Declining malaria transmission results in reductions in total antibodies to merozoite antigens in children. However, some important functional antibodies and antibodies to infected erythrocytes are sustained for many years. This has implications for vaccine development and understanding malaria risk in populations.
Keywords: immunity, Africa, antibodies, complement, phagocytosis
Abstract
Background
We investigated the poorly understood impact of declining malaria transmission on maintenance of antibodies to Plasmodium falciparum merozoite antigens and infected erythrocytes (IEs), including functional immunity.
Methods
In a 3-year longitudinal cohort of 300 Kenyan children, antibodies to different AMA1 and MSP2 alleles of merozoites, IE surface antigens, and antibody functional activities were quantified.
Results
Over a period in which malaria transmission declined markedly, AMA1 and MSP2 antibodies decreased substantially; estimated half-lives of antibody duration were 0.8 year and 1–3 years, respectively. However, 69%–74% of children maintained their seropositivity to AMA1 alleles and 42%–52% to MSP2 alleles. Levels and prevalence of antimerozoite antibodies were consistently associated with increasing age and concurrent parasitemia. Antibodies promoting opsonic phagocytosis of merozoites declined rapidly (half-life, 0.15 years). In contrast, complement-fixing antibodies to merozoites did not decline and antibodies to IE surface antigens expressing virulent phenotypes were much better maintained (half-life, 4–10 years).
Conclusions
A decline in malaria transmission is associated with reduction in naturally acquired immunity. However, loss of immunity is not universal; some key functional responses and antibodies to IEs were better maintained and these may continue to provide some protection. Findings have implications for malaria surveillance and control measures and informing vaccine development.
In areas of moderate to high malaria endemicity, naturally acquired immunity to malaria is characterized by protection against clinical disease and control of high-density parasitemia [1, 2]. Antibodies play a major role in naturally acquired immunity to Plasmodium falciparum malaria [1, 2] and predominantly target the blood stages, including merozoites and infected erythrocytes (IEs). Antimalarial antibodies typically increase with age, exposure, and transmission intensity, and the link between antibody acquisition and the level of malaria exposure has been largely established (reviewed in [3, 4]). As a result of intensified control efforts and other factors (eg, change in malaria policies and practices, changes in users’ and health providers’ behaviors), P. falciparum transmission has declined in many regions in recent years, and these declines have been associated with higher rates and severity of clinical malaria [5–7], which may be attributed to declining naturally acquired immunity in populations. Although there is evidence that declines in malaria transmission are associated with reductions in antibodies to blood-stage antigens [8–10], what is less clear is how rapidly antibody levels to different targets decline in the context of declining transmission, whether significant humoral immune responses are maintained after reductions in transmission, or the impact of changing transmission on functional antibody responses.
Reported estimated half-lives of antibodies to blood-stage malaria antigens range from weeks to years (reviewed in [3]). These data are mainly for merozoite antigens, whereas there are limited data on the maintenance of functional antibody responses associated with protection (eg, opsonization of merozoites for phagocytic clearance and complement fixation on merozoites) [11, 12]. The maintenance and function of antibodies to IE surface antigens or merozoite antigens might be impacted by their different presentation to the immune system, but there are limited data comparing the decay of antibodies to different blood-stage antigens. Also, different kinetics of antibody responses specific for different alleles might give some indication of the relative prevalence and dynamics of circulating parasite strains, and antibody decay rates for different alleles may vary [13, 14]. Greater knowledge on how declining malaria transmission affects maintenance of immunity, especially functional immune responses, is required to identify biomarkers of exposure, evaluate the impact of interventions, and help identify populations at risk (reviewed in [4]), as well as inform the development of long-lasting vaccines.
Here, we examined the impact of declining P. falciparum transmission on the maintenance of antibodies to P. falciparum antigens in a 3-year longitudinal cohort of Kenyan children. We measured antibody responses to 2 representative merozoite antigens, apical membrane antigen 1 (AMA1) and merozoite surface protein 2 (MSP2). The 2 antigens are important targets of naturally acquired antibodies, including functional antibodies, that have been associated with protection against clinical disease in our study population [11, 15, 16], and are established vaccine candidates [17, 18]. We included different alleles of AMA1 and MSP2 to assess patterns of allele-specific antibodies over time. We examined the acquisition of antibody responses in relation to age and parasitemia over time, and calculated antibody decay rates. Furthermore, we determined the maintenance of functional antibodies to merozoites and compared maintenance of antibodies between merozoite and IE surface antigens to determine whether different response types are maintained differently.
METHODS
Study Design and Population
This cohort study was conducted in Ngerenya (Kilifi district, Kenya) [19], where biannual malaria transmission occurs (May–July and November–December), and comprised approximately 300 children aged 0.5–10 years who were followed for 3 years from May 2002 to October 2004. This was an aging cohort (median age, 3.7 years in May 2003 and 5.3 years in October 2004). Venous blood was collected in May (high transmission) and October (low transmission) in 2002–2004 (6 time-points). The same children were seen at most time points and 186 children were present at all 6 sampling points. At each time-point, presence of P. falciparum parasitemia was assessed among all children by light microscopy examination of blood smears. Active malaria case detection was performed weekly; children who were febrile (temperature ≥ 37.5°C) or had a recent history of fever or illness had a blood smear performed. Malaria was defined as any parasitemia with fever in children aged <1 year and a parasitemia ≥2500/µL of blood with fever in children ≥1 year [20]. Ethics approval was obtained from the Ethics Committee of the Kenya Medical Research Institute, and the Alfred Health Human Research and Ethics Committee. Parents/guardians of each participant provided written informed consent.
For cross-sectional analyses of antibody prevalence at each time-point, all available children were included (n = 270–298). For longitudinal analysis of changes in antibody levels over time, only children who were sampled at all time points were included (n = 186). For estimating antibody half-life during a period of minimal malaria transmission, only children who were sampled at all 3 time-points that corresponded to the decline in malaria prevalence (October 2003, May 2004, October 2004), and were aparasitemic at the time of sampling and during the interval periods, were included. From those, a subset of 71 children (who were positive for antibodies to merozoite antigens at October 2003) was selected for analysis of maintenance of functional antibodies and antibodies to IEs.
Antibody Measurements
Immunoglobulin G (IgG) among serum samples was measured by standard enzyme-linked immunosorbent assay, as described previously [21] using recombinant AMA1 (W2mef, HB3, and 3D7 alleles) and MSP2 (3D7 and FC27 alleles), which were expressed in Escherichia coli [15, 22]. For functional antibodies to merozoites, we used intact purified merozoites of the D10 isolate [23]. Merozoite opsonic phagocytosis was performed as described elsewhere [24]. Antibody-mediated fixation of C1q to the surface of merozoites, a biomarker of classical complement activation that leads to inhibition of merozoite invasion and merozoite lysis, was measured as described previously [25]. IgG reactivity to surface antigens of IEs (3D7 and IT4var19 isolates) was evaluated using an established flow cytometry–based approach [26]. Further details are provided in the Supplementary Methods.
Data Analysis
Analyses were performed using Stata Software, versions 13 and 14. Antibody seropositivity threshold was defined as the mean reactivity of negative controls plus 3 standard deviations [27]. Prevalence of antibodies to AMA1 and MSP2 between different age groups and different time-points were compared using the χ2 test. Antibody levels across groups at a single time point were compared using Kruskal–Wallis and Mann–Whitney tests. Multivariate analysis of variance for repeated measures using the Wilks lambda criteria was used to test for differences in antibody levels over time on the T – 1 absolute differences between subsequent measurements. Estimated mean antibody half-lives were determined from linear mixed-effect models as previously described [21].
