Abstract
Dengue virus, primarily transmitted by the Aedes aegypti (L.) mosquito, has rapidly expanded in geographic extent over the past several decades. In some areas, however, dengue fever has not emerged despite established Ae. aegypti populations. The reasons for this are unclear and have sometimes been attributed to socio-economic differences. In 2013 we compared Ae. aegypti adult density and population age structure between two cities in Sonora, Mexico: Hermosillo, which has regular seasonal dengue virus transmission, and Nogales, which has minimal transmission. Larval and pupal abundance was greater in Nogales, and adult density was only higher in Hermosillo during September. Population age structure, however, was consistently older in Hermosillo. This difference in longevity may have been one factor that limited dengue virus transmission in Nogales in 2013, as a smaller proportion of Ae. aegypti females survived past the extrinsic incubation period.
Keywords: Aedes aegypti, dengue, longevity, Mexico
Dengue fever (DF), transmitted principally by Aedes aegypti (L.) (Diptera: Culicidae) mosquitoes, is the most important arboviral disease of the 21st century in terms of human health impacts and economic costs (Suaya et al. 2009, Gubler 2012). An estimated 390 million infections occur each year, with almost 100 million symptomatic cases (Bhatt et al. 2013). The incidence of DF has increased by a factor of 30 since the 1950s ([WHO] World Health Organization 2012) and continues to spread to new areas, including parts of the United States and Europe ([MMWR] Morbidity and Mortality Weekly Report 2010, Tomasello and Schlagenhauf 2013, Wilson and Chen 2015). Yet, some regions remain DF-free despite established Ae. aegypti populations. A better understanding of the complex interactions among environment, vector, humans, and virus is needed to predict the future spread of DF.
Climate—defined here as the long-term average of meteorological conditions—largely limits the distribution of Ae. aegypti on a global scale to the tropics and subtropics (Christophers 1960), but the mosquito’s close association with humans has allowed it to spread and establish far beyond the African region of origin (Tabachnick and Powell 1979). Highly anthropophilic, Ae. aegypti readily enters houses, feeds primarily on human blood, and oviposits in human-made water containers (Christophers 1960, Service 1992). Although Ae. aegypti prefers warm, humid climates, it is active even in arid environments and during extended dry periods when human factors, such as crowded housing and stored water, provide easily accessible bloodmeals and oviposition sites (Focks et al. 1993).
Presence of the vector species is a requirement for dengue virus (DENV) transmission, but vector density alone is not necessarily an accurate predictor of DENV transmission risk in areas with an established vector population (Louis et al. 2014). Longevity of the adult female mosquito plays a major role in determining vectorial capacity. To transmit DENV, the Ae. aegypti female must survive the period between ingesting the infective bloodmeal and viral replication in the salivary glands (i.e., the extrinsic incubation period, or EIP; Focks et al. 1993). The length of the EIP ranges from 7 d in warmer temperatures to 12 d in cooler temperatures (Macdonald 1957, Watts et al. 1987). As Ae. aegypti females do not blood-feed for about 2 d after adult emergence, those surviving beyond 14 d (2 d postemergence plus 12-d EIP) have a higher potential to be DENV vectors than shorter-lived mosquitoes (Kuno 1995, Salazar et al. 2007). Recently, molecular aging of wild-type Ae. aegypti indicated that age structure is associated with the onset and offset of the DENV transmission season in Vietnam (Hugo et al. 2014).
Our study compares the age structure and adult density of Ae. aegypti populations in two cities in northern Sonora, Mexico, during the rainy season (July to September) in 2013 (Fig. 1). The more southern city, Hermosillo (pop. 715,061), has experienced regular seasonal transmission of DENV since 1996 (Ravel et al. 2001). The northern border city of Nogales, Sonora, had no reported locally acquired cases of DF before 2014 (pop. 212,533; [INEGI] Instituto Nacional de Estadistica y Geografía 2015). This is the first study to use molecular methods applied to an extensive sample of wild-caught Ae. aegypti to consider age structure as a factor driving the transmission range of dengue viruses.
Fig. 1.
Study cities in Sonora, Mexico.
