Summary
In this proof-of-concept study, we present the first-in-human data suggesting that systemic administration of technetium Tc 99m tilmanocept (99mTc-tilmanocept) enables noninvasive quantification of arterial CD206+ macrophages. Application of this macrophage-specific molecular imaging strategy highlights high-level aortic 99mTc-tilmanocept uptake in human immunodeficiency virus and a relationship to noncalcified aortic plaque.
Keywords: HIV-associated cardiovascular disease, arterial inflammation, atherosclerosis.
Abstract
Background.
The ability to noninvasively assess arterial CD206+ macrophages may lead to improved understanding of human immunodeficiency virus (HIV)–associated cardiovascular disease.
Methods.
We trialed a novel macrophage-specific arterial imaging technique.
Results.
We demonstrated colocalization between technetium Tc 99m tilmanocept (99mTc-tilmanocept) and CD206+ macrophages ex vivo. In vivo application of 99mTc-tilmanocept single-photon emission computed tomography/computed tomography revealed high-level 99mTc-tilmanocept uptake across 20.4% of the aortic surface volume among HIV-infected subjects, compared with 4.3% among non–HIV-infected subjects (P = .009). Among all subjects, aortic high-level 99mTc-tilmanocept uptake was related to noncalcified aortic plaque volume (r = 0.87; P = .003) on computed tomographic angiography, and this relationship held when we controlled for HIV status.
Conclusion.
These first-in-human data introduce a novel macrophage-specific arterial imaging technique in HIV.
Clinical Trials Registration.
Individuals with human immunodeficiency virus (HIV) infection have an increased risk of myocardial infarction [1, 2], thought to be fueled in part by heightened systemic immune activation [3] and downstream arterial inflammation [4, 5]. Development of targeted immunomodulatory strategies to reduce myocardial infarction risk in HIV requires improved understanding of the relationship between systemic immune activation, arterial inflammation, and atherogenesis/plaque remodeling. We previously assessed aortic fluorodeoxyglucose labeled with fluorine 18 (18F-FDG) uptake seen with positron emission tomography (PET)/computed tomography (CT) as a proxy for arterial inflammation, showing increased uptake in HIV-infected individuals compared with matched controls [6]. However, the 18F-FDG PET/CT technique has limitations: although arterial 18F-FDG uptake on PET/CT tends to track with the number of arterial macrophages [7], it reflects the presence of metabolically active cells and not macrophages per se. More specifically, noninvasive molecular techniques are needed to functionally image arterial inflammation in HIV infection.
We tested the degree of colocalization between the radiopharmaceutical technetium Tc 99m tilmanocept (99mTc-tilmanocept) and CD206+ macrophages in tissue-banked aortic samples of individuals with or without HIV infection. Next, we performed a first-in-human assessment of whether systemic 99mTc-tilmanocept administration could facilitate noninvasive quantification of aortic 99mTc-tilmanocept uptake with single-photon emission computed tomography (SPECT)/CT. 99mTc-tilmanocept (99mTc-diethylenetriaminepentaacetic acid–mannosyldextran) was synthesized to avidly and specifically bind the macrophage mannose receptor, CD206 [8, 9]. This radiopharmaceutical consists of a dextran backbone supporting amine-terminated molecular leashes, which link with mannose moieties (permitting CD206 binding) and moieties of diethylenetriaminepentaacetic acid (permitting labeling with 99mTc) [8, 9] (Supplemental Figure 1). Injected peritumorally or intradermally, 99mTc-tilmanocept has proved useful for preoperative detection of tumor-affected lymph nodes in patients with head and neck cancer [10], breast cancer [11], and melanoma [11]. We hypothesized that subcutaneous administration of 99mTc-tilmanocept would permit SPECT/CT-based quantification of its aortic uptake.
METHODS
Ex Vivo Experiments on Tissue-Banked Aortic Samples From Individuals With or Without HIV
Aortic samples from individuals with (n = 10) or without (n = 10) HIV infection were obtained from the National NeuroAIDS Tissue Consortium and National Disease Research Institute. The density of aortic CD206 macrophage infiltration was compared, and the degree of colocalization of tilmanocept-positive and CD206+ macrophages assessed (Supplemental Methods).
