signs of severe infection. Sick young infants were referred to a tertiary care hospital, with transport provided free of cost. For those infants whose parents refused referral, the physician offered once-daily injections of procaine penicillin and gentamicin. Young infants with isolated fast breathing were invited to participate in the trial and were enrolled if parents consented. Written informed consent was obtained by the study physician in the presence of a witness, and parents/caretakers were given a copy of the consent form. Infants of families who did not provide consent received standard treatment according to IMCI guidelines at the PHC center.
Randomization and Masking
Eligible young infants were randomly assigned to receive 1 of 2 drug regimens: oral amoxicillin (arm A) twice daily for 7 days, or placebo (arm B) twice daily for 7 days. The assignment was based on random numbers generated in Microsoft Excel by a pharmacist and statistician who were not involved in the trial. A block randomization strategy with uneven blocks of 4, 6, 8, and 12 with a 1:1 allocation ratio was used. Self-adhesive labels with unique identifiers and serial numbers were placed on the bottle and the randomization form. The color, consistency, smell, taste, and quantity of the antibiotic and the placebo were identical. Both were stored in powdered form at room temperature and kept refrigerated once reconstituted. Patients and investigators were blinded to the study arm. Novartis Pharmaceuticals and the Clinical Trial Unit of Aga Khan University provided the antibiotic and placebo, respectively. Neither was involved in the design and conduct of this study.
Procedure
The CHW prepared appropriate doses of oral amoxicillin (100 mg/kg/day in 2 divided doses) or of placebo and administered the first treatment in the PHC center and the rest at home to all enrolled young infants using an individual 5-mL disposable spoon for each infant. The CHW then observed each young infant for 10–15 minutes. If the infant vomited, the CHW repeated the dose and observed him/her for another 20 minutes. If the second dose was vomited, the CHW took the infant to the PHC center for assessment by the study physician.
The CHW visited the home of each enrolled infant daily for 8 days to measure respiratory rate, temperature, and pulse oximetry, ascertain treatment failure, provide oral doses, measure treatment adherence, and record adverse events. The morning dose was administered by the CHW, while the evening dose was administered by the parent/caregiver. The study physician examined enrolled young infants on days 3–8 and followed them on days 11 and 14 for possible relapse or death. Additionally, adherence was measured by weighing medicine bottles on days 3 and 8. A patient care coordinator called the CHWs and parents regularly to inquire about doses, timing, and vomiting.
All staff involved in the study received standardized training on study procedures. Ten videos of young infants (with a mix of healthy and sick infants) were shown monthly to ensure the standardization of clinical signs, and refresher training sessions were conducted every 3 months. Supervision and monitoring visits were carried out regularly. Two supervisors monitored day-to-day activities to ensure the quality and consistency of study procedures, and case report forms were checked for errors, consistency, and missing values.
A data safety monitoring board (DSMB) monitored safety of the participants. The trial was stopped after a review and interim analysis by the DSMB (initially blinded, and then unblinded) upon one-third enrollment because of concern about a trend for higher numbers of treatment failures and 2 deaths in the placebo arm as compared to the amoxicillin arm.
Study Outcomes
The primary outcome was treatment failure by day 8, defined as a composite. Treatment failure was defined as development of any one of the following: oxygen saturation <90% on day 3 or anytime until day 8; clinical deterioration, defined as emergence of any sign of being critically ill or severe infection at any time after randomization (as defined in the exclusion criteria); development of serious adverse effects of the study antibiotics (death, organ failure, anaphylactic reaction, severe diarrhea, disseminated and severe rash); hospitalization any time after admission in the study; death anytime within day 1–8 of enrollment. Secondary outcomes were relapse, nonserious adverse events, and death by days 1–14 of enrollment. Relapse was defined as clinical episodes of serious infection between 8 and 14 days of treatment, using the same definition as treatment failure but without deaths, and nonserious adverse events were defined as the occurrence of nondehydrating diarrhea or skin rash. Per protocol analysis was done for both primary and secondary outcomes. The study physicians examined enrolled young infants on days 3, 8, 11, and 14 for assessing treatment failure. In addition, if a CHW suspected treatment failure on her home visit, she brought the infant to the PHC for physician examination. Physician confirmation of treatment failure was not blinded to CHW assessment.
Sample Size and Statistical Analysis
The sample size was calculated on the basis of an a priori equivalence margin of ≤3% in the treatment failure rates between the 2 study groups by day 8. We used the standard formula for detecting equivalence: 2n = 4 (zα+zβ)2p(1-p)/δ2 [16]. We hypothesized that with a proportion of treatment failure in each arm of 4% with α of .05, power of 90%, and anticipated loss to follow-up of 10%, the estimated sample size was 1335 young infants per arm. We used standard case report forms for data collection. The data were double- entered in SQL server database and analyzed using STATA software version 12.
