Confirmation of respiratory syncytial virus (RSV) in the lower respiratory tract is associated with worse clinical outcomes, including increased supplemental oxygen use, decreased supplemental oxygen–free days, and greater mortality rate. Supplemental oxygen–free days as a clinical end point may allow smaller sample sizes for trials evaluating RSV antivirals.
Keywords: Respiratory syncytial virus, pneumonia, hematopoietic cell transplant
Abstract
Background
Clinically meaningful endpoints for respiratory syncytial virus (RSV) treatment trials are lacking for hematopoietic cell transplant (HCT) recipients. We evaluated supplemental oxygen use among HCT recipients with RSV infection.
Methods
Subjects were grouped according to the presence of upper respiratory tract infection (URTI) without lower respiratory tract infection (LRTI), URTI progressing to LRTI, and LRTI at presentation. LRTI was defined as a positive lower respiratory tract sample with or without radiographic abnormality (defined as proven or probable LRTI, respectively) or a positive upper respiratory tract sample with radiographic abnormality (possible LRTI). Supplemental oxygen–free days were defined as any day while alive after diagnosis of RSV infection during which ≤2 L of supplemental oxygen per minute was received.
Results
Among 230 patients, supplemental oxygen use by day 28 after the first diagnosis of RSV infection was lowest in patients presenting with URTI (31 of 197 [16%]). Supplemental oxygen use was lower in patients with possible LRTI (12 of 45 [27%]) than in those with proven/probable LRTI (29 of 42 [69%]). Patients presenting with proven/probable LRTI had a median of 16 fewer supplemental oxygen–free days than those presenting with URTI (P < .0001). Death only occurred among patients with proven/probable LRTI (11 of 42 [26%]).
Conclusions
Confirmation of RSV infection in the lower respiratory tract provides prognostic information that may help prioritize therapies. Supplemental oxygen–free days as a clinical endpoint may allow smaller sample sizes for trials evaluating RSV antivirals.
Respiratory syncytial virus (RSV) infection is associated with significant morbidity and mortality in patients with hematologic malignancy and in hematopoietic cell transplant (HCT) recipients [1, 2]. There are no approved treatments for RSV infection in HCT recipients, although several candidates are in development for treatment of RSV infections in various populations [3–6], including HCT recipients [7, 8]. Historically, mortality rates in HCT recipients with lower respiratory tract infection (LRTI) due to RSV have been as high as 55% [9–11]. However, more-recent studies of subjects with either upper respiratory tract infection (URTI) or LRTI have demonstrated substantially lower mortality rates (5%–10%), which may in part be due to improvements in transplantation practices, including the use of nonmyeloablative conditioning and changes in supportive care practices [12, 13]. Additional factors may reduce the reported mortality rates, such as the lack of clear delineation of disease site (upper versus lower respiratory tract) and the variation in LRTI definition. The strictest definition of LRTI requires detection of RSV in the lower respiratory tract [9, 10]; however, several studies use more-lenient definitions that rely on detection of RSV in the upper respiratory tract alone with LRTI symptoms and/or abnormal radiographic findings, without documentation of RSV in the lower respiratory tract [11, 12, 14]. These variations in the LRTI definition can affect disease prevalence and outcome estimates, variables that influence clinical trial design. To assess this potential impact, we sought to evaluate RSV LRTI, defined by site of virologic detection, and its association with clinical manifestations and outcomes.
Despite heterogeneous definitions of RSV LRTI, mortality rates do appear to have declined over time and mortality as a clinical endpoint is thus not practical for use in clinical trials. Given the identification of several promising antivirals for the treatment of RSV disease, the need to define alternative surrogate endpoints that are clinically meaningful and reflect patient functionality becomes critical.
One such potential end point is the number of days an RSV-infected patient requires supplemental oxygen, a potential clinically meaningful endpoint that may correlate with acute lung injury. Supplemental oxygen use at diagnosis is associated with increased mortality in HCT recipients with RSV LRTI [15]; however, supplemental oxygen use as an outcome has not been previously evaluated. The number of days during which supplemental oxygen was required was evaluated in a recent study of HCT recipients with parainfluenza virus LRTI [16]: during the 28 days after infection diagnosis, subjects with virus detected in the lower respiratory tract (ie, those with proven or probable LRTI) had significantly more days during which supplemental oxygen was required than subjects with LRTI in whom virus was not detected in the lower respiratory tract (ie, those with possible LRTI) [16]. Supplemental oxygen use has not been evaluated for other respiratory viruses, nor has this approach been assessed rigorously as a potential endpoint for clinical trials.
In the present study, we sought to define and compare outcomes, including mortality and days during which supplemental oxygen was required, in subjects with possible, probable, or proven RSV LRTI.
METHODS
Patients and Data Collection
HCT recipients with virologically confirmed RSV infection between January 2003 and January 2015 were included in this study. Clinical data were collected from databases and supplemental review of the medical record. This study was approved by the Institutional Review Board at the Fred Hutchinson Cancer Research Center. Subjects signed informed consent permitting the use of data for research. Subjects with incomplete data on supplemental oxygen use following the diagnosis of RSV infection were excluded.
At our institution, standard practice is to evaluate for lower respiratory tract disease in any HCT recipient with RSV detected in the upper respiratory tract. A patient was considered to have RSV URTI if they had an upper respiratory tract sample that tested positive for RSV and no RSV-positive LRTI specimen or radiographic abnormalities. A patient was considered to have RSV LRTI if they had an RSV-positive lower respiratory tract sample (ie, bronchoalveolar lavage [BAL], a lung biopsy specimen, or an autopsy specimen) with or without radiographic abnormalities (defined as proven or probable LRTI, respectively) or an RSV-positive upper respiratory tract sample with radiographic abnormalities (defined as possible LRTI). The day of RSV LRTI diagnosis was defined as the date when RSV was detected in the lower respiratory tract specimen (for cases of proven/probable LRTI) or the date the radiographic abnormality was detected (for cases of possible LRTI). Virologic confirmation was obtained by direct fluorescent antibody testing, shell vial centrifugation, or conventional viral culture of lower respiratory tract samples; after 2007, patients also had RSV detected by reverse transcription–polymerase chain reaction (PCR) analysis [17].
Subjects were divided into 3 groups. Group 1 comprised patients with URTI at presentation without progression to LRTI; group 2 comprised patients with URTI at presentation with subsequent progression to proven, probable, or possible LRTI; and group 3 comprised patients with proven, probable, or possible LRTI at presentation.
