Summary
Significant heterogeneity in rotavirus infection and disease exists across 8 birth cohorts. Overcrowding and high stool pathogen detection were associated with higher incidence of infection and disease. Studies among older children in diverse settings are needed to understand vaccine performance.
Keywords: children, efficacy, gastroenteritis, rotavirus, vaccine
Abstract
Background
In a multicountry birth cohort study, we describe rotavirus infection in the first 2 years of life in sites with and without rotavirus vaccination programs.
Methods
Children were recruited by 17 days of age and followed to 24 months with collection of monthly surveillance and diarrheal stools. Data on sociodemographics, feeding, and illness were collected at defined intervals. Stools were tested for rotavirus and sera for antirotavirus immunoglobulins by enzyme immunoassays.
Results
A total of 1737 children contributed 22646 surveillance and 7440 diarrheal specimens. Overall, rotavirus was detected in 5.5% (408/7440) of diarrheal stools, and 344 (19.8%) children ever had rotavirus gastroenteritis. Household overcrowding and a high pathogen load were consistent risk factors for infection and disease. Three prior infections conferred 74% (P < .001) protection against subsequent infection in sites not using vaccine. In Peru, incidence of rotavirus disease was relatively higher during the second year of life despite high vaccination coverage.
Conclusions
Rotavirus infection and disease were common, but with significant heterogeneity by site. Protection by vaccination may not be sustained in the second year of life in settings with high burdens of transmission and poor response to oral vaccines.
Before rotavirus vaccines were introduced, despite availability of effective rehydration therapies, rotavirus was estimated to cause nearly half a million deaths annually [1]. Where rotavirus vaccines have been introduced into national programs, a dramatic decrease in hospitalizations due to rotavirus has been reported [2–5]. Reductions in all-cause diarrhea mortality [6, 7] further emphasize the importance of vaccination to prevent severe disease.
Several rotavirus surveillance networks measure the burden of disease and impact following vaccine introduction [8–10], but these are hospital-based and thus capture more severe disease. Given challenges of community-based etiological studies of gastroenteritis, most studies on rotavirus have been cross-sectional; only a few birth cohort studies testing both diarrhea and surveillance samples have been published from low- and middle-income countries (LMICs) in the past 3 decades [11–14]. These geographically distinct studies showed similar incidence of disease [11–13] but remarkable variation in protection afforded by prior infection, a finding subsequently associated with vaccine performance in similar locations [15–17].
The Etiology, Risk Factors, and Interactions of Enteric Infections and Malnutrition and the Consequences for Child Health and Development (MAL-ED) project is a multisite birth cohort study at 8 sites [18]. In this report, we present data on asymptomatic and symptomatic rotavirus infections and compare risk factors across sites. We have demonstrated the effect of rotavirus vaccines on rotavirus disease in settings with routine rotavirus vaccination.
METHODS
The MAL-ED multisite study was conducted from April 2009 to February 2014 in 8 countries with historically high prevalence of childhood diarrhea and malnutrition [18]. Healthy children from the community enrolled within 17 days of birth were followed until 24 months of age at the following locations: Dhaka, Bangladesh (BGD); Fortaleza, Brazil (BRF); Vellore, India (INV); Bhaktapur, Nepal (NEB); Loreto, Peru (PEL); Naushero Feroze, Pakistan (PKN); Venda, South Africa (SAV), and Haydom, Tanzania (TZH). In 3 countries (Brazil, Peru, and South Africa) rotavirus vaccination was provided by the national immunization programs prior to the start of the study. In Tanzania, the vaccine was introduced between December 2012 and January 2013; by then the youngest child in the cohort had crossed 12 months and was hence age ineligible to receive the vaccine.
Appropriate ethical approvals were obtained at all sites. The parent or guardian of every enrolled child provided written informed consent. Details of the study design, descriptions of sites, and surveillance and microbiologic methods have been published previously [19–28].
Data Collection
Household demographics, presence of siblings, maternal characteristics, and other data on the child’s birth and anthropometry were obtained at enrollment [18]. The socioeconomic status (SES) of families was assessed at 6, 12, 18, and 24 months. Anthropometric measurements and vaccination history were collected monthly. Details of illness and child feeding practices were collected during twice-weekly household visits [27].
Surveillance for Diarrhea
Diarrheal episodes were defined by ≥3 loose stools in a 24-hour period and were separated by at least 2 days. Severity was assessed using a modified Vesikari score (mild, 1–4; moderate, 5–8; severe, 9–13; and very severe, 14–17) [27] and the severity score for community diarrhea (CODA) scoring system specifically designed for community-based studies, which includes fever, anorexia, vomiting, frequency, and duration of loose stools [29]; severity scores range from 0 to 15 and episodes were classified as mild (0), moderate (1–6), or severe (≥7).
Stool Collection and Microbiology
Stool samples collected monthly for the first 12 months and at 15, 18, 21, and 24 months, and during all diarrheal episodes till 24 months of age, were preserved, transported, and processed at all sites using harmonized protocols [28]. Rotavirus testing for all specimens used ProSpecT (Oxoid Ltd, Ely, United Kingdom), an enzyme immunoassay to detect the group-specific antigen for rotavirus. A child had rotavirus diarrhea if any diarrheal specimen tested positive for rotavirus. Rotavirus-positive results were considered negative if the stools were collected within 28 days of receiving rotavirus vaccine. Other enteropathogens were assessed using previously reported laboratory methods and overall results have been published [30].
Serum Collection and Testing
About 4–5 mL of blood was collected at 7 and 15 months of age from each child and plasma aliquots were frozen at −20°C. Immunoglobulin G (IgG) and immunoglobulin A (IgA) antibodies against rotavirus were measured by enzyme immunoassay using standardized protocols [31, 32]. A child was considered previously exposed to rotavirus if the IgG or IgA values were >20 U/mL.
Statistical Analysis
Incidence rates with 95% confidence intervals (95% CIs) and median ages for first rotavirus infection and diarrhea were estimated for the entire cohort and each site separately. Poisson multilevel, site- and age-adjusted regression models with random effects to account for within-site and within-child correlations were used to estimate the incidence rate ratios (IRRs) for rotavirus infection and diarrhea, unadjusted and adjusted for sex, weight at first month, history of child death in the family, maternal age at childbirth, education and nutrition status, duration of exclusive breastfeeding, SES, overcrowding, child nutritional status during the first year, and average number of pathogens detected per stool specimen. Kaplan-Meier survival analysis was used to estimate time to first rotavirus infection at each site.
Associations between rotavirus infections, diarrhea, and other exposures were explored using generalized estimating equations specifying a Poisson distribution to obtain IRRs adjusted for age. Cox proportional hazards models were employed to estimate incidence rates and hazard ratios (HRs) for rotavirus infection and diarrhea with subsequent rotavirus infection determined either by stool positivity or serological testing (IgG or IgA >20 units). All analyses were performed using Stata software version 12 (StataCorp, College Station, Texas).
RESULTS
Burden of Rotavirus Infection and Disease
A total of 1737 children were followed until 24 months of age across all sites (Table 1) and contributed 22646 surveillance stools and 7440 diarrheal specimens collected from 9396 diarrheal episodes, with a total follow-up period of 1243351 days.
Table 1.
