Abstract
STUDY QUESTION
Are live birth rates (LBRs) after artificial cycle frozen-thawed embryo transfer (AC-FET) non-inferior to LBRs after modified natural cycle frozen-thawed embryo transfer (mNC-FET)?
SUMMARY ANSWER
AC-FET is non-inferior to mNC-FET with regard to LBRs, clinical and ongoing pregnancy rates (OPRs) but AC-FET does result in higher cancellation rates.
WHAT IS ALREADY KNOWN
Pooling prior retrospective studies of AC-FET and mNC-FET results in comparable pregnancy and LBRs. However, these results have not yet been confirmed by a prospective randomized trial.
STUDY DESIGN, SIZE AND DURATION
In this non-inferiority prospective randomized controlled trial (acronym ‘ANTARCTICA’ trial), conducted from February 2009 to April 2014, 1032 patients were included of which 959 were available for analysis. The primary outcome of the study was live birth. Secondary outcomes were clinical and ongoing pregnancy, cycle cancellation and endometrium thickness. A cost-efficiency analysis was performed.
PARTICIPANT/MATERIALS, SETTING, METHODS
This study was conducted in both secondary and tertiary fertility centres in the Netherlands. Patients included in this study had to be 18–40 years old, had to have a regular menstruation cycle between 26 and 35 days and frozen-thawed embryos to be transferred had to derive from one of the first three IVF or IVF–ICSI treatment cycles. Patients with a uterine anomaly, a contraindication for one of the prescribed medications in this study or patients undergoing a donor gamete procedure were excluded from participation. Patients were randomized based on a 1:1 allocation to either one cycle of mNC-FET or AC-FET. All embryos were cryopreserved using a slow-freeze technique.
MAIN RESULTS AND THE ROLE OF CHANCE
LBR after mNC-FET was 11.5% (57/495) versus 8.8% in AC-FET (41/464) resulting in an absolute difference in LBR of −0.027 in favour of mNC-FET (95% confidence interval (CI) −0.065–0.012; P = 0.171). Clinical pregnancy occurred in 94/495 (19.0%) patients in mNC-FET versus 75/464 (16.0%) patients in AC-FET (odds ratio (OR) 0.8, 95% CI 0.6–1.1, P = 0.25). 57/495 (11.5%) mNC-FET resulted in ongoing pregnancy versus 45/464 (9.6%) AC-FET (OR 0.7, 95% CI 0.5–1.1, P = 0.15). χ2 test confirmed the lack of superiority. Significantly more cycles were cancelled in AC-FET (124/464 versus 101/495, OR 1.4, 95% CI 1.1–1.9, P = 0.02). The costs of each of the endometrial preparation methods were comparable (€617.50 per cycle in NC-FET versus €625.73 per cycle in AC-FET, P = 0.54).
LIMITATIONS, REASONS FOR CAUTION
The minimum of 1150 patients required for adequate statistical power was not achieved. Moreover, LBRs were lower than anticipated in the sample size calculation.
WIDER IMPLICATIONS OF THE FINDINGS
LBRs after AC-FET were not inferior to those achieved by mNC-FET. No significant differences in clinical and OPR were observed. The costs of both treatment approaches were comparable.
STUDY FUNDING/COMPETING INTEREST(S)
An educational grant was received during the conduct of this study. Merck Sharpe Dohme had no influence on the design, execution and analyses of this study. E.R.G. received an education grant by Merck Sharpe Dohme (MSD) during the conduct of the present study. B.J.C. reports grants from MSD during the conduct of the study. A.H. reports grants from MSD and Ferring BV the Netherlands and personal fees from MSD. Grants from ZonMW, the Dutch Organization for Health Research and Development. J.S.E.L. reports grants from Ferring, MSD, Organon, Merck Serono and Schering-Plough during the conduct of the study. F.J.M.B. receives monetary compensation as member of the external advisory board for Merck Serono, consultancy work for Gedeon Richter, educational activities for Ferring BV, research cooperation with Ansh Labs and a strategic cooperation with Roche on automated anti Mullerian hormone assay development. N.S.M. reports receiving monetary compensations for external advisory and speaking work for Ferring BV, MSD, Anecova and Merck Serono during the conduct of the study. All reported competing interests are outside the submitted work. No other relationships or activities that could appear to have influenced the submitted work.
TRIAL REGISTRATION NUMBER
Netherlands trial register, number NTR 1586.
TRIAL REGISTRATION DATE
13 January 2009.
FIRST PATIENT INCLUDED
20 April 2009.
Keywords: assisted reproductive techniques, in vitro fertilization, frozen-thawed embryo transfer, modified natural cycle, artificial cycle
Introduction
It is more than 30 years since Trounson and Mohr reported the successful cryopreservation and thawing of supernumerary human embryos after IVF or IVF–ICSI treatment, and since Zeilmaker reported the first live birth (LB) after frozen embryo transfer (FET) (Trounson and Mohr, 1983; Zeilmaker et al., 1984). The technique introduced by these pioneers has had a profound impact on ART; improving efficacy, encouraging the transfer of fewer embryos into the uterus, and hence reducing complications arising from prematurity (Maheshwari and Bhattacharya, 2013; Fauser et al., 2005). In recent years, improved laboratory techniques and the adoption of single embryo transfer (SET) have led to a rapid increase in the number of FET cycles being performed (Wong et al., 2014). A further rise in the number of FET cycles might be expected if ‘freeze all embryos’ strategies can be shown to reduce the risk of potentially serious complications such as ovarian hyperstimulation syndrome while maintaining or improving live birth rates (LBRs) and perinatal outcomes (Maheshwari et al., 2012). The perceived benefits of FET are already leading to its widespread adoption into clinical practice, but there remains a need for high-quality data on clinical outcomes, and on the optimal means of preparing the endometrium for transfer of the thawed embryo.