Maximum supplemental oxygen use per 24-hour period was recorded for days 0 through 28 following initial RSV presentation, as well as for days 0 through 28 following LRTI diagnosis. Supplemental oxygen use was defined as the delivery of oxygen by any modality, including nasal cannula, mask, noninvasive positive pressure ventilation, or mechanical ventilation, and was recorded if sustained for >4 hours. Supplemental oxygen administered in the context of medication administration and baseline supplemental oxygen use was not recorded. Supplemental oxygen was administered per standardized clinical nursing protocols at our center. A supplemental oxygen–free day was defined as any 24-hour period during which a subject received ≤2 L of supplemental oxygen per minute (sustained for a continuous 48-hour period) while the patient was alive. The impact of death on the number of supplemental oxygen–free days was evaluated using 2 definitions. For definition 1, the day of death and subsequent days up to 28 days following presentation were not considered supplemental oxygen-free days. For definition 2, if a subject died, none of the 28 days were considered supplemental oxygen–free days (ie, the number of supplemental oxygen–free days was 0).
Statistical Analysis
Patients were analyzed on the basis of their first episode of RSV following HCT; for those who presented with or progressed to LRTI, additional analyses were conducted beginning at the time of LRTI diagnosis. The probability of overall survival was estimated using the Kaplan-Meier method; the log-rank test was used to compare mortality curves between groups. Cox proportional hazard regression was used to evaluate unadjusted and adjusted hazard ratios (HRs) for the time to first use of supplemental oxygen and the time to mechanical ventilation. Covariates evaluated as candidate risk factors for inclusion in multivariable models are listed in Table 1 and were selected a priori. Variables with a P value of ≤ 0.2 in univariable analysis were candidates for multivariable models and were retained in the models if they remained significant themselves or modified the effect of another factor (confounder). The median numbers of supplemental oxygen–free days while alive were compared among groups and within groups, divided into those with possible LRTI versus those proven/probable LRTI, by the Wilcoxon rank sum test. Two-sided P values of <.05 were considered to be statistically significant. Sample size estimates were calculated using a 2-sided Pearson χ2 test, for mortality by day 28, and 2-sided t tests, for the number of supplemental oxygen–free days by day 28, with an α of 0.05 and 80% power. All statistical analyses were performed using SAS 9.4 for Windows (SAS Institute, Cary, NC).
Table 1.
Characteristics of Hematopoietic Cell Transplant Recipients With Respiratory Syncytial Virus (RSV) Infection, by Group
| Variables | Whole Cohort (n = 230) | Group 1 (n = 143) | Group 2 (n = 54) | Group 3 (n = 33) |
|---|---|---|---|---|
| Recipient age at transplantation, y | ||||
| Median (IQR) | 50.0 (28.8–59.5) | 48.5 (23.7–58.3) | 51.2 (31.7–61.1) | 51.8 (38.0–61.2) |
| <21 | 46 (20) | 31 (22) | 11 (20) | 4 (12) |
| 21–60 | 128 (56) | 82 (57) | 27 (50) | 19 (58) |
| >60 | 56 (24) | 30 (21) | 16 (30) | 10 (30) |
| Year of transplantation | ||||
| 2003–2010 | 135 (59) | 78 (55) | 38 (70) | 19 (58) |
| 2011–2015 | 85 (37) | 58 (41) | 15 (28) | 12 (36) |
| 2000–2002 | 10 (4) | 7 (5) | 1 (2) | 2 (6) |
| Recipient sex | ||||
| Male | 136 (59) | 86 (60) | 28 (52) | 22 (67) |
| Female | 94 (41) | 57 (40) | 26 (48) | 11 (33) |
| Race | ||||
| White | 151 (66) | 90 (63) | 37 (69) | 24 (73) |
| Other than white | 68 (30) | 46 (32) | 13 (24) | 9 (27) |
| Unknown | 11 (5) | 7 (5) | 4 (7) | … |
| Cell source | ||||
| PBSC | 163 (71) | 104 (73) | 34 (63) | 25 (76) |
| BM/CB | 67 (29) | 39 (27) | 20 (37) | 8 (24) |
| Donor type | ||||
| Allogeneic/unrelated | 171 (74) | 101 (71) | 42 (78) | 28 (85) |
| Autologous | 59 (26) | 42 (29) | 12 (22) | 5 (15) |
| No. of transplantations | ||||
| 1 | 187 (81) | 121 (85) | 40 (74) | 26 (79) |
| 2 | 40 (17) | 21 (15) | 14 (26) | 5 (15) |
| 3 | 3 (1) | 1 (1) | 0 (0) | 2 (6) |
| Morphologic relapse | ||||
| No | 219 (95) | 135 (94) | 52 (96) | 32 (97) |
| Yes | 11 (5) | 8 (6) | 2 (4) | 1 (3) |
| Acute GVHD | ||||
| Grade 0–2 | 133 (58) | 77 (54) | 34 (63) | 22 (67) |
| Grade 3–4 | 32 (28) | 18 (13) | 8 (15) | 6 (18) |
| FEV1 percentage prior to transplantation | ||||
| <80 | 49 (21) | 31 (22) | 12 (22) | 6 (18) |
| ≥80 | 148 (64) | 93 (65) | 32 (59) | 23 (70) |
| Missing | 33 (14) | 19 (13) | 10 (19) | 4 (12) |
| FEV1/FVC prior to transplantation | ||||
| <70 | 37 (16) | 23 (16) | 10 (19) | 4 (12) |
| ≥70 | 162 (70) | 104 (73) | 33 (61) | 25 (76) |
| Missing | 31 (13) | 16 (11) | 11 (20) | 4 (12) |
| DLCO percentage prior to transplantation | ||||
| <80 | 109 (47) | 66 (46) | 25 (46) | 18 (55) |
| ≥80 | 82 (36) | 53 (37) | 18 (33) | 11 (33) |
| Missing | 39 (17) | 24 (17) | 11 (20) | 4 (12) |
| Days to first diagnosis of RSV infection after transplantation, median (IQR) | 128.5 (50–422) | 90.0 (50–407) | 121.5 (48–405) | 152.0 (114–499) |
| Bronchiolitis obliterans prior to RSV infection | ||||
| No | 226 (98) | 143 (100) | 52 (96) | 31 (94) |
| Yes | 4 (2) | 0 (0) | 2 (4) | 2 (6) |
| RSV testing method | ||||
| PCR | 168 (73) | 97 (68) | 44 (81) | 27 (82) |
| Non-PCR | 62 (27) | 46 (32) | 10 (19) | 6 (18) |
| Oxygen use at first diagnosis of RSV infection | ||||
| None | 201 (87) | 140 (98) | 45 (83) | 16 (48) |
| ≤2 L/min | 4 (2) | … | 3 (6) | 1 (3) |
| >2 L/min | 19 (8) | 3 (2) | 6 (11) | 10 (30) |
| MV | 6 (3) | … | … | 6 (18) |
| Steroid use at first diagnosis of RSV infection | ||||
| None | 144 (63) | 95 (66) | 30 (56) | 19 (58) |
| ≤1 mg/kg/day | 68 (30) | 41 (29) | 16 (30) | 11 (33) |
| >1 mg/kg/day | 18 (8) | 7 (5) | 8 (15) | 3 (9) |
Data are no. (%) of subjects, unless otherwise indicated.