Materials and Methods
Study Area
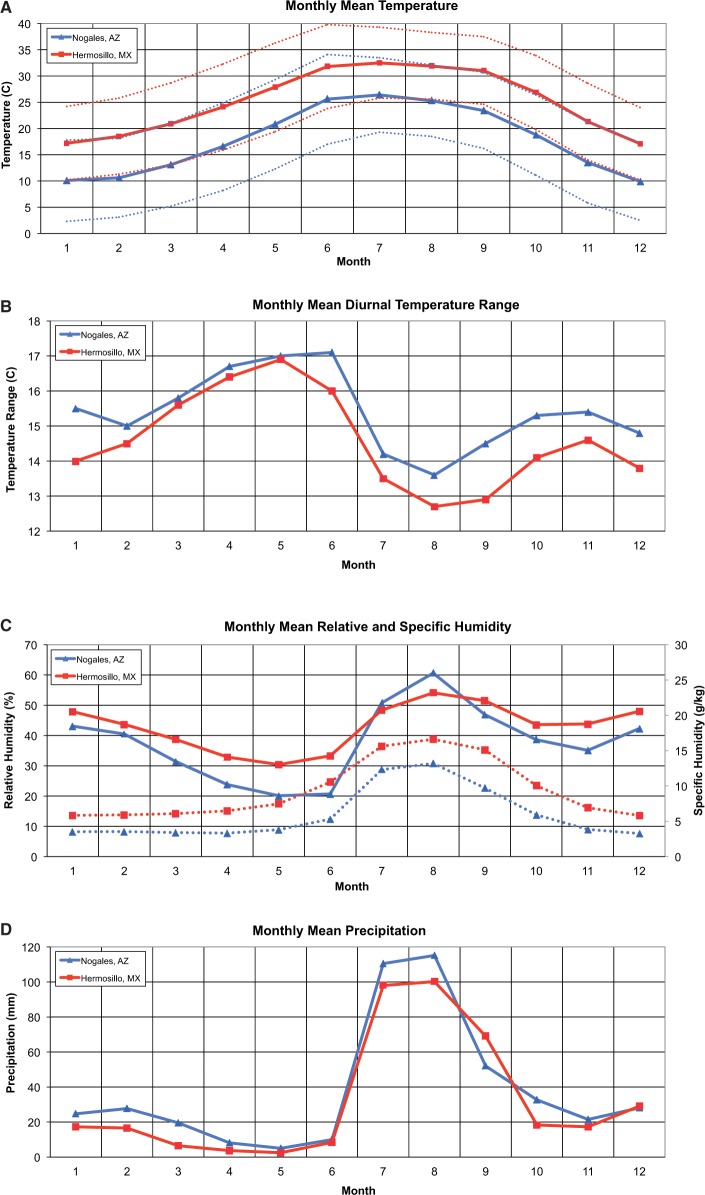
The Sonoran region is characterized by high summer temperatures and low annual rainfall. Nogales, elevation 1,199 m, receives an average of 455 mm of rain annually, with >60% occurring during the summer rainy season from July through September. In August, the average high temperature is 32.1˚C. Hermosillo, elevation 210 m, averages 387 mm of rainfall, with nearly 70% of it falling during the summer rainy season. Hermosillo is warmer than Nogales, with an average August high temperature of 38.3˚C (Servicio Meteorologico Nacional [SMN] 2015). Climatic differences between the two cities are summarized in Fig. 2. Temperature and precipitation data were obtained from the Mexican weather service and are averaged for 1981–2010 (SMN 2015). The means for relative and specific humidity were calculated from daily data obtained from the U.S. National Climatic Data Center, which spanned 1950–1954 and 1976–2009 for Hermosillo and 2002–2008 for Nogales ([NCDC] National Climatic Data Center 2009).
Fig. 2.
Comparison of monthly and annual mean meteorological variables between Nogales (blue) and Hermosillo (red). Panels: Monthly mean (A) temperature (°C; solid lines) and minimum and maximum temperature (°C; dotted lines); (B) diurnal temperature range (°C); (C) relative humidity (%; solid lines) and specific humidity (grams of water vapor per kilogram of air; dotted lines); (D) precipitation (mm).
Socio-Economic and Dengue Profiles
Socio-economic factors related to DF risk were extracted from the INEGI public access database (Table 1). These factors include human population density, age of head of household, proportion of unoccupied houses, access to health care and percentage of the population with access to piped water (Stewart-Ibarra et al. 2014), education (Siqueira et al. 2004), migration or stability of the human population (da Silva-Nunes et al. 2008), and persons per house (Ramos et al. 2008, Braga et al. 2010, Stewart-Ibarra et al. 2014).
Table 1.
2010 Socio-demographic characteristics of Nogales and Hermosillo, Sonora, Mexico
| Socio-demographic characteristics | Nogales | Hermosillo |
|---|---|---|
| Total population | 212,533 | 715,061 |
| Population density (persons/km2) | 5,180 | 5,280 |
| Median age (years) | 24 | 26 |
| Population with no healthcare service (%) | 25.8 | 21.6 |
| Population ≥15 years with no basic education (%) | 25.4 | 22.0 |
| Migration—population ≥5 years living in other state in 2005 (%) | 5.0 | 2.8 |
| Occupied particular houses | 54,742 | 194,096 |
| Persons per house | 3.8 | 3.6 |
| No piped water (%) | 20.1 | 2.6 |
| No indoor toilet (%) | 1.0 | 0.7 |
| No drainage (%) | 2.5 | 1.5 |
| Dirt floor (%) | 3.7 | 3.3 |
| No electricity (%) | 1.2 | 0.7 |
Historical case data in Hermosillo and Nogales, SN, demonstrate regular but low DENV transmission in Hermosillo with periodic outbreaks (Table 2). The period of surveillance data available suggests extremely low seasonal prevalence of DF reported in Nogales. Before 2014, all reported cases of DF in Nogales, SN, were traced to individuals with a travel history to endemic areas.
Table 2.