RESULTS
Declining Malaria Transmission Associated With Decreased Levels of Antibodies to Merozoite Antigens
Antibodies to merozoite antigens AMA1 and MSP2, including different alleles, were measured at each of the 6 cross-sectional surveys from May 2002 to October 2004. In the later part of the study, October 2003 to October 2004, malaria transmission significantly decreased. Parasite prevalence at cross-sectional bleeds dropped from 14.0% (May 2002) to 3.7% (October 2004) (Figure 1A). The incidence of any detectable parasitemia (of any density) was <3% by active surveillance between May and October 2004 (Supplementary Table 2).
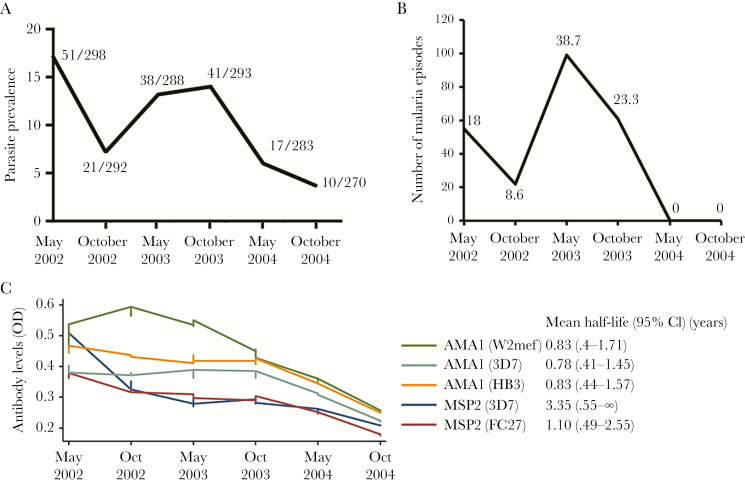
Figure 1.
Decline in parasite prevalence and antibody levels over the study period. A, Decline of prevalence of parasite prevalence at each cross-sectional bleed. At each time-point, presence of Plasmodium falciparum parasitemia was assessed by light microscopy. Data are presented as parasite prevalence (of any density), with the number of parasitemic children over the total number of children tested indicated for each cross-sectional survey time point. B, Number of episodes of malaria at each cross-sectional bleed. Data are presented as number of episodes of malaria (ie, any parasitemia accompanied by fever in children aged <1 year and a parasitemia ≥2500 infected erythrocytes/µL of blood accompanied by fever in children aged ≥1 year) for each time-point. The percentage of children with malaria is indicated for each cross-sectional survey time-point. C, Mean antibody levels to merozoite antigens over the study period. Antibody levels against different alleles of AMA1 and MSP2 were measured by standard enzyme-linked immunosorbent assay. Data are presented as locally weighted scatterplot smoothing curves (the numbers of subjects included at each time point are: May 2002, 298; October 2002, 294; May 2003, 285; October 2003, 294; May 2004, 279; October 2004, 273). The mean half-life of antibodies is shown for each antibody; this was calculated for the period between October 2013 and October 2014 when malaria transmission declined, and included children who were present at each time-point and had no malaria parasitemia detected during that time period. Abbreviations: CI, confidence interval; OD, optical density.
This decline in transmission was accompanied by significant reductions in the prevalence of antibodies to all AMA1 (Table 1) and MSP2 (Table 2) alleles within most age groups over that period (2–8 years for AMA1 and MSP2-FC27; 3 years and 5–7 years for MSP2-3D7). Furthermore, from October 2003 levels of antibodies to all AMA1 and MSP2 alleles significantly decreased between cross-sectional bleeds (Figure 1B; Tables 3 and 4; P < .001).
Table 1.
Seropositivity to AMA1 Alleles by Age at Each Cross-sectional Survey
| Age, y | Seroprevalence (95% CI) | ||||||
|---|---|---|---|---|---|---|---|
| May 2002a | October 2002a | May 2003a | October 2003a | May 2004a | October 2004a | P Valueb | |
| AMA1(W2mef) | |||||||
| 0 | 26.7 (10.6–42.9) | 67.6 (52.3–83) | 26 (9.1–42.9) | 43.5 (22.7–64.3) | 19.1 (1.8–36.4) | 33.4 (8.6–58.2) | .182 |
| 1 | 14.6 (4.5–24.8) | 21.9 (7.3–36.5) | 34.7 (15.9–53.4) | 11.8 (.8–22.9) | 16 (1.3–30.8) | 8.4 (–3.1 to 19.7) | .385 |
| 2 | 33.4 (18.3–48.4) | 48.8 (32.8–64.7) | 11.4 (1.9–20.9) | 15.7 (2.8–28.5) | 6.5 (–2.4 to 15.3) | 3.2 (–3.1 to 9.3) | <.001 |
| 3 | 48.8 (33.3–64.4) | 59 (43.3–74.7) | 34.4 (17.6–51.2) | 43.3 (27–59.5) | 32.5 (17.1–47.8) | 17.9 (3.4–32.4) | .002 |
| 4 | 50 (32.4–67.7) | 76.7 (61.3–92.2) | 40 (24.6–55.5) | 47.7 (32.3–63) | 17.3 (3.2–31.3) | 33.4 (16.1–50.6) | .001 |
| 5 | 61.8 (47.6–75.9) | 71.5 (58.6–84.3) | 48.3 (29.7–66.9) | 46.2 (26.6–65.8) | 45.5 (28.2–62.8) | 42.5 (25.3–59.7) | .007 |
| 6 | 65.8 (50.5–81.2) | 75.9 (60–91.8) | 52.8 (36.2–69.4) | 62 (47–76.9) | 54.2 (33.8–74.7) | 41.7 (21.5–62) | .032 |
| 7 | 74 (55.5–92.4) | 82.9 (70.2–95.6) | 71 (54.7–87.3) | 52 (32–72.1) | 57.9 (42–73.9) | 55.3 (39.2–71.4) | .007 |
| 8c | … | 100 (0–0) | 75 (55.5–94.6) | 81.9 (68.4–95.3) | 72 (54–90.1) | 41.7 (21.5–62) | .005 |
| 9c | … | … | … | … | 81.3 (61.5–101.1) | 72 (54–90.1) | .506 |
| P valued | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | |
| AMA1(HB3) | |||||||
| 0 | 26.7 (10.6–42.9) | 56.8 (40.6–73.1) | 37.1 (18.4–55.7) | 34.8 (14.8–54.8) | 23.9 (5.1–42.6) | 33.4 (8.6–58.2) | .361 |
| 1 | 14.6 (4.5–24.8) | 9.4 (–1 to 19.7) | 34.7 (15.9–53.4) | 14.8 (2.6–26.9) | 12 (–1.1 to 25.1) | 8.4 (–3.1 to 19.7) | .619 |
| 2 | 33.4 (18.3–48.4) | 20.6 (7.7–33.5) | 25 (12.1–38) | 21.9 (7.3–36.5) | 9.7 (–1 to 20.4) | 3.2 (–3.1 to 9.3) | .001 |
| 3 | 46.4 (30.9–61.9) | 46.2 (30.3–62.1) | 31.3 (14.9–47.7) | 43.3 (27–59.5) | 32.5 (17.1–47.8) | 17.9 (3.4–32.4) | .017 |
| 4 | 53.2 (35.5–70.8) | 66.7 (49.5–83.9) | 55 (39.4–70.7) | 45.3 (30–60.6) | 27.6 (11–44.3) | 30 (13.3–46.8) | .002 |
| 5 | 68.1 (54.6–81.7) | 57.2 (43.1–71.3) | 62.1 (44.1–80.2) | 46.2 (26.6–65.8) | 45.5 (28.2–62.8) | 39.4 (22.4–56.4) | .004 |