Abbreviations: BM, bone marrow; CB, cord blood; DLCO diffusing capacity of the lung for carbon monoxide; FEV1, forced expiratory volume, 1 second; FVC, forced vital capacity; GVHD, graft-versus-host disease; IQR, interquartile range; PBSC, peripheral blood stem cell; PCR, polymerase chain reaction.
RESULTS
Patient Characteristics
We identified 230 HCT recipients with RSV infection from 2003 through 2015. The median age at transplant receipt was 50 years (range, 0.5–71.6 years). The median time to initial diagnosis of RSV infection following transplantation was 128.5 days (interquartile range [IQR], 50–422 days). Of 197 patients who presented with URTI, 143 did not progress to LRTI (group 1), and 54 progressed to LRTI (group 2). Thirty-three subjects presented with LRTI (group 3). Comparisons of demographic variables for groups 1, 2, and 3 are shown in Table 1. Of 87 patients who developed or presented with LRTI, infection in 45 (52%) was defined as possible LRTI, and infection in 42 (48%) was defined as probable or proven LRTI. Only 5 probable cases were identified in the whole cohort. The percentage of subjects using >2 L of supplemental oxygen per minute at diagnosis was compared among groups 1, 2, and 3 (Table 2) and was found to be highest in those presenting with LRTI (group 3; 48%). In group 3, 6 patients (18%) required mechanical ventilation at diagnosis.
Table 2.
Supplemental Oxygen Use and Mortality Outcomes Among Hematopoietic Cell Transplant Recipients With Respiratory Syncytial Virus (RSV) Infection, by Site of RSV Detection
| Variable | Groups 1 and 2, URTI, No. (%) (n = 197) | Group 2, LRTI, No. (%) | Group 3, LRTI, No. (%) | Groups 2 and 3, LRTI, No. (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Overall (n = 54) | Possible (n = 27) | Proven/Probable (n = 27) | Overall (n = 33) | Possible (n = 18) | Proven/Probable (n = 15) | Possible (n = 45) | Proven/Probable (n = 42) | ||
| Oxygen use >2 L/min | |||||||||
| At RSV infection diagnosis | 9 (5) | 17 (31) | 3 (11) | 14 (52) | 16 (48) | 6 (33) | 10 (67) | 9 (20) | 24 (57) |
| By day 28 | 31 (16) | 21 (39) | 4 (15) | 17 (63) | 20 (61) | 8 (44) | 12 (80) | 12 (27) | 29 (69) |
| Mechanical ventilation by day 28 | 8 (4) | 8 (15) | 0 (0) | 8 (30) | 9 (27) | 2 (11) | 7 (47) | 2 (4) | 15 (36) |
| Death by day 28 | 4 (2) | 6 (11) | 0 (0) | 6 (22) | 5 (15) | 0 (0) | 5 (33) | 0 (0) | 11 (26) |
Abbreviations: LRTI, lower respiratory tract infection; URTI, upper respiratory tract infection.
Supplemental Oxygen Use and Need for Mechanical Ventilation Following Diagnosis of RSV Infection
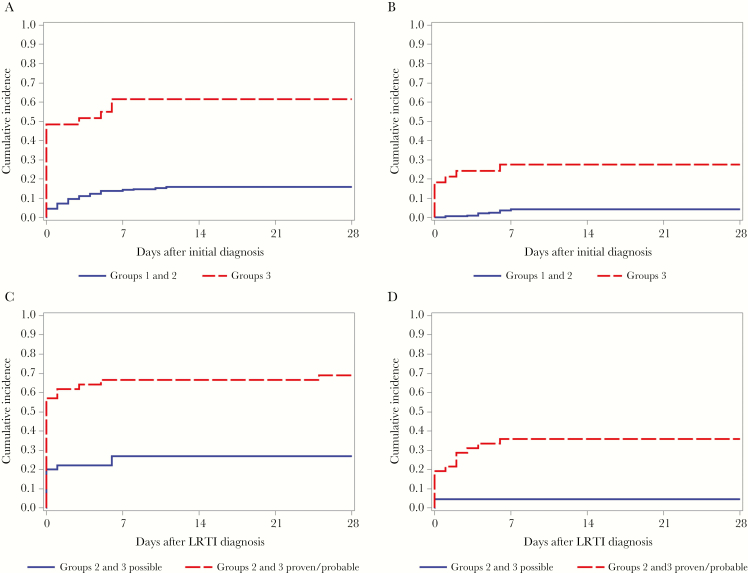
The proportion of patients who required any supplementation with >2 L of oxygen per minute by day 28 after diagnosis of RSV infection was lowest for patients who presented with URTI (16% for groups 1 and 2) and highest among those who presented with LRTI (61% for group 3; Table 2). In cumulative incidence curves (Figure 1A and 1B), patients presenting with LRTI were more likely to require supplemental oxygen or mechanical ventilation than patients presenting with URTI (P < .0001 and P < .0001, respectively, by the log-rank test). After LRTI diagnosis, the need for supplementation with >2 L of oxygen per minute and mechanical ventilation by day 28 was higher in subjects who presented with LRTI than those who had progressed from URTI (61% and 27%, respectively, in group 3 vs 39% and 15%, respectively, in group 2). Further comparison between patients with possible LRTI versus those with proven/probable LRTI from groups 2 and 3 showed a higher need for supplemental oxygen use (69% vs 27%) and a higher need for mechanical ventilation (36% vs 4%) in patients with proven/probable LRTI versus those with possible LRTI. In cumulative incidence curves (Figure 1C and 1D), supplemental oxygen use and mechanical ventilation from the time of LRTI were significantly higher for patients with proven/probable LRTI than for those with possible LRTI (P < .0001 and P = .0003, respectively, by the log-rank test).