Incidence of DF cases in Hermosillo and Nogales, per 100,000 person years
| Year | Hermosillo | Nogales |
|---|---|---|
| 2006 | 22.6 | 1.4 |
| 2007 | 15.4 | 0.5 |
| 2008 | 92.0 | No cases reported |
| 2009 | 22.2 | 1.9 |
| 2010 | 504.0 | 1.9 |
| 2011 | 26.3 | 1.0 |
| 2012 | 12.3 | No cases reported |
| 2013 | 33.1 | 1.8 |
Incidence is calculated based on reported DF cases (probable and confirmed cases are both included). Data were obtained from the Sonoran Health Department. Calculations were made using the 2010 census for total population in Hermosillo (715,061) and Nogales (212,533). During 2006–2013, all reported cases in Nogales were traced to travel to DF-endemic areas.
Mosquito Sampling
Adults
In each city, mosquitoes were collected from outdoor sites associated with a residence. Indoor placement was not feasible, although traps were placed next to houses, often in doorways or under porches, to maximize the potential for attracting mosquitoes from inside the homes. To ensure independence of sampling and geographic dispersion, trapping sites were located at least 500 m apart. Sixteen sites were selected in Hermosillo and 15 sites in Nogales. Trapping sites were identified for each city with random points at least 1 km away from each other generated using ArcGIS. A 500-m buffer was designated around each of the points and snowball sampling through study affiliates was conducted to identify a secure residence within the 500-m buffer to place a trap.
Mosquito adults were collected at each site once per month during the rainy season (July through September). Each month, mosquitoes were collected during a 4-d period using a BG Sentinel trap (Bioagents AG, Germany) baited with octenol and a synthetic lure designed to imitate human skin odors (Williams et al. 2006). Mosquitoes were removed alive at least once daily and brought to the laboratory where they were identified to species, sexed, and frozen at −80˚C until dissected. Adult Ae. aegypti females were counted for each trap to determine vector density.
Immatures
Houses surrounding trap locations were surveyed for mosquito larval habitat to determine presence of container-breeding mosquito species, relative abundance of Ae. aegypti larvae and pupae in proximity to trapping locations, and the types of containers providing Ae. aegypti immature habitat. At each adult trapping site, four nearby houses were selected for larval surveillance within 20–100 m from the trapping site, one in each direction (north, south, east, and west). Larval collections were conducted twice in the same houses, once in late July through early August (early collection) and once in late August through early September (late collection). Team members searched the entire outdoor premise for water-holding containers, and noted which containers were positive for water and for immature mosquitoes. When mosquito-positive containers were found, all pupae and a sample of larvae were collected, preserved in alcohol, and identified to species at the University of Arizona.
Mosquito Age and Parity Assessments
Field-caught female Ae. aegypti adults were assessed for parity and age using ovary tracheation and transcript expression of an age-sensitive gene (Scp1), respectively. Ovary tracheation is used to determine if an adult female mosquito has taken at least one bloodmeal and developed a batch of eggs. Mosquitoes that have never taken a bloodmeal (nulliparous) have ovaries with tightly coiled trachea. In parous mosquitoes, the ovary tracheae are permanently uncoiled. Ovary tracheation allows rapid, accurate differentiation between younger and older mosquitoes, as nulliparous females have usually lived <5 d since emergence while parous females are usually >5 d old (Joy et al. 2012). Ovaries from all collected females were dissected in 1× phosphate-buffered saline, dried, and examined under a compound microscope at 400× magnification to determine parity.
To refine our assessment of mosquito age, we analyzed relative expression levels of the gene Sarcoplasmic calcium-binding protein 1 (Scp1). Scp1 is highly expressed in Ae. aegypti females shortly after adult emergence, and expression declines in a predictable pattern as the mosquito ages (Joy et al. 2012). In earlier work using lab and semifield-reared mosquitoes of known ages, we accurately classified mosquitoes into one of three age classes: <5 d old (non-DENV vectors), 5–14 d old (unlikely DENV vectors), and >14 d old (possible DENV vectors) (Joy et al. 2012). Quantification of Scp1 transcript expression was restricted to parous mosquitoes, given that nulliparous mosquitoes were consistently age graded as non-DENV vectors (i.e., <5 d; unpublished data). Up to 10 randomly selected parous females were age-graded for each trapping event as previously described (Joy et al. 2012). Following dissection, total RNA was isolated (RNeasy kit, Qiagen, Valencia, CA) and cDNA (High capacity cDNA kit, Life Technologies, Grand Island, NY) generated from the head and thorax of each Ae. aegypti female. Quantitative real-time PCR (qPCR) was used to quantify expression of Scp1 transcript and the RPS17 housekeeping gene. The ratio of Scp1 to RPS17 expression was used to classify the individual mosquito into one of the three age classes.
Statistical Methods
Differences in Ae. aegypti Density
To determine whether there was a difference in Ae. aegypti density between Nogales and Hermosillo, two-sample t-tests on log-transformed counts of adult female Ae. aegypti were conducted for each month. The log transformation improved assumptions of normality (Shapiro–Wilk W tests, P values >0.15) and homogeneity of variance (Bartlett tests, P values >0.55). Differences in house index (percentage of houses with at least one container of Ae. aegypti larvae or pupae) between the two cities were determined using a Fisher’s exact test.
