Abstract
Antibodies that mediate antibody-dependent cellular cytotoxicity (ADCC) against avian influenza virus subtypes, including H7N9 and H5N1, have been detected in human sera. Using NK cell activation and NK cytotoxicity assays, we compared ADCC-mediating antibodies (ADCC-Abs) in sera collected from healthy infants, children and adults against H7N9 virus–infected cells and recombinant hemagglutinin (HA), neuraminidase (NA), and nucleoprotein (NP) proteins. High titers of ADCC-Abs against H7N9 virus–infected cells were detected in sera from adults and children but not infants. ADCC-Abs titers directed against H7N9 HA or NA proteins. Further analysis showed that ADCC-Abs titers were significantly higher toward H7N9 NP, as compared with H7N9 HA or NA proteins, and correlated strongly with ADCC-Abs titers against H7N9 virus–infected cells. Indeed, ADCC-Abs to NPs of seasonal H1N1 and H3N2 viruses correlated strongly with ADCC-Abs to H7N9 NP, suggesting that seasonal influenza infections and vaccinations may induce these cross-reactive antibodies. Targeting ADCC-Abs to internal proteins may be a potential mechanism of universal vaccine design.
Keywords: antibody-dependent cellular cytotoxicity, ADCC, influenza virus, antibody, nucleoprotein.
Since the first reported cases in 2013, avian influenza A H7N9 viruses have caused sporadic outbreaks in China. To date, there have been 571 laboratory-confirmed cases and 212 deaths, with transmission mostly occurring through contact with infected poultry [1]. Because the disease in poultry is asymptomatic, the control and containment of H7N9 influenza virus in live bird markets is difficult. The virus represents a potential pandemic risk to humans because of the apparent absence of preexisting immunity in the population and the continued circulation of the H7N9 influenza virus in poultry. Although the seroprevalence of neutralizing antibodies to the H7N9 virus is low, there are influenza vaccine strategies that can generate a robust H7N9-specific antibody response [2–4].
It is well established that influenza-specific neutralizing antibodies, targeting the surface hemagglutinin (HA) glycoprotein provide robust protection from infection. Recent studies have aimed to understand the role of nonneutralizing effector functions in influenza immunity. These functions include antibody-dependent phagocytosis (ADP) [5, 6], antibody-dependent complement fixation [7], and antibody-dependent cellular cytotoxicity (ADCC) [8]. Of these, ADCC has been shown to be essential to the protective ability of HA stem-specific monoclonal antibodies in mice challenged with influenza virus [9–11], and higher ADCC-mediating antibody (ADCC-Ab) titers have been associated with lower viral titers and reduced disease severity in humans experimentally infected with influenza virus [12].
There have been conflicting reports on the presence and role of cross-reactive H7N9-specific ADCC-Abs in healthy humans. Previous analysis of human sera from healthy adults and pooled human intravenous immunoglobulin detected cross-reactive ADCC-Abs against H5 HA but not H7 HA protein [13]. However, recent studies using an NK cell–mediated cytotoxicity assay found high titers of ADCC-Abs directed against H7N9 and H5N1 virus–infected cells in sera from healthy adults and children [14]. Potential explanations for these somewhat contradictory results are that differences in the assays to measure ADCC-Abs may influence the ability to detect ADCC-Abs against H7N9 virus (virus-infected cells or purified HA protein as targets) or that ADCC-Abs are directed at viral proteins other than HA. We and others have shown that ADCC-Abs to internal proteins including nucleoprotein (NP) and matrix 1 (M1) can be generated following influenza vaccination/infection [5, 12]. To examine this issue further, we retested sera from infants, children, and adults that we had previously examined in NK cell cytotoxicity assays [14] in the NK cell activation assays and compared ADCC-Ab responses to both H7N9 virus-infected cells and a range of recombinant influenza proteins searching for the influenza A protein that induced these cross-reactive ADCC-Abs to avian influenza viruses.
METHODS
Serum Samples
The serum samples used in this study were described previously [14]. Briefly, adult serum samples were obtained from 16 healthy adults (aged 18–63 years) prior to receipt of a licensed influenza vaccine. Sera from 10 infants (aged 9 months–1 years) and 52 children (aged 2–17 years) were purchased from a commercial company and were all collected from subjects in the United States.
Viruses and Recombinant Influenza Proteins
Egg-grown stocks of influenza A/Anhui/01/2013 (H7N9) virus were used for infected-cell ADCC assays under biosafety level 3 conditions at the National Institutes of Health (NIH). The following recombinant proteins were purchased: A/Anhui/01/2013 (H7N9) HA, NA, NP, seasonal influenza viruses NPs from A/Puerto Rico/08/34 (H1N1), A/California/07/2009 (H1N1pdm09), and A/Hong Kong/1/1968 (H3N2) influenza viruses. The pairwise sequence identity between NP proteins of seasonal influenza viruses (H1N1, H1N1pdm09, and H3N2) and H7N9 NP is 93.1% (performed using Geneious software).