Figure 1.
A and B, Unadjusted cumulative incidence curves for time to first use of supplemental oxygen (A) and time to mechanical ventilation (B) at day 28 following initial diagnosis of respiratory syncytial virus (RSV) infection, categorized by group. P < .0001, by the log-rank test (A and B). C and D, Unadjusted cumulative incidence curves for time to first use of supplemental oxygen (C) and time to mechanical ventilation (D) at day 28 following diagnosis of RSV lower respiratory tract infection (LRTI) in groups 2 and 3, categorized by possible versus proven/probable LRTI. P < .0001, by the log-rank rest (C); P = .0003, by the log-rank test (D).
Risk factors for the time to first use of supplemental oxygen were evaluated in univariate modes among all patients from the time of initial diagnosis (Supplementary Table 1). Of the variables evaluated, only age at transplantation, LRTI (as a time-dependent variable), steroid use in the 2 weeks prior to diagnosis of RSV infection, and PCR use as the testing platform were associated with the outcome. In a multivariate model (Table 3), all variables except PCR as a testing method remained significant risk factors for supplemental oxygen use. Risk factors for mechanical ventilation were also evaluated in univariate and multivariate models, and only LRTI as a time-dependent risk factor remained significant (Supplementary Tables 2 and 3).
Table 3.
Multivariate Analysis of Risk Factors for Time to First Use of Supplemental Oxygen During the 28 Days After Diagnosis of Syncytial Virus (RSV) Infection
| Covariate | HR (95% CI) | P |
|---|---|---|
| Recipient age at transplantation, y | ||
| <21 | 1 | |
| 21–60 | 4.87 (1.47–16.2) | .01 |
| >60 | 5.74 (1.64–20.1) | .006 |
| Steroid use at diagnosis | ||
| None | 1 | |
| ≤1 mg/kg/day | 1.92 (1.05–3.52) | .035 |
| >1 mg/kg/day | 2.39 (1.02–5.61) | .045 |
| LRTI as time-dependent covariate | ||
| No | 1 | |
| Yes | 7.63 (4.33–13.4) | <.001 |
| RSV testing method | ||
| Non-PCR | 1 | |
| PCR | 1.47 (.71–3.05) | .303 |
Data are for 230 hematopoietic cell transplant recipients.
Abbreviations: CI, confidence interval; HR, hazard ratio; LRTI, lower respiratory tract infection; PCR, polymerase chain reaction.
Supplemental Oxygen–Free Days Following RSV Infection Diagnosis
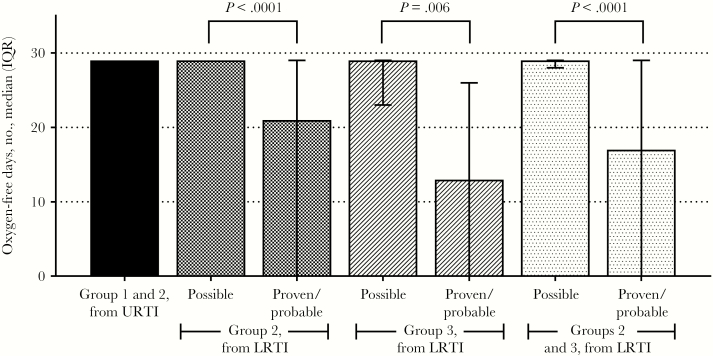
Median numbers of supplemental oxygen–free days (and associated interquartile ranges) while alive, per definition 1, are shown in Figure 3. The median number of supplemental oxygen–free days after URTI diagnosis for patients in groups 1 and 2 was 29 (IQR, 29–29 days). Group 3 patients with proven/probable LRTI were alive and free of supplemental oxygen use for a median of 16 days less than those in groups 1 and 2 from initial diagnosis (13 vs 29 days; P < .0001). Further analysis of groups 2 and 3 from the start of LRTI indicated that the number of supplemental oxygen–free days was significantly influenced by how LRTI was defined. Group 2 and 3 patients with possible LRTI had significantly more supplemental oxygen–free days than patients with probable/proven LRTI (29 days [IQR, 28–29 days] vs 17 days [IQR, 0–29 days]; P < .0001). Of the 9 subjects who died by day 28, only 3 had any supplemental oxygen–free days in that period. Thus, calculation of supplemental oxygen–free days per definition 2 did not change medians for any groups (Supplementary Table 4).
Figure 3.
Supplemental oxygen–free days while alive following respiratory syncytial virus (RSV) infection. Bars represent interquartile ranges (IQRs).