| 6 | 79 (65.8–92.2) | 55.2 (36.7–73.7) | 61.2 (44.9–77.4) | 57.2 (42–72.4) | 54.2 (33.8–74.7) | 37.5 (17.7–57.4) | .004 |
| 7 | 74 (55.5–92.4) | 77.2 (63–91.4) | 83.9 (70.7–97.1) | 52 (32–72.1) | 52.7 (36.5–68.8) | 52.7 (36.5–68.8) | .002 |
| 8c | … | 100 (0–0) | 85 (68.9–101.2) | 84.9 (72.4–97.4) | 80 (64–96.1) | 45.9 (25.4–66.3) | .002 |
| 9c | … | … | … | … | 75 (53–97.1) | 76 (58.9–93.2) | .943 |
| P valued | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | |
| AMA1(3D7) | |||||||
| 0 | 23.4 (7.9–38.8) | 48.7 (32.3–65.1) | 29.7 (12.1–47.3) | 26.1 (7.7–44.6) | 23.9 (5.1–42.6) | 26.7 (3.4–50) | .384 |
| 1 | 8.4 (.4–16.3) | 3.2 (–3.1 to 9.3) | 34.7 (15.9–53.4) | 20.6 (6.8–34.5) | 8 (–3 to 19) | 12.5 (–1.1 to 26.1) | .386 |
| 2 | 35.9 (20.6–51.3) | 20.6 (7.7–33.5) | 13.7 (3.4–24) | 18.8 (5–32.6) | 13 (.9–25) | 6.3 (–2.4 to 14.9) | .002 |
| 3 | 46.4 (30.9–61.9) | 35.9 (20.6–51.3) | 28.2 (12.3–44.1) | 37.9 (22–53.8) | 29.8 (14.8–44.8) | 17.9 (3.4–32.4) | .027 |
| 4 | 40.7 (23.3–58) | 63.4 (45.8–81) | 47.5 (31.8–63.3) | 40.5 (25.4–55.6) | 17.3 (3.2–31.3) | 33.4 (16.1–50.6) | .017 |
| 5 | 63.9 (49.9–77.8) | 49 (34.8–63.2) | 51.8 (33.2–70.4) | 46.2 (26.6–65.8) | 39.4 (22.4–56.4) | 30.4 (14.4–46.3) | .003 |
| 6 | 71.1 (56.4–85.8) | 55.2 (36.7–73.7) | 50 (33.4–66.7) | 57.2 (42–72.4) | 41.7 (21.5–62) | 33.4 (14–52.7) | .004 |
| 7 | 65.3 (45.3–85.3) | 68.6 (53–84.3) | 77.5 (62.4–92.5) | 52 (32–72.1) | 50 (33.9–66.2) | 50 (33.9–66.2) | .022 |
| 8c | … | 100 (0–0) | 75 (55.5–94.6) | 87.9 (76.6–99.3) | 76 (58.9–93.2) | 41.7 (21.5–62) | .003 |
| 9c | … | … | … | … | 81.3 (61.5–101.1) | 72 (54–90.1) | .506 |
| P valued | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | |
All samples included in analysis.
Abbreviation: CI, confidence interval.
aCross-sectional survey when blood was collected.
b P values calculated using a χ2 test for trend for immunoglobulin G (IgG) prevalence in the same age group at different cross-sectional bleeds (P ≤ .05 indicated in bold type).
cMissing values are due to a lack of children in that age group.
d P values calculated using a χ2 test for trend for IgG prevalence in different age groups at the same cross-sectional bleed (P ≤ .05 indicated in bold type).
Table 2.
Seropositivity to MSP2 Alleles by Age at Each Cross-sectional Survey
| Age, y | Seroprevalence (95% CI) | ||||||
|---|---|---|---|---|---|---|---|
| May 2002a | October 2002a | May 2003a | October 2003a | May 2004a | October 2004a | P Valueb | |
| MSP2(3D7) | |||||||
| 0 | 10 (–1 to 21) | 2.8 (–2.7 to 8.1) | 3.8 (–3.6 to 11) | 0 (0–0) | 0 (0–0) | 6.7 (–6.5 to 19.8) | .246 |
| 1 | 10.5 (1.7–19.2) | 3.2 (–3.1 to 9.3) | 3.9 (–3.8 to 11.5) | 0 (0–0) | 4 (–3.9 to 11.9) | 4.2 (–4.1 to 12.4) | .150 |
| 2 | 15.4 (3.9–27) | 0 (0–0) | 6.9 (–.8 to 14.4) | 3.2 (–3.1 to 9.3) | 6.5 (–2.4 to 15.3) | 3.2 (–3.1 to 9.3) | .156 |
| 3 | 31.8 (17.3–46.2) | 10.3 (.6–20) | 0 (0–0) | 16.3 (4.2–28.4) | 13.6 (2.3–24.8) | 3.6 (–3.5 to 10.7) | .013 |
| 4 | 18.8 (5–32.6) | 13.4 (1–25.8) | 20 (7.4–32.7) | 19.1 (7–31.2) | 6.9 (–2.6 to 16.4) | 10 (–1 to 21) | .249 |
| 5 | 49 (34.5–63.5) | 14.3 (4.4–24.3) | 13.8 (1–26.7) | 3.9 (–3.8 to 11.5) | 9.1 (–1 to 19.1) | 6.1 (–2.3 to 14.4) | <.001 |
| 6 | 47.4 (31.3–63.6) | 27.6 (11–44.3) | 27.8 (12.9–42.7) | 31 (16.8–45.2) | 8.4 (–3.1 to 19.7) | 0 (0–0) | <.001 |
| 7 | 52.2 (31.3–73.2) | 20 (6.5–33.6) | 22.6 (7.6–37.7) | 28 (10–46.1) | 23.7 (10–37.5) | 10.6 (.6–20.5) | .009 |
| 8c | … | 0 (0–0) | 30 (9.4–50.7) | 27.3 (11.8–42.8) | 28 (10–46.1) | 25 (7.3–42.8) | .860 |
| 9c | … | … | … | … | 37.5 (12.9–62.2) | 28 (10–46.1) | .529 |
| P valued | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | |
| MSP2(FC27) | |||||||
| 0 | 13.4 (1–25.8) | 5.5 (–2.1 to 12.9) | 7.5 (–2.8 to 17.6) | 4.4 (–4.3 to 13) | 4.8 (–4.7 to 14.2) | 0 (0–0) | .113 |
| 1 | 18.8 (7.6–30) | 6.3 (–2.4 to 14.9) | 7.7 (–2.8 to 18.2) | 0 (0–0) | 0 (0–0) | 8.4 (–3.1 to 19.7) | .012 |
| 2 | 12.9 (2.2–23.5) | 10.3 (.6–20) | 6.9 (–.8 to 14.4) | 3.2 (–3.1 to 9.3) | 6.5 (–2.4 to 15.3) | 0 (0–0) | .026 |
| 3 | 31.8 (17.3–46.2) | 15.4 (3.9–27) | 9.4 (–1 to 19.7) | 24.4 (10.3–38.4) | 16.3 (4.2–28.4) | 3.6 (–3.5 to 10.7) | .026 |
| 4 | 18.8 (5–32.6) | 40 (22.1–58) | 20 (7.4–32.7) | 21.5 (8.9–34.1) | 6.9 (–2.6 to 16.4) | 10 (–1 to 21) | .024 |
| 5 | 23.5 (11.2–35.7) | 18.4 (7.4–29.4) | 20.7 (5.7–35.8) | 23.1 (6.5–39.7) | 9.1 (–1 to 19.1) | 6.1 (–2.3 to 14.4) | .031 |
| 6 | 31.6 (16.6–46.7) | 20.7 (5.7–35.8) | 27.8 (12.9–42.7) | 26.2 (12.7–39.8) | 0 (0–0) | 4.2 (–4.1 to 12.4) | .003 |
| 7 | 39.2 (18.7–59.7) | 34.3 (18.3–50.4) | 35.5 (18.3–52.7) | 20 (4–36.1) | 10.6 (.6–20.5) | 10.6 (.6–20.5) | .000 |
| 8c | … | 0 (0–0) | 50 (27.5–72.6) | 45.5 (28.2–62.8) | 36 (16.8–55.3) | 12.5 (–1.1 to 26.1) | .012 |
| 9c | … | … | … | … | 50 (24.6–75.5) | 36 (16.8–55.3) | .381 |
| P valued | .033 | .004 | <.001 | <.001 | <.001 | .001 | |
All samples included in analysis.