Differences in Age Structure
Logistic regression for binomial counts was used to determine differences in the proportion of parous adult Ae. aegypti females and the proportion surviving to Age Class 3 (>14 d). In both cases, explanatory variables in the regression models were city, month, and the interaction between these factors. To evaluate relative transmission risk, we used adult female mosquito counts and age structure information to calculate the relative number of possible vectors at each trapping site for each month (i.e., relative number of vectors = density of females × proportion of females >14 d). A negative binomial regression model was used to assess differences in relative number of possible vectors (Ae. aegypti >14 d) between cities, after accounting for month. Explanatory variables included in this model were city, month, and the interaction between these factors. Following this analysis, a bivariate negative binomial regression model was used to compare relative transmission risk among months in each city by modeling the number of Ae. aegypti females in Age Class 3 per trap by city. Negative binomial regression models are useful for modeling count data that are overdispersed (Lawless 1987).
Results
Adult Ae. aegypti Density
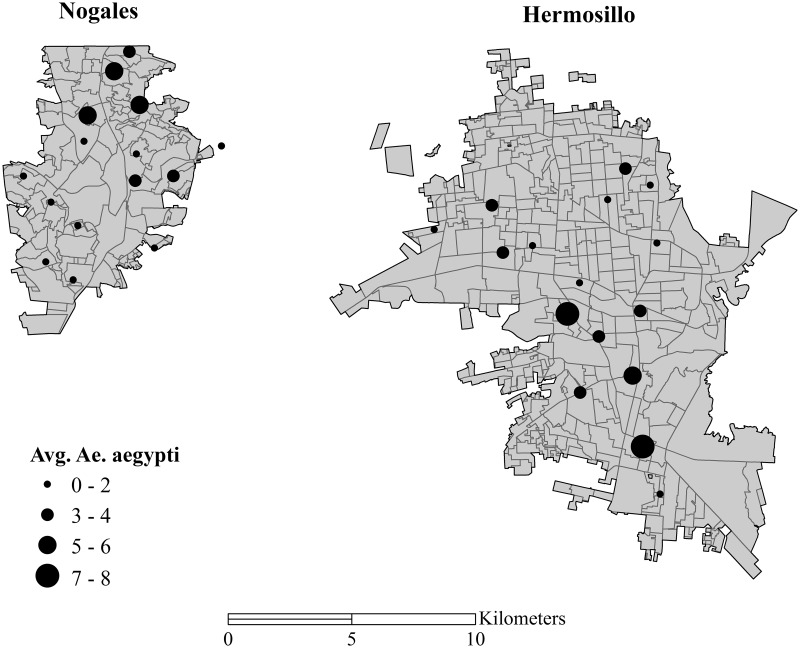
Adult Ae. aegypti density varied between months in both cities, but density did not differ significantly between cities (Fig. 3). A total of 826 Ae. aegypti females were trapped during the study period from both cities. In Nogales, a total of 371 Ae. aegypti females were trapped; average count of female Ae. aegypti per trap night was 2.1 (total females/trap/night). Over the course of the season, average female count per trap per night was 1.6 during July, 2.4 during August, and 2.2 during September. In Hermosillo, a total of 455 female Ae. aegypti were trapped for an average of 2.4 Ae. aegypti females per trap per night. Over the course of the season, average female count per trap per night was 1.2 during July, 1.4 during August, and 4.6 during September. Adult female density did not differ significantly between Hermosillo and Nogales in July (t = 1.20, P = 0.24) and August (t = 0.91, P = 0.37), but there was a trend for higher density in Hermosillo than Nogales during September (t = 1.90, P = 0.07).
Fig. 3.
Distribution of mosquito trapping sites and mean number of Ae. aegypti females collected per trap night from July through September in each city.
Immature Ae. aegypti Indices
In Hermosillo, surveys of the outside premises of 64 houses for Ae. aegypti larval habitat found a total of 19 containers with mosquito larvae or pupae (any species), seven in the early collection and 12 in the late collection. The majority of larvae and pupae were Ae. aegypti (18 out of 19 inhabited containers). The house index (percentage of houses with at least one container with larvae or pupae) in Hermosillo for Ae. aegypti was 9% (n = 6) in both August and September. Culex quinquefasciatus larvae were found in two containers. In Nogales, surveys of the outside premises of 60 houses identified a total of 158 mosquito-positive containers, 92 during August and 66 during September. Among the mosquito-positive containers, 85 contained Ae. aegypti, 49 contained Cx. quinquefasciatus, and 71 contained a third species, Aedes epactius. Almost half the mosquito-positive containers included more than one species (40% contained two species, 5% contained all three species.) The house index for Ae. aegypti in Nogales was 43% (n = 26) during August and 40% (n = 24) during September. The house index was significantly different between cities for both collection periods (P < 0.0001).