Mortality Following RSV Infection Diagnosis
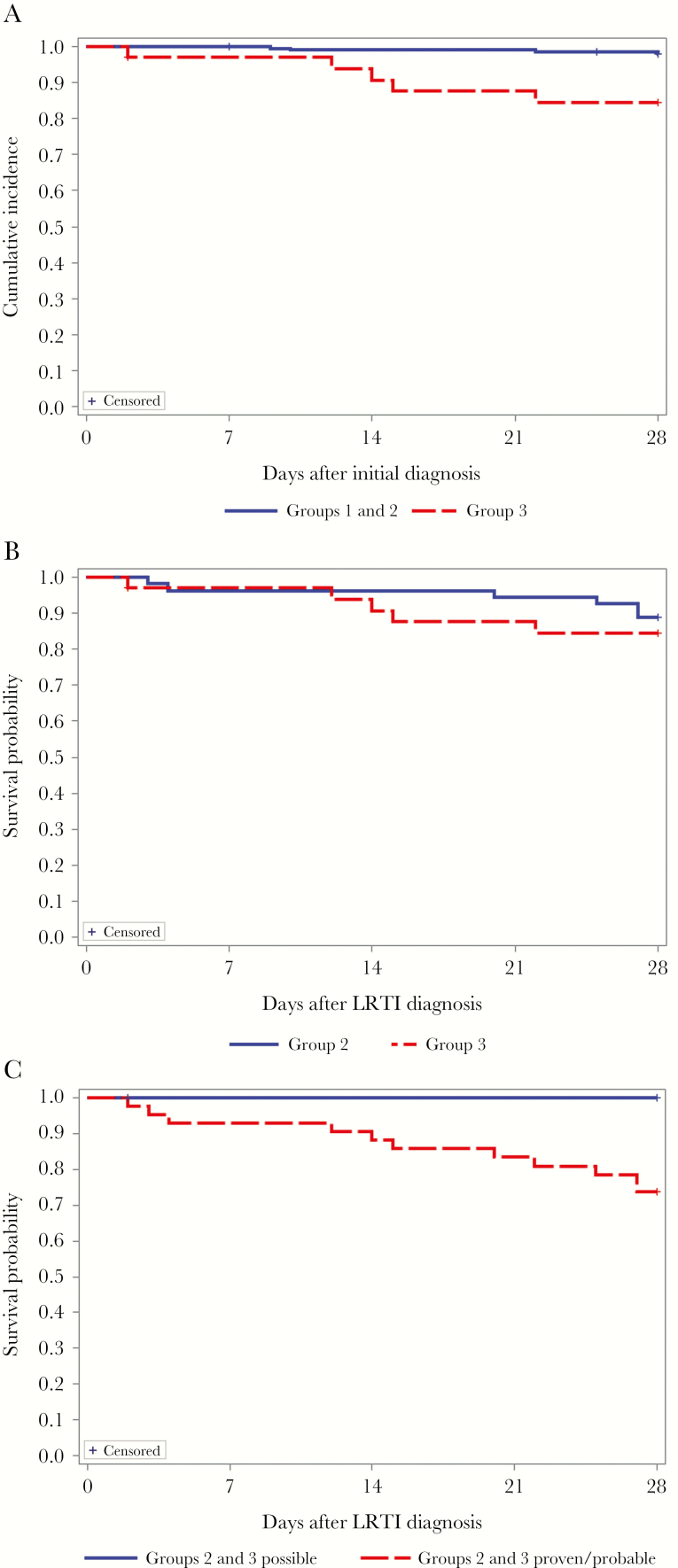
Death only occurred for patients in groups 2 and 3 (11% and 15%, respectively, during the 28 days following LRTI; Table 2). A Kaplan-Meier curve for mortality for all subjects from the time of initial diagnosis (URTI for groups 1 and 2 and LRTI for group 3) is shown in Figure 2A; curves differed significantly (P = .0002, by the log-rank test). Curves for mortality from the time of LRTI diagnosis (groups 2 and 3) are shown in Figure 2B and 2C. No difference was noted in patients with LRTI, whether or not they had progressed from URTI or presented with LRTI (P = .54, by the log-rank test; Figure 2B). The frequency of death was, however, influenced by the definition of LRTI. There were no deaths among patients defined as having possible LRTI; deaths were only observed in group 2 and 3 patients with probable or proven LRTI (26%; Table 2 and Figure 2C).
Figure 2.
Unadjusted Kaplan-Meier curves for overall survival at day 28 for groups 1 and 2 versus group 3 following initial diagnosis of respiratory syncytial virus (RSV) infection (P = .0002, by the log-rank test; A), for group 2 versus group 3 following RSV LRTI (P = .514, by the log-rank test; B), and for groups 2 and 3 possible versus groups 2 and 3 proven/probable (the log-rank test could not be performed because of a lack of events in possible group; C).
Sample Size Modeling
Given the mortality rates observed in this cohort and the potential for supplemental oxygen–free days to be considered as a relevant endpoint in clinical trials enrolling patients who develop RSV LRTI, we evaluated the differences in sample size requirements between mortality and supplemental oxygen–free day endpoints. Table 4 provides sample size estimates comparing mortality and number of supplemental oxygen–free days by day 28 for subjects developing or presenting with proven/probable LRTI (proven/probable from groups 2 and 3). Based on a baseline 28-day mortality rate of 26% in subjects with proven/probable LRTI, a reduction by a conservative estimate of 10% (to a 28-day mortality rate of 16%) would require a sample size of 221 subjects per arm (with a type 1 error of 5% and a power of 80%). In contrast, a conservative increase in the number of supplemental oxygen–free days from 16 (the observed mean number of supplemental oxygen–free days in patients with proven/probable LRTI) to 22 would require 64 subjects per arm. Additional sample size calculations for more and less conservative changes in mortality and number of supplemental oxygen–free days are shown in Table 4, with similarly lower sample sizes required in the supplemental oxygen–free day endpoint.
Table 4.
Sample Size Estimates for Use of Supplemental Oxygen–Free Days as an End Point in Clinical Trials of Proven/Probable Respiratory Syncytial Virus Lower Respiratory Tract Infection in Hematopoietic Cell Transplant Recipients
| End Point | ||
|---|---|---|
| Day 28 mortality rate, reduction from baseline,b % | Odds Ratio | Recipients/Arm, No.a |
| 2.5 | 0.874 | 4677 |
| 5 | 0.757 | 1128 |
| 10 | 0.542 | 260 |
| 15 | 0.352 | 105 |
| 20 | 0.182 | 52 |
| Oxygen-free days by day 28, increase from baseline,c no. | Effect Sized | Recipients/Arm, No.e |
| 2 | 0.164 | 586 |
| 4 | 0.328 | 147 |
| 6 | 0.492 | 66 |
| 8 | 0.656 | 38 |
| 10 | 0.82 | 25 |
Estimates were made on the assumptions of a type 1 error of 5% and a power of 80%.
aCalculated by a 2-sided Pearson χ2 test.
bThe baseline value was 26%.
cThe baseline value was 15 days.
dCalculated as the increase from baseline, divided by a SD of 12.2.
eCalculated by a 2-sided test.
DISCUSSION
In the present study, we established that clinical outcomes, including mortality and supplemental oxygen–free days, are significantly worse in HCT recipients with RSV detected in the lower respiratory tract (ie, those with probable/proven LRTI) than in patients with RSV detected in the upper respiratory tract only (ie, those with URTI and possible LRTI). Death due to any cause only occurred in patients with proven/probable LRTI. We also demonstrated significant reductions in the number of supplemental oxygen–free days for patients with proven/probable LRTI as compared to those with possible LRTI. These data support the use of supplemental oxygen–free days as a potential clinical endpoint that could be associated with significant reductions in sample size.