High-Throughput NK Cell Activation Assay
Sera from subjects were assessed for ADCC using a modified flow-based assay as previously described [12]. Briefly, a 96-well enzyme-linked immunosorbent assay plate was coated with 400 ng/well of recombinant protein or bovine serum albumin (for measuring background NK cell activation) overnight at 4°C. Following washing, plates were incubated with diluted human sera (from 1:20–1:81920) for 2 hours at 37°C. Following washing, plates were incubated with 100–500000 NK-92 cells stably expressing human CD16/GFP (176V; NK92-CD16/GFP, kindly provided by Kerry Campbell at Fox Chase Cancer Center) for 5 hours at 37°C, 10% carbon dioxide. After incubation, cells were stained with CD107a APC-Cy7 (clone H4A3) and fixed with 10% paraformaldehyde. Cells were analyzed by flow cytometry, and the endpoint titer of ADCC-Ab was defined as the highest dilution of serum inducing CD107a expression from NK cells at a level that was at least twice the background level in antigen-negative wells.
When virus-infected target cells were used, A549 cells were infected with egg-grown stock of influenza A/Anhui/01/2013 (H7N9) virus at an multiplicity of infection of 10 in OptiMEM media supplemented with TPCK Trypsin overnight at 37°C in a biosafety level 3 at the NIH. Following incubation, cells were trypsinized, washed, and incubated with NK92-CD16 cells at an effector to target ratio of 1:3 (typically 100000 NK-92-CD16 cells to 300000 infected A549 cells) and serum at serial dilutions (1:10–1:5120) for 5 at 37°C, 10% carbon dioxide. Processing and analysis were performed as above. Background activation was set as the activation of NK92-CD16 cells with virus-infected cells but without sera.
Statistical Analysis
Statistical analyses used Prism GraphPad, version 6. Data were analyzed by the nonparametric unpaired Mann–Whitney U tests. Linear correlation analysis was performed with log10 transformed ADCC-Ab titers.
RESULTS
Comparison of the Ability of NK Cell Activation Assay and Traditional Cytotoxicity Assays for Detection of H7N9-Specific Antibody-Dependent Cellular Cytotoxicity–Mediating Antibodies in Healthy Subjects
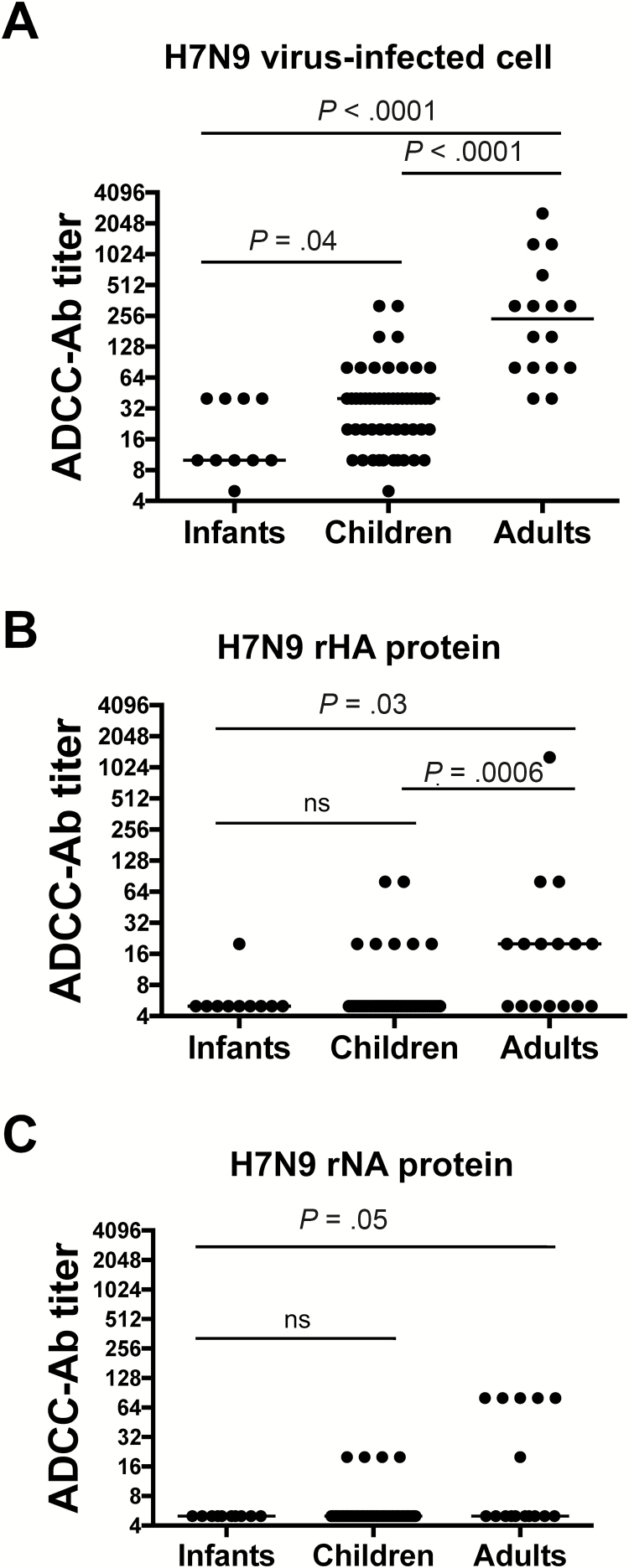
In recent studies, we have shown that high titers of cross-reactive H5N1 and H7N9 ADCC-Abs are present in healthy children and adults who were unlikely to have been exposed to avian influenza viruses [14]. These observations were contrary to a previous study in which we showed that, although some cross-reactive H5 HA ADCC-Abs were found in healthy adults [15], only undetectable or low levels of cross-reactive ADCC-Abs to H7 HA were detected in intravenous immunoglobulin and healthy adult sera [2, 13]. To discern whether this discrepancy was due to differences in assays, we measured ADCC-Abs with a high-throughput NK cell activation assay [12] in sera from infants, children, and adults that had been previously tested for ADCC-Abs to H7N9 virus–infected cells in a traditional ADCC cytotoxicity assay [14]. Indeed, a similar trend in