Burden of Rotavirus Infection and Disease in the First 2 Years of Life in the MAL-ED Cohort
| Dhaka, Bangladesh | Fortaleza, Brazila | Vellore, India | Bhaktapur, Nepal | Loreto, Perua | Naushero Feroze, Pakistan | Venda, South Africaa | Haydom, Tanzania |
Overall | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Children followed to 24 mo |
213 | 167 | 228 | 228 | 198 | 252 | 240 | 211 | 1737 | |
| Incidence of rotavirus infection per 100 child-years (95% confidence interval) | Year 1 | 70.17 (59.46–82.27) |
3.07 (1.12–6.80) |
21.62 (16.12–28.43) |
30.43 (23.86–38.28) |
33.74 (26.26–42.73) |
35.43 (28.6–43.49) |
13.78 (9.53–19.33) |
33.31 (26.07–41.97) |
30.77 (28.2–33.5) |
| Year 2 | 33.34 (26.19–41.87) |
6.56 (3.44–11.39) |
12.69 (8.66–17.98) |
18.35 (13.40–24.57) |
60.30 (50.1–72.0) |
17.88 (13.15–23.79) |
10.15 (6.65–14.87) |
17.54 (12.47–24.02) |
21.72 (19.6–24.1) |
|
| Incidence of rotavirus diarrhea per 100 child-years | Year 1 | 40.85 (32.84–50.26) |
0.61 (0.03–3.03) |
11.26 (7.45–16.38) |
15.88 (11.29–21.74) |
9.86 (6.11–15.12) |
18.73 (13.87–24.77) |
0.89 (0.15–2.94) |
6.37 (3.54–10.62) |
13.46 (11.17–15.29) |
| Year 2 | 23.34 (17.46–30.6) |
2.38 (0.75–5.75) |
6.12 (3.48–10.03) |
14.85 (10.45–20.52) |
25.77 (19.33–33.70) |
9.35 (6.06–13.80) |
1.27 (0.32–3.45) |
1.95 (0.62–4.70) |
10.54 (9.1–12.2) |
|
| Median (IQR){range} age at first rotavirus infection, d | 241 (157–336.5) {6–654} |
539 (306–641) {37–738} |
301 (189–386) {20–684} |
304 (197–459) {41–713} |
376 (215–566.5) {1–744} |
252 (153–389) {29–731} |
334 (212–461) {22–738} |
251 (149–370) {28–736} |
282 (179–437) {1–744} |
|
| Median (IQR) {range} age at first rotavirus diarrhea, d | 270 (183–384) {23–654} |
490 (430–539) {37–738} |
301.5 (223–379) {20–702} |
348 (214–493) {101–713} |
458 (343–579) {101–744} |
270 (169–384) {40–729} |
405 (262–413) {228–445} |
246 (182–355) {82–548} |
327 (210–450.5) {20–744} |
|
| Children exposed by serologyb at 7 mo, No. (%) | 169 (89.4) | 126 (96.2) | 205 (90) | 205 (94.4) | 182 (95.8) | 202 (82.1) | 192 (94.1) | 105 (81.4) | 1384 (90.87) |
|
| Children exposed by serologyb at 15 mo, No. (%) | 175 (94.6) | 106 (79) | 192 (85) | 172 (78.2) | 121 (78.6) | 209 (87.1) | 192 (87.3) | 97 (85.8) | 1255 (84.97) |
|
Abbreviations: IQR, interquartile range; MAL-ED, Etiology, Risk Factors, and Interactions of Enteric Infections and Malnutrition and the Consequences for Child Health and Development.
aSites using monovalent rotavirus vaccine in their national immunization program.
bImmunoglobulin G or immunoglobulin A >20 units/mL.
Rotavirus was detected in 930 of 30086 (3.1%) specimens. Thirty-eight positive samples were considered negative as the stools were collected within 28 days following immunization. Exact date of immunization was not available for 2 children (1 each from the Brazilian and Peruvian sites). Of the remaining 36 positives, 30.5% (11/36) were detected within 14 days of vaccination and all were surveillance stool specimens. A total of 892 stool positives were included in the analyses. Of the 1737 children, 659 (37.9%) were ever infected with rotavirus and only 344 (19.8%) ever had acute diarrhea associated with rotavirus. Rotavirus was detected in 5.5% (408/7440) of diarrheal stool specimens. Despite the low rates of stool detection, serology showed that 91% of the study cohort had IgA or IgG antibodies >20 units by 7 months. In sites using vaccines (BRF, PEL, SAV), >94% of children were rotavirus seropositive at 7 months, whereas in sites not using vaccine (BGD, INV, NEB, PKN, and TZH), 81.5%–90% of children had antibodies (Table 1).
Proportions of children receiving at least 1 dose of rotavirus vaccine were 88% (147/167), 96.2% (231/240), and 98% (194/198) and those receiving 2 doses were 71.8% (120/147), 85.8% (206/240), and 96.9% (192/198) in BRF, SAV, and PEL, respectively. Adherence to vaccination schedule varied across the 3 sites using rotavirus vaccine with 44.9%, 71.2%, and 64.6% of the children receiving 2 doses of vaccine within a +14-day window of scheduled age in BRF, PEL, and SAV respectively. None of the children in other sites had received the vaccine.
The proportion of children identified as infected by stool antigen detection was significantly higher in countries without vaccination (478/1132 [42.2%] vs 181/605 [29.9%] with vaccination; P < .001). The proportion infected was significantly higher in BGD (63.8% [136/213]) and PEL (58.5% [116/198]), compared with PKN (41.2% [104/252]), NEB (41.2% [94/228]), TZH (39.3% [83/211]), INV (26.7% [61/228]), and SAV (20.8% [50/240]) and was the least in BRF (8.9% [15/167]).
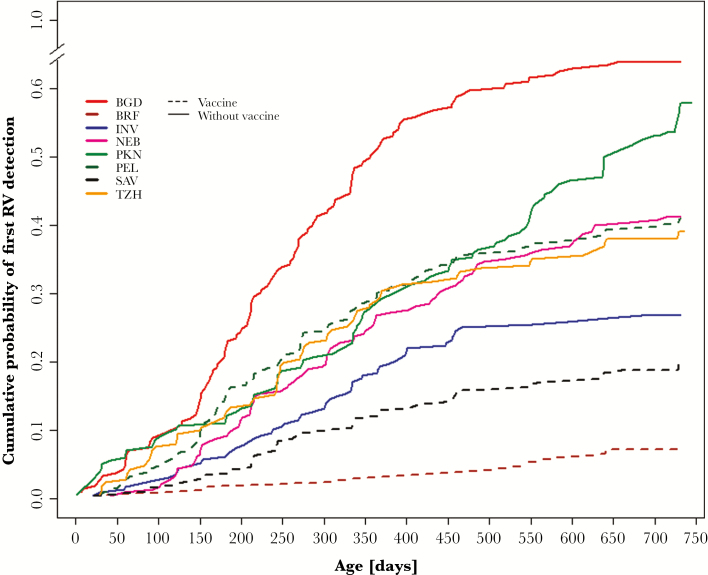
Repeat or multiple infections based on stool testing were seen in only 178 (10%) children, with the highest proportion in BGD (27.2% [58/213]), followed by PEL (22.7% [45/198]). The incidence of rotavirus infection during infancy across all sites was 30.77 per 100 child-years, higher than in the second year of life (21.72 per 100 child-years). This pattern was consistent across all sites except BRF and PEL. The incidence of rotavirus infection during the first year of life was highest in BGD and was least in BRF (Table 1; Supplementary Figure 1). Surprisingly, in the second year of life, PEL had the highest incidence rate, despite high vaccination coverage (96.9% [192/198]). The site in Peru experienced higher rotavirus season during the latter part of the study, with overall incidence of rotavirus infection being 31.28, 32.59, 43.79, 89.66, and 235.64 for the years 2010, 2011, 2012, 2013, and 2014, respectively.