RSV infection in HCT recipients has historically been associated with significant mortality, with death rates of up to 55% in patients with LRTI [9–11]. However, mortality rates have been declining, presumably because of changes in transplantation practices, such as declining use of myeloablative regimens and improved supportive care practices. Additionally, previous studies of RSV in HCT recipients have used different definitions of LRTI which may account for wide variations in endpoints, including progression to LRTI, supplemental oxygen use, and mortality [1, 10, 11, 15, 18]. Current guidelines also include possible LRTI in recommendations for management of RSV [19]. Furthermore, differences exist among transplant centers in the use of BAL for diagnosing LRTI due to respiratory viruses. In the present study, we sought to characterize clinical outcomes both by the clinical status at the time of presentation to care (ie, URTI [group 1], URTI with progression to LRTI [group 2], and LRTI [group 3]) and by the site of virologic detection (URTI only, possible LRTI, probable LRTI, or proven LRTI). We found substantial differences in supplemental oxygen use, the number of supplemental oxygen–free days, and mortality between subjects with and those without RSV detected in the lower respiratory tract. Our data demonstrate that subjects with possible LRTI required very little supplemental oxygen and had no deaths and that they are more similar to subjects with URTI only, regardless of initial presentation. Conversely, patients with proven/probable LRTI are the only subjects who died and had significantly higher use of supplemental oxygen. These data are consistent with a previous study evaluating parainfluenza virus LRTI in HCT recipients, in which outcomes in possible LRTI cases were significantly better than in proven/probable LRTI cases [16]. Taken together, these data suggest that a less conservative approach to defining LRTI without determining whether RSV is truly in the lower respiratory tract (in cases where imaging findings are abnormal) may be clinically misleading and contributes to the finding that mortality rates appear to have decreased among patients with a diagnosis of RSV LRTI.
The number of supplemental oxygen–free days as an endpoint was also evaluated in the current study. Supplemental oxygen use correlates with acute lung injury, which, in turn, is associated with mortality in HCT recipients [15, 20, 21]. Additionally, supplemental oxygen use typically requires hospitalization; thus, the number of supplemental oxygen–free days represent a composite endpoint that takes into account disease severity, need for hospitalization, and mortality. Our data showed significant decreases in supplemental oxygen–free days in subjects with any LRTI as compared to those with URTI, and further decreases were seen in subjects with proven/probable LRTI as compared to those with possible LRTI. Ventilator-free days were first defined in the 1990s and then introduced to the ARDSNet in 2002 by Schoenfeld et al [22, 23], and they have been used as an end point extensively in the intensive care unit literature. Several studies, including randomized controlled trials [24–27] and limited studies of viral infections such as enteroviruses [28], influenza virus [29, 30], and herpes viruses [31] in critically ill patients, have since used this end point. Our definition of supplemental oxygen–free days is consistent with Schoenfeld’s original definition for ventilator-free days in acute respiratory distress syndrome [23], with 2 notable exceptions. First, given the low rates of mechanical ventilation in this cohort (7% overall), our study was not sufficiently powered to evaluate ventilator-free days and thus evaluated supplemental oxygen–free days. Generally, patients receiving >2 L of supplementary oxygen per minute require hospital admission, and thus an increasing number of supplemental oxygen–free days still represents significant healthcare cost savings. Second, the effect of death was evaluated both with the traditional definition (wherein death at any point during the 28-day period gives a score of 0 supplemental oxygen–free days for that subject) and with a definition that allows any days prior to death during which no supplemental oxygen was used to be counted as supplemental oxygen–free days. This second definition was used, given the high levels of competing risk of death in transplant recipients. Ultimately, only 3 subjects had any supplemental oxygen–free days prior to death, and thus mean and median supplemental oxygen–free days did not vary greatly with use of either definition.
Numbers of supplemental oxygen–free days were significantly different among subjects, based on their initial presentation (groups 1, 2, and 3), as well as by the classification of LRTI (possible vs proven/probable; Figure 3). The lowest median number of supplemental oxygen–free days occurred in patients presenting with proven/probable LRTI (13 days), followed by subjects with proven/probable LRTI regardless of presentation (17 days). In contrast, subjects with possible LRTI in either group 2 or 3 had a median of 29 supplemental oxygen–free days, the same as subjects with URTI only. These data, along with mortality outcomes outlined above, suggest that the prognosis of HCT recipients with RSV detected in the upper respiratory tract and radiographic abnormalities requires sampling of the lower respiratory tract (eg, BAL) to determine whether proven LRTI is present. Confirmation of RSV LRTI with BAL can provide useful prognostic information and may help stratify patients by risk and help prioritize expensive and intensive therapeutic options. Currently, ribavirin is the only antiviral available for specific treatment of RSV disease [10, 11, 15, 32]; however, cost remains a prohibitive barrier to its use, with an estimated cost of greater than $250 000 for a 10-day course [33]. Risk stratification based on confirmation of probable/proven LRTI may assist in treatment considerations for HCT recipients with RSV. In addition, several antivirals are in phase 2 trials for treatment of RSV infections in various populations [5, 34–37], including HCT recipients [7, 8], and risk stratification may help develop rational prevention and treatment strategies for immunocompromised patients with these agents.
Based on outcome data from the current study, we calculated estimated sample size requirements to compare the 28-day mortality rate and number of supplemental oxygen–free days by day 28 as endpoints in subjects with proven/probable LRTI (Table 4). Supplemental oxygen–free days have the potential to significantly reduce the per arm sample size, compared with a traditional mortality endpoint, and could potentially aid in designing clinical trials with feasible sample sizes while maintaining a clinically relevant endpoint. The most common criticisms of use of ventilator-free days as an endpoint is lack of consensus definitions and high levels of heterogeneity, thus making comparisons among studies difficult [38–41]. Because this is the first-time that supplemental oxygen–free days has been evaluated for RSV infection in HCT recipients and because this is a single center study, further studies that implement strict criteria for evaluating the need for supplemental oxygen and apply consistent definitions are needed. In addition, accurate diagnosis of proven/probable LRTI may also play a significant role in enriching clinical trial recruitment for patients who are most likely to experience the adverse outcome, thus also reducing the sample size.
