Abstract
The functioning of the supply chain may be a driving factor behind the development of human immunodeficiency virus (HIV) drug resistance (HIVDR) in many low- and middle-income countries (LMICs). Additionally, the effectiveness of supply chains will likely impact the scale-up of both viral-load monitoring and HIVDR testing. This article describes the complexities of global supply chains relevant for LMICs and presents early data on stock-outs and drug substitutions in several countries supported by the US President’s Emergency Plan for AIDS Relief. Supply chain systems will need to be strengthened to minimize interruptions as new antiretroviral therapy regimens are introduced and to facilitate adoption of new laboratory technologies.
Keywords: drug resistance, antiretroviral, supply chains, stock outs, logistics
Weaknesses or disruptions in antiretroviral (ARV) supply chains are frequently underestimated contributors to the development of human immunodeficiency virus (HIV) drug resistance (HIVDR), particularly in low- and middle-income countries (LMICs). Although stock-outs (when a health facility runs out of a specific ARV product) are recognized as a contributor to HIVDR [1], stock shortages (when a site has a limited quantity of a product, causing healthcare workers to either dispense a smaller amount than what was prescribed [eg, 2 weeks of ARVs] or temporarily substitute components of ARV regimens) are also programmatically relevant, yet rarely reported in peer-reviewed literature. Most patients in LMICs have initiated HIV treatment using nonnucleoside reverse-transcriptase inhibitor (NNRTI)–based regimens, which are particularly susceptible to emergent HIVDR from stock interruption due to their long half-lives [2]. Weak health systems and supply chains, coupled with infrequent viral-load (VL) monitoring, have hampered the ability of patients to routinely adhere to antiretroviral therapy (ART) and for healthcare providers to detect HIVDR early. As a result, HIVDR appears to be increasing in sub-Saharan Africa [3]. Early warning indicator (EWI) surveys [4], performed by countries to identify risk for emergent HIVDR, demonstrate higher rates of drug stock-outs (percentages of sites with ≥1 ARV drug out of stock in the reporting year) in LMIC settings, particularly Eastern (33.1%), Western (23.9%), and Central Africa (21.9%), compared with 2.9% in all other global regions [5]. Although many patient-level factors contribute to the lower rates of retention and on-time drug pick-up in LMICs, in a systematic review, 16.1% of patients cited drug stock-outs as a barrier to ART adherence. Additionally, 17.5% cited distance to clinic as a barrier [6], which becomes problematic when stock shortages lead providers to give <1 month supply of ARVs to patients to mitigate drug stock-outs.
THE COMPLEXITY OF GLOBAL SUPPLY CHAINS
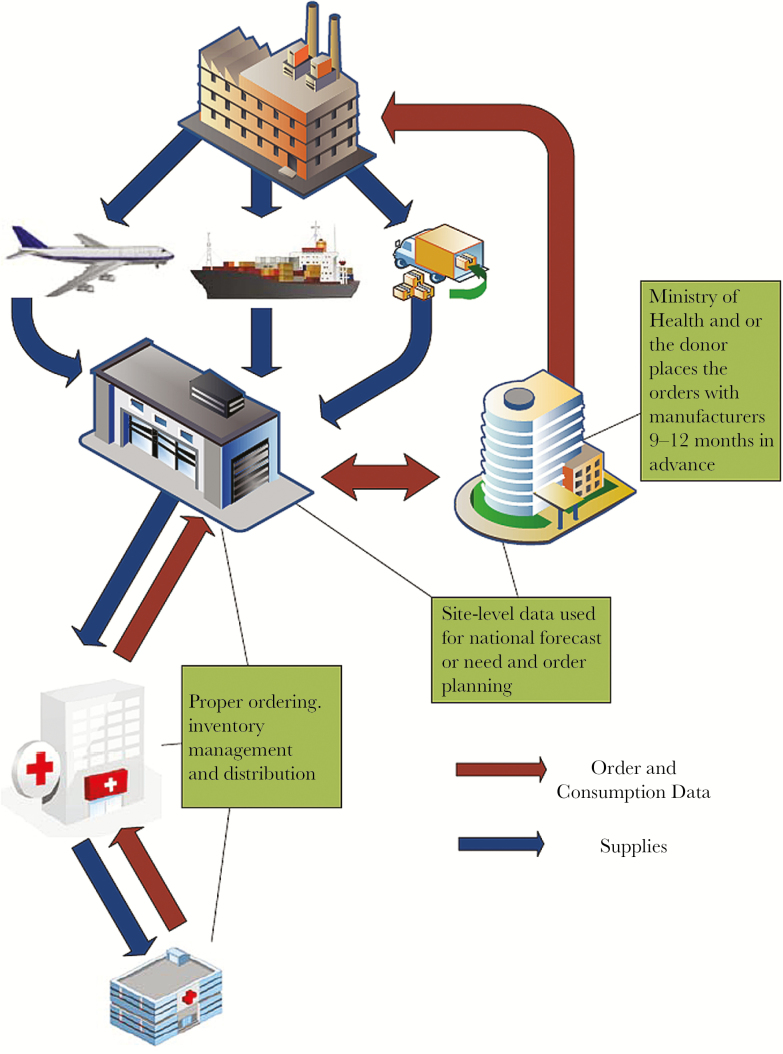
Global ART supply chains in LMICs are complicated, with multiple stress points and stakeholders that need to be successfully coordinated to ensure on-time delivery of ARVs to facilities and patients. Supply-chain management encompasses the planning and management of multiple activities, including quantification and forecasting; procurement; importation and regulatory approval; delivery; proper storage at multiple intermediate points; and tracking and management of ARV stock levels and expiry. Importantly, it also includes coordination with donors, ARV manufacturers (and their suppliers), third-party providers, and customers [Figure 1] [7]. Accuracy and timeliness of all steps are required for the system to function. In many LMICs, it can take 2–6 months from the placement of an ARV order until it arrives and clears customs and an additional 4–6 months for the order to travel from a central warehouse to intermediate points and to the final service delivery point. This means programs need to have estimated their need 9–12 months in advance, secured the necessary financing, established the procurement vehicle, placed the order, and completed any independent quality control testing needed. As throughout the health sector in LMICs, well-trained logistics specialists are in short supply and frequently leave for higher-paying opportunities in the private sector.