For primary analysis, we included infants who met predefined per protocol criteria based on treatment adherence and follow-up. Per protocol status determination and inclusion in analysis was based on adequate follow-up for outcome assessment and adherence to treatment. Adequacy of follow-up for primary outcome assessment was defined as any of (i) all clinical follow-up visits on days 2–8 completed; (ii) all follow-up visits before treatment failure completed; (iii) clinical follow-up completed on days 2 and 3 and at least 3 follow-ups from days 4 to 8, and vital status on day 8 is known. Treatment adherence was determined to be adequate if the infant received 100% of doses of scheduled antibiotics on all 7 days or by the time of treatment failure; or if infant received 100% of scheduled antibiotics on the first 2 days of therapy and at least 70% of all scheduled doses of each antibiotic on days 3–7 or by the time of treatment failure. We reported risk difference of proportions of treatment failure with a 95% confidence interval (CI) in both arms of the study.
All deaths and serious adverse events, including anaphylactic reaction, severe dehydration caused by diarrhea, or a new onset of severe rash, were reported to the Ethics Review Committee within 48 hours of their occurrence. This information was also provided to the DSMB on a regular basis.
The trial was approved by the Ethics Review Committee of the Aga Khan University, Karachi. The trial registration number is NCT01533818.
RESULTS
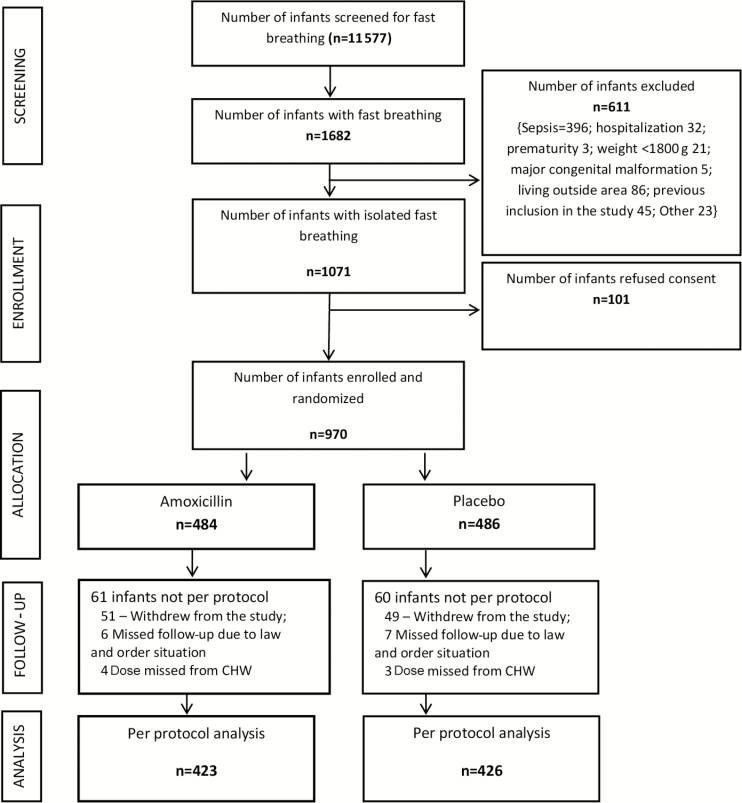
During the period 4 June 2012 to 29 May 2014, 11 577 young infants were screened for fast breathing (Figure 1), and 1682 (14.5%) infants with fast breathing were assessed for eligibility. Six hundred eleven (36.3%) infants were excluded. Of the eligible 1071 young infants, 970 participated in the trial; 486 received placebo and 484 received amoxicillin. The baseline characteristics were similar across study arms (Table 1). Four hundred twenty-six (44%) infants were aged <7 days, 53.8% were male, and 46.1% were female. A total of 849 (87.6%) infants fulfilled the criteria for per protocol analysis, 426 in the placebo arm and 423 in the amoxicillin arm.
Figure 1.
Trial interim flowchart. Abbreviation: CHW, community health worker.
Table 1.