Human Subjects Study
Study Design
Six HIV-infected subjects were recruited and enrolled. Major entry criteria included age ≥18 years, documented HIV infection, current use of antiretroviral therapy without regimen changes within the last 3 months, and subclinical atherosclerosis at CT angiography. Major exclusion criteria included known current or prior clinical cardiovascular disease, current treatment with prescription systemic steroids or anti-inflammatory/immunosuppressant medical therapies, any recent statin therapy, and estimated glomerular filtration rate <60 mL/min/1.73 m2. Subsequently, for comparison, non−HIV-infected subjects without known current or prior clinical cardiovascular disease were recruited. Seven non−HIV-infected subjects were screened to enroll 3 with cardiovascular risk profiles (sex, age, and Framingham risk score) similar to those in the HIV-infected group. These subjects did not have prior CT angiographic data available, but all 3 demonstrated subclinical atherosclerosis at the CT angiography performed as part of the study. Subjects underwent 99mTc-tilmanocept SPECT/CT, CT angiography, and detailed immune/metabolic phenotyping. The study, approved by Partners HealthCare Institutional Review Board and registered at clinical trials.gov (NCT02542371), began in August 2015 and was completed in July 2016. All subjects provided written informed consent.
99mTc-Tilmanocept SPECT/CT
99mTc-tilmanocept SPECT/CT was performed using a Symbia T6 SPECT/CT system (Siemens). A total 99mTc-tilmanocept dose of <2 mCi (50 μg) (1.37–1.85 mCi) was injected subcutaneously by a physician trained in nuclear medicine. The dose range was derived by subtracting the residual radioactivity in the syringe post injection from the total radioactivity in the syringe prior to injection. The subcutaneous route was chosen because it is a Food and Drug Administration–approved route of administration for this radiopharmaceutical, albeit for a different indication. For details on imaging technique and analysis, see Supplemental Methods. In brief, muscle was chosen as an analytic reference region, owing to low and consistent activity across subjects and groups, to control for nonspecific tissue and blood-pool uptake.
For each subject, tilmanocept activity (uptake) was normalized to a mean muscle activity of 1. A CT-guided volume of interest was drawn to cover the entirety of the aorta visible in the SPECT field of view (aortic root, arch, descending aorta to the liver base). To assure specificity of aortic analyses, “high-level” tilmanocept uptake was defined as any voxel with activity (uptake) at ≥5 times muscle activity. This level of tilmanocept uptake was roughly equivalent to the mean activity observed in the liver (Supplemental Figure 2). For each subject, we calculated the total volume within the aortic volume of interest that was at or above the “high-level” activity threshold, along with the percentage of the total volume at or above that level.
CT Angiography
CT angiography of the coronary arteries and thoracic aorta was performed with a Somatom Definition Flash 128-slice dual-source CT scanner (Siemens Medical Solutions), according to Society of Cardiac Computed Tomography guidelines [12] (Supplemental Methods). Techniques for assays and flow cytometric analyses are described in the Supplemental Methods.
Statistical Analysis
For ex vivo experiments, t tests were used. For the human study, the primary end point was aortic 99mTc-tilmanocept uptake at SPECT/CT. Our initial goal was to determine feasibility of 99mTc-tilmanocept SPECT/CT imaging in humans. A secondary aim was to compare aortic 99mTc-tilmanocept uptake among HIV-infected versus non−HIV-infected subjects. Continuous parameters were assessed for normality and presented as mean (standard deviation [SD]) or median (interquartile range [IQR]) values. Between-group comparisons were made using t, Wilcoxon rank sum
, or χ2 tests, as appropriate. Correlations between aortic 99mTc-tilmanocept uptake and CT angiographic/metabolic/immune parameters were assessed using Pearson or Spearman correlation coefficients, as appropriate. Statistical tests were 2 sided and performed using JMP software (version 11; SAS Institute).