Abbreviation: CI, confidence interval.
aCross-sectional survey when blood was collected.
b P values calculated using a χ2 test for trend for immunoglobulin G (IgG) prevalence in the same age group at different cross-sectional bleeds (P ≤ .05 indicated in bold type).
cMissing values are due to a lack of children in that age group.
d P values calculated using a χ2 test for trend for IgG prevalence in different age groups at the same cross-sectional bleed (P ≤ .05 indicated in bold type).
Table 3.
Levels of Antibodies to AMA1 by Age Group and Parasitemia Status
| AMA1 (W2mef Allele) | All Samples | Aparasitemic | Parasitemic | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 y | 1–3 y | 4–6 y | 7–10 y | P Valuea | 0 y | 1–3 y | 4–6 y | 7–10 y | P Valuea | 0 y | 1–3 y | 4–6 y | 7–10 y | P Valuea | ||
| May 2002b | No.c | 30 | 128 | 117 | 23 | 30 | 109 | 93 | 15 | 0 | 19 | 24 | 8 | |||
| Median | 0.04 | 0.01 | 0.24 | 0.24 | <.001 d | 0.04 | -0.01 | 0.10 | 0.44 | <.001 d | 0.00 | 0.65 | 0.89 | 1.30 | .710 | |
| IQR | (–0.01 to 0.18) | (–0.02 to 0.17) | (0.03–1.18) | (0.1–1.9) | (–0.01 to 0.18) | (–0.03 to 0.09) | (0.02–0.59) | (0.13–1.67) | (0–0) | (0.11–1.67) | (0.43–2.02) | (0.06–2.19) | ||||
| October 2002b | No.c | 37 | 110 | 108 | 36 | 36 | 105 | 99 | 31 | 1 | 5 | 9 | 5 | |||
| Median | 0.70 | 0.17 | 0.66 | 0.66 | <.001 d | 0.73 | 0.17 | 0.63 | 1.60 | <.001 d | –0.04 | 1.27 | 1.29 | 0.86 | .433 | |
| IQR | (0.1–1.43) | (0.02–0.47) | (0.2–1.66) | (0.51–2.37) | (0.12–1.48) | (0.01–0.39) | (0.18–1.63) | (0.49–2.34) | (–0.04 to –0.04) | (1.13–2.2) | (0.27–2.19) | (0.66–2.47) | ||||
| May 2003b | No.c | 27 | 102 | 105 | 51 | 26 | 93 | 88 | 40 | 1 | 9 | 17 | 11 | |||
| Median | 0.01 | 0.01 | 0.07 | 0.07 | <.001 d | 0.01 | 0.00 | 0.05 | 0.30 | <.001 d | –0.04 | 0.04 | 0.58 | 2.21 | .013 d | |
| IQR | (–0.03 to 0.13) | (–0.03 to 0.1) | (0–0.73) | (0.08–2.06) | (–0.02 to 0.13) | (–0.02 to 0.07) | (0–0.46) | (0.07–1.51) | (–0.04 to –0.04) | (–0.04 to 0.3) | (0.12–1.53) | (0.19–2.34) | ||||
| October 2003b | No.c | 23 | 103 | 110 | 58 | 23 | 93 | 91 | 45 | 0 | 10 | 18 | 13 | |||
| Median | 0.08 | 0.02 | 0.14 | 0.14 | <.001 d | 0.08 | 0.01 | 0.07 | 0.31 | <.001 d | 0.00 | 1.08 | 1.73 | 2.19 | .123 | |
| IQR | (0.02–0.24) | (–0.01 to 0.1) | (0.02–0.78) | (0.07–1.92) | (0.02–0.24) | (–0.01 to 0.05) | (0.01–0.49) | (0.07–1.18) | (0–0) | (0.21–1.71) | (0.44–2.13) | (1.61–2.26) | ||||
| May 2004b | No.c | 21 | 93 | 86 | 79 | 21 | 88 | 84 | 69 | 0 | 5 | 2 | 10 | |||
| Median | 0.00 | 0.00 | 0.05 | 0.05 | <.001 d | 0.00 | 0.00 | 0.05 | 0.24 | <.001 d | 0.00 | 1.10 | 0.22 | 1.72 | .117 | |
| IQR | (–0.02 to 0.08) | (–0.02 to 0.05) | (0.01–0.28) | (0.06–1.31) | (–0.02 to 0.08) | (–0.02 to 0.04) | (0.01–0.28) | (0.03–1.02) | (0–0) | (0.58–1.55) | (0.1–0.34) | (0.98–2.03) | ||||
| October 2004b | No.c | 15 | 84 | 87 | 87 | 15 | 80 | 87 | 81 | 0 | 4 | 0 | 6 | |||
| Median | 0.02 | –0.01 | 0.03 | 0.03 | <.001 d | 0.02 | –0.02 | 0.03 | 0.15 | <.001 d | 0.00 | 0.36 | 0.00 | 1.12 | .286 | |
| IQR | (–0.01 to 0.21) | (–0.03 to 0.02) | (–0.02 to 0.26) | (0.02–0.88) | (–0.01 to 0.21) | (–0.03 to 0.01) | (–0.02 to 0.26) | (0.01–0.75) | (0–0) | (0.26–0.48) | (0–0) | (0.2–1.5) | ||||
Abbreviation: IQR, interquartile range.
a P values calculated using a Kruskal–Wallis test (P ≤ .05 indicated in bold type).
bCross-sectional survey.
cNumber of samples tested by enzyme-linked immunosorbent assay. Data for the 3D7 and HB3 alleles of AMA1 are provided in the Supplementary Materials.
d P ≤ .05 when comparing antibody levels between children aged 1–3 years and children aged 7–10 years using a Mann–Whitney U test (P values not shown).
Table 4.