In order to provide an optimal uterine environment for the implanting embryo, and to synchronize the endometrium with the developmental stage of the embryo, several methods of endometrium preparation have been developed. In natural cycle frozen-thawed embryo transfer (NC-FET), detection of ovulation is the marker for timing of thawing and transfer. The moment of ovulation can be estimated based on the detection of the luteinizing hormone (LH) surge in either urine or blood (constituting ‘true’ NC-FET) or after triggering ovulation of the dominant follicle using human chorionic gonadotrophin (hCG) (‘modified’ NC-FET). Despite the different approaches to determining the moment of ovulation, both methods have been shown to result in comparable pregnancy rates (Ghobara and Vandekerckhove, 2008; Groenewoud et al., 2013). Artificial cycle frozen-thawed embryo transfer (AC-FET) mimics the natural menstruation cycle by the administration of consecutive estrogen and progesterone. Follicular phase estrogen supplementation allows the endometrium to proliferate while suppressing the formation of the dominant follicle, and hence premature ovulation and luteinization which could render the endometrium asynchronous with the implanting embryo. Complete suppression is, however, not guaranteed. Up to 5% cycle cancellation due to development of a dominant follicle has been reported (Sathanandan et al., 1991; Simon et al., 1998). Adding progesterone mimics the shift from follicular to secretory phase, allowing the planning of embryo thawing and transfer.
A systematic review has interrogated the available studies addressing the optimal means of preparing the endometrium to receive frozen-thaw embryos, but conclusions were limited due to a lack of randomized controlled trials (RCTs) (Ghobara and Vandekerckhove, 2008). Moreover, neither cancellation rates nor cost-efficiency were reported in the retrospective studies analysed. In order to address these significant gaps in the literature, the present open label, non-inferiority multicentre RCT, entitled ‘Cryo-thawed embryo transfer: natural versus artificial cycle. An open label, non-inferiority multicentre trial (ANTARCTICA trial)’ was undertaken. In addition to assessing clinical treatment outcomes, this study was also designed to compare cancellation rates and cost-efficiency of the two endometrium preparation methods tested.
Materials and Methods
Study design and participants
The study protocol was approved by the Medical Ethics Committee of the Isala Clinics in Zwolle and by the institutional review boards of the participating centres. The second and last authors assume responsibility for the completeness and accuracy of the data and analyses and for the fidelity of the study to the protocol. The trial was registered at the Netherlands trial registry (number NTR 1586) and the protocol has previously been published (Groenewoud et al., 2012b).
From February 2009 to April 2014, eligible patients undergoing frozen-thawed embryo transfer in 17, both secondary and tertiary, fertility clinics in the Netherlands were invited to participate. Study inclusion criteria included age 18–40 and an ovulatory cycle of 26–35 days duration. Frozen embryos to be transferred were to originate from the patient's first three IVF or ICSI treatment cycles. Finally, patients had to be willing to sign an informed consent. Exclusion criteria included any contra-indication to estrogen or progesterone supplementation (e.g. prior thrombosis, prior or current hormone sensitive malignancy, porphyria) and anatomical uterine anomalies. Patients undergoing a gamete donor procedure were also excluded except those patients affected by or be the carrier of a genetic disease. Included patients participated in just one study treatment cycle. The first patient was included in the study on 20th of April 2009. Follow-up ended on 1 August 2015 following delivery of the last included patient.
Randomization and masking
Stratified randomization with variable block sizes (ranging 2–12) was used in order to achieve a balanced 1:1 allocation. Stratification was based on the origin of the frozen embryos (IVF versus ICSI) and fertility clinic. To ensure allocation concealment, a web-based randomization module using a computerized list was used. The nature of the treatment interventions precluded blinding of patients and treating physicians.
Procedures
Patients undergoing modified NC-FET (mNC-FET) attended for ultrasound evaluation of the dominant follicle from Day 10 to 12 of their menstrual cycle. Ultrasound monitoring continued until the dominant follicle reached 16–20 mm in diameter. When the follicle had reached a size indicating maturity, hCG (5000 IU Pregnyl® or 250 mg Ovitrelle®, Merck, Kenilworth, USA) was given subcutaneously to trigger ovulation. No minimal endometrial thickness to precede treatment was appointed in the protocol and no additional endocrine monitoring was performed. Patients did not receive luteal support.
In AC- FET cycles, oral estrogen (progynova® 2 mg, three times daily; Bayer, Leverkusen, Germany) was commenced on the first or second day of the cycle with the aim of supporting endometrial proliferation and suppressing follicle growth. After 12–14 days, vaginal ultrasound examination was performed to confirm that no dominant follicle had emerged and to measure endometrial thickness. When the endometrial thickness reached ≥8 mm, vaginal micronized progesterone 200 mg three times daily [Lutinus® (Ferring, Saint-Prex, Switzerland) or Utrogestan® (Besins Healthcare, Brussels, Belgium)] was administered and embryo thawing and transfer was planned. If the endometrial thickness was considered inadequate, the estrogen dosage was raised to 8 mg daily and ultrasound examination was repeated after 1 week. If the endometrium remained <8 mm, the FET treatment cycle was cancelled. In cases where a dominant follicle emerged, serum LH and progesterone were determined to rule out luteinization. If LH concentrations were <13 IU and progesterone levels <15 nmol/l, luteinization was deemed not to have occurred and FET was performed.
All participating centres used slow-freeze cryopreservation technique to cryopreserve the supernumerary embryos after initial treatment. Both cleavage as well as blastocyst stage embryos were allowed for transfer in this study. Criteria on which embryos should be considered for cryopreservation or guidelines on the developmental stage at the moment of cryopreservation were not included in the study protocol. The timing of thawing and transferring was based on the developmental stage at the time of freezing. In cleavage stage embryos, thawing was performed on the fourth or fifth day after hCG injection or progesterone initiation. Blastocyst embryos were thawed on the sixth day after hCG injection or progesterone initiation. Transfer was performed on the day of or the day after thawing. Embryo scoring was performed after thawing according to standard validated morphological characteristics. This standard was based on the ESHRE Istanbul consensus on embryo assessment (Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology, 2011). Embryo quality was graded as ‘good’, ‘reasonable’, ‘moderate’ or ‘poor’ according to the number of cells, degree of fragmentation and renewed development of the embryo. All participating centres applied the same agreed criteria for grading. A maximum of three embryos could be transferred.