RESULTS
Ex Vivo Experiments on Tissue-Banked Aortic Samples From Individuals With or Without HIV Infection
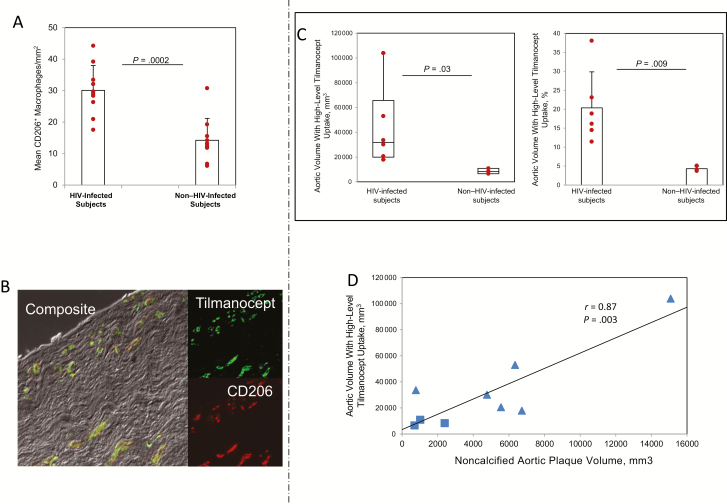
The mean number of CD206+ macrophages was significantly higher in aortic sections from individuals with HIV infection than in those from individuals without HIV infection (mean [SD], 30.1 [7.9] vs 14.2 [7.0] macrophages per square millimeter; P < .001; Figure 1A). The mean (SD) percentage of CD206+ tilmanocept-positive macrophages in aortic samples from HIV-infected versus non−HIV-infected subjects was 89.1% (6.3%) versus 86.3% (6.8%), respectively, reflecting a high degree of colocalization (Figure 1B). Aortic samples from both groups featured a low percentage of cells that were positive for tilmanocept but negative for CD206 (mean [SD], 3.1% [1.8%] for HIV vs 3.3% [2.1%] for non-HIV).
Figure 1.
Ex vivo experiments on tissue-banked aortic samples from individuals with or without human immunodeficiency virus (HIV) infection and in vivo aortic high-level technetium Tc 99m tilmanocept (99mTc-tilmanocept) uptake and relationship to aortic noncalcified plaque in both groups of subjects. A, In tissue-banked aortic samples, the mean number of CD206+ macrophages per square millimeter was significantly higher among HIV-infected individuals (n = 10) than among non−HIV-infected individuals (n = 10) (P = .0002). B, Double-label immunofluorescence using a CD206 antibody and tilmanocept-Alexa Fluor 488 revealed a high and similar degree of colocalization of tilmanocept and CD206 in sections from HIV-infected (n = 10) and non−HIV-infected (n = 10) subjects (mean [standard deviation (SD)], 89.1% [6.3%] vs 86.3% [6.8%], respectively). The mean (SD) percentages of CD206+ tilmanocept-negative macrophages and CD206− tilmanocept-positive macrophages in HIV-infected (n = 10) versus non−HIV-infected (n = 10) subjects were 7.8% (7.0%) versus 10.4% (6.2%) and 3.1% (1.8%) versus 3.3% (2.1%), respectively. C, Aortic volume with high-level 99mTc-tilmanocept uptake was significantly increased in HIV-infected (n = 6) compared with non−HIV-infected (n = 3) subjects (P = .03). Results represent medians and interquartile ranges, with whiskers showing minimum and maximum values. Aortic volume with high-level 99mTc-tilmanocept uptake is shown to facilitate assessment of the relationship with total noncalcified coronary atherosclerotic plaque volume. The percentage of aortic volume with high-level 99mTc-tilmanocept uptake was significantly increased among HIV-infected (n = 6) compared with non−HIV-infected (n = 3) subjects (P = .009). Results represent means with SDs. D, Regression analysis demonstrating a significant relationship between aortic volume with high-level 99mTc-tilmanocept uptake at single-photon emission computed tomography/computed tomography and noncalcified aortic plaque volume (Pearson correlation coefficient [r] = 0.87; P = .003). HIV-infected subjects (n = 6) are represented as triangles, and non−HIV-infected control subjects (n = 3) as squares.
Human Subjects Study
Baseline Cardiometabolic Parameters
Study subjects were men with a mean (SD) age of 58 (5) years. No subject received statin therapy within 1 year of the study. The Framingham risk score did not differ significantly between groups (9% for HIV vs 8% for non-HIV; P = .55). Among HIV-infected subjects, the mean (SD) duration since HIV diagnosis was 23.3 (8.1) years, the median log10 viral load was 1.4 copies/mL (IQR, 1.3–2.8 copies/mL), and the mean (SD) CD4+ cell count was 534/μL (138/μL) (Supplemental Table 1).