Levels of Antibodies to MSP2 by Age Group and Parasitemia Status
| MSP2 (3D7 Allele) | All Samples | Aparasitemic | Parasitemic | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 y | 1–3 y | 4–6 y | 7–10 y | P Valuea | 0 y | 1–3 y | 4–6 y | 7–10 y | P Valuea | 0 y | 1–3 y | 4–6 y | 7–10 y | P Valuea | ||
| May 2002b | No.c | 30 | 128 | 117 | 23 | 30 | 109 | 93 | 15 | 0 | 19 | 24 | 8 | |||
| Median | 0.04 | 0.13 | 0.33 | 0.33 | <.001 d | 0.04 | 0.10 | 0.28 | 0.29 | <.001 d | 0.00 | 0.89 | 1.14 | 1.76 | .640 | |
| IQR | (0.02–0.13) | (0.03–0.4) | (0.12–1.09) | (0.11–1.29) | (0.02–0.13) | (0.03–0.3) | (0.12–0.82) | (0.11–0.87) | (0–0) | (0.58–1.58) | (0.16–2.08) | (0.33–2.43) | ||||
| October 2002b | No.c | 37 | 110 | 108 | 36 | 36 | 105 | 99 | 31 | 1 | 5 | 9 | 5 | |||
| Median | 0.04 | 0.05 | 0.14 | 0.14 | <.001 d | 0.04 | 0.04 | 0.13 | 0.12 | <.001 d | 0.01 | 0.62 | 0.21 | 1.65 | .193 | |
| IQR | (0.01–0.1) | (0.02–0.09) | (0.06–0.34) | (0.07–0.47) | (0.02–0.1) | (0.02–0.08) | (0.06–0.33) | (0.07–0.34) | (0.01–0.01) | (0.04–0.77) | (0.14–0.54) | (0.5–2.43) | ||||
| May 2003b | No.c | 27 | 102 | 105 | 51 | 26 | 93 | 88 | 40 | 1 | 9 | 17 | 11 | |||
| Median | 0.09 | 0.04 | 0.13 | 0.13 | <.001 d | 0.10 | 0.04 | 0.10 | 0.14 | <.001 d | 0.06 | 0.12 | 0.73 | 1.16 | .048 d | |
| IQR | (0.01–0.23) | (0.01–0.1) | (0.05–0.43) | (0.07–0.72) | (0.01–0.23) | (0.01–0.09) | (0.04–0.31) | (0.06–0.31) | (0.06–0.06) | (0.04–0.26) | (0.2–1.39) | (0.2–2.56) | ||||
| October 2003b | No.c | 23 | 103 | 110 | 58 | 23 | 93 | 91 | 45 | 10 | 18 | 13 | 0 | |||
| Median | 0.08 | 0.06 | 0.13 | 0.13 | <.001 d | 0.08 | 0.06 | 0.10 | 0.18 | <.001 d | 0.43 | 0.49 | 1.77 | 0.00 | .426 | |
| IQR | (0.04–0.19) | (0.02–0.25) | (0.05–0.41) | (0.08–0.56) | (0.04–0.19) | (0.01–0.16) | (0.04–0.28) | (0.07–0.44) | (0.29–1.03) | (0.32–0.75) | (0.32–2.17) | (0–0) | ||||
| May 2004b | No.c | 21 | 93 | 86 | 79 | 21 | 88 | 84 | 69 | 0 | 5 | 2 | 10 | |||
| Median | 0.01 | 0.04 | 0.08 | 0.08 | <.001 d | 0.01 | 0.03 | 0.08 | 0.17 | <.001 d | 0.00 | 0.22 | 0.49 | 0.43 | .809 | |
| IQR | (0–0.02) | (0–0.1) | (0.04–0.24) | (0.06–0.6) | (0–0.02) | (0–0.09) | (0.04–0.23) | (0.04–0.47) | (0–0) | (0.2–1.53) | (0.25–0.72) | (0.17–2.14) | ||||
| October 2004b | No.c | 15 | 84 | 87 | 87 | 15 | 80 | 87 | 81 | 0 | 4 | 0 | 6 | |||
| Median | 0.02 | 0.04 | 0.07 | 0.07 | <.001 d | 0.02 | 0.03 | 0.07 | 0.13 | <.001 d | 0.00 | 0.45 | 0.00 | 1.63 | .088 | |
| IQR | (0.01–0.06) | (0.01–0.08) | (0.03–0.14) | (0.05–0.45) | (0.01–0.06) | (0.01–0.07) | (0.03–0.14) | (0.05–0.37) | (0–0) | (0.13–1.33) | (0–0) | (1.17–2.31) | ||||
Abbreviation: IQR, interquartile range.
a P values calculated using a Kruskal–Wallis test (P ≤ .05 indicated in bold type).
bCross-sectional survey.
cNumber of samples tested by enzyme-linked immunosorbent assay. Data on antibodies to the FC27 allele of MSP2 are provided in the Supplementary Materials.
d P ≤ .05 when comparing antibody levels between children aged 1–3 years and children aged 7–10 years using a Mann–Whitney U test (P values not shown).
Despite declining transmission and immune responses, AMA1 and MSP2 antibody prevalence and levels were consistently higher among older children at every time point (P < .001 to P = .033; P < .001, respectively; Tables 1 and 2), such that median antibody levels were significantly higher among 7- to 10-year-olds compared with 1- to 3-year-olds (P < .001; Tables 2 and 3; S3, S4, and S5). Similar associations with age were observed for antibody responses to all alleles of AMA1 and MSP2 and to schizont protein extract used as a proxy for P. falciparum blood-stage exposure (Supplementary Table 6). The prevalence of antibodies to MSP2-3D7 was higher than to the MSP2-FC27 allele, reflecting the moderately higher prevalence of MSP2-3D7 genotype infections in the study population [28].
At each time point, children with parasitemia (including any density) at time of sampling had higher levels of antibodies to all alleles of AMA1 (Tables 3 and 4; Supplementary Table 3) and MSP2 (Table 4; Supplementary Table 5) compared to aparasitemic children (P < .01). This suggests that ongoing exposure to infection helped maintain higher levels in the cohort, and the declining transmission and exposure therefore resulted in declining antibody levels. Associations between antibodies and increasing age were observed in aparasitemic children, but less consistently among parasitemic children. Similar results were observed for antibody responses to all alleles of MSP2 and AMA1 and to schizont extract (Supplementary Table 6).
Decay Rates of Antibodies to Merozoite Antigens With Declining Malaria Transmission
We examined the rate of decline of levels and prevalence of antibodies to AMA1 and MSP2 between October 2003 and October 2004 as malaria transmission declined. While there was interindividual variation and fluctuation over time, overall cohort antibody levels to all AMA1 and MSP2 alleles significantly declined in all age groups during this interval (Figure 1B; Tables 1 and 2). We estimated the mean half-life of antibodies to AMA1 or MSP2 between October 2003 and October 2004 [21] in children who were seropositive at the October 2003 contact point and had no recorded parasitemia at the time of sample collection or during this 12-month period of follow-up (Figure 1B). Estimated half-lives of antibodies to the different AMA1 alleles were similar at around 0.8 years (range, 0.78–0.83 years) and comparable to antibodies to MSP2 FC27 allele (1.1 years), but substantially shorter than that of antibodies to MSP2 3D7 allele (3.4 years). No significant differences in decay rates were found between age groups, although confidence intervals were wide.
Despite declining malaria transmission, many children among the whole cohort maintained their seropositive status for antibodies to AMA1 and/or MSP2 (Table 5); 69%–73% of children who were seropositive at October 2003 maintained seropositivity to each AMA1 allele at October 2004, but overall antibody reactivity had declined substantially. For MSP2 alleles, 42%–52% who were positive at October 2003 remained seropositive at October 2004. Not all children had declining antibodies during this period. For example, for AMA1-W2mef, 28.5% of children showed no significant decline between October 2003 to May 2004 (33.8% and 31% for 3D7 and HB3 alleles, respectively); for MSP2, proportions were 32.1% and 27.8% for 3D7 and FC27 alleles, respectively.
Table 5.
Maintenance of Antibodies to AMA1 and MSP2 Between October 2003 and October 2004
| Seropositive, % (No.)a | |||
|---|---|---|---|
| Allele | October 2003 | October 2004 | Maintained Seropositivityb |
| AMA1(W2mef) | 45.3% (124) | 36.5% (100) | 73.4% (91) |
| AMA1(HB3) | 44.5% (122) | 36.1% (99) | 73.8% (90) |
| AMA1(3D7) | 43.8% (120) | 34.7% (95) | 69.2% (83) |
| MSP2(3D7) | 15.3% (42) | 9.1% (25) | 52.4% (22) |
| MSP2(FC27) | 19.0% (52) | 9.5% (26) | 42.3% (22) |
aAll children present at October 2003 and October 2004 cross-sectional bleeds (n = 274).
bPercentage of seropositive individuals in October 2003 who maintained their seropositive status in October 2004.
Maintenance of Functional Antibodies to Merozoites and Antibodies to Infected Erythrocyte Surface Antigens
We evaluated maintenance of functional antibodies to merozoites as malaria transmission declined. We studied antibodies that promote opsonic phagocytosis of merozoites and antibodies that fix complement on the surface of merozoites as these 2 functional measures of merozoite antibodies have been associated with protective immunity in longitudinal studies of children [24, 25, 29]. We measured antibody levels over the last 12 months of the study (October 2003 to October 2004) among children who had no recorded episode of parasitemia during that period.