Treatment costs were collected using a web-based survey. All patients included in the study received individual passwords and login codes. The survey collected data on number of visits, distance travelled during treatment, mode of transport and number of days taking a leave of absence or sick leave. Patients also could declare costs they had made that were not reimbursed by the healthcare insurance but were related to treatment (e.g. pregnancy tests). Since healthcare insurances cover fertility treatment in the Netherlands, the tariffs for monitoring of treatment and the laboratory phase were obtained from the Dutch College of Healthcare Insurances. This college also provides guidelines on calculating further costs per treatment.
Outcomes and statistical analysis
The primary outcome measure of this non-inferiority trial was LB. Secondary outcomes were clinical pregnancy rate (CPR) and ongoing pregnancy rate (OPR), cancellation rate, cost-efficiency, endometrium thickness and the occurrence of serious adverse events. Analysis of the primary efficacy variable was based on the normal approximation to the binomial distribution and was performed in accordance with the principles of intention-to-treat (ITT). A non-inferiority trial aims to demonstrate that an intervention is not worse than the comparator by more than a pre-specified, small amount or non-inferiority margin (Committee for Medicinal Products for Human Use and Efficacy Working Party and Committee for Release for Consultation, 2006). In this study, an appropriate choice of margin provides assurance that the intervention (AC-FET) is not substantially inferior to the comparator (mNC-FET) in LBR. In practice, most researchers select a non-inferiority margin to retain 80–85% of the clinical effect of the active control (Kaul and Diamond, 2006). Two studies reporting LB were published prior to the design of the ANTARCTICA trial (Loh and Leong, 1999; Kawamura, 2007). The average LBR reported in these studies was 35%. Considering that a non-inferiority threshold should retain 80% of the clinical effect of the control treatment, non-inferiority could be inferred when the margin of difference is less than −7%. In order to demonstrate non-inferiority at this level, with at least 80% power, one-sided type I error of 2.5% and a true event rate of 20%, the sample size estimate was 1030 patients. Anticipating a 10% drop-out rate, the total number required was 1150 patients.
The primary end-point was analysed based on the absolute risk difference and its confidence interval (CI). Since all patients received the designated treatment and no patients were lost to follow-up after starting treatment our per-protocol analyses and intention to treat analyses are identical. Non-inferiority of AC-FET to mNC-FET was considered to be established if the lower limit of the 95% CI of the difference in LBR between AC-FET and mNC-FET was shown to lie above the non-inferiority margin of −7%. This is equivalent to performing a one-sided hypothesis test at the 0.025 level of significance, based on the null hypothesis that AC-FET is inferior to mNC-FET. If the 95% CI for the difference not only lies above the non-inferiority margin, but also above zero, superiority of AC-FET over mNC-FET will be concluded in terms of statistical significance at the 2-sided, 5% level (P < 0.05). For the baseline variables and secondary end-points, superiority testing was conducted using logistic regression, χ2 statistics, Fisher exact tests, Mann–Whitney U-tests or Student t-tests depending on the research question to be addressed, type and distribution of data and sample size. Subgroup-analyses were performed to rule out hospital-related differences. For all analyses, a two-sided alpha of 5% was applied.
Cost-efficiency analyses were performed calculating cost per patient per treatment. Differences in costs between NC-FET and AC-FET were tested based on a Student t-test using bootstrapping (5000 times). Secondary, incremental cost-effectiveness ratio (ICER) was computed by comparing the cost of mNC-FET and AC-FET. A four quadrants scatterplot of the cost-effectiveness analyses plane was calculated to obtain insight in the uncertainty surrounding the point estimate of the ICER. This was based on 5000 times bootstrap resampling, and accompanying 95% CI.
Results
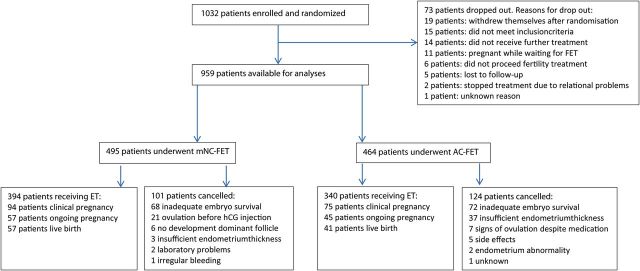
Figure 1 displays the flowchart of participant flow. Over a 5-year period (February 2009–April 2014) 1032 patients were included in this study. At the completion of the study, 73 randomized patients had dropped out, leaving 959 patients for both the per-protocol and the modified ITT analyses. Reasons for dropout are summarized in Fig. 1. Remaining patients received treatment according to study group allocation, resulting in 495 patients (51.6%) receiving mNC-FET and 464 (48.4%) receiving AC-FET. Baseline characteristics are presented in Table I. Mean age at the time of inclusion was 33.5 years and mean duration of subfertility was 3.0 years. Three hundred and eighty-three patients were primarily infertile (39.9%). The main indication for ART was severe male factor in 41.4% of patients followed by unexplained subfertility (20.6%) and tubal factor (13.7%). 55.6% of patients had undergone ICSI treatment in their initial cycle. No patients undergoing FET after a donor gamete procedure were included. Twenty-seven point 5% (259/959) patients had received 1 IVF or IVF–ICSI treatment prior to the treatment from which the cryopreserved embryos transferred in this study derived and 17.3% (163/959) had experienced more than one treatment (Table I). The average number of FET cycles performed prior to their inclusion in the ANTARCTICA study was 0.8. Fresh embryo transfer resulted in pregnancy in 242 out of 959 patients (25.2%). In the trial most patients received a single embryo FET (573/734, 78.1%), in 239 of these patients this was an elective SET. Two patients received a triple embryo transfer, the remainder 159 patients (21.7%) a double embryo transfer. Average embryo survival rate was 72%, the average embryo quality score was reasonable to good. Most thawed and transferred embryos were cleavage stage embryos (92.4%). With the exception of duration of cryopreservation, no significant differences were observed in the baseline characteristics between both treatments.
Figure 1.