ADCC-Ab titers to H7N9 virus–infected cells was observed when sera were tested by both assays. Significantly higher titers of ADCC-Abs to H7N9 virus–infected cells were observed in sera from adults (median titer = 240) and children (median titer = 40) compared with infants (median titer = 10) (Figure 1A). The H7N9 virus–infected cell ADCC-Ab titers correlated with the increasing age, supporting the hypothesis that H7N9 ADCC-Abs are generated throughout life following influenza infections and/or vaccinations (R2 = .45; P < .0001) (Supplementary Figure 1). There was a significant correlation between ADCC-Ab titers measured by the traditional cytotoxicity and NK cell activation assay (R2 = .49; P < .0001) (Supplementary Figure 2). These data support the conclusion that cross-reactive ADCC-Abs against the H7N9 virus are present in healthy adults and children and suggest that they may only be detected using H7N9 virus–infected cells rather than recombinant H7 HA protein.
Figure 1.
Antibody-dependent cellular cytotoxicity–mediating antibodies (ADCC-Abs) that recognize A(H7N9) virus–infected cells in sera of healthy subjects do not directly target H7N9 hemagglutinin (HA) or neuraminidase (NA) proteins. ADCC-Ab titers were measured against A/Anhui/01/2013 H7N9 virus–infected A549 cells (A), A/Anhui/01/2013 rHA protein (B), or A/Anhui/01/2013 rNA protein (C) in sera from infants (n = 10), children (n = 52), and adults (n = 16). Bars indicate median titers. Titers were compared using separate Mann–Whitney U tests (**P < .05; ns = not statistically significant).
H7N9 Hemagglutinin and Neuraminidase Proteins not being the Major Targets of Cross-Reactive H7N9-Specific Antibody-Dependent Cellular Cytotoxicity–Mediating Antibodies in Healthy Subjects
Because the surface glycoproteins HA and neuraminidase (NA) are highly expressed on the surface of virus-infected cells, we wanted to determine whether the response to H7N9 virus–infected cells was due to ADCC-Abs directed against the H7 HA and/or N9 NA. To do so, we measured ADCC-Abs titers in sera from infants, children, and adults against recombinant H7 HA and N9 NA proteins by the NK cell activation assay. The titer of H7N9 HA-specific ADCC-Abs in adults and children was much lower than ADCC-Abs to H7N9 virus–infected cells (median levels of 20 and 5 compared with median titers of 240 and 40, respectively) (Figure 1A and 1B). Additionally, the titer of H7N9 NA-specific ADCC-Abs in adults and children was also much lower than ADCC-Abs to H7N9 virus–infected cells (Figure 1C). We confirmed that NA-specific ADCC-Abs could be measured in sera from subjects vaccinated with H7N9 live attenuated vaccine and inactivated subunit vaccine in a prime-boost strategy (data not shown). Although H7N9 ADCC-Abs can be detected in sera from healthy subjects using H7N9 virus–infected cells, they cannot be detected using recombinant HA or NA protein, suggesting that the HA and NA glycoproteins are likely not the targets of cross-reactive H7N9-specific ADCC-Abs in healthy subjects.