This study has several limitations. First, this is a retrospective review, and the use of BAL to evaluate LRTI is largely based on protocols at our center, which recommend BAL when RSV is detected in the upper respiratory tract and radiographic abnormalities are present. However, the decision to pursue bronchoscopy is ultimately left to the attending physician, and thus some potential probable or proven cases may have been missed. Despite this inherent limitation in capturing the proven/probable LRTI cases, we demonstrated differences in clinical outcomes between groups. Additionally, the definition of a supplemental oxygen–free day as one during which supplementation with ≤2 L of oxygen per minute was required relies on subjective clinical decisions that may not have a physiological correlate. However, the requirement of a sustained 48-hour period without use of >2 L of supplemental oxygen per minute to be deemed an supplemental oxygen–free day should account for this inherent variability.
In conclusion, our data demonstrate that patients with RSV detected in the lower respiratory tract (ie, those with proven/probable LRTI) had worse outcomes than patients with RSV detected in the upper respiratory tract only despite the presence of radiographic abnormalities (ie, those with possible LRTI). The separation of LRTI into these groups could be useful in future outcomes research; however, validation of these results with other sites and protocols will be important. One potential application would be in cross-validating the immunodeficiency scoring index suggested for use in HCT recipients with RSV infection, to help develop consensus definitions [14]. Furthermore, by using a more conservative approach to defining LRTI and implementing supplemental oxygen–free days as a surrogate clinical endpoint, we believe clinical trials may be able to use smaller sample sizes, thereby facilitating more-rapid development of novel agents to treat RSV infections in HCT recipients.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. We thank Zach Stednick for help with data collection and management.
A. W. designed and performed the research, collected data, analyzed data, and wrote the manuscript; L. K., J. Y., S. O., and G. S. C. collected data and critically reviewed the manuscript; H. X. and W. L. performed statistical analyses, generated tables and figures, and critically reviewed the manuscript; T. W., J. W. C., and J. A. E. contributed to the analysis plan and critically reviewed the manuscript; and J. A. E. and M. B. designed and performed the research, analyzed data, provided resources, and wrote the manuscript.
Financial support. This work was supported by the National Institutes of Health (grants National Heart, Lung, and Blood Institute K24 HL093294-06 [to M. B.] and National Institute of Allergy and Infectious Diseases K23 AI114844-02 [to A. W.]), Gilead Sciences (investigator-initiated study grant to M. B.), and the Seattle Children’s Center for Clinical and Translational Research Clinical Research Scholar’s Program (to A.W.).
Potential conflicts of interest. M. B. received research support from Aviragen Therapeutics and Gilead Sciences and served as a consultant for Gilead Sciences, Aviragen Therapeutics, Humabs Biomed, and Ablynx. J. A. E. received research support from Gilead Sciences, Alios BioPharma, GlaxoSmithKline, and Pfizer and served as a consultant for Gilead Sciences. G. S. C. has served as a consultant for Gilead Sciences. T. R. W. and J. W. C. are employees of Gilead Sciences. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Ljungman P, Ward KN, Crooks BN et al. . Respiratory virus infections after stem cell transplantation: a prospective study from the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant 2001; 28:479–84. [DOI] [PubMed] [Google Scholar]
- 2. Boeckh M. The challenge of respiratory virus infections in hematopoietic cell transplant recipients. Br J Haematol 2008; 143:455–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. DeVincenzo JP, Whitley RJ, Mackman RL et al. . Oral GS-5806 activity in a respiratory syncytial virus challenge study. N Engl J Med 2014; 371:711–22. [DOI] [PubMed] [Google Scholar]
- 4. DeVincenzo JP, McClure MW, Symons JA et al. . Activity of oral ALS-008176 in a respiratory syncytial virus challenge study. N Engl J Med 2015; 373:2048–58. [DOI] [PubMed] [Google Scholar]
- 5. Gottlieb J, Zamora MR, Hodges T et al. . ALN-RSV01 for prevention of bronchiolitis obliterans syndrome after respiratory syncytial virus infection in lung transplant recipients. J Heart Lung Transplant 2016; 35:213–21. [DOI] [PubMed] [Google Scholar]
- 6. DeVincenzo J, Lambkin-Williams R, Wilkinson T et al. . A randomized, double-blind, placebo-controlled study of an RNAi-based therapy directed against respiratory syncytial virus. Proc Natl Acad Sci U S A 2010; 107:8800–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gilead Sciences. GS-5806 in hematopoietic cell transplant recipients with respiratory syncytial virus infection of the upper respiratory tract Bethesda, MD: National Library of Medicine; https://clinicaltrials.gov/ct2/show/NCT02254408. Accessed 28 July 2017. [Google Scholar]
- 8. Gilead Sciences. GS-5806 in hematopoietic cell transplant recipients with respiratory syncytial virus (RSV) infection of the lower respiratory tract Bethesda, MD: National Library of Medicine; https://clinicaltrials.gov/ct2/show/NCT02254421. Accessed 28 July 2017. [Google Scholar]
- 9. Renaud C, Xie H, Seo S et al. . Mortality rates of human metapneumovirus and respiratory syncytial virus lower respiratory tract infections in hematopoietic cell transplantation recipients. Biol Blood Marrow Transplant 2013; 19:1220–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Waghmare A, Campbell AP, Xie H et al. . Respiratory syncytial virus lower respiratory disease in hematopoietic cell transplant recipients: viral RNA detection in blood, antiviral treatment, and clinical outcomes. Clin Infect Dis 2013; 57:1731–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shah DP, Ghantoji SS, Shah JN et al. . Impact of aerosolized ribavirin on mortality in 280 allogeneic haematopoietic stem cell transplant recipients with respiratory syncytial virus infections. J Antimicrob Chemother 2013; 68:1872–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pilie P, Werbel WA, Riddell J 4th, Shu X, Schaubel D, Gregg KS. Adult patients with respiratory syncytial virus infection: impact of solid organ and hematopoietic stem cell transplantation on outcomes. Transpl Infect Dis 2015; 17:551–7. [DOI] [PubMed] [Google Scholar]
- 13. Anderson NW, Binnicker MJ, Harris DM et al. . Morbidity and mortality among patients with respiratory syncytial virus infection: a 2-year retrospective review. Diagn Microbiol Infect Dis 2016; 85:367–71. [DOI] [PubMed] [Google Scholar]