Patient flowchart. mNC, modified natural cycle; AC, artificial cycle; FET, frozen embryo transfer.
Table I.
Baseline characteristics (data are number (%) or mean (SD)).
| Overall | Type of frozen embryo transfer cycle |
||
|---|---|---|---|
| Modified natural | Artificial | ||
| Treatment allocation | 959 (100%) | 495 (51.6%) | 464 (48.4%) |
| Age at randomization (years) | 33.5 (4.0) | 33.3 (4.0) | 33.8 (4.0) |
| Age at ovum pickup (years) | 32.9 (4.1) | 33.1 (4.1) | 33.1 (4.1) |
| Duration of subfertility (years) | 3.0 (2.3) | 2.9 (2.2) | 3.1 (2.5) |
| Fertility status | |||
| Primary subfertility | 383 (39.9%) | 196 (39.6%) | 191 (41.2%) |
| Parity | |||
| 0 | 555 (57.9%) | 284 (57.4%) | 271 (58.4%) |
| 1 | 353 (36.8%) | 188 (38.0%) | 165 (35.6%) |
| >2 | 51 (5.3%) | 23 (4.6%) | 28 (6.0%) |
| Initial treatment | |||
| IVF | 426 (44.4%) | 231 (46.7%) | 195 (42.0%) |
| IVF–ICSI | 533 (55.6%) | 264 (53.3%) | 269 (58.0%) |
| Outcome initial treatment | |||
| Live birth | 242 (25.2%) | 116 (23.4%) | 127 (27.4%) |
| Number of diagnoses | |||
| One diagnosis | 879 (91.7%) | 452 (91.3%) | 427 (92.0%) |
| Two diagnoses | 80 (8.3%) | 43 (8.7%) | 37 (8.0%) |
| Diagnoses | |||
| Unknown | 211 (20.6%) | 113 (21.3%) | 98 (19.9%) |
| Severe male subfertility | 424 (41.4%) | 216 (40.7%) | 208 (42.2%) |
| Moderate male subfertility | 129 (12.6%) | 55 (10.4%) | 74 (15.0%) |
| Tubal factor | 140 (13.7%) | 79 (14.9%) | 61 (12.4%) |
| Endometriosis Stage 1–2 | 40 (3.9%) | 23 (4.3%) | 17 (3.5%) |
| Endometriosis Stage 3-4 | 24 (2.4%) | 13 (2.5%) | 11 (2.2%) |
| Hormonal factor | 29 (2.8%) | 20 (3.8%) | 9 (1.8%) |
| Cervical factor | 9 (0.9%) | 3 (0.6%) | 6 (1.2%) |
| Other | 17 (1.7%) | 8 (1.5%) | 9 (1.8%) |
| Number of IVF or IVF–ICSI treatment prior study ET | |||
| 0 | 521 (55.2%) | 274 (56.1%) | 247 (54.3%) |
| 1 | 259 (27.5%) | 127 (26.0%) | 132 (29.0%) |
| 2 | 141 (15.0%) | 73 (15.0%) | 68 (14.9%) |
| 3 | 22 (2.3%) | 14 (2.9%) | 8 (1.8%) |
| Number of FET prior to study ET | |||
| 0 | 334 (34.9%) | 184 (37.2%) | 150 (32.5%) |
| 1 | 509 (53.2%) | 255 (51.5%) | 254 (55.1%) |
| 2 | 75 (7.8%) | 35 (7.1%) | 40 (8.7%) |
| >3 | 38 (4.5%) | 21 (4.2%) | 17 (3.7%) |
| Duration of cryopreservation (years) | 0.71 (1.0) | 0.65 (0.90) | 0.76 (1.0) |
| Survival (%) | 72 | 74 | 70 |
| Developmental stage at cryopreservation | |||
| Cleavage stage (Day 3 or 4) | 886 (92.4%) | 454 (91.7%) | 432 (93.1%) |
| Blastocyste stage (Day 5) | 73 (7.6%) | 41 (8.3%) | 32 (6.9%) |
| Number of embryos transferred | 1.0 (0.6) | 1.0 (0.58) | 0.96 (0.61) |
| Embryo quality score | |||
| Good | 348 (40.0%) | 182 (39.0%) | 166 (40.9%) |
| Reasonable | 288 (33.0%) | 158 (33.8%) | 130 (32.0%) |
| Moderate | 196 (22.5%) | 102 (21.8%) | 94 (23.2%) |
| Poor | 41 (4.7%) | 25 (5.4%) | 16 (3.9%) |
| Endometrial thickness (mm) | 9.0 (1.8) | 9.0 (2.0) | 8.9 (1.6) |
The LBR per started treatment cycle after mNC-FET was 11.5% (57/495) versus 8.8% in AC-FET (41/464). Considering the absolute difference in LBR of 2.7% in favour of mNC-FET and the 95% CI (−0.065 to +0.012; P = 0.171) non-inferiority of the AC-FET compared with mNC-FET was concluded (Fig. 2). A χ2 test confirmed the lack of superiority (P = 0.17). Analyses of the secondary outcomes showed no significant differences with regard to clinical and ongoing pregnancy (CPR: 94/495 (19.0%) versus 75/464 (16.2%), odds ratio (OR) 0.8, 95% CI 0.6–1.1, P = 0.25; OPR: 57/495 (11.5%) versus 45/464 (9.7%) OR 0.7, 95% CI 0.5–1.1, P = 0.15). LB and pregnancy rates per embryo transfer are summarized in Table II. Endometrial thickness was not significantly different between the treatment groups (9 versus 8.9 mm, P = 0.61). Overall 23.5% of treatment cycles were cancelled. Table III summarizes the reasons for cancellation and the frequency of their occurrence. The main reason for cancellation was insufficient embryo survival 62.2% of cases followed by insufficient endometrial thickness in 17.8% and premature ovulation before hCG injection in 9.3% of cases. In AC-FET significantly more cycles were cancelled compared with mNC-FET (see Table III). The difference in cancellation rates can be ascribed mainly to more cancellation due to insufficient endometrial thickness in AC-FET (3 in mNC-FET (due to protocol violations) versus 37 in AC-FET, OR 13.9, 95% CI 4.4–46.7, P < 0.01). No serious adverse events were reported. Subgroup-analyses showed no influence of hospital-related factors.