Similar to infection, the incidence of rotavirus diarrhea was significantly higher in infancy (13.46 per 100 child-years) than in the second year (10.54 per 100 child-years) (P = .014). SAV and BRF had low incidence of rotavirus diarrhea over 2 years of follow-up, but in PEL, incidence of rotavirus diarrhea (25.77 per 100 child years) in the second year was significantly higher (P < .001) than in infancy (Table 1; Supplementary Figure 1). Two-year burden of rotavirus infection and diarrhea were significantly higher in sites not using vaccine (Table 2).
Table 2.
Burden of Rotavirus Infection and Disease in Sites With and Without Rotavirus Vaccine
| Rotavirus Infection and Disease | Incidence Rate per 100 Child-years (95% CI) | |||
|---|---|---|---|---|
| Sites Using Rotavirus Vaccine | Sites not Using Rotavirus Vaccine (BGD, INV, NEB, PKN, and TZH) | Overall | ||
| PEL | BRF, SAV | |||
| Rotavirus infection during first 2 years | 47.07a (40.6–54.3) | 8.96 (7.05–11.24) | 28.71b (26.55–31.01) | 26.20 (24.53–27.97) |
| Rotavirus diarrhea during first 2 years | 17.84a (13.9–22.4) | 1.26 (.64–2.25) | 14.78b (13.25–16.45) | 11.98 (10.86–13.19) |
| Hospitalized rotavirus diarrhea during first 2 years | 0.25 (.013–1.27) | … | 1.08b (.70–1.58) | 0.73 (.48–1.06) |
Abbreviations: BGD, Dhaka, Bangladesh; BRF, Fortaleza, Brazil; CI, confidence interval; INV, Vellore, India; NEB, Bhaktapur, Nepal; PEL, Loreto, Peru; PKN, Naushero Feroze, Pakistan; SAV, Venda, South Africa; TZH, Haydom, Tanzania.
a P < .005, comparing PEL with BRF and SAV.
b P < .005, comparing sites not using vaccine with ones using vaccine.
The median ages of rotavirus infection and diarrhea for the entire cohort were 282 (interquartile range [IQR], 179–437) days and 327 (IQR, 210–450) days, respectively. Children were slightly older when first infected or at their first rotavirus diarrhea in sites with rotavirus vaccine (Table 1; Figure 1). In sites with vaccination, 0.5% (3/605), 2.1% (13/605), and 6.3% (38/605) of children were infected in the first week, the first month, and by 6 months of age, respectively. No rotavirus was detected in diarrheal stools in the first month whereas 0.8% (5/605) of children had rotavirus diarrhea between 2 and 6 months of age. In sites not using vaccine, 0.3% (3/1132) of infants were infected in the first week, 1.6% (18/1132) in the first month, and 14.2% (161/1132) by 6 months. The proportion of children who had rotavirus diarrhea were 0, 0.3% (3/1132), and 5.8% (66/1132) in the first week, the first month, and the first 6 months of age, respectively.
Figure 1.
Survival curves for age at first rotavirus infection across the MAL-ED sites. Abbreviations: BGD, Dhaka, Bangladesh; BRF, Fortaleza, Brazil; INV, Vellore, India; NEB, Bhaktapur, Nepal; PEL, Loreto, Peru; PKN, Naushero Feroze, Pakistan; RV, rotavirus; SAV, Venda, South Africa; TZH, Haydom, Tanzania.
Clinical Features of Rotavirus Gastroenteritis
Across all sites, the severity of rotavirus diarrhea (median, 4 [IQR, 3–6]) was higher than for nonrotavirus diarrhea (median, 3 [IQR, 2–5]) (P < .001) as assessed using the modified Vesikari score. For hospitalized rotavirus gastroenteritis, the median severity score was 6 (IQR, 5–8) (Supplementary Table 1; Supplementary Figure 2). Six percent of episodes (25/408) required hospitalization, with 17.6% of hospitalizations in Tanzania. Of 16 children with severe rotavirus gastroenteritis in sites with vaccination, 15 were in PEL and 13 of 15 (86.6%) had received 2 doses of rotavirus vaccine.
The median duration of the first rotavirus diarrheal episode was 4 (IQR, 1–35) days; 1 (IQR, 1–4) day in SAV and 4 (IQR, 1–12) days in BGD. Across the sites, 54% (220/408) of rotavirus diarrheal episodes were associated with vomiting, 5.4% (22/408) with fever, and 1.9% (8/408) with blood in stools.
Rotavirus gastroenteritis was seasonal. In the Asian sites except PKN, 2 peaks were observed: 1 in December and January, corresponding to winter, and 1 in July during the monsoon. PEL and TZH also had a higher burden during the monsoon (March to July) and lower rotavirus gastroenteritis during dry seasons (August to February) (Supplementary Figure 3).
Predictors of Rotavirus Infection and Disease
In site- and age-adjusted multivariate analysis, higher weight at the first month conferred significant protection against rotavirus infection (IRR, 0.838 [95% CI, .714–.984]; P = .031). Increasing maternal age, lower SES, and presence of overcrowding in the family were associated with higher incidence of infections but were not statistically significant. The average number of all enteric pathogens detected in stool specimens (bacteria, parasites, and viruses) was associated with significantly higher incidence of rotavirus infection (IRR, 1.512 [95% CI, 1.277–1.792]; P < .001; Table 3).
Table 3.