Figure 1.
Multiple steps and logistics management points with complex antiretroviral supply chains.
Proper storage, distribution, and accounting at multiple service delivery points is critical; it is not uncommon to have facility-level stock-outs while the country is stocked sufficiently at a national level [8]. Many sites in LMICs rely on paper-based information systems and have limited communication and transportation infrastructure, hindering the ability to adapt quickly when problems arise. This becomes even more challenging when global ARV guidelines change (shifting first- or second-line regimens) or when rapid VL scale-up leads to an increase in the numbers of patients needing second- or third-line therapy. Manufacturers of the various active pharmaceutical ingredients in India and China and the finished dosage formulators need extra lead time to produce sufficient quantities of quality ARVs to meet global demand.
Complexities only increase with pediatric ARVs. With the continued scale-up of Option B+, the number of children living with HIV is expected to further decrease, which will impact an already fragile pediatric ARV market. Antiretroviral dosing for children varies by age and weight, requiring different formulations, which frequently results in small, fragmented orders across multiple products and manufacturers [9] that must then be distributed across many facilities [10]. Furthermore, children are less often included in early warning indicator monitoring and HIVDR surveys, which may mask an emerging problem.
MONITORING SUPPLY CHAIN EXECUTION: THE US PRESIDENTS EMERGENCY PLAN FOR AIDS RELIEF EXPERIENCE
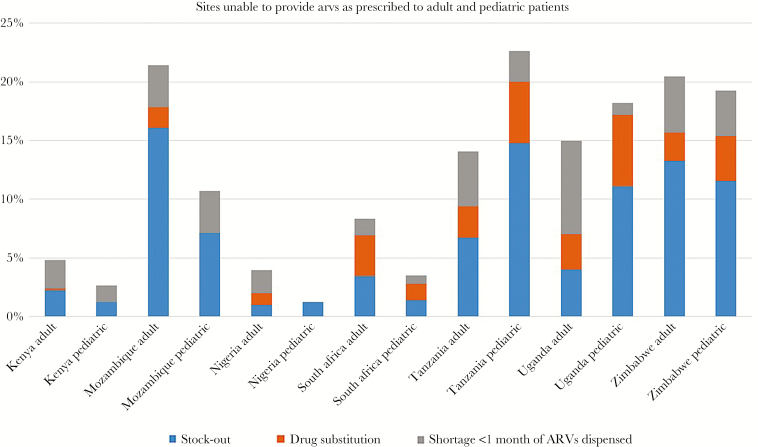
The US Presidents Emergency Plan for AIDS Relief (PEPFAR), responsible for the procurement of >$2.8 billion in ARVs from 2005 to 2016, supports several supply chain data collection and monitoring activities, including the Site Improvement Monitoring Tool (SIMS), National Supply Chain Assessments (NSCAs), and Logistics Management Information Systems. Although not a nationally representative sample, SIMS data are collected through visits to PEPFAR-supported sites that meet a “high-volume” threshold, frequently accounting for a substantial number of patients served through HIV programs. These assessments collect data on whether a patient has been turned away (within the previous 3 months) without receiving their prescribed medication, has received a substitute medication due to a stock-out, or has received <1 month of their prescribed medication (drug shortage). These graduated responses have been reported in numerous countries in Africa [11]. PEPFAR-supported countries that conducted >50 SIMS assessments between January 2016 and February 2017 reported significant variability in stock-outs and shortages. For example, in Nigeria, only 4% of sites were unable to fill the prescribed medication compared with 21% and 22% of sites in Mozambique and Zimbabwe, respectively. Rates of drug shortages (especially in combination with substitutions) were surprisingly high. [Figure 2]. The NSCAs provide a more in-depth, nationally representative assessment but are performed less frequently; however, the most recent NSCA in Mozambique found similar rates of drug stock-outs and shortages [12].