Anti-H7N9 Nucleoprotein Reactive Antibody-Dependent Cellular Cytotoxicity–Mediating Antibodies Detected in Sera of Healthy Subjects
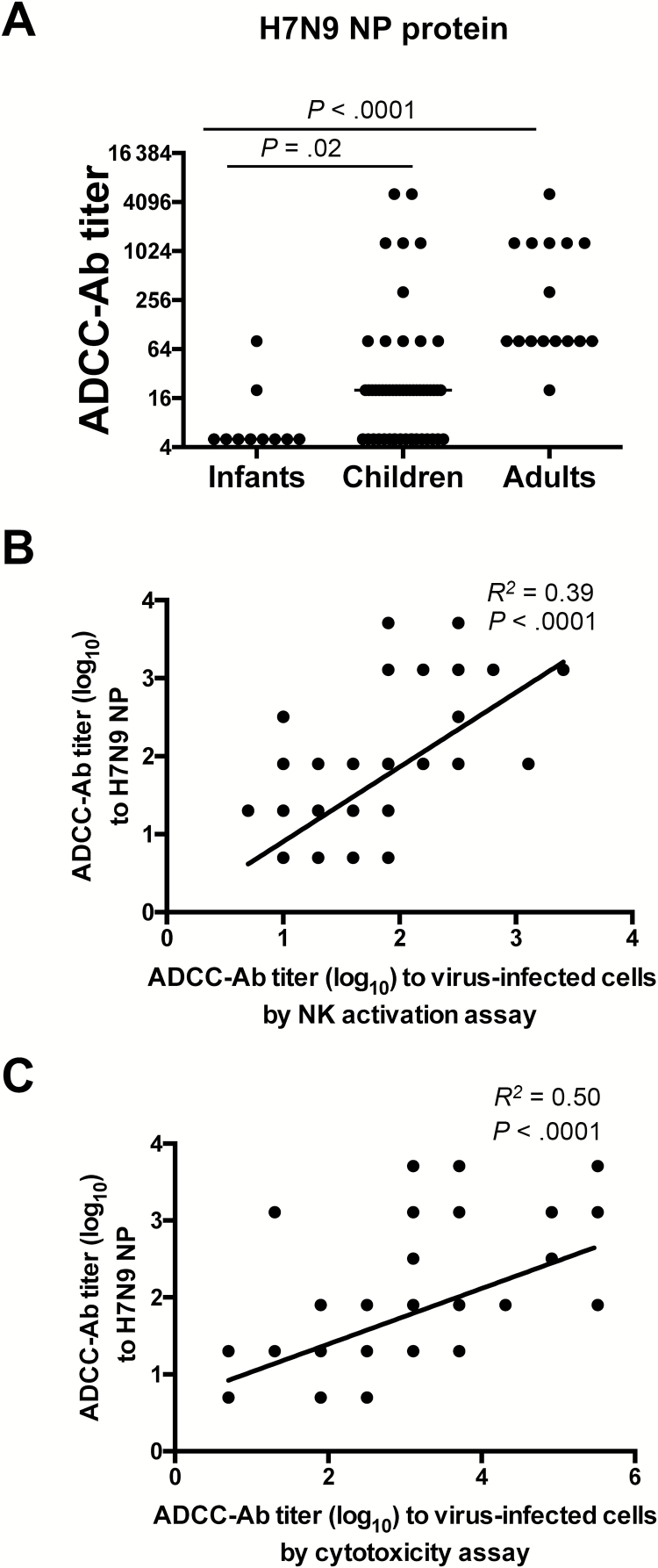
Our studies have suggested that cross-reactive ADCC-Abs following vaccination and infection can be generated to conserved internal proteins such as NP and M1 [5, 12]. Therefore, we measured ADCC-Abs to H7N9 NP in the sera from infants, children, and adults and found significantly higher titers of H7N9 NP-reactive ADCC-Abs in sera from adults and children than infants (P < .001) (Figure 2A). Further, the H7N9 NP-reactive ADCC-Ab titers correlated with ADCC-Ab titers measured using H7N9 virus–infected cells by either the NK cell activation assay (R2 = .39; P < .0001) (Figure 2B) or cytotoxicity assay (R2 = .50; P < .0001) (Figure 2C), suggesting that cross-reactive ADCC-Abs measured against H7N9 virus–infected cells may be targeting the H7N9 NP.