Risk Factors for Rotavirus Infection and Disease in the MAL-ED Cohort
| Risk Factor | Rotavirus Infection | Rotavirus Diarrhea | ||||
|---|---|---|---|---|---|---|
| IRR | (95% CI) | P Value | IRR | (95% CI) | P Value | |
| Female child | 0.944 | (.815–1.093) | .439 | 0.887 | (.712–1.105) | .285 |
| Weight at first month (kg) | 0.838a | (.714–.984) | .031 | 0.850 | (.668–1.081) | .185 |
| Presence of sibling | 0.954 | (.798–1.14) | .605 | 0.884 | (.677–1.153) | .362 |
| Any child death in the family | 0.906 | (.722–1.137) | .395 | 0.845 | (.597–1.195) | .34 |
| Maternal age (y) | 1.011 | (.997–1.026) | .117 | 1.012 | (.991–1.034) | .275 |
| Maternal education up to fifth grade | 0.97 | (.815–1.156) | .734 | 0.779 | (.597–1.017) | .066 |
| Maternal undernutrition (BMI <18.5 kg/m2) | 0.804 | (.624–1.036) | .092 | 0.787 | (.537–1.153) | .219 |
| Exclusively breastfed for <4 months | 0.810a | (.667–.983) | .033 | 0.893 | (.665–1.200) | .453 |
| Low socioeconomic status (<33rd centile of WAMI score) | 1.022 | (.842–1.24) | .827 | 1.227 | (.918–1.640) | .168 |
| Overcrowding in the family (>2 people per room) | 1.174 | (.983–1.403) | .077 | 1.401a | (1.072–1.830) | .014 |
| Child ever stunted during first year (LAZ < –2 SD at any time point during first year of life) |
0.932 | (.782–1.110) | .431 | 0.800 | (.614–1.043) | .099 |
| Child ever wasted during first year (WHZ < –2 SD at any time point during first year of life) |
0.979 | (.79–1.212) | .843 | 1.138 | (.826–1.566) | .429 |
| Child ever underweight during first year (WAZ < –2 SD at any time point during first year of life) |
0.931 | (.756–1.147) | .503 | 0.846 | (.620–1.154) | .291 |
| Average number of pathogens per stool sample | 1.512a | (1.277–1.792) | .0001 | 1.451a | (1.122–1.875) | .005 |
Abbreviations: BMI, body mass index; CI, confidence interval; IRR, incidence rate ratio; LAZ, length-for-age z score; MAL-ED, Etiology, Risk Factors, and Interactions of Enteric Infections and Malnutrition and the Consequences for Child Health and Development; SD, standard deviation; WAMI, access to improved water and sanitation, assets, maternal education, and household income; WAZ, weight-for-age z score; WHZ, weight-for-height z score.
aStatistically significant at P < .05 using a multilevel, country- and age-adjusted Poisson regression analysis.
Among the predictors of rotavirus infection, only 2 overlapped with rotavirus diarrhea. Living in crowded households (IRR, 1.401 [95% CI, 1.072–1.830]; P = .014) and high pathogen detection in stools (IRR, 1.451 [95% CI, 1.122–1.875]; P = .005) were significantly associated with higher incidence of rotavirus diarrhea (Table 3; Supplementary Table 2). Overcrowding was also associated with a significantly higher risk of multiple rotavirus diarrheal episodes in sites without vaccine (odds ratio, 4.16 [95% CI, 1.36–12.74]; P < .005).
Surprisingly, presence of siblings was associated with some protection against rotavirus diarrhea in Asian sites without rotavirus vaccination (BGD, INV, NEB, and PKN) with an IRR of 0.728 (95% CI, .552–.959; P < .05), whereas this was associated with higher risk in South American sites (BRF and PEL) with immunization (Supplementary Table 2).
On initial analysis, breastfeeding did not protect against rotavirus diarrhea; in Asian sites not using vaccine, the adjusted incidence rate ratio was 0.69 (P < .001) in children exclusively breastfed for <4 months (Supplementary Table 2). Because BGD had both the longest duration of exclusive breastfeeding as well as the highest rates of rotavirus infection and disease, additional analyses excluding BGD were conducted, and exclusive breastfeeding status was still not significantly associated with protection from rotavirus diarrhea during the first 24 months (IRR, 0.79 [95% CI, .52–1.19]; P = .260) (Supplementary Table 3). During the first 6 months (183 days) of life, the incidence rate of rotavirus disease was higher (8.43 per 100 child-years [95% CI, 5.71–12.03]; P = .08) among children exclusively breastfed for <25% (up to 45 days) of the time compared with 4.28 and 2.83 per 100 child-years in children exclusively breastfed for 25%–74% (46–135 days) and >74% (>135 days) of the time (Supplementary Table 4).
Protection From Infection and Disease Conferred by Prior Rotavirus Infection in Sites Without Vaccination
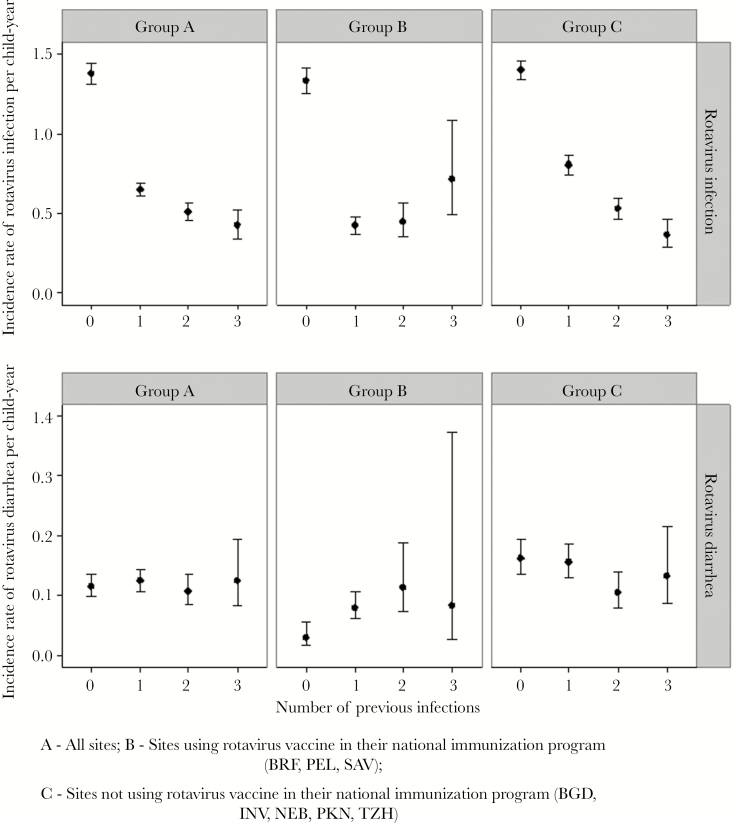
Prior rotavirus infections offered significant protection against subsequent infections (Figure 2). In sites without vaccination, children with 1, 2, and 3 infections had 43%, 62%, and 74% protection from subsequent infection (P < .01) (Table 4). Protection against disease was not significant, but this may be because of the small number of events. In sites with vaccination, there was reduction in infection following rotavirus vaccination or infection. Site-specific incidence rates and HRs are presented in Supplementary Tables 5 and 6. Across all sites, severity of rotavirus gastroenteritis among children with a first infection resulting in diarrhea was significantly higher (median CODA score = 5) (P = .013) compared with previously infected children (median CODA score = 4) (Supplementary Table 1).
Figure 2.
Incidence rates of subsequent rotavirus infection and diarrhea in the MAL-ED cohort. Abbreviations: BGD, Dhaka, Bangladesh; BRF, Fortaleza, Brazil; INV, Vellore, India; NEB, Bhaktapur, Nepal; PEL, Loreto, Peru; PKN, Naushero Feroze, Pakistan; SAV, Venda, South Africa; TZH, Haydom, Tanzania.
Table 4.