Parity and Age Structure
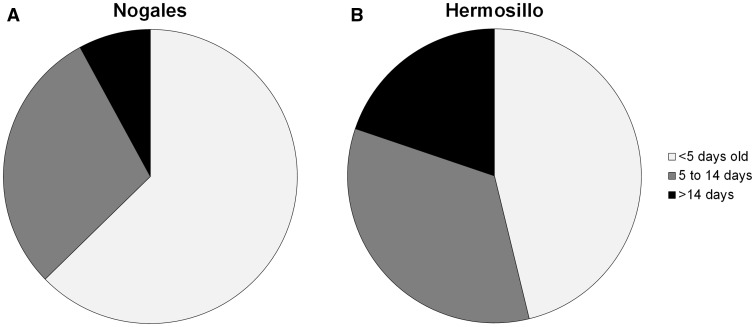
In both cities, the majority of Ae. aegypti females collected during each month were parous (Hermosillo: July = 62%, August = 73%, September = 68%; Nogales: July = 68%, August = 71%, September = 57%). There were no significant differences for parity between cities or among months (Table 3). In contrast, the age structure as measured by Scp1 gene expression showed a distinctly older mosquito population in Hermosillo than in Nogales (Fig. 4). Overall, a significantly higher percentage of Ae. aegypti females were in Age Class 3 (>14 d old—possible vectors) in Hermosillo relative to Nogales (Table 4 and Fig. 5). The relative proportion of Ae. aegypti in the oldest age class did not differ by month and the interaction between month and city was not significant (Table 3). The relative risk of encountering potential vector Ae. aegypti females (calculated as the number of females collected multiplied by the proportion in Age Class 3 per site) was significantly higher in Hermosillo as compared to Nogales in September but not during July or August (Table 4).
Table 3.
Differences in parity and proportion of mosquitoes in Age Class 3 (>14 d old) between cities
| Parity | df | Likelihood-ratio chi-square | P-value |
|---|---|---|---|
| City | 1 | 0.39 | 0.53 |
| Month | 2 | 3.88 | 0.14 |
| City*Month | 2 | 2.44 | 0.30 |
| Age class | |||
| City | 1 | 11.96 | 0.0005 |
| Month | 2 | 1.83 | 0.40 |
| City*Month | 2 | 0.17 | 0.92 |
Fig. 4.
Proportion of total parous Ae. aegypti female adults in each of the three age classes for Nogales (A) and Hermosillo (B).
Table 4.
Relative risk of potential disease vectors (number of female Ae. aegypti age > 14 d) in Hermosillo relative to Nogales by month
| Adult Ae. aegypti > 15 d | |||
|---|---|---|---|
| Month of comparison | Relative risk | Relative risk (95% CI) | P-value |
| July | 1 | 2.25 (0.53, 9.43) | 0.27 |
| Aug. | 1 | 2.67 (0.62, 11.30) | 0.18 |
| Sept. | 1 | 5.05 (1.59, 16.03) | 0.006 |
Negative binomial regression was used to compare calculated numbers of female Ae. aegypti that were > 14 d between Nogales and Hermosillo in July, August, and September. There were no significant differences in the relative trap counts of Ae. aegypti females that were >14 d old in July or August, but counts were significantly higher in September in Hermosillo as compared to Nogales.
Fig. 5.
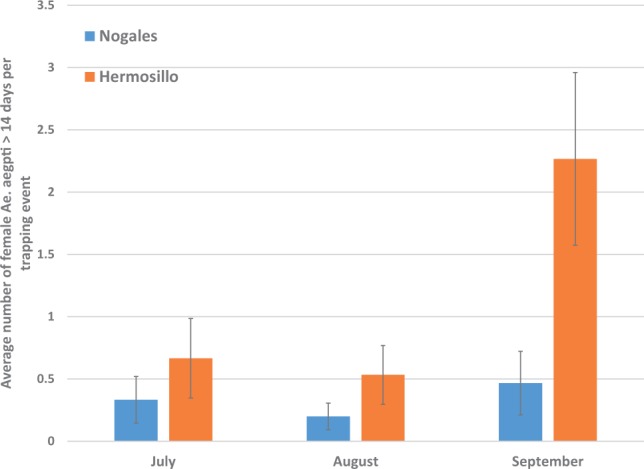
Relative risk of potential disease vectors (number of female Ae. aegypti age >14 d) in Hermosillo and Nogales.
Discussion
Aedes aegypti populations exhibited a significant difference in age structure between the two cities. Both the proportion and relative number of Ae. aegypti females over 14 d old was higher in Hermosillo than in Nogales, SN. This is the first study to demonstrate a difference in age structure between Ae. aegypti populations in cities with disparate levels of DENV transmission. There was also a trend of increasing adult Ae. aegypti density in Hermosillo relative to Nogales during September, which, when coupled with greater longevity, may explain not only the difference in DENV between the two cities, but the peak of transmission in September in the state of Sonora (El Sistema Nacional de Vigilancia Epidemiológica 2013).