Figure 2.
Absolute risk reduction and 95% CIs. mNC, modified natural cycle; AC, artificial cycle; FET, frozen embryo transfer.
Table II.
Outcomes per embryo transfer.
| Overall | Type of frozen embryo transfer cycle |
OR (95% CI) | P-value | ||
|---|---|---|---|---|---|
| Modified natural | Artificial | ||||
| Clinical pregnancy/ET | 167/734 (22.8%) | 94/394 (23.9%) | 75/340 (22.1%) | 0.8 (0.64–1.27) | 0.6 |
| Ongoing pregnancy/ET | 101/734 (13.8%) | 57/394 (14.5%) | 45/340 (13.2%) | 0.8 (0.52–1.22) | 0.3 |
| Live birth/ET | 98/734 (13.4%) | 57/394 (14.5%) | 41/340 (12.1%) | 0.8 (0.53–1.25) | 0.3 |
Table III.
Reasons for cancellation.
| Overall | Type of frozen embryo transfer cycle |
OR (95% CI) | P-value | ||
|---|---|---|---|---|---|
| Modified natural | Artificial | ||||
| Cancellation | 225 | 101 | 124 | 1.4 (1.1–1.9) | 0.02 |
| Inadequate survival of embryo | 140 (62.2%) | 68 (67.3%) | 72 (58.1%) | 0.6 (0.39–1.2) | 0.15 |
| Insufficient endometrium thickness | 40 (17.8%) | 3 (3.0%) | 37 (29.8%) | 13.9 (4.1–46.7) | <0.01 |
| Ovulation prior to hCG injection | 21 (9.3%) | 21 (20.8%) | 0 (0%) | 0.1 (0.04–0.38) | <0.01 |
| Signs of ovulation despite medication | 7 (3.1%) | 0 (0%) | 7 (5.6%) | — | — |
| No development of dominant follicle | 6 (2.7%) | 6 (5.9%) | 0 (0%) | — | — |
| Side effects | 5 (2.2%) | 0 (0%) | 5 (4.0%) | — | — |
| Endometrium abnormalities (e.g. spotting) | 3 (1.3%) | 1 (1.0%) | 2 (1.6%) | — | — |
| Laboratory problems | 2 (0.9%) | 2 (2.0%) | 0 (0%) | — | — |
| Unknown reason | 1 (0.4%) | 0 (0%) | 1 (0.8%) | — | — |
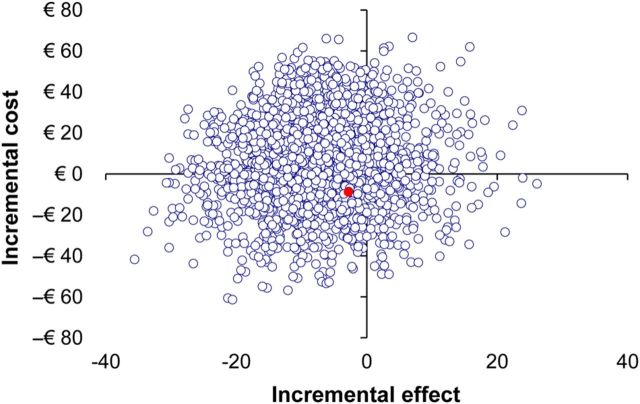
Out of the 959 included patients, 252 (26.2%) completed the questionnaire concerning costs of treatment. Table IV presents the costs incorporated in the cost-efficiency analyses including price per unit. Analysis by Student's t-test showed no significant difference in costs per treatment entity (mNC-FET €617.50 per cycle versus AC-FET €625.73, P = 0.54). In only a small portion of patients (21.8%) participating in the survey, treatment resulted in pregnancy (55 out of 252 patients). While these numbers meant no robust calculations of costs per pregnancy could be performed, the ICER was calculated. The additional cost per 1% increment in pregnancy rate in AC-FET cycles was just € 1 (95% CI −€ 18–€ 16) above the cost per 1% increment achieved using mNC-FET. Figure 3 shows the CE plane displaying the distribution of individual calculated ICERs. The distribution of the ICERs in the CE plane is in accordance with the practically equal costs of both treatments.
Table IV.
Costs analysed in the cost-efficiency analysis.
| Direct costs |
Indirect costs |
|||
|---|---|---|---|---|
| Within healthcare | Cost for monitoring of treatment | € 286·62 | — | |
| Cost for thawing and transferring of embryos | € 204·42 | |||
| Medication: | ||||
| Natural cycle | € 22·78 | |||
| Artificial cycle | ||||
| Pregnant | € 60·38 | |||
| Not pregnant | € 16·94 | |||
| Extra consults other than consults fertility centre: | ||||
| General practitioner | € 28 | |||
| Specialist | € 64 | |||
| Specialist university hospital | € 129 | |||
| Outside healthcare | Cost made by patient not reimbursed by insurance | Variable | Cost due to loss of labour productivity (e.g. leave of absence or sickness): | |
| Travel expenses | € 0·20/km | |||
| Parking fee | € 3·00 | Man | € 32.46/h | |
| Woman | € 25.94/h | |||
Figure 3.
Cost-effectiveness plane based on bootstrap analysis displaying differences in LBR compared with differences in cost between modified natural cycle and artificial cycle frozen embryo transfer. The red dot represents the actual difference in LBR and costs.
Discussion
Based on the presented data, it can be concluded that AC-FET is not inferior to mNC-FET with regard to LBR. Moreover, the costs of the two endometrium preparation methods are comparable. Following AC-FET, however, more cycles were cancelled, mainly due to insufficient endometrial thickness. Given that AC-FET is not inferior to mNC-FET both methods for endometrial preparation can be offered to patients awaiting FET. Factors such as patients' preference as well as logistics of individual fertility clinics should be decisive in choosing a certain method.