Incidence Rates and Hazard Ratios for Protection With Prior Rotavirus Infection
| No. of Previous Rotavirus Infections | Subsequent Rotavirus Infection | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | Countries Using Vaccine | Countries not Using Vaccine | ||||||||||
| Events | IR | Unadjusted HR | Adjusted HR (95% CI) |
Events | IR | Unadjusted HR | Adjusted HR (95% CI) | Events | IR | Unadjusted HR | Adjusted HR (95% CI) | |
| 0 | 1657 | 1.376 | 571 | 1.333 | 1086 | 1.399 | ||||||
| 1 | 929 | 0.649 | 0.471 | 0.439a (.401–.481) | 248 | 0.425 | 0.319 | 0.286a (.241–.338) | 681 | 0.802 | 0.573 | 0.531a (.478–.590) |
| 2 | 307 | 0.509 | 0.369 | 0.304a (.261–.355) | 68 | 0.450 | 0.337 | 0.271a (.205–.358) | 239 | 0.528 | 0.377 | 0.319a (.266–.383) |
| ≥3 | 92 | 0.424 | 0.307 | 0.222a (.176–.280) | 26 | 0.719 | 0.539 | 0.360a (.238–.545) | 66 | 0.365 | 0.260 | 0.197a (.150–.258) |
| Subsequent Rotavirus Diarrhea | ||||||||||||
| 0 | 139 | 0.115 | 13 | 0.030 | 126 | 0.162 | ||||||
| 1 | 178 | 0.124 | 1.077 | 1.178 (.916–1.515) | 46 | 0.079 | 2.601 | 1.305 (.564–3.022) | 132 | 0.156 | 0.958 | 1.161 (.889–1.516) |
| 2 | 64 | 0.106 | 0.918 | 0.795 (.561–1.125) | 17 | 0.112 | 3.703 | 1.254 (.490–3.210) | 47 | 0.104 | 0.640 | 0.750 (.509–1.105) |
| ≥3 | 27 | 0.124 | 1.077 | 0.673 (.409–1.106) | 3 | 0.083 | 2.733 | 0.495 (.114–2.152) | 24 | 0.133 | 0.816 | 0.774 (.456–1.315) |
Age- and site-adjusted incidence rates and hazard ratios.
Abbreviations: CI, confidence interval; HR, hazard ratio; IR, incidence rate.
a P < .001.
DISCUSSION
This is the largest-ever study to examine rotavirus infection and disease in LMIC children using a standardized approach and the first multicountry study. Although rotavirus is a leading cause of gastroenteritis where rotavirus vaccines are not in use, there is significant heterogeneity in infection and disease by site.
Serology showed that by 7 months of age, >80% of children at all sites had been exposed to rotavirus, but the rates of rotavirus infection and disease in the MAL-ED study by stool testing are much lower than from other birth cohorts [11–13]. In a previous study from similar settings in Vellore, India, which employed fortnightly stool sample testing, the overall incidence of rotavirus infection and diarrhea have been estimated to be 0.99 and 0.25 per child-year, respectively, during a 3-year follow-up between 2002 and 2006 [13]. In this study, rotavirus-positive results from stools were considered negative if collected within 28 days of receiving vaccine, which might have resulted in slight underestimation of the rates during infancy in sites using vaccine.
This may be due to the very restricted time window for stool collection, which resulted in stools being analyzed for only 79.2% of diarrheal episodes. Approximately 1 in 5 unvaccinated children had rotavirus gastroenteritis in the first 2 years of life in the MAL-ED cohorts, compared to at least 1 in 3 in Guinea-Bissau, Mexico, and India in earlier cohorts [11–13]. Among the 5 sites without vaccine, BGD had twice the incidence of infection and diarrhea of any other site in infancy and the second year of life (except incidence of diarrhea in the second year in NEB), but analysis of the relative contributions of rotavirus to disease reported attributable fractions of 9.6% in infancy and 6.0% in the second year of life for BGD, and higher attributable fractions for NEB and TZH in the second year of life [30].
There were striking differences between sites that administered rotavirus vaccination and those that did not, with the vaccine clearly preventing rotavirus infection and diarrhea in infancy. In all sites with vaccination, the time to first infection and first diarrhea was greater. In PEL, the rates of infection and diarrhea in the second year were high throughout the study period despite high rates of vaccine uptake and tight compliance to the recommended schedule at 2 and 4 months of age, indicating that vaccine-induced protection was not sustained beyond infancy. Prior reported longitudinal population-based studies in the periurban areas of Lima demonstrate internally consistent incidence rates with a median incidence rate of 30.9 per 100 child-years in the prevaccine era, lower than that observed in this study [33]. The higher-than-expected incidence is in part due to the site, which has intense enteropathogen exposure. Although we previously reported an attributable fraction of 2.9% for rotavirus in the second year in PEL, this was triple that in infancy [30], suggesting that the vaccine did have a favorable impact on the population for a time following vaccination. While the vaccine is intended to protect against severe rotavirus gastroenteritis, the high proportion of children with rotavirus gastroenteritis and the number of cases of severe gastroenteritis and hospitalizations in PEL suggest a lack of sustained protection by vaccination at that site. This site also exhibited high rates of nonresponsiveness to oral polio vaccine (Pan, unpublished data), demonstrating that this is not likely due to cold chain or vaccine failure of the Rotarix vaccine, but an impaired capacity of children to fully respond to oral vaccines. The proportion of study children belonging to lower socioeconomic strata was higher in Peru (12.6%) as compared to the Brazilian (nil) and South African (3.5%) sites. Similar findings have been reported from Malawi where, after vaccine introduction and with high coverage, a reduction in rotavirus hospital admission rate and detection rate in infants was observed along with a shift in the age distribution of rotavirus cases [34]. The shift in the incidence of this severe illness from infancy to later childhood can still be regarded as a success, as older children are less likely to die from diarrhea.
As previously shown in other studies, episodes of rotavirus diarrhea were more severe than nonrotavirus diarrhea, and in all sites, disease was more common in cooler months, with Asian sites showing a second peak associated with rainfall [35–37]. Among factors contributing to rotavirus infection and disease, being infected with other pathogens and living in crowded homes were associated with greater risk demonstrating the critical role of the environment in pathogen exposure. It is difficult to explain why presence of siblings could decrease risk of infection in some Asian sites; this could partly be due to greater exposure among mothers with more children, resulting in higher maternal antibodies protecting the newborn during infancy. The role of exclusive breastfeeding in protection from rotavirus infection or diarrhea could not be demonstrated (Table 3; Supplementary Tables 2 and 3), and this finding has previously been reported from India [13]. An earlier study from Bangladesh has reported that the exclusivity of breastfeeding confers protection in the first year and not in the second year of life [38].
In sites not using vaccine, protection conferred by prior infection was seen, confirming findings of previous birth cohorts [11–13], but surprisingly, it was not possible to measure protection against rotavirus diarrhea. The general trend was toward decreasing incidence of diarrhea with each subsequent rotavirus infection except in INV, where there was an increased incidence of diarrhea in children with 3 prior infections, but this was based on data from only 4 children (Supplementary Table 4).