Baseline Characteristics of All Enrolled Young Infants
| Characteristic | Amoxicillin (n = 484) | Placebo (n = 486) |
|---|---|---|
| Age, day(s) | ||
| Mean ± SD | 15.4 ± 16.2 | 16.3 ± 16.9 |
| 0–6 | 216 (44.6%) | 210 (43.2%) |
| 7–28 | 169 (34.9%) | 159 (32.7%) |
| 29–59 | 99 (20.5%) | 117 (24.1%) |
| Sex | ||
| Male | 262 (54.1%) | 260 (53.5%) |
| Female | 222 (45.9%) | 226 (46.5%) |
| Weight, gm | 3202.7 ± 651.4 | 3234.7 ± 690.8 |
| Parity | ||
| Primipara | 120 (24.8%) | 113 (23.3%) |
| Multipara | 362 (74.8%) | 373 (76.8%) |
| Unknown | 1 (0.2%) | 0 (0.0%) |
| Place of delivery | ||
| Home | 195 (40.3%) | 203 (41.8%) |
| Hospital/clinic | 285 (58.9%) | 276 (56.8%) |
| Other | 2 (0.4%) | 2 (0.4%) |
| Unknown | 2 (0.4%) | 2 (0.4%) |
| Delivery attended by | ||
| Skilled birth attendant | 289 (59.7%) | 287 (59.1%) |
| Unskilled birth attendant | 190 (39.3%) | 196 (40.3%) |
| Unknown | 5 (1.0%) | 3 (0.6%) |
| Breastfeeding | ||
| Exclusively breastfed | 416 (86.3%) | 419 (86.2%) |
| Not exclusively breastfed | 60 (12.5%) | 66 (13.6%) |
| Unknown | 6 (1.2%) | 1 (0.2%) |
| Respiratory rate, breaths/min | 72.8 ± 12.3 | 73 ± 13.2 |
| Temperature, °C | 36.9 ± 0.4 | 36.9 ± 0.5 |
| Oxygen saturation, % | 96.1 ± 2.4 | 96.0 ± 2.5 |
Data are presented as No. (%) or mean ± SD.
In the placebo arm, 25 (5.9%) young infants had treatment failure by day 8; this number was 12 (2.8%) in the amoxicillin arm. The risk difference between the proportion of treatment failure across the 2 treatment arms was 3.1 (95% CI, 0.3–5.8) with an upper CI limit above the prespecified equivalence margin (Table 2). Two deaths were observed by day 14 in the placebo arm compared with none in the amoxicillin arm. The reasons for treatment failure are shown in Table 2.
Table 2.
Primary and Secondary Treatment Outcomes in Per Protocol Young Infants
| Outcome | Amoxicillin (n = 423) |
Placebo (n = 426) |
Risk Difference (95% CI) |
|---|---|---|---|
| Treatment failure by day 8 | 12 (2.8%) | 25 (5.8%) | 3.1% (95% CI, 0.3 to 5.8)a |
| Death | 0 | 2 (0.5%) | |
| Hospitalization | 0 (0.0%) | 1 (0.2%) | |
| Low oxygen saturation | 2 (0.5%) | 2 (0.5%) | |
| Severe chest indrawing | 3 (0.7%) | 6 (1.5%) | |
| Poor feeding | 3 (0.7%) | 2 (0.5%) | |
| Hyperthermia | 4 (1.0%) | 10 (2.4%) | |
| Hypothermia | 0 | 1 (0.2%) | |
| Convulsions | 0 | 1 (0.2%) | |
| Nonfatal relapse; risk difference | 15 (3.6%) | 17 (4.1%) | 0.5% (95% CI, –0.2 to 7.2) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviation: CI, confidence interval.
a P value .04.
One hundred twenty-one infants not adherent to the protocol were also followed on days 8, 11, and 14 of enrollment. All these infants were alive by day 14 of follow-up.
Adverse events were rare. Four infants in each arm had a higher frequency of loose stools as reported by the caregiver, and 1 infant (in the placebo arm) had an onset of generalized rash. The frequency of relapse was 15 of 423 (3.6%) in the amoxicillin arm and 17 of 426 (4.1%) in the placebo arm (risk difference, 0.5 [95% CI, –0.2 to 7.2]).
DISCUSSION
This study in a double-blind, randomized, placebo-controlled trial failed to show equivalence of placebo to oral amoxicillin in young infants with isolated fast breathing, no hypoxemia, and no other WHO-defined clinical sign of severe illness. On the contrary, the study suggests that such infants benefit from amoxicillin therapy given in primary care settings in areas with high newborn mortality. An open-label trial from Africa on the management of isolated fast breathing in young infants [17] has previously reported that oral amoxicillin is equivalent to injectable therapy.
