Abstract
Insect-specific flaviviruses (ISFVs) commonly infect vectors of mosquito-borne arboviruses. To investigate whether infection with an ISFV might affect mosquito flight behavior, we quantified flight behavior in Culex pipiens L. naturally infected with Culex flavivirus (CxFV). We observed a significant reduction in the scotophase (dark hours) flight activity of CxFV-positive mosquitoes relative to CxFV-negative mosquitoes, but only a marginal reduction in photophase (light hours) flight activity, and no change in the circadian pattern of flight activity. These results suggest that CxFV infection alters the flight activity of naturally infected Cx. pipiens most dramatically when these vectors are likely to be host seeking and may therefore affect the transmission of medically important arboviruses.
Keywords: Culex flavivirus, Culex pipiens, flight activity, mosquito behavior, insect-specific flavivirus
Insect-specific flaviviruses (ISFVs; family Flaviviridae ; genus Flavivirus ) are distinct from medically important flaviviruses and are of interest because they co-occur in vectors of arthropod-borne viruses, such as West Nile virus (WNV; Kuno 2007 , Blitvich et al. 2009 , Kent et al. 2010 , Newman et al. 2011 , Bolling et al. 2012 ). Recent studies suggest that ISFVs may affect vector competence or vectorial capacity for arboviruses ( Kent et al. 2010 , Bolling et al. 2012 ). Culex species mosquitoes are important vectors of WNV, which is maintained in an enzootic, mosquito–bird transmission cycle ( Colpitts et al. 2012 ). ISFVs have been identified in Culex mosquito populations globally, but the effects of ISFVs on arbovirus transmission have not been extensively examined ( Hoshino et al. 2007 , Morales-Betoulle et al. 2008 , Cook et al. 2009 , Blitvich et al. 2009 , Bolling et al. 2011 ).
Culex flavivirus (CxFV) is an ISFV that infects Culex species mosquitoes ( Hoshino et al. 2007 ). CxFV was first identified and isolated in Japan in 2007 and has since been detected in Culex populations globally (e.g., Blitvich et al. 2009 , Cook et al. 2009 , Bolling et al. 2011 ). CxFV is primarily transovarially transmitted and has been isolated from all mosquito tissues examined ( Saiyasombat et al. 2011 , Bolling et al. 2012 ). Recent studies of CxFV ecology and coinfection with WNV have generated conflicting results. In a fine-scale study of Culex pipiens L. from a WNV “hot spot” in suburban Chicago, CxFV and WNV coinfected individual Culex mosquitoes and WNV-positive mosquito pools were four times more likely than WNV-negative pools to also be positive for CxFV ( Newman et al. 2011 ). Conversely, at a broader spatial scale, no association was identified in Culex quinquefasciatus Say ( Kent-Crockett et al. 2012 ). Similarly, experimental studies of transmission have generated equivocal results: Kent et al. (2010) found that Cx. quinquefasciatus coinoculated with CxFV transmitted WNV more efficiently. However, Bolling et al. (2012) identified early suppression of WNV infection in Cx. pipiens naturally infected with CxFV. To our knowledge, there are no studies of the potential effects of CxFV infection on mosquito behavior, a factor that could influence arbovirus infection and transmission.
Virus infection may alter mosquito motility or feeding behavior ( Berry et al. 1986 , Platt et al. 1997 , Lee et al. 2000 ). For example, in Aedes aegypti (L.) infected with dengue virus, locomotor activity was increased compared with uninfected controls ( Lima-Camara et al. 2011 ). Conversely, when Aedes trivittatus Coquillett were infected with trivittatus virus, there was no significant difference in spontaneous flight activity compared with uninfected controls ( Berry et al. 1987 ). In Culex tarsalis Coquillett infected with Western equine encephalitis virus, spontaneous flight activity and longevity were decreased, reducing vectorial capacity ( Lee et al. 2000 ). The direction and magnitude of such effects therefore appear to differ by system such that either an increase or decrease in subsequent arboviral transmission might be predicted.
To better understand the effects of CxFV infection on Cx. pipiens behavior, we examined the circadian patterns of flight activity of Cx. pipiens naturally infected with CxFV and compared them with age-matched CxFV-negative mosquitoes. We predicted that CxFV infection would affect mosquito flight activity in ways that could subsequently influence arbovirus transmission.
Materials and Methods
Mosquitoes
C x . pipiens mosquitoes were collected as unhatched egg rafts once per week between September and October, 2013 from two locations in the western suburbs of Chicago, IL (village of Alsip; 41.6706° N, 87.7322° W). Egg rafts were hatched separately, and larvae were reared on a diet of Tetramin slurry ad libitum. Insectary chamber conditions were maintained at 27°C, 80% humidity, and a photoperiod of 16:8 (L:D) h, with a 90-min crepuscular period at the beginning and end of each light cycle (approximating the photoperiod in Chicago during summer). After pupal emergence, adults were identified, maintained in modified soup cartons, and fed a 0.3 M sucrose solution.