Figure 2.
H7N9-specific antibody-dependent cellular cytotoxicity–mediating antibodies (ADCC-Abs) are generated toward the H7N9 nucleoprotein. ADCC-Ab titers against recombinant A/Anhui/01/2013 (H7N9) nucleoprotein (NP) in sera from infants (n = 10), children (n = 52), and adults (n = 16) (A). Linear correlation analysis of ADCC-Ab titers against H7N9 NP and H7N9 virus-infected cells measured by NK cell activation assay (B) or cytotoxicity assay (cytotoxicity assay results have been published elsewhere [14]) (C). Bars indicate median titers. Titers were compared using separate Mann–Whitney U tests (**P < .05; ns = not statistically significant). In (B) and (C) some dots represent >2 samples.
Cross-Reactive H7N9 Nucleoprotein-Specific Antibody-Dependent Cellular Cytotoxicity–Mediating Antibodies Correlating Strongly With Seasonal Nucleoprotein-Specific Antibody-Dependent Cellular Cytotoxicity–Mediating Antibodies
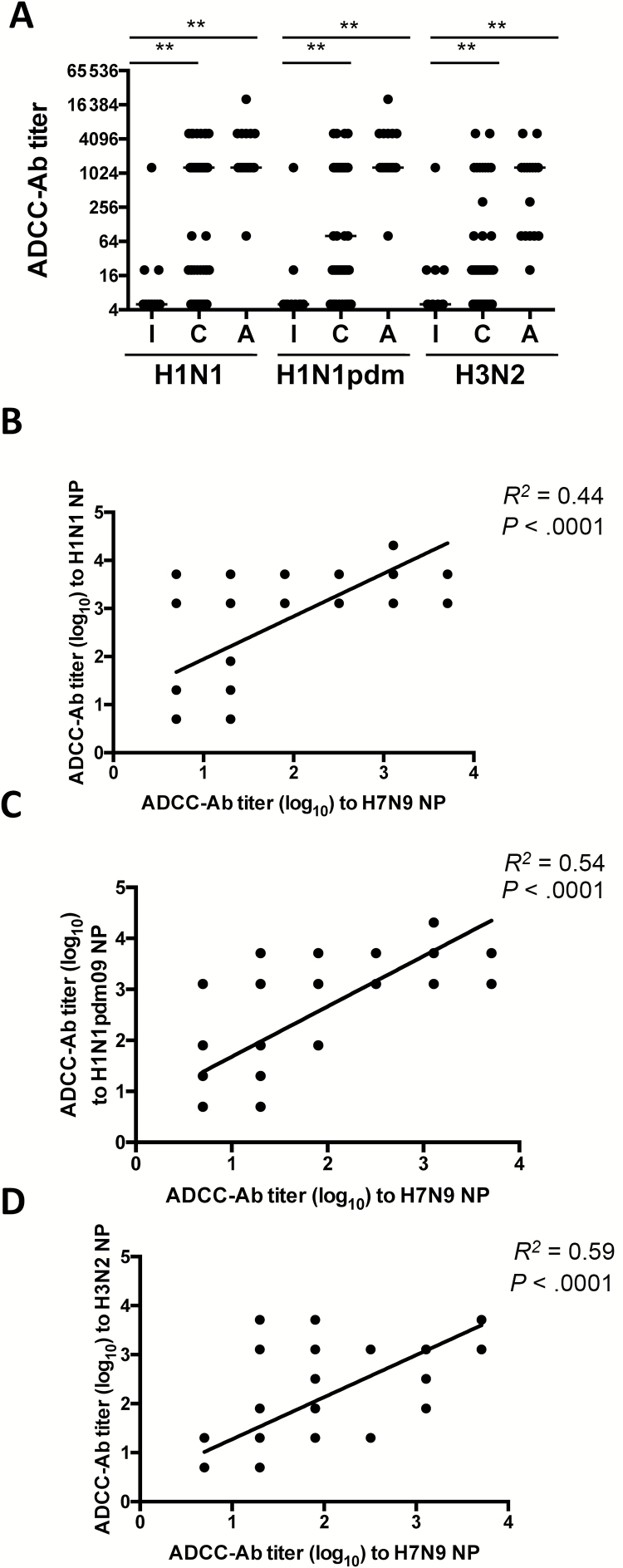
We tested sera collected from subjects in the United States who were unlikely to be infected with or vaccinated against the H7N9 influenza virus. Given the high degree of similarity between amino acid sequences of the NP protein among influenza A viruses, we hypothesized that the detection of H7N9 NP-reactive antibodies is likely the result of cross-reactivity with the NP of seasonal influenza viruses. We therefore measured ADCC-Abs against NPs from A/Puerto Rico/08/34 (H1N1), A/California/07/2009 (H1N1pdm09), and A/Hong Kong/1/1968 (H3N2) in sera from the different age groups. We observed high titers of NP-reactive ADCC-Abs in adults and children against all 3 influenza NPs tested (Figure 3A). They were all significantly higher in children and adults in comparison with infants (P < .05, separate Mann–Whitney U tests) (Figure 3A). The H1N1, H1N1pmd09, and H3N2 NP-specific ADCC-Ab titers in subjects correlated significantly with H7N9 NP-specific ADCC-Ab titers (R2 = .44, P < .0001; R2 = .54, P < .0001; R2 = .59, P < .0001, respectively) (Figure 3B–D). Due to the sequence conservation among influenza NPs, we conclude that NP-specific ADCC-Ab titers induced by seasonal influenza vaccination or infection during early life likely cross-react with H7N9 NP and contribute to the ADCC-Ab response to virus-infected cells.