Overall, this study demonstrates the value of using standardized protocols to assess infectious disease heterogeneity across populations. Despite the sites being chosen to be disadvantaged communities, the differences in incidence of infection and disease were surprising for a ubiquitous viral pathogen. Although no formal analyses of vaccine effectiveness could be carried out owing to the high coverage of vaccination, the apparent lack of protection by the rotavirus vaccine in the second year of life in the Peruvian site is an observation that has not been previously reported in Latin America. To understand vaccine performance across a range of disease severity, rotavirus disease should be investigated in older children in community settings and in diverse social and geographic environments.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
APPENDIX. MAL-ED NETWORK INVESTIGATORS
| Last Name | First Name | Institution | Role in the MAL-ED Network |
|---|---|---|---|
| Acosta | Angel Mendez | A.B. PRISMA, Iquitos, Peru | Data management |
| Chavez | Cesar Banda | A.B. PRISMA, Iquitos, Peru | Laboratory |
| Flores | Julian Torres | A.B. PRISMA, Iquitos, Peru | Laboratory |
| Olotegui | Maribel Paredes | A.B. PRISMA, Iquitos, Peru | Study coordinator |
| Pinedo | Silvia Rengifo | A.B. PRISMA, Iquitos, Peru | Nutrition |
| Trigoso | Dixner Rengifo | A.B. PRISMA, Iquitos, Peru | Laboratory |
| Vasquez | Angel Orbe | A.B. PRISMA, Iquitos, Peru | Psychologist, cognitive development |
| Ahmed | Imran | Aga Khan University, Naushahro Feroze, Pakistan | Data management |
| Alam | Didar | Aga Khan University, Naushahro Feroze, Pakistan | Laboratory, nutrition |
| Ali | Asad | Aga Khan University, Naushahro Feroze, Pakistan | Vaccine response |
| Bhutta | Zulfiqar A | Aga Khan University, Naushahro Feroze, Pakistan | Pakistan site PI |
| Qureshi | Shahida | Aga Khan University, Naushahro Feroze, Pakistan | Laboratory, microbiology |
| Shakoor` | Sadia | Aga Khan University, Naushahro Feroze, Pakistan | Microbiology |
| Soofi | Sajid | Aga Khan University, Naushahro Feroze, Pakistan | Operations, surveillance |
| Turab | Ali | Aga Khan University, Naushahro Feroze, Pakistan | Operations, surveillance, nutrition |
| Yousafzai | Aisha K | Aga Khan University, Naushahro Feroze, Pakistan | Cognitive development |
| Zaidi | Anita KM | Aga Khan University, Naushahro Feroze, Pakistan | Pakistan site co-PI, microbiology |
| Bodhidatta | Ladaporn | AFRIMS, Bangkok, Thailand | Microbiology |
| Mason | Carl J | AFRIMS, Bangkok, Thailand | Nepal site PI, vaccine response |
| Babji | Sudhir | Christian Medical College, Vellore, India | Microbiology supervisor |
| Bose | Anuradha | Christian Medical College, Vellore, India | Nutrition |
| John | Sushil | Christian Medical College, Vellore, India | India site co- PI |
| Kang | Gagandeep | Christian Medical College, Vellore, India | India site PI |
| Kurien | Beena | Christian Medical College, Vellore, India | Cognitive development supervisor |
| Muliyil | Jayaprakash | Christian Medical College, Vellore, India | Epidemiology |
| Mohan | Venkata Raghava | Christian Medical College, Vellore, India | Data management |
| Ramachandran | Anup | Christian Medical College, Vellore, India | Biochemistry, nutrition |
| Rose | Anuradha | Christian Medical College, Vellore, India | Epidemiology |
| Pan | William | Duke University, Durham, NC, USA | DCC, biostatistician |
| Ambikapathi | Ramya | FIC, NIH, Bethesda, MD, USA | DCC, nutrition |
| Carreon | Danny | FIC, NIH, Bethesda, MD, USA | DCC, SES and data management |
| Charu | Vivek | FIC, NIH, Bethesda, MD, USA | DCC, data management |
| Dabo | Leyfou | FIC, NIH, Bethesda, MD, USA | DCC, data management |
| Doan | Viyada | FIC, NIH, Bethesda, MD, USA | DCC, data management |
| Graham | Jhanelle | FIC, NIH, Bethesda, MD, USA | DCC, data management |
| Hoest | Christel | FIC, NIH, Bethesda, MD, USA | DCC, vaccine response |
| Knobler | Stacey | FIC, NIH, Bethesda, MD, USA | Senior scientific program director |
| Lang | Dennis | FIC, NIH, Bethesda, MD, USA | Senior program coordinator |
| McCormick | Benjamin | FIC, NIH, Bethesda, MD, USA | DCC, computational biology |
| McGrath | Monica | FIC, NIH, Bethesda, MD, USA | DCC, QA/QC lead, microbiology, epidemiology |
| Miller | Mark | FIC, NIH, Bethesda, MD, USA | PI |
| Mohale | Archana | FIC, NIH, Bethesda, MD, USA | DCC, data management |
| Nayyar | Gaurvika | FIC, NIH, Bethesda, MD, USA | DCC, data management |
| Psaki | Stephanie | FIC, NIH, Bethesda, MD, USA | DCC, SES |
| Rasmussen | Zeba | FIC, NIH, Bethesda, MD, USA | DCC, cognitive development, epidemiology |
| Richard | Stephanie | FIC, NIH, Bethesda, MD, USA | DCC, surveillance, epidemiology |
| Seidman | Jessica | FIC, NIH, Bethesda, MD, USA | DCC program manager, gut function, cognitive development, vaccine response |
| Wang | Vivian | FIC, NIH, Bethesda, MD, USA | DCC, data management |
| Blank | Rebecca | FNIH, Bethesda, MD, USA | Scientific program manager |
| Gottlieb | Michael | FNIH, Bethesda, MD, USA | PI |
| Tountas | Karen | FNIH, Bethesda, MD, USA | Scientific program manager |
| Amour | Caroline | Haydom Lutheran Hospital, Haydom, Tanzania | Laboratory |
| Mduma | Estomih | Haydom Lutheran Hospital, Haydom, Tanzania | Tanzania site co-PI, field site manager |
| Ahmed | Tahmeed | icddr-b, Dhaka, Bangladesh | Bangladesh site PI, nutrition |
| Ahmed | AM Shamsir | icddr-b, Dhaka, Bangladesh | Field supervisor |
| Dinesh | Mondol | icddr-b, Dhaka, Bangladesh | Microbiology supervisor |
| Tofail | Fahmida | icddr-b, Dhaka, Bangladesh | Cognitive development |
| Haque | Rashidul | icddr-b, Dhaka, Bangladesh | Bangladesh site co-PI |
| Hossain | Iqbal | icddr-b, Dhaka, Bangladesh | Coordinator, case control |
| Islam | Munirul | icddr-b, Dhaka, Bangladesh | Nutrition |
| Mahfuz | Mustafa | icddr-b, Dhaka, Bangladesh | Field supervisor |
| Chandyo | Ram Krishna | IOM, Tribuhvan University, Kathmandu, Nepal | Field manager, nutrition |
| Shrestha | Prakash Sunder | IOM, Tribuhvan University, Kathmandu, Nepal | Nepal site co-PI |
| Shrestha | Rita | IOM, Tribuhvan University, Kathmandu, Nepal | Psychologist |
| Ulak | Manjeswori | IOM, Tribuhvan University, Kathmandu, Nepal | Clinical supervisor |
| Black | Robert | JHU, Baltimore, MD, USA | Epidemiology |
| Caulfield | Laura | JHU, Baltimore, MD, USA | JHU site PI, Nutrition |
| Checkley | William | JHU, Baltimore, MD, USA | DCC, epidemiology, statistician |
| Chen | Ping | JHU, Baltimore, MD, USA | Data management |