Areas of 99mTc-Tilmanocept Uptake at SPECT/CT
Among all subjects, significantly increased 99mTc-tilmanocept uptake (relative to muscle) was visualized in the kidneys, liver, and aorta (Supplemental Figure 2). 99mTc-tilmanocept is known to be renally cleared. Liver uptake was similar among HIV-infected and non−HIV-infected subjects (median [IQR] liver-to-muscle ratio 5.7 [5.5−8.1] vs 5.1 [4.2−5.8], respectively; P = .37).
Aortic 99mTc-Tilmanocept Uptake at SPECT/CT
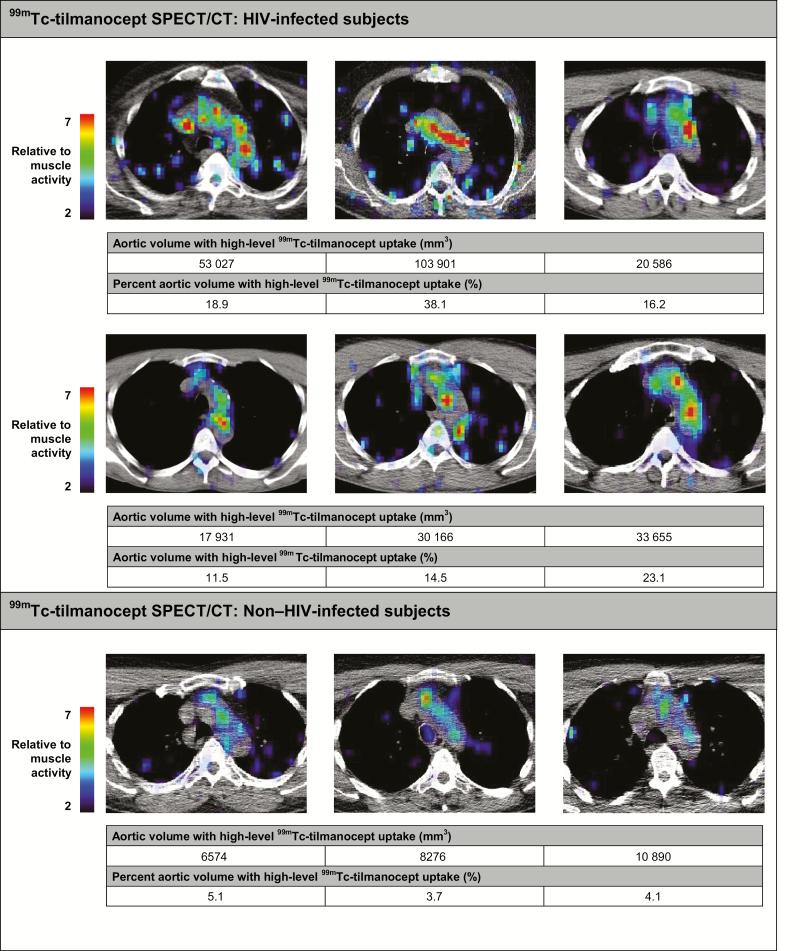
Among all study subjects, aortic 99mTc-tilmanocept uptake was observed (Figure 2). Overall, the aortic volume with high-level 99mTc-tilmanocept uptake was significantly higher in the HIV-infected than in the non−HIV-infected subjects (median uptake [IQR], 31 910 [19922−65745] vs 8276 [6574−10890] mm3; P = .03), as was the percentage of aortic volume with high-level 99mTc-tilmanocept uptake (mean [SD], 20.4% [9.5%] vs 4.3% [0.7%]; P = .009; Figure 1C).
Figure 2.