Figure 3.
Antibody-dependent cellular cytotoxicity–mediating antibodies (ADCC-Abs) to H7N9 nucleoprotein (NP) correlate strongly with ADCC-Abs to seasonal influenza NP. ADCC-Ab titer against recombinant NP from A/Puerto Rico/8/1934 (H1N1), A/California/07/2009 (H1N1pdm09), or A/Hong Kong/1/1968 (H3N2) in sera from infants (I, n = 10), children (C, n = 52), and adults (A, n = 16) (A). Linear correlation analysis of H7N9 NP ADCC-Ab titers versus titers to NPs of A/Puerto Rico/8/1934 (H1N1, B), A/California/07/2009 (H1N1pdm09, C), or A/Hong Kong/1/1968 (H3N2, D). Bars indicate median titers. Titers were compared using separate Mann–Whitney U tests (**P < .05; ns = not statistically significant). In B–D, some dots represent >2 samples.
DISCUSSION
The generation of ADCC-Abs following influenza vaccination and infection has been extensively characterized in humans. ADCC-Abs in humans have been classically measured by cytotoxicity assays [16–18] using infected target cells and healthy donor effector cells. In contrast, newer assays use NK cell activation as a surrogate marker for ADCC-Ab effector function, where ADCC-Abs can be assessed by CD107a/IFNγ expression or a reporter assay read-out [10, 15, 19]. It is possible that the use of subtly different assays for measuring ADCC-Abs in humans may lead to differences in the sensitivity of ADCC-Ab detection. High titers of H7N9-reactive ADCC-Abs were detected in the sera of healthy adults and children using the cytotoxicity assay [14] but not using the NK cell activation assay [2, 13]; these discrepancies may reflect the inability of NK cell activation assays to able to detect such antibodies. However, we show here that ADCC-Abs are detected against H7N9 virus–infected cells using a high-throughput NK cell activation assay. Additionally, there is a strong correlation between ADCC-Ab titers measured by the 2 assays. Interestingly, we could not detect H7N9-reactive ADCC-Ab using recombinant HA or NA proteins but detected robust responses to both H7N9 virus–infected cells and the H7N9 NP protein. The H7N9 NP being the target of ADCC-Abs may explain the inability to detect ADCC-Abs to H7N9 in previous studies. Measuring ADCC-Abs in sera targeting only surface proteins may miss ADCC-Abs directed against internal viral proteins, such as NP. Our findings highlight the importance of measuring ADCC-Abs using virus-infected cells rather than immobilized recombinant HA protein or HA-expressing cell-lines.
Recent data suggest that ADCC-Abs to internal viral proteins such as NP and M1 are induced by influenza infections and vaccination. Seasonal inactivated influenza vaccine has been recently shown to induce ADCC-Abs to NP in children, likely because there is NP in some inactivated vaccine formulations [12, 20]. Also, influenza infection in adults induces ADCC-Abs to both M1 and NP proteins [5]. Numerous studies have shown that NP is expressed on the cell surface as well as in the cytoplasm and nucleus of virus-infected cells and can be bound on the surface by anti-NP antibodies in serum [21–24]. It is unknown whether there is an active mechanism by which NP is translocated to the surface of virus-infected cells and whether this is a receptor-mediated interaction. Passive transfer or induction of anti-NP antibodies in mice has been found to provide robust homologous and heterologous protection [21, 25, 26]. Studies that have attempted to understand the mechanism of anti-NP antibody-mediated protection using in vitro neutralization have been unsuccessful, and anti-NP antibodies are consistently found to be nonneutralizing [21, 24]. Our in vitro ADCC assays show that sera from healthy humans containing anti-NP antibodies can induce robust NK cell activation against NP and virus-infected cells. It has been speculated that effector cells such as NK cells can become activated through binding of NP-specific antibodies on virus-infected cells and will likely not only induce killing of the virus-infected cell but also provide an antiviral environment through the secretion of cytokines such as IFNγ [5, 27]. It is unknown whether this proinflammatory environment leads to protection or immunopathology. It is plausible that NP antibodies function in vivo through ADCC activity; passive transfer studies in Fc-knockout mice may be useful to confirm this hypothesis.