The current study is the first multicentre RCT of substantial size comparing LBR in mNC-FET with AC-FET. Furthermore, it is the first RCT addressing cancellation rates and cost-efficiency. Based on the outcomes of previously published studies and systematic reviews a non-inferiority design was adopted. As stated before, the non-inferiority threshold was chosen so that 80% of the LBR after mNC-FET would be retained. Given a reported average LBR of 35% a non-inferiority threshold of 7% was adopted (Loh and Leong, 1999; Kawamura, 2007). The minimal clinical important difference (MCID) of 7.5% was also based on these studies. Since small changes in the MCID and non-inferiority threshold can have major consequences for the conclusions of a study, it is essential that a non-inferiority threshold is recorded in the study protocol prior to the start of a study. Even though both thresholds were recorded in the official study protocol the published version of the protocol did not refer to the non-inferiority threshold of 7% but to the MCID of 7.5% (Groenewoud et al., 2012b). This could lead to the inference that the sample size calculation was based on the 7.5% MCID, whereas it was based on a non-inferiority threshold of 7% as recorded in the official protocol. Trials with a non-inferiority design are sometimes regarded as being inferior to a superiority design. Since the guidelines for constructing, analysing and reporting of non-inferiority trials were closely followed, the design of the study does not in our view diminish the validity of the results of our study.
Of the planned 1150 patients, only 959 were ultimately included, randomized and analysed. Difficulties in motivating eligible patients were the primary cause of this shortfall. After starting the trial in 2009, more liberal cryopreservation and elective SET policies were introduced. As the embryo quality criteria for freezing loosened, fewer were considered to be fit for transfer, leading to higher cancellation rates due to inadequate embryo post-thaw survival. These were higher than the 10% dropout anticipated, leaving fewer patients for the final analyses. In order to achieve 1150 participants at the rate of recruitment being maintained, a 12-month extension would have been necessary. However, the available resources excluded such an extension, and given the high numbers already recruited, and the importance of making the study findings available while still reflecting ongoing clinical practice (as slow-freeze techniques remain widely used), it was decided to stop recruitment in April 2014. Since no data safety monitoring board was installed at the beginning of the study, the decision to terminate the study was made by the main investigators. An interim analysis was not anticipated in the initial study protocol and was therefore not performed before ending recruitment.
There are a number of methodological limitations to the study that should be taken into account when interpreting the findings. The present trial had an open label design but this was inherent to the nature of the study interventions, as blinding was not possible. The low response rate to our request for data for the cost-efficiency analyses (26.2%) was disappointing, but the analysis of cost-efficiency on a large sample was aided by bootstrapping. With this consideration and confirmation that the baseline characteristics of the patients participating did not differ from those not participating, the probability of substantial non-response bias is small, and the result can be considered valid and representative of the total study population. In the cost-efficiency analysis costs to both the patients and to the healthcare system were incorporated ensuring completeness. The Dutch healthcare insurance system uses fixed prices, based on actual cost-price, for each treatment. The results of the cost-efficiency analyses can therefore be considered valid and can be generalized to other countries. Since no information on eligible patients not included in the trial is available it is not possible to fully rule out selection bias. However, given the number of patients included in this trial as well as participation of both secondary and tertiary clinics, the patients included can be considered to represent a cross-section of fertility patients, limiting the risk of selection bias. Multicentre trials are often characterized by a degree of heterogeneity in treatment approach reflecting those in daily practice, and differences in the detail of cryopreservation and thaw procedures could have resulted in a variation in LB and pregnancy rates. However, the LB and pregnancy rates were not found to vary significantly between participating hospitals. Many clinics have adopted vitrification over slow freezing as the former appears to offer improved embryo survival and quality (Li et al., 2014; Debrock et al., 2015; Zhu et al., 2015). However, the focus of the interventions studied was endometrial preparation, and receptivity is not altered by the method of cryopreservation. The conclusions of the present study are therefore applicable to FET cycles after both cryopreservation methods.
The LBR, CPR and OPR reported in this study appear to be lower compared with those given in previous published studies (Groenewoud et al., 2013). This may reflect the number of embryos transferred as the differences diminished when calculating outcomes per embryo (Groenewoud et al., 2013). LBR also depends greatly on the quality of embryos selected for cryopreservation. Since embryo selection criteria were not described in any of the other studies no comparison on post-freeze morphology could be made. Hospital-related factors were analysed and despite some variation in LBR the treating hospital was no confounding factor for LB. Another contributing factor to the low LBR might be the number of cleavage stage embryo thawed and transferred (92.6%). A movement away from the use of cleavage stage embryos as best practice seems to be taking place. Cryopreservation of cleavage stage embryos, however, can be still justified by the significant higher overall cumulative LBR (Glujovsky et al., 2012). In the present study, overall embryo survival was 72% which is consistent with other reports using the ‘slow-freeze’ cryopreservation technique (Edgar and Gook, 2012).
True NC-FET (tNC-FET) offers patients the convenient possibility of home urine testing for the onset of the LH surge. False positive or negative testing, due to substantial inter-patient and cycle variation in LH surge amplitude and shape, can lead to default planning of thawing and transferring (Miller and Soules, 1996; Park et al., 2007). Performing regular ultrasound and endocrine monitoring avoids possible irregularities but reverses the convenience of tNC-FET and increases costs. Awaiting the LH surge leads to an uncertain planning which can be bothersome for both patients as well as laboratories. By adopting mNC-FET monitoring of the dominant follicle is secured and some adjustment in planning is possible, hCG injection can be delayed 1 or 2 days depending on the dominant follicle size. Since previous studies showed no effect on pregnancy rates of preovulatory progesterone elevation as well as LH surges prior to hCG injection no extensive endocrine monitoring of the cycle was performed (Groenewoud et al., 2012a; Lee et al., 2014). The positive effect of luteal support on CPR and OPR remains debatable, therefore no luteal phase support was given in NC-FET (Kyrou et al., 2010; Bjuresten et al., 2011; Lee et al., 2013). Failure to detect ovulation for adequate planning of thawing and transferring in NC-FET, which results in cycle cancellation, is often cited as a disadvantage of NC-FET. In this study, 6.2% of cycles in NC-FET were cancelled for reasons other than insufficient survival after thawing, others, however, have reported up to 13% cancellations (Hill et al., 2010). However, in the present study cancellation rates in AC-FET were higher, mainly due to failure to meet strict criteria with regard to endometrial thickness. In mNC-FET no minimal endometrial thickness for continuation of treatment was defined since endometrium thickness in this arm of the study depended on endogenous estrogen alone. However, in mNC-FET three treatment cycles were cancelled because of insufficient endometrium thickness. These cancellations should be regarded as protocol violations. The difference in cancellation criteria between mNC-FET and AC-FET with regard to endometrium thickness can be defended by the suggestion that AC-FET benefits from a thicker endometrium (El-Toukhy et al., 2008). However, the true clinical significance of a thin endometrium for outcomes after each preparation regimens, and the merit of cancellation unless specific criteria are met, requires further study. Treatment was cancelled because of intolerable side effects in five patients receiving AC-FET. Side effects reported varied between headache, nausea and increase in weight. No thromboembolic events were reported. No side effects were reported in the mNC-FET arm of the study.