Aortic technetium Tc 99m tilmanocept (99mTc-tilmanocept) single-photon emission computed tomography (SPECT)/computed tomography (CT) in human immunodeficiency virus (HIV)–infected and non−HIV-infected subjects. Axial cross-sections of the aorta from 99mTc-tilmanocept SPECT/CT scans are shown for HIV-infected (n = 6) and non−HIV-infected (n = 3) subjects. Aortic volume and the percentage of aortic volume with high-level 99mTc-tilmanocept uptake (>5 times muscle 99mTc-tilmanocept uptake) are displayed for each subject. The adjacent color scale indicates aortic 99mTc-tilmanocept uptake relative to muscle 99mTc-tilmanocept uptake, with red representing areas of high relative 99mTc-tilmanocept uptake. Abbreviation: 99mTc, metastable technetium isotope.
Aortic 99mTc-Tilmanocept Uptake in Relation to Atherosclerotic Plaque at CT Angiography
HIV-infected subjects had an increased volume of total aortic plaque (mean [SD], 10623.2 [6746.7] vs 2271.3 [1148.5] mm3; P = .03) and noncalcified aortic plaque (attenuation <130 HU) (6541.0 [4697.3] vs 1371.0 [900.2] mm3; P = .04), compared with age-matched non−HIV-infected subjects with similar Framingham risk scores. Among all study subjects, there was a significant relationship between aortic volume with high-level 99mTc-tilmanocept uptake and noncalcified aortic atherosclerotic plaque volume (r = 0.87; P = .003), and this relationship held when HIV status was controlled for (overall model, R2 = 0.76; overall P = .01; P = .02 for noncalcified plaque volume; Figure 1D).
Aortic 99mTc-Tilmanocept Uptake in Relation to Immune Phenotype
HIV-infected subjects, in general, had higher levels of systemic monocyte activation markers, as well as higher absolute numbers of monocyte subsets, higher absolute CD8+ T-cell counts, and relatively lower absolute CD4+ T-cell counts (Supplemental Table 2, Supplemental Table 3). Among all subjects, aortic volume with high-level 99mTc-tilmanocept uptake related to levels of soluble CD14 (P = .72; P = .03), absolute number of CD14+CD16− monocytes (P = .77; P = .02), absolute CD8+ T-cell count (P = .73; P = .02), and absolute CD8+PD1+ T-cell count (P = .70; P = .04). In analogous univariate analyses performed separately among HIV-infected and non−HIV-infected subjects, relationships between these systemic immune parameters and aortic volume of high-level 99mTc-tilmanocept uptake did not achieve statistical significance.
DISCUSSION
We present first-in-human data applying a novel CD206+ macrophage-specific arterial imaging strategy, 99mTc-tilmanocept SPECT/CT, among individuals with HIV. Given the tilmanocept specificity for binding CD206+ macrophages confirmed in our ex vivo studies, aortic 99mTc-tilmanocept uptake quantified by SPECT/CT may be inferred to reflect in situ arterial CD206+ macrophage density. In our subjects, systemic administration of 99mTc-tilmanocept via subcutaneous injection resulted in markedly increased 99mTc-tilmanocept uptake in the aorta, liver, and kidneys. Aortic uptake of 99mTc-tilmanocept was particularly striking. Indeed, among the HIV-infected subjects with low Framingham risk score, high-level 99mTc-tilmanocept uptake was detectable across 20.4% of the aortic surface volume. In contrast, among non−HIV-infected subjects with similar Framingham risk scores, high-level 99mTc-tilmanocept uptake was detectable across only 4.3% of the aortic surface volume. Moreover, we show that aortic volume with high-level 99mTc-tilmanocept uptake relates robustly to noncalcified aortic atherosclerotic plaque volume, even after controlling for HIV status, highlighting a potential link between arterial CD206+ macrophage density and atherogenesis.
Recent research lends biologic plausibility to the hypothesis that CD206+ macrophages, such as those tagged by 99mTc-tilmanocept, are relevant to atherogenesis. Indeed, analysis of coronary artery sections from sudden cardiac death victims has revealed that CD206+ macrophages are particularly abundant in “unstable” thin-capped fibroatheromas [13]. The traditional macrophage classification paradigm (developed from in vitro studies) sorts CD206+ macrophages into the M2 phenotype—an “alternatively activated” class working to counterbalance the proinflammatory effects of “classically activated” M1 macrophages. However, macrophage behavior in vivo defies rigid classification. Significant heterogeneity exists within the canonical M1/M2 macrophage categories, including, for example, a subclass of M2 macrophages, M2b, producing high-levels of proinflammatory cytokines [14]. Moreover, macrophages demonstrate plasticity in vivo, switching phenotypes in response to locally encountered microenvironmental cues [14]. Our findings suggest a greater relative abundance of CD206+ macrophages in the aortas of HIV-infected versus non−HIV infected individuals, and further highlight a relationship between aortic high-level tilmanocept uptake and aortic noncalcified atherosclerotic plaque. Further research is needed to elucidate whether arterial CD206+ macrophages contribute to or compensate for in situ arterial inflammation/atherogenesis in HIV infection.