| Kosek | Margaret | JHU, Baltimore, MD, USA | Peru site PI, gut function |
| Lee | Gwenyth | JHU, Baltimore, MD, USA | Nutrition, gut function |
| Yori | Pablo Peñataro | JHU, Baltimore, MD, USA | Data management |
| Murray-Kolb | Laura | Pennsylvania State University, University Park, PA, USA | Lead, cognitive development |
| Schaefer | Barbara | Pennsylvania State University, University Park, PA, USA | DCC, cognitive development, psychometrics |
| Pendergast | Laura | Temple University, Philadelphia, PA, USA | DCC, cognitive development, psychometrics |
| Abreu | Claudia | Universidade Federal do Ceara, Fortaleza, Brazil | Study coordinator |
| Bindá | Alexandre | Universidade Federal do Ceara, Fortaleza, Brazil | Laboratory |
| Costa | Hilda | Universidade Federal do Ceara, Fortaleza, Brazil | Psychologist |
| Di Moura | Alessandra | Universidade Federal do Ceara, Fortaleza, Brazil | Nurse study coordinator |
| Filho | Jose Quirino | Universidade Federal do Ceara, Fortaleza, Brazil | DCC, data management |
| Leite | Álvaro | Universidade Federal do Ceara, Fortaleza, Brazil | Pediatrician |
| Lima | Aldo | Universidade Federal do Ceara, Fortaleza, Brazil | Brazil site PI |
| Lima | Noelia | Universidade Federal do Ceara, Fortaleza, Brazil | Pediatrician |
| Lima | Ila | Universidade Federal do Ceara, Fortaleza, Brazil | Laboratory |
| Maciel | Bruna | Universidade Federal do Ceara, Fortaleza, Brazil | Nutrition |
| Moraes | Milena | Universidade Federal do Ceara, Fortaleza, Brazil | Nutrition |
| Mota | Francisco | Universidade Federal do Ceara, Fortaleza, Brazil | Pediatrician |
| Oria | Reinaldo | Universidade Federal do Ceara, Fortaleza, Brazil | Brazil site co-PI |
| Quetz | Josiane | Universidade Federal do Ceara, Fortaleza, Brazil | Laboratory |
| Soares | Alberto | Universidade Federal do Ceara, Fortaleza, Brazil | Data management |
| Svensen | Erling | University of Bergen, Norway; Haydom Lutheran Hospital, Haydom, Tanzania | Tanzania site PI, cognitive development |
| Tor | Strand | University of Bergen, Norway | Consultant, nutrition |
| Patil | Crystal | University of Illinois, Urbana-Champaign, IL, USA | Nutrition |
| Bessong | Pascal | University of Venda, Thohoyandou, South Africa | South Africa site PI |
| Mahopo | Cloupas | University of Venda, Thohoyandou, South Africa | Nutrition |
| Mapula | Angelina | University of Venda, Thohoyandou, South Africa | Psychology supervisor, cognitive development |
| Nesamvuni | Cebisa | University of Venda, Thohoyandou, South Africa | Nutrition |
| Nyathi | Emanuel | University of Venda, Thohoyandou, South Africa | Data management |
| Samie | Amidou | University of Venda, Thohoyandou, South Africa | Laboratory supervisor |
| Barrett | Leah | UVA, Charlottesville, VA, USA | Study coordinator |
| Gratz | Jean | UVA, Charlottesville, VA, USA | Laboratory, Tanzania site |
| Guerrant | Richard | UVA, Charlottesville, VA, USA | UVA PI, surveillance, cognitive development |
| Houpt | Eric | UVA, Charlottesville, VA, USA | Microbiology, Tanzania site development |
| Olmsted | Liz | UVA, Charlottesville, VA, USA | Financial manager |
| Petri | William | UVA, Charlottesville, VA, USA | UVA co-PI, vaccine response |
| Platts-Mills | James | UVA, Charlottesville, VA, USA | DCC, microbiology |
| Scharf | Rebecca | UVA, Charlottesville, VA, USA | Cognitive development |
| Shrestha | Binob | Walter Reed/AFRIMS Research Unit, Kathmandu, Nepal | Data management |
| Shrestha | Sanjaya Kumar | Walter Reed/AFRIMS Research Unit, Kathmandu, Nepal | Nepal site co-PI, gut function |
Abbreviations: AFRIMS, Armed Forces Research Institute of Medical Sciences; DCC, Data Coordinating Center; FIC, Fogarty International Center; FNIH, Foundation for the National Institutes of Health; icddr,b, International Centre for Diarrhoeal Disease Research, Bangladesh; IL, Illinois; IOM, Institute of Medicine; JHU, Johns Hopkins University; MD, Maryland; NC, North Carolina; NIH, National Institutes of Health; PA, Pennsylvania; PI, Principal Investigator; SES, socioeconomic status; USA, United States of America; UVA, University of Virginia; VA, Virginia.
Notes
Acknowledgments. The authors thank the staff, parents, and children of the MAL-ED sites for their contributions.
Financial support. The MAL-ED study is carried out as a collaborative project supported by the Bill & Melinda Gates Foundation, the Foundation for the National Institutes of Health, and the Fogarty International Center, National Institutes of Health.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Tate JE, Burton AH, Boschi-Pinto C et al. . 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12:136–41. [DOI] [PubMed] [Google Scholar]
- 2. Yen C, Armero Guardado JA, Alberto P et al. . Decline in rotavirus hospitalizations and health care visits for childhood diarrhea following rotavirus vaccination in El Salvador. Pediatr Infect Dis J 2011; 30:S6–S10. [DOI] [PubMed] [Google Scholar]
- 3. Molto Y, Cortes JE, De Oliveira LH et al. . Reduction of diarrhea-associated hospitalizations among children aged < 5 years in Panama following the introduction of rotavirus vaccine. Pediatr Infect Dis J 2011; 30:S16–20. [DOI] [PubMed] [Google Scholar]
- 4. Field EJ, Vally H, Grimwood K, Lambert SB. Pentavalent rotavirus vaccine and prevention of gastroenteritis hospitalizations in Australia. Pediatrics 2010; 126:e506–12. [DOI] [PubMed] [Google Scholar]
- 5. Yen C, Tate JE, Wenk JD, Harris JM 2nd, Parashar UD. Diarrhea-associated hospitalizations among US children over 2 rotavirus seasons after vaccine introduction. Pediatrics 2011; 127:e9–e15. [DOI] [PubMed] [Google Scholar]
- 6. Richardson V, Hernandez-Pichardo J, Quintanar-Solares M et al. . Effect of rotavirus vaccination on death from childhood diarrhea in Mexico. N Engl J Med 2010; 362:299–305. [DOI] [PubMed] [Google Scholar]
- 7. do Carmo GM, Yen C, Cortes J et al. . Decline in diarrhea mortality and admissions after routine childhood rotavirus immunization in Brazil: a time-series analysis. PLoS Med 2011; 8:e1001024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Anderson EJ, Rupp A, Shulman ST, Wang D, Zheng X, Noskin GA. Impact of rotavirus vaccination on hospital-acquired rotavirus gastroenteritis in children. Pediatrics 2011; 127:e264–70. [DOI] [PubMed] [Google Scholar]
- 9. Buttery JP, Lambert SB, Grimwood K et al. . Reduction in rotavirus-associated acute gastroenteritis following introduction of rotavirus vaccine into Australia’s National Childhood vaccine schedule. Pediatr Infect Dis J 2011; 30:S25–9. [DOI] [PubMed] [Google Scholar]