Antibodies with opsonic phagocytosis activity declined rapidly with a half-life of just 0.15 years (95% reference range, 0.09–0.4 years), significantly shorter than the half-lives of antibodies to individual merozoite antigens (Figure 2A). Despite declining levels, the prevalence of phagocytosis-promoting antibodies remained consistently high, from 92.3% in October 2003 to 89.4% in October 2004, suggesting that some level of functional opsonic phagocytosis activity is retained for long periods of time.
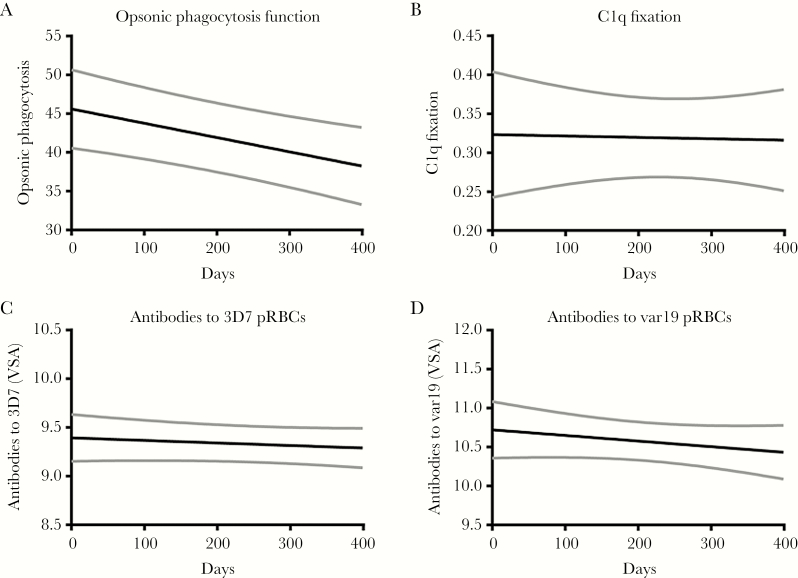
Figure 2.
Decline in levels of functional antibodies to merozoites and antibodies to infected erythrocyte (IE) surface antigens over the study period. In a subset of 70 samples, antibody function was measured at 3 time-points (October 2003, May 2004, and October 2004). The predicted mean antibody levels (and 95% confidence intervals [CIs] shown in gray) over time are shown. Day 0 represents October 2003 with days of follow-up indicated on the x-axis. Mean functional antibody half-lives were calculated from the fixed-effects slope component of a mixed-effects model and are represented in years. Antibody half-life for each antibody measure is as follows: A, Opsonic phagocytosis of merozoites, 0.15 (95% reference range, .09–0.40). B, Complement (C1q) fixation on merozoites (95% reference range, 0.70–∞). C, Antibodies to surface antigens of 3D7 IEs, 10.5 (95% reference range, 3.2–∞). D, Antibodies to surface antigens A4var19 IEs 4.0 (95% reference range, 1.4–∞). Abbreviations: pRBC, parasitised red blood cells; VSA, variant surface antigens.
In contrast, levels of complement-fixing antibodies did not appreciably decline (0.7–∞) (Figure 2B). Accordingly, the proportion of children seropositive for complement-fixing antibodies remained constant from 78% in October 2003 to 81.3% in October 2004. However, there was a wide range of observed responses with some individuals exhibiting a measurable decline and others remaining stable throughout. No significant correlations were found between the measures of functional antibodies to merozoites, consistent with the different rates of decline.
We measured levels of antibodies to surface antigens of IEs given they could be maintained differently to merozoite antigens due to their different presentation to the immune system (Figure 2C and 2D). We previously reported that these antibodies are acquired in an age-dependent manner [12, 24] in our study population and predominantly target PfEMP1 [30, 31]. We included 2 genetically distinct isolates expressing PfEMP1 types associated with virulent phenotypes and disease pathogenesis: (i) Isolate IT4var19 expresses var19 (containing a DC8 arrangement) which mediates adhesion to cerebral endothelial cells; (ii) a 3D7 isolate we identified expresses group A var genes (including a DC13 arrangement) associated with virulent properties (Supplementary Table 1). Antibodies to IE surface antigens expressed by both isolates were relatively stable as malaria transmission declined (half-lives, 10.5 [95% reference range, 3.2–∞] and 4 [95% reference range, 1.4–∞] years for 3D7 and IT4var19 IEs, respectively).
Collectively, these results show that while total levels of antibodies to individual merozoite antigens are sensitive to changes in malaria transmission, specific antibody functional activity is better maintained, especially their capacity to fix complement. Antibodies to IEs expressing PfEMP1 variants associated with disease pathogenesis also appear to be better maintained.
DISCUSSION
There is concern that recent reductions in malaria transmission in many endemic countries [6, 32–35] may lead to rapid declines in naturally acquired immunity, leaving many at increased risk of malaria and severe complications. Declining P. falciparum prevalence in our cohort was accompanied by decreasing antibodies to 2 key merozoite antigens, AMA1 and MSP2; this was consistent across different alleles of MSP2 and AMA1. In the context of low parasite prevalence and absence of clinical malaria, most children maintained seropositivity to AMA1, whereas fewer children retained seropositivity for MSP2. The broad similarity in estimated half-lives of antibodies to AMA1 and MSP2 (approximately 9 months and 1–3 years, respectively, with overlapping confidence intervals) suggests that differences in maintenance of seropositivity could be related to lower starting levels of antibodies to MSP2.
Different kinetics of antibody responses specific for different alleles might give some indication of the relative prevalence and dynamics of circulating parasite strains [13, 14]. Antibody responses to AMA1-W2mef increased from May 2002 before declining and reaching levels comparable to the other 2 AMA1 alleles by October 2003. This coincided with an increase in the incidence of parasitemia (of any density) and clinical malaria between May 2002 and May 2003; P. falciparum strains carrying a W2mef-like AMA1 allele may have been more prevalent during that time. The higher prevalence of antibodies to MSP2-3D7 is consistent with infections with this genotype being moderately more prevalent.
Rather than determining absolute decay rates of antibodies in the absence of any exposure, we estimated antibody maintenance in a practical setting where malaria transmission had dramatically reduced, an increasingly common situation globally. Maintenance of antibodies was highly variable among individuals, from rapid decay to no decline. Overall estimated half-lives of 1–3 years for antibodies to AMA1 or MSP2 indicate that antibodies to merozoite antigens decline relatively quickly; however, such decay rates and the wide variation in antibody maintenance among individuals imply that detectable levels of these antibodies could be maintained for several years. Our findings are largely consistent with studies in pregnant women [21] and a small study of adults in Southeast Asia [36]. In contrast, some studies in African children reported rapid declines in antibodies to merozoite antigens when measured immediately after treatment for acute malaria [37, 38]. Given antibodies initially decay rapidly following an acute infection, estimates of antibody half-lives will be much shorter if measured following an acute episode than measured in uninfected subjects as we have done [3, 39, 40]. Published evidence suggests impairment in the induction of B-cell memory to malaria [41], which may partly explain the lack of sustained responses.