Consistent with our findings, a recent study comparing LBR in mNC-FET and FET after mild ovarian stimulation, reported no significant difference in LBR (Peeraer et al., 2015). Clinics and patients can therefore base their preference on other factors, such as personal convenience. FET is increasingly displacing fresh embryo as the main source of embryo transfers and is therefore becoming a key element of ART. The present study is the first large RCT comparing mNC-FET with AC-FET and the findings can offer a significant contribution to guiding clinical practice in FET cycles.
Authors' roles
E.R.G., B.J.K., N.S.M. and B.J.C. initiated the study and drafted the protocol. All authors beside E.R.G., B.J.K. and N.S.M. were responsible for conducting the study at the local hospital including acquisition of data. E.R.G. and B.J.K. performed statistical analyses. E.R.G., B.J.K., N.S.M. and B.J.C. were responsible for interpretation of data. E.R.G. drafted the article with input of B.J.K., B.J.K. and N.S.M., all authors performed revision of intellectual content of the final version of the article.
Funding
Funding for this study was provided by an unrestricted educational grant was awarded by Merck Sharp Dohme (MSD). MSD had no input or influence on the realization of the study protocol or execution of the study. Nor did MSD play any role in the analysis and interpretation of the data as well as the preparation and approval of this manuscript. N.S.M. is supported by the National Institute for Health Research, Biomedical Research Centres (NIHR BRC) Southampton in Nutrition.
Conflict of interest
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: E.R.G. received an education grant by Merck Sharpe Dohme (MSD) during the conduct of the present study. B.J.C. reports grants from MSD during the conduct of the study. A.H. reports grants from MSD and Ferring BV the Netherlands and personal fees from MSD. Grants from ZonMW, the Dutch Organization for Health Research and Development. J.S.E.L. reports grants from Ferring, MSD, Organon, Merck Serono and Schering-Plough during the conduct of the study. F.J.M.B. receives monetary compensation as member of the external advisory board for Merck Serono, consultancy work for Gedeon Richter, educational activities for Ferring BV, research cooperation with Ansh Labs and a strategic cooperation with Roche on automated anti Mullerian hormone assay development. N.S.M. reports receiving monetary compensations for external advisory and speaking work for Ferring BV, MSD, Anecova and Merck Serono during the conduct of the study. All reported competing interests are outside the submitted work. No other relationships or activities that could appear to have influenced the submitted work.
Acknowledgements
We thank the women who participated in this study. We thank all participating hospitals and their staff for their contribution to this study, as well as the research nurses and office members of the Dutch Consortium for their hard work and dedication (www.studies-obsgyn.nl).
References
- Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting .Hum Reprod 2011;26:1270–1283. [DOI] [PubMed] [Google Scholar]
- Bjuresten K, Landgren BM, Hovatta O, Stavreus-Evers A. Luteal phase progesterone increases live birth rate after frozen embryo transfer .Fertil Steril 2011;95:534–537. [DOI] [PubMed] [Google Scholar]
- Committee for Medicinal Products for Human Use, Efficacy Working Party and Committee for Release for Consultation. Committee for Medicinal Products for Human Use (CHMP) guideline on the choice of the non-inferiority margin .Stat Med 2006;25:1628–1638. [DOI] [PubMed] [Google Scholar]
- Debrock S, Peeraer K, Fernandez Gallardo E, De Neubourg D, Spiessens C, D'Hooghe TM. Vitrification of cleavage stage Day 3 embryos results in higher live birth rates than conventional slow freezing: a RCT. Hum Reprod 2015;30:1820–1830. [DOI] [PubMed] [Google Scholar]
- Edgar DH, Gook DA. A critical appraisal of cryopreservation (slow cooling versus vitrification) of human oocytes and embryos .Hum Reprod Update 2012;18:536–554. [DOI] [PubMed] [Google Scholar]
- El-Toukhy T, Coomarasamy A, Khairy M, Sunkara K, Seed P, Khalaf Y, Braude P. The relationship between endometrial thickness and outcome of medicated frozen embryo replacement cycles .Fertil Steril 2008;89:832–839. [DOI] [PubMed] [Google Scholar]
- Fauser BC, Devroey P, Macklon NS. Multiple birth resulting from ovarian stimulation for subfertility treatment .Lancet 2005;365:1807–1816. [DOI] [PubMed] [Google Scholar]
- Ghobara T, Vandekerckhove P. Cycle regimens for frozen-thawed embryo transfer .Cochrane Database Syst Rev 2008;23:CD003414. [DOI] [PubMed] [Google Scholar]
- Glujovsky D, Blake D, Farquhar C, Bardach A. Cleavage stage versus blastocyst stage embryo transfer in assisted reproductive technology .Cochrane Database Syst Rev 2012;7:CD002118. [DOI] [PubMed] [Google Scholar]
- Groenewoud ER, Kollen BJ, Macklon NS, Cohlen BJ. Spontaneous LH surges prior to HCG administration in unstimulated-cycle frozen-thawed embryo transfer do not influence pregnancy rates .Reprod Biomed Online 2012a;24:191–196. [DOI] [PubMed] [Google Scholar]