Figure 2.
US Presidents Emergency Plan for AIDS Relief site-level assessments of supply-chain performance. Proportion of sites with an antiretroviral stock-out, shortage, or drug substitution. Abbreviation: ARV, antiretroviral.
Suboptimal coordination between donors and host country government and limited human resources and infrastructure have been cited as causes for stock-outs [13, 14]. Anecdotally, some countries maintain a system whereby sites are rewarded for avoiding stock-outs. Although this provides an incentive for good logistics management and reporting, it can lead to perverse incentives to ration ARVs when stocks are low.
INTRODUCTION OF NEW ANTIRETROVIRAL REGIMENS AND LABORATORY SUPPLY CHAIN CHALLENGES
Standardized first-line regimens allow for simplification of treatment algorithms for providers and patients, as well as bulk purchasing to obtain the lowest price. Consistent with World Health Organization guidelines, most patients in PEPFAR-supported countries take NNRTI-based regimens. However, with more effective and less toxic ARVs on the horizon, there may soon be a large-scale transition in recommended regimens [15]. With increased VL testing and identification of ART failure, access to resistance testing may also be needed, particularly for patients requiring third-line therapy. Both factors will place strain on current systems, requiring substantial planning and coordination among facilities, national logistics systems, regulators, and manufacturers.
For years, global HIV programs focused on performing CD4+ testing and rationing treatment to the most ill to use limited budgets wisely. In scaling CD4+ testing in LMICs, laboratory system limitations, including weak sample referral networks, broken supply chains, inadequate human resources, limited infrastructure, and a lack of patient tracking systems, became apparent [16, 17]. Global guidance to treat all, irrespective of CD4+, count, and monitor therapy using VL [18], has switched the focus to ensuring accurate diagnosis and building VL capacity. However, issues that affect VL scale-up are even more daunting than those affecting CD4+ monitoring. These include inconsistent pricing, restrictive reagent cold-chain requirements, short reagent shelf lives, and lack of adequate service maintenance coverage. Ad hoc equipment placement has resulted in excess capacity in some laboratories and wasted resources [19–21]. Lack of accurate supply plans based on consumption data and minimal transparency on pricing and service offered by manufacturers and licensed distributors can result in stock-outs, expiry, and equipment breakdowns [22]. Placement of instruments must take into consideration the entire network capacity and sample referral systems. Reagent rental, leasing, or bundled pricing agreements rather than capital procurement can ensure service maintenance coverage for all instruments. These challenges will be intensified with the introduction of HIVDR testing, an inevitable next step in laboratory capacity building in LMICs to optimize ART options for patients on second- and third-line ART.
CONCLUSIONS
Breakdowns in complex supply chains are a contributing factor, particularly in LMICs, to the development of HIVDR. Better forecasting and use of programmatic and procurement data, particularly as new ART regimens are introduced, are integral to preventing stock-outs and shortages. Additionally, supply chain systems to support laboratory testing need to be strengthened to help scale-up VL monitoring and prepare for the eventual implementation of individual-level HIVDR testing.
Notes
Disclaimer. The content in this document are those of the authors and do not necessarily reflect the views of the United States Agency for International Development or the United States Government.
Funding. The United States Agency for International Development (USAID) Office of HIV/AIDS is supported by the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR). The content of this publication is solely the responsibility of the authors and does not represent the official views of USAID or PEPFAR.