The list of clinical signs recommended by WHO for identifying severe illness covers a range from mild to severe, including isolated fast breathing, movement only with stimulation, and convulsions. Recently issued WHO guidelines for management of possible serious bacterial infection where referral is not feasible recommend that oral amoxicillin be used in young infants 7–59 days of age with the single sign of fast breathing [18]. Previous studies in Asia have shown that oral antibiotics were beneficial for neonates and young infants with fast breathing [19]. The present study reinforces previous findings using a placebo-controlled design and extends the age range to include 0–7 days.
Amoxicillin therapy for isolated fast breathing may provide a benefit because fast breathing can be an early sign of lower respiratory infection in areas with the high force of infection exposing the vulnerable newborn, or because other clinical signs of sepsis are subtle and may be missed early in the presentation. Unpublished data from the Aetiology of Neonatal Sepsis in South Asia (ANISA) study from this site reveal high rates of nasopharyngeal pneumococcal colonization in this population even in the first week of life.
In this study, 1071 of 11 577 (9.2%) young infants screened for fast breathing were detected to have isolated fast breathing either by a CHW during a home visit or by a caregiver who sought care for the infant at the PHC center. The finding that 9% of young infants seen need to be treated with amoxicillin in such settings will inevitably raise questions about driving antimicrobial resistance through excessive antimicrobial use. This concern must be balanced against newborn pneumonia contributing to thousands of child deaths annually. This study was conducted before pneumococcal conjugate vaccines were introduced in Pakistan’s routine childhood immunization program. It is conceivable that high vaccine coverage will provide herd protection to the newborn and decrease neonatal pneumonia rates. The indirect effect of pneumococcal conjugate vaccine on newborn pneumococcal colonization should be documented.
This study has 3 main strengths. First, it was a community- based, double-blind, placebo-controlled trial with adequate adherence and follow-up. Second, high quality was maintained and ensured by physician-confirmed treatment outcome, and by conducting monthly standardization of clinical signs and close monitoring and supervision of all health workers. Third, a high proportion of infants in the first week of life were enrolled (44%).
There were also some important limitations. First, due to resource constraints, blood or nasopharyngeal secretions were not taken to ascertain etiology, and thus diagnosis was based solely on clinical signs. Second, the number of enrollments estimated at the beginning of the study was not achieved, as the study was prematurely stopped by the DSMB due to safety concerns because of trend of higher treatment failures in the placebo arm and 2 deaths vs no deaths in the amoxicillin arm and lower treatment failure rates (2.8% vs 5.9%). Third, this study was done on term young infants with isolated fast breathing without hypoxemia, and results cannot be generalized to all young infants in high-mortality settings. Application of these findings to other settings will require operationalizing pulse oximetry in primary healthcare as an essential tool in making treatment and referral decisions. Fourth, physician confirmation of treatment failure was not blinded to CHW assessment. However, we believe this did not bias the study findings because both CHWs and physicians were blinded to the allocation of amoxicillin vs placebo.
CONCLUSIONS
The results of this study indicate that placebo is not equivalent to oral amoxicillin in full-term young infants presenting with isolated fast breathing without hypoxemia or other signs of clinical illness. It suggests that treatment with oral antibiotics is beneficial and that this can be carried out in an ambulatory setting. This study has significant public health implications, particularly in communities where the acceptance of referral may be poor. Multiple factors may influence the feasibility of hospitalizing a young infant, including poverty, distance from the hospital, and cultural barriers (eg, lack of permission by elders). Treatment with oral amoxicillin in ambulatory settings may save lives by improving compliance with treatment; it may also reduce the burden on the healthcare system and reduce the cost of transportation, medicines, and medical supplies. The present study was initiated prior to the publication of new guidelines on the treatment of possible severe bacterial infection in situations where referral is not feasible. Results support and contribute to the new recommendations.
Notes
Acknowledgments. The authors recognize the contributions of the patients who volunteered to participate in this study as well as study physicians; Benazir Baloch, Khairunnisa Karimi, Bilquis Mehboob, Shahla Naeem, Rakshanda Fahim, Irum Nizam, Ghazala Shaikh, Rubina Rafiq; community health workers; the data management team; and drivers. We also acknowledge Shamim Ahmed Qazi and Cathy Wolfheim for assistance in editing this manuscript.
Author contributions. S. S. T. designed and conducted the trial, interpretation of data, and preparation of the first draft of the manuscript; A. A. M. and S. S. supervised data collection, interpretation of the data, and participated in drafting of the manuscript; O. P., A. Z., and I. A. supervised trial design, data analysis, and revising manuscript drafts; I. A. and Y. S. designed the statistical methodologies of the trial and data analysis.