In this study we showed that H7N9-reactive ADCC-Abs are present in healthy subjects and these antibodies are likely primarily induced to the internal viral protein, NP. There are some limitations to our observations in this study. First, we did not analyze ADCC-Abs to other internal viral proteins such as M1 or M2, which are also highly conserved among influenza A viruses. However, the amount of M1 protein on the cell surface has been shown to be 10-fold lower than the level of NP protein [23] and therefore may be less likely to be the target of H7N9-reactive ADCC-Abs. Anti-M2 antibodies were undetectable in less than half of adults [28]. Second, we can only speculate that seasonal influenza infections and vaccination are the source of cross-reactive H7N9 ADCC-Abs because we have not tested for H7N9-reactive antibodies before and after seasonal vaccination/infection in this study. However, previous studies found that children vaccinated with seasonal inactivated influenza vaccines produce NP-reactive ADCC-Abs [12]. Third, the in vivo protective capacity of H7N9 NP-reactive ADCC-Abs in the absence of neutralizing antibodies is unclear and requires further investigation.
In summary, we have confirmed the presence of H7N9-cross-reactive ADCC-Abs to H7N9 virus–infected cells, which can be detected using the NK cell activation assay with H7N9 virus–infected cells as targets. These H7N9-cross-reactive ADCC-Abs likely target the H7N9 NP and may be generated throughout life by repeated seasonal influenza A virus infections and vaccination. Their role may be important as part of the immune response to emerging influenza viruses when preexisting immune responses to the HA and NA proteins of novel infecting subtypes are absent.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Financial support. This research was supported in part by the Intramural Research Program of NIAID, NIH, and NIAID Centers of Excellence for Influenza Research and Surveillance (CEIRS) contract HHSN272201400005C. S. J. is supported by a National Health and Medical Research Council (NHMRC), Australia Early Career Fellowship (APP1072127).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. WHO risk assessment of human infections with avian influenza A(H7N9) virus. http://www.who.int/influenza/human_animal_interface/influenza_h7n9/RiskAssessment_H7N9_23Feb20115.pdf Accessed 27 Septemeber 2016. [Google Scholar]
- 2. Sobhanie M, Matsuoka Y, Jegaskanda S, et al. Evaluation of the safety and immunogenicity of a candidate pandemic live attenuated influenza vaccine (pLAIV) against influenza A(H7N9). J Infect Dis 2016; 213:922–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jackson LA, Campbell JD, Frey SE, et al. Effect of varying doses of a monovalent H7N9 influenza vaccine with and without AS03 and MF59 adjuvants on immune response: a randomized clinical trial. JAMA 2015; 314:237–46. [DOI] [PubMed] [Google Scholar]
- 4. Rudenko L, Isakova-Sivak I, Naykhin A, et al. H7N9 live attenuated influenza vaccine in healthy adults: a randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Infect Dis 2016; 16:303–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vanderven HA, Ana-Sosa-Batiz F, Jegaskanda S, et al. What lies beneath: antibody dependent natural killer cell activation by antibodies to internal influenza virus proteins. EBioMedicine 2016; 8:277–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huber VC, Lynch JM, Bucher DJ, Le J, Metzger DW. Fc receptor-mediated phagocytosis makes a significant contribution to clearance of influenza virus infections. J Immunol 2001; 166:7381–8. [DOI] [PubMed] [Google Scholar]
- 7. Terajima M, Cruz J, Co MD, et al. Complement-dependent lysis of influenza a virus-infected cells by broadly cross-reactive human monoclonal antibodies. J Virol 2011; 85:13463–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jegaskanda S, Reading PC, Kent SJ. Influenza-specific antibody-dependent cellular cytotoxicity: toward a universal influenza vaccine. J Immunol 2014; 193:469–75. [DOI] [PubMed] [Google Scholar]
- 9. DiLillo DJ, Tan GS, Palese P, Ravetch JV. Broadly neutralizing hemagglutinin stalk-specific antibodies require FcγR interactions for protection against influenza virus in vivo. Nat Med 2014; 20:143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Impagliazzo A, Milder F, Kuipers H, et al. A stable trimeric influenza hemagglutinin stem as a broadly protective immunogen. Science 2015; 349:1301–6. [DOI] [PubMed] [Google Scholar]