Supplement sponsorship. This work is part of a supplement sponsored by the National Institute of Allergy and Infectious Disease, NIH, and the Centers for Disease Control and Prevention.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. HIV Drug Resistance Surveillance Guidance: 2015 update. Geneva, Switzerland: World Health Organization; 2015. http://www.who.int/hiv/pub/drugresistance/hiv-drug-resistance-2015- update/en/. [Google Scholar]
- 2. Parienti JJ, Das-Douglas M, Massari V et al. Not all missed doses are the same: sustained NNRTI treatment interruptions predict HIV rebound at low-to-moderate adherence levels. PLoS One 2008; 3:e2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rhee SY, Blanco JL, Jordan MR et al. Geographic and temporal trends in the molecular epidemiology and genetic mechanisms of transmitted HIV-1 drug resistance: an individual-patient- and sequence-level meta-analysis. PLoS Med 2015; 12:e1001810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization. Meeting Report on Assessment of World Health Organization HIV Drug Resistance Early Warning Indicators: Report of the Early Advisory Indicator Panel Meeting, 11–12 August 2011. Geneva, Switzerland: World Health Organization; 2012. http://www.who.int/hiv/pub/meetingreports/ewi_meeting_report/en/. [Google Scholar]
- 5. World Health Organization. Global Report on Early Warning Indicators of HIV Drug Resistance. Geneva, Switzerland: World Health Organization; 2016. http://www.who.int/hiv/pub/drugresistance/ewi-hivdr-2016/en/. [Google Scholar]
- 6. Shubber Z, Mills EJ, Nachega JB et al. Patient-reported barriers to adherence to antiretroviral therapy: a systematic review and meta-analysis. PLoS Med 2016; 13:e1002183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mentzer JT, Stank TP, Esper TL. Supply chaing management and its relationship to logistics, marketing, production, and operations management. J Bus Logist 2008; 29: 31–46. [Google Scholar]
- 8. Wagenaar BH, Gimbel S, Hoek R et al. Stock-outs of essential health products in Mozambique—longitudinal analyses from 2011 to 2013. Trop Med Int Health 2014; 19:791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. World Health Organization. Policy Brief: IATT Paediatric ARV Formulary and Limited-Use List: 2016 Update http://apps.who.int/medicinedocs/en/d/Js23120en/.
- 10. Paediatric ARV Procurement Working Group. Consolidated Paediatric ARV Planned for Procurement by the Consortium, 2016–2017. Geneva, Switzerland: World Health Organization; 2016. http://www.who.int/hiv/amds/amds2016-ppt-PAPWG.pdf?ua=1 [Google Scholar]
- 11. Messou E, Anglaret X, Duvignac J et al. Antiretroviral treatment changes in adults from Côte d’Ivoire: the roles of tuberculosis and pregnancy. AIDS 2010; 24:93–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bartram K, Chovitz B, Clancy E et al. Mozambique national supply chain assessment results: a review of the public health supply chain in Mozambique 2013. http://pdf.usaid.gov/pdf_docs/pa00m48w.pdf.
- 13. Schouten EJ, Jahn A, Ben-Smith A et al. Antiretroviral drug supply challenges in the era of scaling up ART in Malawi. J Int AIDS Soc 2011; 14(suppl 1):S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yu D, Souteyrand Y, Banda MA, Kaufman J, Perriëns JH. Investment in HIV/AIDS programs: does it help strengthen health systems in developing countries? Global Health 2008; 4:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ripin D, Prabhu VR. A cost-savings analysis of a candidate universal antiretroviral regimen. Curr Opin HIV AIDS 2017; 12:403–7. [DOI] [PubMed] [Google Scholar]
- 16. Birx D, de Souza M, Nkengasong JN. Laboratory challenges in the scaling up of HIV, TB, and malaria programs: the interaction of health and laboratory systems, clinical research, and service delivery. Am J Clin Pathol 2009; 131:849–51. [DOI] [PubMed] [Google Scholar]
- 17. Thairu L, Katzenstein D, Israelski D. Operational challenges in delivering CD4 diagnostics in sub-Saharan Africa. AIDS Care 2011; 23:814–21. [DOI] [PubMed] [Google Scholar]
- 18. World Health Organization. Guideline on When to Start Antiretroviral Therapy and on Pre-exposure Prophylaxis for HIV. Geneva, Switerland: World Health Organizaiton; 2015. http://www.who.int/hiv/pub/guidelines/earlyrelease-arv/en/. [PubMed] [Google Scholar]
- 19. Habiyambere V, Ford N, Low-Beer D et al. Availability and use of HIV monitoring and early infant diagnosis technologies in WHO member states in 2011–2013: analysis of annual surveys at the facility level. PLoS Med 2016; 13:e1002088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kilmarx PH, Simbi R. Progress and challenges in scaling up laboratory monitoring of HIV treatment. PLoS Med 2016; 13:e1002089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Williams J, Umaru F, Edgil D, Kuritsky J. Progress in harmonizing tiered HIV laboratory systems: challenges and opportunities in 8 African countries. Glob Health Sci Pract 2016; 4:467–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Medecins Sans Frontieres. Making viral load routine: part II. 2016. https://www.msfaccess.org/content/report-making-viral-load-routine. [Google Scholar]