Recognizing the potential importance of CD206+ (mannose receptor–bearing) macrophages to atherogenesis, Tahara et al [13] previously applied 2-deoxy-2-18F-fluoro-D-mannose (18F-FDM) PET in a study of rabbits with or without atherosclerosis. Increased in vivo aortic 18F-FDM uptake was demonstrated in atherosclerotic animals. Among the atherosclerotic rabbits, this uptake was highest in aortic regions featuring vulnerable atherosclerotic plaque and was correlated with density of arterial macrophages, characterized by staining with RAM-11 (a monoclonal antibody reacting with a rabbit macrophage cytoplasmic antigen). The 18F-FDM technique, to date trialed only in animals, differs from our technique insofar as it probably characterizes predominantly cellular mannose uptake [13], whereas ours characterizes cells bearing the mannose receptor. Comparative studies of 18F-FDM PET and 99mTc-tilmanocept SPECT/CT are required to establish the relative merits of each technique, including sensitivity and specificity for quantifying arterial CD206+ macrophage infiltration in humans.
This proof-of concept study has limitations, including lack of aortic tissue from the same human subjects who underwent in vivo 99mTc-tilmanocept SPECT/CT and a small sample size (precluding intragroup analyses of correlations between aortic high-level tilmanocept uptake and systemic immune parameters). Study strengths include the first-in-human application of a novel, noninvasive molecular imaging technique targeting CD206+ arterial wall macrophages. This technique may be usefully applied in larger cohorts to provide insights into specific systemic inflammatory pathways promoting in situ arterial inflammation and atherogenesis in HIV infection and to track arterial responses to targeted systemic anti-inflammatory therapies.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Author contributions. M. V. Z. and S. K. G. designed the overall study, and M. Q. W., T. B., and G. E. F. contributed to the design and analyses of the data from 99mTc-tilmanocept SPECT/CT. M. T. L., B. F., and U. H. provided CT data. T. H. B. provided enzyme-linked immunosorbent assay data on immune markers. T. H. B., J. W., P. A., and K. C. W. provided flow cytometric data. J. W. and K. C. W. provided data from experiments on banked aortic samples. M. T. and A. M. recruited the subjects and performed study visits. M. V. Z, M. T., L. S., and S. K. G. performed primary study analysis. F. C. and B. A. contributed to discussions about optimal dosing and administration of 99mTc-tilmanocept. S. K. G. had full access to the data in the study and had final responsibility for the decision to submit the manuscript for publication.
Acknowledgments. We thank the nursing staff at the Massachusetts General Hospital Clinical Research Center, as well as the individuals who participated in this study.
Disclaimer. The study funders had no role in the conduct of the study; collection, management, analysis, and interpretation of the data; preparation of the manuscript; and decision to submit the manuscript for publication. Navidea Biopharmaceuticals is the preclinical and clinical developer of the agent 99mTc-tilmanocept and its Alexa Fluor 488 conjugates. Navidea Biopharmaceuticals reviewed the manuscript before submission, but submission was not contingent on approval by Navidea Biopharmaceuticals. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of Navidea Biopharmaceuticals, the NeuroAIDS Tissue Consortium, or the National Institutes of Health.