- Groenewoud ER, Macklon NS, Cohlen BJ, ANTARCTICA trial study group. Cryo-thawed embryo transfer: natural versus artificial cycle. A non-inferiority trial. (ANTARCTICA trial). BMC Womens Health 2012b;12:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewoud ER, Cantineau AE, Kollen BJ, Macklon NS, Cohlen BJ. What is the optimal means of preparing the endometrium in frozen-thawed embryo transfer cycles? A systematic review and meta-analysis .Hum Reprod Update 2013;19:458–470. [DOI] [PubMed] [Google Scholar]
- Hill MJ, Miller KA, Frattarelli JL. A GnRH agonist and exogenous hormone stimulation protocol has a higher live-birth rate than a natural endogenous hormone protocol for frozen-thawed blastocyst-stage embryo transfer cycles: an analysis of 1391 cycles .Fertil Steril 2010;93:416–422. [DOI] [PubMed] [Google Scholar]
- Kaul S, Diamond GA. Good enough: a primer on the analysis and interpretation of non-inferiority trials .Ann Intern Med 2006;145:62–69. [DOI] [PubMed] [Google Scholar]
- Kawamura T. Clinical outcomes of two different endometrial preparation methods for cryopreserved embryo transfer in patients with a normal menstrual cycle .Reprod Med Biol 2007;6:53–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyrou D, Fatemi HM, Popovic-Todorovic B, Van den Abbeel E, Camus M, Devroey P. Vaginal progesterone supplementation has no effect on ongoing pregnancy rate in hCG-induced natural frozen-thawed embryo transfer cycles .Eur J Obstet Gynecol Reprod Biol 2010;150:175–179. [DOI] [PubMed] [Google Scholar]
- Lee VC, Li RH, Ng EH, Yeung WS, Ho PC. Luteal phase support does not improve the clinical pregnancy rate of natural cycle frozen-thawed embryo transfer: a retrospective analysis .Eur J Obstet Gynecol Reprod Biol 2013;169:50–53. [DOI] [PubMed] [Google Scholar]
- Lee VC, Li RH, Chai J, Yeung TW, Yeung WS, Ho PC, Ng EH. Effect of preovulatory progesterone elevation and duration of progesterone elevation on the pregnancy rate of frozen-thawed embryo transfer in natural cycles .Fertil Steril 2014;101:1288–1293. [DOI] [PubMed] [Google Scholar]
- Li Z, Wang YA, Ledger W, Edgar DH, Sullivan EA. Clinical outcomes following cryopreservation of blastocysts by vitrification or slow freezing: a population-based cohort study .Hum Reprod 2014;29:2794–2801. [DOI] [PubMed] [Google Scholar]
- Loh SK, Leong NK. Factors affecting success in an embryo cryopreservation programme .Ann Acad Med Singapore 1999;28:260–265. [PubMed] [Google Scholar]
- Maheshwari A, Bhattacharya S. Elective frozen replacement cycles for all: ready for prime time? Hum Reprod 2013;28:6–9. [DOI] [PubMed] [Google Scholar]
- Maheshwari A, Pandey S, Shetty A, Hamilton M, Bhattacharya S. Obstetric and perinatal outcomes in singleton pregnancies resulting from the transfer of frozen-thawed versus fresh embryos generated through in vitro fertilization treatment: a systematic review and meta-analysis .Fertil Steril 2012;98:368–377.e1-9. [DOI] [PubMed] [Google Scholar]
- Miller PB, Soules MR. The usefulness of a urinary LH kit for ovulation prediction during menstrual cycles of normal women .Obstet Gynecol 1996;87:13–17. [DOI] [PubMed] [Google Scholar]
- Park SJ, Goldsmith LT, Skurnick JH, Wojtczuk A, Weiss G. Characteristics of the urinary luteinizing hormone surge in young ovulatory women .Fertil Steril 2007;88:684–690. [DOI] [PubMed] [Google Scholar]
- Peeraer K, Couck I, Debrock S, De Neubourg D, De Loecker P, Tomassetti C, Laenen A, Welkenhuysen M, Meeuwis L, Pelckmans S et al. Frozen-thawed embryo transfer in a natural or mildly hormonally stimulated cycle in women with regular ovulatory cycles: a RCT .Hum Reprod 2015;30:2552–2562. [DOI] [PubMed] [Google Scholar]
- Sathanandan M, Macnamee MC, Rainsbury P, Wick K, Brinsden P, Edwards RG. Replacement of frozen-thawed embryos in artificial and natural cycles: a prospective semi-randomized study .Hum Reprod 1991;6:685–687. [DOI] [PubMed] [Google Scholar]
- Simon A, Hurwitz A, Zentner BS, Bdolah Y, Laufer N. Transfer of frozen-thawed embryos in artificially prepared cycles with and without prior gonadotrophin-releasing hormone agonist suppression: a prospective randomized study .Hum Reprod 1998;13:2712–2717. [DOI] [PubMed] [Google Scholar]
- Trounson A, Mohr L. Human pregnancy following cryopreservation, thawing and transfer of an eight-cell embryo .Nature 1983;305:707–709. [DOI] [PubMed] [Google Scholar]
- Wong KM, Mastenbroek S, Repping S. Cryopreservation of human embryos and its contribution to in vitro fertilization success rates .Fertil Steril 2014;102:19–26. [DOI] [PubMed] [Google Scholar]
- Zeilmaker GH, Alberda AT, van Gent I, Rijkmans CM, Drogendijk AC. Two pregnancies following transfer of intact frozen-thawed embryos .Fertil Steril 1984;42:293–296. [DOI] [PubMed] [Google Scholar]
- Zhu HY, Xue YM, Yang LY, Jiang LY, Ling C, Tong XM, Zhang SY. Slow freezing should not be totally substituted by vitrification when applied to Day 3 embryo cryopreservation: an analysis of 5613 frozen cycles .J Assist Reprod Genet 2015;32:1371–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]