- 14. Shah DP, Ghantoji SS, Ariza-Heredia EJ et al. . Immunodeficiency scoring index to predict poor outcomes in hematopoietic cell transplant recipients with RSV infections. Blood 2014; 123:3263–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Seo S, Campbell AP, Xie H et al. . Outcome of respiratory syncytial virus lower respiratory tract disease in hematopoietic cell transplant recipients receiving aerosolized ribavirin: significance of stem cell source and oxygen requirement. Biol Blood Marrow Transplant 2013; 19:589–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Seo S, Xie H, Campbell AP et al. . Parainfluenza virus lower respiratory tract disease after hematopoietic cell transplant: viral detection in the lung predicts outcome. Clin Infect Dis 2014; 58:1357–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kuypers J, Wright N, Morrow R. Evaluation of quantitative and type-specific real-time RT-PCR assays for detection of respiratory syncytial virus in respiratory specimens from children. J Clin Virol 2004; 31:123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim YJ, Guthrie KA, Waghmare A et al. . Respiratory syncytial virus in hematopoietic cell transplant recipients: factors determining progression to lower respiratory tract disease. J Infect Dis 2014; 209:1195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hirsch HH, Martino R, Ward KN, Boeckh M, Einsele H, Ljungman P. Fourth European Conference on Infections in Leukaemia (ECIL-4): guidelines for diagnosis and treatment of human respiratory syncytial virus, parainfluenza virus, metapneumovirus, rhinovirus, and coronavirus. Clin Infect Dis 2013; 56:258–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rowan CM, Smith LS, Loomis A et al. ; Investigators of the Pediatric Acute Lung Injury and Sepsis Network Pediatric acute respiratory distress syndrome in pediatric allogeneic hematopoietic stem cell transplants: a multicenter study. Pediatr Crit Care Med 2017; 18:304–9. [DOI] [PubMed] [Google Scholar]
- 21. Seo S, Renaud C, Kuypers JM et al. . Idiopathic pneumonia syndrome after hematopoietic cell transplantation: evidence of occult infectious etiologies. Blood 2015; 125:3789–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bernard GR, Artigas A, Brigham KL et al. . The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994; 149:818–24. [DOI] [PubMed] [Google Scholar]
- 23. Schoenfeld DA, Bernard GR; ARDS Network Statistical evaluation of ventilator-free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Crit Care Med 2002; 30:1772–7. [DOI] [PubMed] [Google Scholar]
- 24. The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000; 342:1301–8. [DOI] [PubMed] [Google Scholar]
- 25. Willson DF, Thomas NJ, Markovitz BP et al. ; Pediatric Acute Lung Injury and Sepsis Investigators Effect of exogenous surfactant (calfactant) in pediatric acute lung injury: a randomized controlled trial. JAMA 2005; 293:470–6. [DOI] [PubMed] [Google Scholar]
- 26. Nguyen NQ, Besanko LK, Burgstad C et al. . Delayed enteral feeding impairs intestinal carbohydrate absorption in critically ill patients. Crit Care Med 2012; 40:50–4. [DOI] [PubMed] [Google Scholar]
- 27. Papazian L, Forel JM, Gacouin A et al. ; ACURASYS Study Investigators Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med 2010; 363:1107–16. [DOI] [PubMed] [Google Scholar]
- 28. Chi CY, Khanh TH, Thoa le PK et al. . Milrinone therapy for enterovirus 71-induced pulmonary edema and/or neurogenic shock in children: a randomized controlled trial. Crit Care Med 2013; 41:1754–60. [DOI] [PubMed] [Google Scholar]
- 29. Oh DK, Lee MG, Choi EY et al. ; Korean Society of Critical Care Medicine H1N1 collaborative Low-tidal volume mechanical ventilation in patients with acute respiratory distress syndrome caused by pandemic influenza A/H1N1 infection. J Crit Care 2013; 28:358–64. [DOI] [PubMed] [Google Scholar]
- 30. Delaney JW, Pinto R, Long J et al. ; Canadian Critical Care Trials Group H1N1 Collaborative The influence of corticosteroid treatment on the outcome of influenza A(H1N1pdm09)-related critical illness. Crit Care 2016; 20:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Coisel Y, Bousbia S, Forel JM et al. . Cytomegalovirus and herpes simplex virus effect on the prognosis of mechanically ventilated patients suspected to have ventilator-associated pneumonia. PLoS One 2012; 7:e51340. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32. Shah DP, Ghantoji SS, Mulanovich VE, Ariza-Heredia EJ, Chemaly RF. Management of respiratory viral infections in hematopoietic cell transplant recipients. Am J Blood Res 2012; 2:203–18. [PMC free article] [PubMed] [Google Scholar]
- 33. Chemaly RF, Aitken SL, Wolfe CR, Jain R, Boeckh MJ. Aerosolized ribavirin: the most expensive drug for pneumonia. Transpl Infect Dis 2016; 18:634–6. [DOI] [PubMed] [Google Scholar]
- 34. Gilead Sciences. Efficacy, pharmacokinetics, and safety of gs-5806 in hospitalized adults with respiratory syncytial virus (RSV) infection Bethesda, MD: National Library of Medicine; https://clinicaltrials.gov/ct2/show/NCT02135614. Accessed 28 July 2017. [Google Scholar]
- 35. Gilead Sciences. GS-5806 in lung transplant (LT) recipients with respiratory syncytial virus (RSV) infection Bethesda, MD: National Library of Medicine; https://clinicaltrials.gov/ct2/show/NCT02534350. Accessed 28 July 2017. [Google Scholar]
- 36. Alios Biopharma. A study of ALS-008176 in infants hospitalized with RSV Bethesda, MD: National Library of Medicine; https://clinicaltrials.gov/ct2/show/NCT02202356. Accessed 28 July 2017. [Google Scholar]
- 37. Zamora MR, Budev M, Rolfe M et al. . RNA interference therapy in lung transplant patients infected with respiratory syncytial virus. Am J Respir Crit Care Med 2011; 183:531–8. [DOI] [PubMed] [Google Scholar]
- 38. Spragg RG, Bernard GR, Checkley W et al. . Beyond mortality: future clinical research in acute lung injury. Am J Respir Crit Care Med 2010; 181:1121–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Moss M. Mortality is the only relevant outcome in ARDS: yes. Intensive Care Med 2015; 41:141–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Contentin L, Ehrmann S, Giraudeau B. Heterogeneity in the definition of mechanical ventilation duration and ventilator-free days. Am J Respir Crit Care Med 2014; 189:998–1002. [DOI] [PubMed] [Google Scholar]
- 41. Blackwood B, Clarke M, McAuley DF, McGuigan PJ, Marshall JC, Rose L. How outcomes are defined in clinical trials of mechanically ventilated adults and children. Am J Respir Crit Care Med 2014; 189:886–93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.