Financial support This work was supported by Navidea Biopharmaceuticals (investigator-initiated grant to S. K. G.) and by the National Institutes of Health (NIH; grant R01NS40237 to K. C. W., grants M01-RR-01066 and 1 UL1 RR025758-01 to the Harvard Clinical and Translational Science Center from the National Center for Research Resources, grant NIH DKP30 045061, and phase 1 small business innovative research grant R43HL127846 to Navidea Biopharmaceuticals); the Harvard Catalyst/ Harvard Clinical and Translational Sciences Center (medical research investigator training award to M. V. Z.; National Center for Research Resources and the National Center for Advancing Translational Sciences, NIH award 8KL2TR000168-05); NIH (T32DK 007028 to M. T.); NIH (grant T32EB 013180 to M. Q. W.); and the American Roentgen Ray Society (scholarship to M. T. L.). Experiments on tissue were provided by the NeuroAIDS Tissue Consortium and the National Disease Research Institute, supported by NIH funding through the National Institute of Mental Health and the National Institute of Neurological Disorders and Stroke (grants U24MH100930 to the Texas NeuroAIDS Research Center and U24MH100928 to the California NeuroAIDS Tissue Network).
Potential conflicts of interest. M. V. Z. participated in a scientific advisory board meeting for Roche Diagnostics and received grant support from Gilead Sciences, both unrelated to this study. U. H. has received grant support from HeartFlow, Siemens Healthcare, Genzyme, and the American College of Radiology Imaging Network and personal fees from the American Heart Association, all unrelated to this study. K. C. W. served on the scientific advisory board and was paid by Macrophage Therapeutics, unrelated to this study. S. K. G. served as a paid consultant to Gilead Sciences, Theratechnologies, Bristol-Myers Squibb, NovoNordisk, Merck, Navidea, and AstraZeneca and received grant support from Amgen, Bristol-Myers Squibb, Gilead Sciences, Kowa Pharmaceuticals, and Theratechnologies, all unrelated to this study. F. C. and B. A. are employees of Navidea Biopharmaceuticals. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab 2007; 92:2506–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Freiberg MS, Chang CC, Kuller LH, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med 2013; 173:614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wada NI, Jacobson LP, Margolick JB, et al. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS 2015; 29:463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hsue PY, Deeks SG, Hunt PW. Immunologic basis of cardiovascular disease in HIV-infected adults. J Infect Dis 2012; 205:S375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zanni MV, Schouten J, Grinspoon SK, Reiss P. Risk of coronary heart disease in patients with HIV infection. Nat Rev Cardiol 2014; 11:728–41. [DOI] [PubMed] [Google Scholar]
- 6. Subramanian S, Tawakol A, Burdo TH, et al. Arterial inflammation in patients with HIV. JAMA 2012; 308:379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tawakol A, Migrino RQ, Bashian GG, et al. In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol 2006; 48:1818–24. [DOI] [PubMed] [Google Scholar]
- 8. Vera DR, Wallace AM, Hoh CK. [99mTc]MAG(3)-mannosyl-dextran: a receptor-binding radiopharmaceutical for sentinel node detection. Nucl Med Biol 2001; 28:493–8. [DOI] [PubMed] [Google Scholar]
- 9. Wallace AM, Hoh CK, Vera DR, Darrah DD, Schulteis G. Lymphoseek: a molecular radiopharmaceutical for sentinel node detection. Ann Surg Oncol 2003; 10:531–8. [DOI] [PubMed] [Google Scholar]
- 10. Agrawal A, Civantos FJ, Brumund KT, et al. [99mTc]tilmanocept accurately detects sentinel lymph nodes and predicts node pathology status in patients with oral squamous cell carcinoma of the head and neck: results of a phase III multi- institutional trial. Ann Surg Oncol 2015; 22:3708–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leong SP, Kim J, Ross M, et al. A phase 2 study of 99mTc-tilmanocept in the detection of sentinel lymph nodes in melanoma and breast cancer. Ann Surg Oncol 2011; 18:961–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abbara S, Arbab-Zadeh A, Callister TQ, et al. SCCT guidelines for performance of coronary computed tomographic angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr 2009; 3:190–204. [DOI] [PubMed] [Google Scholar]
- 13. Tahara N, Mukherjee J, de Haas HJ, et al. 2-deoxy-2-[18F]fluoro-D-mannose positron emission tomography imaging in atherosclerosis. Nat Med 2014; 20:215–9. [DOI] [PubMed] [Google Scholar]
- 14. Chinetti-Gbaguidi G, Colin S, Staels B. Macrophage subsets in atherosclerosis. Nat Rev Cardiol 2015; 12:10–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.