- 10. Kang G, Arora R, Chitambar SD et al. ; Indian Rotavirus Strain Surveillance Network Multicenter, hospital-based surveillance of rotavirus disease and strains among Indian children aged <5 years. J Infect Dis 2009; 200:S147–53. [DOI] [PubMed] [Google Scholar]
- 11. Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR et al. ; Human Rotavirus Vaccine Study Group Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med 2006; 354:11–22. [DOI] [PubMed] [Google Scholar]
- 12. Fischer TK, Valentiner-Branth P, Steinsland H et al. . Protective immunity after natural rotavirus infection: a community cohort study of newborn children in Guinea-Bissau, West Africa. J Infect Dis 2002; 186:593–7. [DOI] [PubMed] [Google Scholar]
- 13. Gladstone BP, Ramani S, Mukhopadhya I et al. . Protective effect of natural rotavirus infection in an Indian birth cohort. N Engl J Med 2011; 365:337–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. O’Ryan ML, Lucero Y, Prado V et al. . Symptomatic and asymptomatic rotavirus and norovirus infections during infancy in a Chilean birth cohort. Pediatr Infect Dis J 2009; 28:879–84. [DOI] [PubMed] [Google Scholar]
- 15. Linhares AC, Velázquez FR, Pérez-Schael I et al. ; Human Rotavirus Vaccine Study Group Efficacy and safety of an oral live attenuated human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in Latin American infants: a randomised, double-blind, placebo-controlled phase III study. Lancet 2008; 371:1181–9. [DOI] [PubMed] [Google Scholar]
- 16. Armah GE, Sow SO, Breiman RF et al. . Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet 2010; 376:606–14. [DOI] [PubMed] [Google Scholar]
- 17. Zaman K, Dang DA, Victor JC et al. . Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet 2010; 376:615–23. [DOI] [PubMed] [Google Scholar]
- 18. The MAL-ED Network Investigators. The MAL-ED Study: A multinational and multidisciplinary approach to understand the relationship between enteric pathogens, malnutrition, gut physiology, physical growth, cognitive development, and immune responses in infants and children up to 2 years of age in resource-poor environments. Clin Infect Dis 2014; 59:S193–206. [DOI] [PubMed] [Google Scholar]
- 19. Ahmed T, Mahfuz M, Islam MM et al. . The MAL-ED cohort study in Mirpur, Bangladesh. Clin Infect Dis 2014; 59:S280–06. [DOI] [PubMed] [Google Scholar]
- 20. Bessong P, Nyathi E, Mahopo C, Netshandama V. Development of the Dzimauli community in Vhembe district, Limpopo province of South Africa for the MAL-ED cohort study. Clin Infect Dis 2014; 59:S317–24. [DOI] [PubMed] [Google Scholar]
- 21. John SM, Thomas RJ, Kaki S et al. . Establishment of the MAL-ED birth cohort study site in Vellore, southern India. Clin Infect Dis 2014; 59:S295–9. [DOI] [PubMed] [Google Scholar]
- 22. Lima A, Oriá RB, Soares AM et al. . Geography, population, demography, socioeconomic, anthropometry, and environmental status in the MAL-ED cohort and case-control study sites in Fortaleza, Ceará, Brazil. Clin Infect Dis 2014; 59:S287–94. [DOI] [PubMed] [Google Scholar]
- 23. Mduma ER, Gratz J, Patil C et al. . The Etiology, Risk Factors and Interactions of Enteric Infections and Malnutrition and the Consequences for Child Health and Development Study (MAL-ED): description of the Tanzanian site. Clin Infect Dis 2014; 59:S325–30. [DOI] [PubMed] [Google Scholar]
- 24. Shrestha PS, Shrestha SK, Bodhidatta L et al. . Bhaktapur, Nepal: the MAL-ED birth cohort study in Nepal. Clin Infect Dis 2014; 59:S300–03. [DOI] [PubMed] [Google Scholar]
- 25. Turab A, Soofi SB, Ahmed I et al. . Demographic, socioeconomic, and health characteristics of the MAL-ED network study site in rural Pakistan. Clin Infect Dis 2014; 59:S304–9. [DOI] [PubMed] [Google Scholar]
- 26. Yori PP, Lee G, Olórtegui MP et al. . Santa Clara de Nanay: the MAL-ED cohort in Peru. Clin Infect Dis 2014; 59:S310–16. [DOI] [PubMed] [Google Scholar]
- 27. Richard SA, Barrett LJ, Guerrant RL, Checkley W; MAL-ED Network Investigators Disease surveillance methods used in the 8-site MAL-ED cohort study. Clin Infect Dis 2014; 59:S220–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Houpt E, Gratz J, Kosek M et al. . Microbiologic methods utilized in the MAL-ED cohort study. Clin Infect Dis 2014; 59:S225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee G, Peñataro Yori P, Paredes Olortegui M et al. . An instrument for the assessment of diarrhoeal severity based on a longitudinal community-based study. BMJ Open 2014; 4:e004816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Platts-Mills JA, Babji S, Bodhidatta L et al. . Pathogen-specific burdens of community diarrhoea in developing countries: a multisite birth cohort study (MAL-ED). Lancet Glob Health 2015; 3:e564–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ward RL, Bernstein DI, Smith VE et al. . Rotavirus immunoglobulin a responses stimulated by each of 3 doses of a quadrivalent human/bovine reassortant rotavirus vaccine. J Infect Dis 2004; 189:2290–3. [DOI] [PubMed] [Google Scholar]
- 32. Kompithra RZ, Paul A, Manoharan D et al. . Immunogenicity of a three dose and five dose oral human rotavirus vaccine (RIX4414) schedule in south Indian infants. Vaccine 2014; 32:A129–33. [DOI] [PubMed] [Google Scholar]
- 33. Ehrenkranz P, Lanata CF, Penny ME, Salazar-Lindo E, Glass RI. Rotavirus diarrhea disease burden in Peru: the need for a rotavirus vaccine and its potential cost savings. Rev Panam Salud Publica 2001; 10:240–8. [DOI] [PubMed] [Google Scholar]
- 34. Bar-Zeev N, Kapanda L, Tate JE et al. ; VacSurv Consortium Effectiveness of a monovalent rotavirus vaccine in infants in Malawi after programmatic roll-out: an observational and case-control study. Lancet Infect Dis 2015; 15:422–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sowmyanarayanan TV, Ramani S, Sarkar R et al. . Severity of rotavirus gastroenteritis in Indian children requiring hospitalization. Vaccine 2012; 30:A167–72. [DOI] [PubMed] [Google Scholar]
- 36. Levy K, Hubbard AE, Eisenberg JN. Seasonality of rotavirus disease in the tropics: a systematic review and meta-analysis. Int J Epidemiol 2009; 38:1487–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jagai JS, Sarkar R, Castronovo D et al. . Seasonality of rotavirus in South Asia: a meta-analysis approach assessing associations with temperature, precipitation, and vegetation index. PLoS One 2012; 7:e38168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Clemens J, Rao M, Ahmed F et al. . Breast-feeding and the risk of life-threatening rotavirus diarrhea: prevention or postponement? Pediatrics 1993; 92:680–5. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.