While previous studies indicate the importance of infrastructure in driving differences in DENV transmission, infrastructure appears unlikely to explain the disparate DENV transmission in Nogales and Hermosillo. Nogales has more limited access to piped water, poor storm water drainage, and lower levels of education than Hermosillo—factors that have been related to increased DF risk in other locations (Siqueira et al. 2004, Siqueira et al. 2008, Schmidt et al. 2011). While Hermosillo is a larger urban area, Nogales has a population size and density that should be sufficient to sustain DENV transmission (Schmidt et al. 2011). Indicators that are related to poverty, such as households with a dirt floor, no electricity, and no indoor toilet were marginally higher in Nogales than in Hermosillo. There was a slightly higher proportion of the population with access to healthcare in Hermosillo than in Nogales, which could lead to differential reporting. However, an analysis of surveillance data in the state of Sonora actually found an increased number of reported cases of DF in municipalities with lesser access to health care (Reyes-Castro 2015).
Habitat favorable for larvae and pupae was more abundant in Nogales, possibly explained by the poorer infrastructure in Nogales noted above. Though the number of households inspected for larvae and pupae was relatively small, significant differences were noted between the two cities. Almost half the houses surveyed in Nogales were positive for Ae. aegypti larvae each month, whereas <10% of houses surveyed in Hermosillo were positive. The greater relative abundance of mosquito-positive containers in Nogales indicates that the absence of DENV transmission there cannot be due to a lack of immature habitat. By contrast, adult vector density was more similar in the two cities during most of the rainy season. Average trap counts of adult Ae. aegypti females were higher in Nogales than Hermosillo during July and August, but this difference was not significant. During September, average trap counts in Hermosillo more than tripled while trap counts did not increase in Nogales. Because adult densities were relatively comparable between the two cities but larvae and pupae incidence was greater in Nogales, there may be important cryptic or indoor habitats in Hermosillo that were not detected in this study, or survival of larvae to adulthood may be greater in Hermosillo than Nogales.
The assessment of age structure using relative expression of the Scp1 gene clearly revealed a larger proportion of older, possible vector mosquitoes in Hermosillo than Nogales. This difference remained consistent from July through September. Adult mosquito counts rose in Hermosillo relative to Nogales during September, resulting in an increase in the relative risk of encountering an adult female older than 14 d old in Hermosillo during this month. Interestingly, this rise in the relative risk of encountering vectors coincided with the onset of the DF transmission season in Hermosillo in 2013 (El Sistema Nacional de Vigilancia Epidemiológica 2013).
If the age structure patterns observed in 2013 in Nogales and Hermosillo prove constant across years, this raises the question of causation. Why are the mosquitoes living longer in Hermosillo? One potential factor is climate. Mean Nogales nighttime minimum temperatures are below 20˚C during July through September, well below the optimal temperature for adult survival, while in Hermosillo minimum temperatures are about 25˚C (Christophers 1960, Lansdowne and Hacker 1975). Additionally, the mean July–September diurnal temperature range (DTR) in Nogales is 14.1˚C, about 1˚C larger than in Hermosillo. Studies of Ae. aegypti under fluctuating temperatures have observed negative effects of large DTR on life-history traits and vector competence (Lambrechts et al. 2011, Mohammed and Chadee 2011, Carrington et al. 2013). The DTR in both Nogales and Hermosillo drops during July and August, and during September the DTR stays lower in Hermosillo but begins to rise in Nogales (Fig. 2B). This divergence in climate between the two cities coincides with the onset of dengue transmission in Hermosillo. Humidity may also play a role. There is evidence that higher relative humidity promotes better survival of Ae. aegypti (Reiskind and Lounibos 2009). While Nogales experiences higher rainfall averages than Hermosillo, it has generally lower relative and specific humidity. Climate could also play an important role in length of the EIP, which together with vector survival can strongly influence vectorial capacity. A warmer climate in Hermosillo could shorten the EIP, while the increased relative and/or specific humidity could extend vector life span enough to facilitate transmission.
We found intriguing and marked differences in population age structure between Ae. aegypti populations in Nogales and Hermosillo. Additional years of sampling will be needed to determine the consistency of these patterns. In this arid region at the fringe of DENV transmission, established Ae. aegypti populations do not necessarily confer high infection risk. Factors that provide a suitable habitat for population establishment are not necessarily favorable for survival of Ae. aegypti into adulthood past the EIP required for transmission to occur. This is the first evidence from the field showing that female Ae. aegypti survival differs between cities with and without local DENV transmission, indicating that the role of vector survival in the expansion of DENV should be further explored.
Acknowledgments
Funding support for this work was provided by grant R01AI091843 from NIH-NIAID. We would like to thank Dr. Rodrigo Medellin Legorreta of the Institute de Ecologia, UNAM, for his valuable assistance with collecting permits. We also thank the following individuals for their work on this project: Rosa Elena Cuevas Ruiz, Cindy Ochoa Cuevas, Silvia Ojeda Torres, Emmanuel Bernal, and Daniel Williamson. This study is dedicated to the memory of Lupita Inzunza, a gifted scientist and colleague.