Financial support. S. S. T. was supported by the National Institute of Health’s Fogarty International Center (1 D43 TW007585-01) during the conduct of this study. The study costs were funded by the Department of Paediatrics and Child Health, Aga Khan University.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Dedication. To my Father Prof. Pirbhulal Tikmani and mother Asha Devi (Revti).
References
- 1. Lawn JE, Blencowe H, Oza S, et al. Lancet Every Newborn Study Group Every Newborn: progress, priorities, and potential beyond survival. Lancet 2014; 384:189–205. [DOI] [PubMed] [Google Scholar]
- 2. Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, et al. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet 2014; 385:11. [DOI] [PubMed] [Google Scholar]
- 3. Young Infants Clinical Signs Study Group. Clinical signs that predict severe illness in children under age 2 months: a multicentre study. Lancet 2008; 371:135–42. [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization. Integrated Management of Childhood Illness: chart booklet. Geneva, Switzerland: WHO, 2014. [Google Scholar]
- 5. Kim SY. Neonatal respiratory distress: recent progress in understanding pathogenesis and treatment outcomes. Korean J Pediatr 2010; 53:1–6. [Google Scholar]
- 6. Kasap B, Duman N, Ozer E, Tatli M, Kumral A, Ozkan H. Transient tachypnea of the newborn: predictive factor for prolonged tachypnea. Pediatr Int 2008; 50(1):81–4. [DOI] [PubMed] [Google Scholar]
- 7. Hermansen CL, Lorah KN. Respiratory distress in the newborn. Am Fam Physician 2007; 76:987–94. [PubMed] [Google Scholar]
- 8. Rodrigo I. Respiratory distress in the newborn. Child Health 2004; 33:82–4. [Google Scholar]
- 9. Gozal D, Colin AA, Daskalovic YI, Jaffe M. Environmental overheating as a cause of transient respiratory chemoreceptor dysfunction in an infant. Pediatrics 1988; 82:738–40. [PubMed] [Google Scholar]
- 10. Misra S, Weber M. Clinico-epidemiological profile and validation of symptoms and signs of severe illness in young infants (<60 days) reporting to a district hospital. Indian Pediatr 2007; 44:751–9. [PubMed] [Google Scholar]
- 11. Mazzi E, Bartos AE, Carlin J, Weber MW, Darmstadt GL; Bolivia Clinical Signs Study Group Clinical signs predicting severe illness in young infants (<60 days) in Bolivia. J Trop Pediatr 2010; 56:307–16. [DOI] [PubMed] [Google Scholar]
- 12. Deorari AK, Chellani H, Carlin JB, et al. Clinicoepidemiological profile and predictors of severe illness in young infants (<60 days) reporting to a hospital in North India. Indian Pediatr 2007; 44:739–48. [PubMed] [Google Scholar]
- 13. Owais A, Sultana S, Stein AD, Bashir NH, Awaldad R, Zaidi AK. Why do families of sick newborns accept hospital care? A community-based cohort study in Karachi, Pakistan. J Perinatol 2011; 31:586–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hazir T, Nisar YB, Abbasi S, et al. Comparison of oral amoxicillin with placebo for the treatment of World Health Organization–defined nonsevere pneumonia in children aged 2–59 months: a multicenter, double-blind, randomized, placebo-controlled trial in Pakistan. Clin Infect Dis 2010;59:8. [DOI] [PubMed] [Google Scholar]
- 15. Zaidi AK, Tikmani SS, Sultana S, et al. Simplified antibiotic regimens for the management of clinically diagnosed severe infections in newborns and young infants in first-level facilities in Karachi, Pakistan: study design for an outpatient randomized controlled equivalence trial. Pediatr Infect Dis J 2013; 32(suppl 1):S19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Friedman LM, Furberg CD, DeMets DL. Fundamentals of clinical trials. 4th ed New York: Springer-Verlag; 2010. [Google Scholar]
- 17. Tshefu A, Lokangaka A, Ngaima S, et al. Oral amoxicillin compared with injectable procaine benzylpenicillin plus gentamicin for treatment of neonates and young infants with fast breathing when referral is not possible: a randomised, open-label, equivalence trial. Lancet 2015; 385:1758–66. [DOI] [PubMed] [Google Scholar]
- 18. World Health Organization. Guidelines: managing possible serious bacterial infection in young infants when referral is not feasible. Geneva, Switzerland: WHO, 2015. [PubMed] [Google Scholar]
- 19. Zaidi AK, Ganatra HA, Syed S, et al. Effect of case management on neonatal mortality due to sepsis and pneumonia. BMC Public Health 2011; 11(suppl 3):S13. [DOI] [PMC free article] [PubMed] [Google Scholar]