- 11. DiLillo DJ, Palese P, Wilson PC, Ravetch JV. Broadly neutralizing anti-influenza antibodies require Fc receptor engagement for in vivo protection. J Clin Invest 2016; 126:605–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jegaskanda S, Luke C, Hickman HD, et al. Generation and protective ability of influenza virus-specific antibody-dependent cellular cytotoxicity in humans elicited by vaccination, natural infection, and experimental challenge. J Infect Dis 2016; 214:945–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jegaskanda S, Vandenberg K, Laurie KL, et al. Cross-reactive influenza-specific antibody-dependent cellular cytotoxicity in intravenous immunoglobulin as a potential therapeutic against emerging influenza viruses. J Infect Dis 2014; 210:1811–22. [DOI] [PubMed] [Google Scholar]
- 14. Terajima M, Co MD, Cruz J, Ennis FA. High antibody-dependent cellular cytotoxicity antibody titers to H5N1 and H7N9 avian influenza A viruses in healthy US adults and older children. J Infect Dis 2015; 212:1052–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jegaskanda S, Job ER, Kramski M, et al. Cross-reactive influenza-specific antibody-dependent cellular cytotoxicity antibodies in the absence of neutralizing antibodies. J Immunol 2013; 190:1837–48. [DOI] [PubMed] [Google Scholar]
- 16. Hashimoto G, Wright PF, Karzon DT. Antibody-dependent cell-mediated cytotoxicity against influenza virus-infected cells. J Infect Dis 1983; 148:785–94. [DOI] [PubMed] [Google Scholar]
- 17. Hashimoto G, Wright PF, Karzon DT. Ability of human cord blood lymphocytes to mediate antibody-dependent cellular cytotoxicity against influenza virus-infected cells. Infect Immun 1983; 42:214–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Quinnan GV, Ennis FA, Tuazon CU, et al. Cytotoxic lymphocytes and antibody-dependent complement-mediated cytotoxicity induced by administration of influenza vaccine. Infect Immun 1980; 30:362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Henry Dunand CJ, Leon PE, Huang M, et al. Both neutralizing and non-neutralizing human H7N9 influenza vaccine-induced monoclonal antibodies confer protection. Cell Host Microbe 2016; 19:800–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ahmed SS, Volkmuth W, Duca J, et al. Antibodies to influenza nucleoprotein cross-react with human hypocretin receptor 2. Sci Transl Med 2015; 7:294ra105. [DOI] [PubMed] [Google Scholar]
- 21. Fujimoto Y, Tomioka Y, Takakuwa H, et al. Cross-protective potential of anti-nucleoprotein human monoclonal antibodies against lethal influenza A virus infection. J Gen Virol 2016; 97:2104–16. [DOI] [PubMed] [Google Scholar]
- 22. Bullido R, Gómez-Puertas P, Albo C, Portela A. Several protein regions contribute to determine the nuclear and cytoplasmic localization of the influenza A virus nucleoprotein. J Gen Virol 2000; 81(Pt 1):135–42. [DOI] [PubMed] [Google Scholar]
- 23. Yewdell JW, Frank E, Gerhard W. Expression of influenza A virus internal antigens on the surface of infected P815 cells. J Immunol 1981; 126:1814–9. [PubMed] [Google Scholar]
- 24. Bodewes R, Geelhoed-Mieras MM, Wrammert J, et al. In vitro assessment of the immunological significance of a human monoclonal antibody directed to the influenza a virus nucleoprotein. Clin Vaccine Immunol 2013; 20:1333–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Carragher DM, Kaminski DA, Moquin A, Hartson L, Randall TD. A novel role for non-neutralizing antibodies against nucleoprotein in facilitating resistance to influenza virus. J Immunol 2008; 181:4168–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. LaMere MW, Lam HT, Moquin A, et al. Contributions of antinucleoprotein IgG to heterosubtypic immunity against influenza virus. J Immunol 2011; 186:4331–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hassane M, Paget C. “Universal flu vaccine”: Can NK cell-mediated ADCC tip the scales? EBioMedicine 2016; 8:18–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhong W, Reed C, Blair PJ, Katz JM, Hancock K, Influenza serology working G. Serum antibody response to matrix protein 2 following natural infection with 2009 pandemic influenza A(H1N1) virus in humans. J Infect Dis 2014; 209:986–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.