We complemented observations on the dynamics of antibody levels by providing important new data on maintenance of functional antibodies to merozoite antigens on which very little is currently known. We measured antibodies that mediate opsonic phagocytosis and complement fixation on merozoites as these have emerged as likely mechanisms of immunity and promising functional correlates of immunity [12, 24, 25, 29]. Functional complement-fixing antibodies to merozoites were better maintained than total antibodies to merozoite antigens. This may suggest that complement fixation requires only low levels of antibodies and antibody levels must decline below a certain threshold before functional activity is lost. Alternatively, complement fixation might be mediated by specific antibodies that are better maintained over time due to their role in immunity. Functional activity is dependent on multiple antibody properties, such as affinity, subclass, allotype, glycosylation, and epitope specificity, and not just total antibody levels [42]. In contrast, opsonic phagocytosis-promoting antibodies rapidly declined, suggesting this functional response is more sensitive to decrease in antibody levels. However, most children remained positive for opsonic phagocytosis activity over the 12-month period even though overall activity did decline quickly. While antibody decay was estimated among children who had no parasitemia detected during surveillance using microscopic evaluation of blood smears, it is possible that we missed some parasitemic events; data on parasitemia detected using more sensitive PCR methods were not available. Our findings indicate that some forms of immunity may be better maintained, and highlight the need for assessing antibody function in population studies rather than simply antibody levels. The sustained persistence of some key elements of immunity is consistent with epidemiologic evidence suggesting that some level of immunity may be maintained for extended periods after decline or interruption malaria transmission [3].
Our previous studies showed that the prevalence of growth-inhibitory antibodies in this cohort was low [43]; therefore, these were not evaluated. However, we previously showed that growth-inhibitory antibodies were higher at times of higher malaria transmission [43]. It was not possible to investigate the relationship between declining immunity and subsequent risk of malaria in this study. Prior studies [15, 44] have suggested that there is a threshold magnitude of antibodies required to mediate immunity. Our results finding a decline in total antibodies to merozoites suggest that susceptibility to malaria is likely to have increased in children as a result of this decline.
Antibody acquisition longevity may also vary according to antigen specificity and parasite life-stage [21, 38, 45]; therefore, we compared maintenance of antibodies to merozoite antigens vs IE surface antigens. For our studies, we used isolates that expressed PfEMP1 variants associated with disease pathogenesis as we reasoned that antibodies to these variants are likely to have a role in immunity. Levels of antibodies to IE surface antigens were better maintained than levels of antibodies to merozoite surface antigens despite a lower seropositivity at the beginning of the study. This may be due to their different presentation to the immune system and suggests that some individuals can maintain effective antibody-mediated immunity to IE surface antigens. We have previously reported that the majority (>80%) of antibody reactivity to IEs targets PfEMP1 [30, 31]; however, a minor proportion of antibody reactivity may target other surface antigens. IE surface antibodies correlate with functional opsonic phagocytosis activity [31], a mechanism thought to be important in mediating IE clearance. Our results suggest inherent differences in maintenance of antibodies to antigens of IEs vs merozoites; they also suggest that generating long-lived immune responses to malaria is possible. Similarly, we previously found antibodies to PfEMP1 had much slower decay rates than antibodies to merozoite antigens in pregnant women [21]. Relatively short-lived antibodies suggest an inherent weakness in naturally acquired immunity to merozoites. Understanding differences in antibody maintenance between antibodies to merozoites and IEs may yield important insights to guide vaccine design. Antibodies may be valuable biomarkers to enhance malaria surveillance [4]. Understanding acquisition and maintenance of antibodies is crucial in assessing their utility in serosurveillance approaches. Our results suggest that antibodies to AMA1 and MSP2 are relatively sensitive biomarkers of changes in malaria transmission, consistent with findings in other populations [46, 47]. On the other hand, we found that antibodies to IEs expressing virulent phenotypes, isolates A4va19 [ 48] and 3D7 [ 49], were less affected by changes in malaria transmission.
In conclusion, by evaluating antibodies to merozoite antigens, IE surface antigens, and functional antibodies in a longitudinal cohort of children, we have provided significant new insights into the impact of declining malaria on immunity. The data provide estimates of decay rate of antibodies in a practical context of declining population malaria transmission. Furthermore, the findings indicate that certain elements of immunity may persist for extended periods following reduction in transmission. There was a decline in the prevalence and levels of antibodies to merozoite antigens AMA1 and MSP2 associated with falling prevalence of P. falciparum among children. Opsonic phagocytosis-promoting antibodies decayed more rapidly following declining transmission, whereas complement-fixing antibodies to merozoites or antibodies to IE surface antigens were much better maintained. Understanding the great variability among individuals in antibody maintenance may be very valuable for informing vaccine and biomarker development and implementation. With upscaling of malaria control and progress toward elimination in some regions, results from this study provide important insights on how immunity may be affected by changes in malaria transmission intensity and provide information to aid selection of biomarkers for monitoring transmission and identifying susceptible populations or groups for targeted interventions.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. We thank the children, parents, and guardians who participated in the study, field staff and clinic staff involved, and the staff the Centre for Geographic Medicine, KEMRI, for their valuable support and assistance. We also thank Robin Anders (LaTrobe University, Australia) for providing recombinant AMA1 and MSP2 proteins. This article is published with permission from the Director of KEMRI.
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This work was supported by the National Health and Medical Research Council of Australia (program grant to J. G. B.; senior research fellowship to J. G. B.; and Infrastructure for Research Institutes Support Scheme Grant); Australia Research Council (Future Fellowship to F. J. I. F.); Wellcome Trust; and the Victorian State Government Operational Infrastructure Support.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Marsh K, Kinyanjui S. Immune effector mechanisms in malaria. Parasite Immunol 2006; 28:51–60. [DOI] [PubMed] [Google Scholar]
- 2. Doolan DL, Dobaño C, Baird JK. Acquired immunity to malaria. Clin Microbiol Rev 2009; 22:13–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fowkes FJ, Boeuf P, Beeson JG. Immunity to malaria in an era of declining malaria transmission. Parasitology 2016; 143:139–53. [DOI] [PubMed] [Google Scholar]
- 4. Elliott SR, Fowkes FJ, Richards JS, Reiling L, Drew DR, Beeson JG. Research priorities for the development and implementation of serological tools for malaria surveillance. F1000Prime Rep 2014; 6:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Griffin JT, Ferguson NM, Ghani AC. Estimates of the changing age-burden of Plasmodium falciparum malaria disease in sub-Saharan Africa. Nat Commun 2014; 5:3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ceesay SJ, Casals-Pascual C, Erskine J et al. . Changes in malaria indices between 1999 and 2007 in The Gambia: a retrospective analysis. Lancet 2008; 372:1545–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brasseur P, Badiane M, Cisse M, Agnamey P, Vaillant MT, Olliaro PL. Changing patterns of malaria during 1996–2010 in an area of moderate transmission in southern Senegal. Malar J 2011; 10:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Diop F, Richard V, Diouf B et al. . Dramatic declines in seropositivity as determined with crude extracts of Plasmodium falciparum schizonts between 2000 and 2010 in Dielmo and Ndiop, Senegal. Malar J 2014; 13:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Migot F, Chougnet C, Raharimalala L, Astagneau P, Lepers JP, Deloron P. Human immune responses to the Plasmodium falciparum ring-infected erythrocyte surface antigen (Pf155/RESA) after a decrease in malaria transmission in Madagascar. Am J Trop Med Hyg 1993; 48:432–9. [DOI] [PubMed] [Google Scholar]