References Cited
- Bhatt S., Gething P. W., Brady O. J., Messina J. P., Farlow A. W., Moyes C. L., Drake J. M., Brownstein J. S., Hoen A. G., Sankoh O., et al. 2013.The global distribution and burden of dengue. Nature 496: 504–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga C., Luna C. F., Martelli C. M., de Souza W. V., Cordeiro M. T., Alexander N., de Albuquerque Mde F., Junior J. C., Marques E. T. 2010.Seroprevalence and risk factors for dengue infection in socio-economically distinct areas of Recife, Brazil. Acta Trop. 113: 234–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington L. B., Seifert S. N., Armijos M. V., Lambrechts L., Scott T. W. 2013.Reduction of Aedes aegypti vector competence for dengue virus under large temperature fluctuations. Am. J. Trop. Med. Hyg. 88: 689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christophers S. R. 1960.. Aëdes aegypti (L.) the yellow fever mosquito; its life history, bionomics, and structure, University Press, Cambridge England. [Google Scholar]
- da Silva-Nunes M., de Souza V. A., Pannuti C. S., Speranca M. A., Terzian A. C., Nogueira M. L., Yamamura A. M., Freire M. S., da Silva N. S., Malafronte R. S., et al. 2008.Risk factors for dengue virus infection in rural Amazonia: Population-based cross-sectional surveys. Am. J. Trop. Med. Hyg. 79: 485–494. [PubMed] [Google Scholar]
- El Sistema Nacional de Vigilancia Epidemiológica. 2013.Casos nuevos de Fiebre por dengue (A90) por mes de ocurrencia Estados Unidos Mexicanos 2013. D.G.d. Epidemiologia. Secretaria de Salud, Mexico City, MX. [Google Scholar]
- Focks D. A., Haile D. G., Daniels E., Mount G. A. 1993.Dynamic life table model for Aedes aegypti (Diptera: Culicidae): Simulation results and validation. J. Med. Entomol. 30: 1018–1028. [DOI] [PubMed] [Google Scholar]
- Gubler D. J. 2012.. The economic burden of dengue. Am. J. Trop. Med. Hyg. 86: 743–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugo L. E., Jeffery J. A., Trewin B. J., Wockner L. F., Nguyen T. Y., Nguyen H. L., Nghia Le T., Hine E., Ryan P. A., Kay B. H. 2014.Adult survivorship of the dengue mosquito Aedes aegypti varies seasonally in central Vietnam. PLoS Negl. Trop. Dis. 8: e2669.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (INEGI) Instituto Nacional de Estadistica y Geografía. 2015.Informacion Nacional, por entidad federativa y municipiones - Sonora. G. e. I. INEGI Instituto Nacional de Estadística, Mexico City. [Google Scholar]
- Joy T. K., Jeffrey Gutierrez E. H., Ernst K., Walker K. R., Carriere Y., Torabi M., Riehle M. A. 2012.Aging field collected Aedes aegypti to determine their capacity for dengue transmission in the southwestern United States. PLoS ONE 7: e46946.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno G. 1995.. Review of the factors modulating dengue transmission. Epidemiol. Rev. 17: 321–335. [DOI] [PubMed] [Google Scholar]
- Lambrechts L., Paaijmans K. P., Fansiri T., Carrington L. B., Kramer L. D., Thomas M. B., Scott T. W. 2011.Impact of daily temperature fluctuations on dengue virus transmission by Aedes aegypti. Proc. Natl. Acad. Sci. USA. 108: 7460–7465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansdowne C., Hacker C. S. 1975.The effect of fluctuating temperature and humidity on the adult life table characteristics of five strains of Aedes aegypti. J. Med. Entomol. 11: 723–733. [DOI] [PubMed] [Google Scholar]
- Lawless J. F. 1987.. Negative binomial and mixed Poisson regression. Can. J. Stat. 15: 209–225. [Google Scholar]
- Louis V. R., Phalkey R., Horstick O., Ratanawong P., Wilder-Smith A., Tozan Y., Dambach P. 2014.Modeling tools for dengue risk mapping - a systematic review. Intl. J. Health Geogr. 13: 50.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald G. 1957.. The epidemiology and control of malaria, Oxford University Press, London. [Google Scholar]
- (MMWR) Morbidity and Mortality Weekly Report. 2010.Locally acquired dengue–Key West, Florida, 2009-2010. MMWR Morb. Mortal. Wkly. Rep. 59: 577–581. [PubMed] [Google Scholar]
- Mohammed A., Chadee D. D. 2011.Effects of different temperature regimens on the development of Aedes aegypti (L.) (Diptera: Culicidae) mosquitoes. Acta Trop. 119: 38–43. [DOI] [PubMed] [Google Scholar]
- (NCDC) National Climatic Data Center. 2009. Global Surface Summary of the Day. National Climatic Data Center, NESDIS, NOAA, U.S. Department of Commerce. (https://data.noaa.gov/dataset/global-surface-summary-of-the-day-gsod) (accessed 9 april 2009).