- 10. Wong J, Hamel MJ, Drakeley CJ et al. . Serological markers for monitoring historical changes in malaria transmission intensity in a highly endemic region of Western Kenya, 1994–2009. Malar J 2014; 13:451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Beeson JG, Drew DR, Boyle MJ, Feng G, Fowkes FJ, Richards JS. Merozoite surface proteins in red blood cell invasion, immunity and vaccines against malaria. FEMS Microbiol Rev 2016; 40:343–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Teo A, Feng G, Brown GV, Beeson JG, Rogerson SJ. Functional antibodies and protection against blood-stage malaria. Trends Parasitol 2016; 32:887–98. [DOI] [PubMed] [Google Scholar]
- 13. Cavanagh DR, Elhassan IM, Roper C et al. . A longitudinal study of type-specific antibody responses to Plasmodium falciparum merozoite surface protein-1 in an area of unstable malaria in Sudan. J Immunol 1998; 161:347–59. [PubMed] [Google Scholar]
- 14. Giha HA, Staalsoe T, Dodoo D et al. . Nine-year longitudinal study of antibodies to variant antigens on the surface of Plasmodium falciparum-infected erythrocytes. Infect Immun 1999; 67:4092–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stanisic DI, Richards JS, McCallum FJ et al. . Immunoglobulin G subclass-specific responses against Plasmodium falciparum merozoite antigens are associated with control of parasitemia and protection from symptomatic illness. Infect Immun 2009; 77:1165–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Polley SD, Conway DJ, Cavanagh DR et al. . High levels of serum antibodies to merozoite surface protein 2 of Plasmodium falciparum are associated with reduced risk of clinical malaria in coastal Kenya. Vaccine 2006; 24:4233–46. [DOI] [PubMed] [Google Scholar]
- 17. Thera MA, Doumbo OK, Coulibaly D et al. . Safety and immunogenicity of an AMA1 malaria vaccine in Malian children: results of a phase 1 randomized controlled trial. PLoS One 2010; 5:e9041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Genton B, Betuela I, Felger I et al. . A recombinant blood-stage malaria vaccine reduces Plasmodium falciparum density and exerts selective pressure on parasite populations in a phase 1-2b trial in Papua New Guinea. J Infect Dis 2002; 185:820–7. [DOI] [PubMed] [Google Scholar]
- 19. Nyakeriga AM, Troye-Blomberg M, Dorfman JR et al. . Iron deficiency and malaria among children living on the coast of Kenya. J Infect Dis 2004; 190:439–47. [DOI] [PubMed] [Google Scholar]
- 20. Mwangi TW, Ross A, Snow RW, Marsh K. Case definitions of clinical malaria under different transmission conditions in Kilifi district, Kenya. J Infect Dis 2005; 191:1932–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fowkes FJ, McGready R, Cross NJ et al. . New insights into acquisition, boosting, and longevity of immunity to malaria in pregnant women. J Infect Dis 2012; 206:1612–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Anders RF, Crewther PE, Edwards S et al. . Immunisation with recombinant AMA-1 protects mice against infection with Plasmodium chabaudi. Vaccine 1998; 16:240–7. [DOI] [PubMed] [Google Scholar]
- 23. Boyle MJ, Wilson DW, Richards JS et al. . Isolation of viable Plasmodium falciparum merozoites to define erythrocyte invasion events and advance vaccine and drug development. Proc Natl Acad Sci U S A 2010; 107:14378–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Osier FH, Feng G, Boyle MJ et al. . Opsonic phagocytosis of Plasmodium falciparum merozoites: mechanism in human immunity and a correlate of protection against malaria. BMC Med 2014; 12:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Boyle MJ, Reiling L, Feng G et al. . Human antibodies fix complement to inhibit Plasmodium falciparum invasion of erythrocytes and are associated with protection against malaria. Immunity 2015; 42:580–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Beeson JG, Mann EJ, Elliott SR et al. . Antibodies to variant surface antigens of Plasmodium falciparum-infected erythrocytes and adhesion inhibitory antibodies are associated with placental malaria and have overlapping and distinct targets. J Infect Dis 2004; 189:540–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Persson KE, McCallum FJ, Reiling L et al. . Variation in use of erythrocyte invasion pathways by Plasmodium falciparum mediates evasion of human inhibitory antibodies. J Clin Invest 2008; 118:342–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Färnert A, Williams TN, Mwangi TW et al. . Transmission-dependent tolerance to multiclonal Plasmodium falciparum infection. J Infect Dis 2009; 200:1166–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hill DL, Eriksson EM, Li Wai Suen CS et al. . Opsonising antibodies to P. falciparum merozoites associated with immunity to clinical malaria. PLoS One 2013; 8:e74627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chan JA, Howell KB, Langer C et al. . A single point in protein trafficking by Plasmodium falciparum determines the expression of major antigens on the surface of infected erythrocytes targeted by human antibodies. Cell Mol Life Sci 2016; 73:4141–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chan JA, Howell KB, Reiling L et al. . Targets of antibodies against Plasmodium falciparum-infected erythrocytes in malaria immunity. J Clin Invest 2012; 122:3227–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. World Health Organization. World malaria report 2012. Geneva, Switzerland: WHO, 2012. [Google Scholar]
- 33. Snow RW, Marsh K. Malaria in Africa: progress and prospects in the decade since the Abuja declaration. Lancet 2010; 376:137–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. O’Meara WP, Mangeni JN, Steketee R, Greenwood B. Changes in the burden of malaria in sub-Saharan Africa. Lancet Infect Dis 2010; 10:545–55. [DOI] [PubMed] [Google Scholar]
- 35. Okiro EA, Alegana VA, Noor AM, Snow RW. Changing malaria intervention coverage, transmission and hospitalization in Kenya. Malar J 2010; 9:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wipasa J, Suphavilai C, Okell LC et al. . Long-lived antibody and B cell memory responses to the human malaria parasites, Plasmodium falciparum and Plasmodium vivax. PLoS Pathog 2010; 6:e1000770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kinyanjui SM, Conway DJ, Lanar DE, Marsh K. IgG antibody responses to Plasmodium falciparum merozoite antigens in Kenyan children have a short half-life. Malar J 2007; 6:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Akpogheneta OJ, Duah NO, Tetteh KK et al. . Duration of naturally acquired antibody responses to blood-stage Plasmodium falciparum is age dependent and antigen specific. Infect Immun 2008; 76:1748–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. White MT, Griffin JT, Akpogheneta O et al. . Dynamics of the antibody response to Plasmodium falciparum infection in African children. J Infect Dis 2014; 210:1115–22. [DOI] [PubMed] [Google Scholar]
- 40. Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med 2007; 357:1903–15. [DOI] [PubMed] [Google Scholar]
- 41. Portugal S, Tipton CM, Sohn H et al. . Malaria-associated atypical memory B cells exhibit markedly reduced B cell receptor signaling and effector function. Elife 2015; 4. doi:10.7554/eLife.07218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Irani V, Guy AJ, Andrew D, Beeson JG, Ramsland PA, Richards JS. Molecular properties of human IgG subclasses and their implications for designing therapeutic monoclonal antibodies against infectious diseases. Mol Immunol 2015; 67:171–82. [DOI] [PubMed] [Google Scholar]
- 43. McCallum FJ, Persson KE, Mugyenyi CK et al. . Acquisition of growth-inhibitory antibodies against blood-stage Plasmodium falciparum. PLoS One 2008; 3:e3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Murungi LM, Kamuyu G, Lowe B et al. . A threshold concentration of anti-merozoite antibodies is required for protection from clinical episodes of malaria. Vaccine 2013; 31:3936–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McCallum FJ, Persson KE, Fowkes FJ et al. . Differing rates of antibody acquisition to merozoite antigens in malaria: implications for immunity and surveillance. J Leukoc Biol 2017; 101:913–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stanisic DI, Fowkes FJ, Koinari M et al. . Acquisition of antibodies against Plasmodium falciparum merozoites and malaria immunity in young children and the influence of age, force of infection, and magnitude of response. Infect Immun 2015; 83:646–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Helb DA, Tetteh KK, Felgner PL et al. . Novel serologic biomarkers provide accurate estimates of recent Plasmodium falciparum exposure for individuals and communities. Proc Natl Acad Sci U S A 2015; 112:E4438–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Avril M, Brazier AJ, Melcher M, Sampath S, Smith JD. DC8 and DC13 var genes associated with severe malaria bind avidly to diverse endothelial cells. PLoS Pathog 2013; 9:e1003430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chan JC , Stanisic DI , Duffy MF, . et al. Patterns of protective associations differ for antibodies to P.falciparum-infected erythrocytes and merozoites in immunity against malaria in children. Eur J Immunol 2017; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.