- Ramos M. M., Mohammed H., Zielinski-Gutierrez E., Hayden M. H., Lopez J. L., Fournier M., Trujillo A. R., Burton R., Brunkard J. M., Anaya-Lopez L., et al. 2008.Epidemic dengue and dengue hemorrhagic fever at the Texas-Mexico border: Results of a household-based seroepidemiologic survey, December 2005. Am. J. Trop. Med. Hyg. 78: 364–369. [PubMed] [Google Scholar]
- Ravel S., Monteny N., Velasco Olmos D., Escalante Verdugo J., Cuny G. 2001.A preliminary study of the population genetics of Aedes aegypti (Diptera: Culicidae) from Mexico using microsatellite and AFLP markers. Acta Trop. 78: 241–250. [DOI] [PubMed] [Google Scholar]
- Reiskind M. H., Lounibos L. P. 2009.Effects of intraspecific larval competition on adult longevity in the mosquitoes Aedes aegypti and Aedes albopictus. Med. Vet. Entomol. 23: 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-Castro P. 2015.. Dynamics of dengue transmission in the arid region of Sonora. PhD, University of Arizona, Mexico. [Google Scholar]
- Salazar M. I., Richardson J. H., Sanchez-Vargas I., Olson K. E., Beaty B. J. 2007.Dengue virus type 2: Replication and tropisms in orally infected Aedes aegypti mosquitoes. BMC Microbiol. 7: 9.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt W. P., Suzuki M., Thiem V. D., White R. G., Tsuzuki A., Yoshida L. M., Yanai H., Haque U., Tho Le H., Anh D. D., et al. 2011.Population density, water supply, and the risk of dengue fever in Vietnam: Cohort study and spatial analysis. PLoS Med. 8: e1001082.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Service M. W. 1992.. Importance of ecology in Aedes aegypti control. Southeast Asian J. Trop. Med. Public Health 23: 681–690. [PubMed] [Google Scholar]
- Siqueira J. B., Maciel I. J., Barcellos C., Souza W. V., Carvalho M. S., Nascimento N. E., Oliveira R. M., Morais-Neto O., Martelli C. M. 2008.Spatial point analysis based on dengue surveys at the household level in central Brazil. BMC Public Health 8: 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siqueira J. B., Martelli C. M., Maciel I. J., Oliveira R. M., Ribeiro M. G., Amorim F. P., Moreira B. C., Cardoso D. D., Souza W. V., Andrade A. L. 2004.Household survey of dengue infection in central Brazil: Spatial point pattern analysis and risk factors assessment. Am. J. Trop. Med. Hyg. 71: 646–651. [PubMed] [Google Scholar]
- (SMN) Servicio Meteorologico Nacional. 2015. Normales Climatologicas. Servicio Meteorologico Nacional, Mexico City, Mexico. (http://smn.cna.gob.mx/es/climatologia/informacion-climatologica) (accessed 13 April 2015).
- Stewart-Ibarra A. M., Munoz A. G., Ryan S. J., Ayala E., Borbor-Cordova M. J., Finkelstein J. L., Mejia R., Ordonez T., Recalde-Coronel G., Rivero K. 2014.Spatiotemporal clustering, climate periodicity, and social-ecological risk factors for dengue during an outbreak in Machala, Ecuador, in 2010. BMC Infect. Dis. 14: 610.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suaya J. A., Shepard D. S., Siqueira J. B., Martelli C. T., Lum L. C., Tan L. H., Kongsin S., Jiamton S., Garrido F., Montoya R., et al. 2009.Cost of dengue cases in eight countries in the Americas and Asia: A prospective study. Am. J. Trop. Med. Hyg. 80: 846–855. [PubMed] [Google Scholar]
- Tabachnick W. J., Powell J. R. 1979.A world-wide survey of genetic variation in the yellow fever mosquito, Aedes aegypti. Genet. Res. 34: 215–229. [DOI] [PubMed] [Google Scholar]
- Tomasello D., Schlagenhauf P. 2013.Chikungunya and dengue autochthonous cases in Europe, 2007-2012. Travel Med. Infect. Dis. 11: 274–284. [DOI] [PubMed] [Google Scholar]
- Watts D. M., Burke D. S., Harrison B. A., Whitmire R. E., Nisalak A. 1987.Effect of temperature on the vector efficiency of Aedes aegypti for dengue 2 virus. Am. J. Trop. Med. Hyg. 36: 143–152. [DOI] [PubMed] [Google Scholar]
- (WHO) World Health Organization. 2012.Global strategy for dengue prevention and control 2012-2020, pp. 1–5. World Health Organization, Geneva, Switzerland. [Google Scholar]
- Williams C. R., Long S. A., Russell R. C., Ritchie S. A. 2006.Field efficacy of the BG-Sentinel compared with CDC backpack aspirators and CO2-baited EVS traps for collection of adult Aedes aegypti in Cairns, Queensland, Australia. J. Am. Mosq. Control Assoc. 22: 296–300. [DOI] [PubMed] [Google Scholar]
- Wilson M. E., Chen L. H. 2015.Dengue: Update on epidemiology. Curr. Infect. Dis. Rep. 17: 457.. [DOI] [PubMed] [Google Scholar]