Pairs of emerged adult mosquitoes from each original egg raft were tested for CxFV using a nested reverse transcriptase-polymerase chain reaction (RT-PCR) with pan-flavivirus and CxFV-specific primers (following Newman et al. 2011 ). Prior studies by Saiyasombat et al. (2011) and testing of Cx. pipiens from our study site suggest a 100% efficiency of transovarial transmission for CxFV; therefore, all mosquitoes derived from egg rafts that tested CxFV positive were considered CxFV positive, and all mosquitoes derived from egg rafts that tested CxFV negative were considered CxFV negative for the purposes of assigning them to treatment groups (infection status later confirmed; see below).
Measurement of Flight Activity
Twelve CxFV-positive and 12 CxFV-negative, 4- to 5-d-old female mosquitoes were anesthetized using CO 2 and transferred to an acoustic flight activity chamber ( Rowley et al. 1987 , Keating et al. 2013 ) in individual reagent bottles, with position in the chamber assigned at random. The flight chamber allows light penetration, and measures flight activity by recording mosquito wing beats (see Rowley et al. 1987 for design). Activity was detected by individual microphones and scored as “1” or “0” at 1-s intervals.
The experiment was conducted twice, with different cohorts of age-matched CxFV-positive and CxFV-negative mosquitoes. A photoperiod of 16:8 (L:D) h was maintained with hard transitions between light and dark. Activity chamber experiments were performed at ambient temperature (∼22°C). Mosquitoes were allowed to acclimate to chamber conditions for 12 h prior to data collection. Flight activity was averaged hourly for each individual over 120 h (5 d; average hourly flight activity, abbreviated “flight activity” hereafter). Flight activity was calculated for each group (CxFV positive and CxFV negative), as well as for photophase (light phase in cycle) and scotophase (dark phase in cycle) to identify differences in circadian patterns. Resulting values were compared using Mann–Whitney U -tests. Circadian activity patterns were compared between groups using repeated-measures ANOVA with the lme4 package ( Bates et al. 2014 ) in R version 3.0.3 ( R Core Team 2014 ) with hours as the repeated variable. Mosquitoes that died in the chamber prior to the conclusion of the experiment ( n = 2) were excluded.
Confirmation of CxFV Infection Status
Following experiments, individual mosquitoes were tested to confirm CxFV infection status as described above (see “Mosquitoes” and Newman et al. 2011 ).
Results
CxFV infection strongly affected scotophase flight activity, but only marginally affected photophase flight activity. Average scotophase flight activity was significantly lower for 22 CxFV-positive mosquitoes compared with 24 CxFV-negative mosquitoes ( Table 1 ). Conversely, there was only a marginal difference in average photophase flight activity between CxFV-positive mosquitoes and CxFV-negative mosquitoes ( Table 1 ). Repeated-measures ANOVA indicated that flight activity differed significantly by infection status (CxFV positive or CxFV negative) at each time point (hour), with a significant interaction between infection status and time point: the average activity peaks of CxFV-positive mosquitoes decreased more steeply than the average activity peaks of CxFV-negative mosquitoes ( Table 2 ).
Table 1.
Average flight activity of CxFV-positive and CxFV-negative Cx. pipiens during scotophase and photophase
| Mosquitoes | Avg. activity (s/h) | n | U | P |
|---|---|---|---|---|
| Scotophase | ||||
| CxFV+ | 51.99 | 22 | ||
| CxFV− | 148.20 | 24 | 327708 | <0.0001 * |
| Photophase | ||||
| CxFV+ | 4.11 | 22 | ||
| CxFV− | 5.24 | 24 | 1575656 | 0.05 * |
Nonparametric, Mann–Whitney U -test results are shown.
*Statistical significance.
Table 2.
Repeated-measures ANOVA on the flight activity of 22 CxFV-positive and 24 CxFV-negative Cx. pipiens
| Variable | df | SS | MS | F | P |
|---|---|---|---|---|---|
| Group a | 1 | 1631123 | 1631123 | 5.18 | <0.05 * |
| Time point b | 119 | 28832853 | 242293 | 29.17 | <0.0001 * |
| Group::time point | 119 | 6390408 | 53701 | 6.47 | <0.0001 * |
a Group—CxFV-positive or CxFV-negative mosquitoes.
b Time point—repeated variable of “hours” for each mosquito in each group.
* Statistical significance.
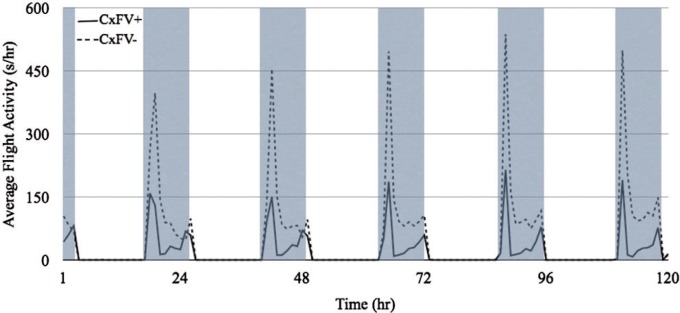
Overall circadian activity patterns were similar between CxFV-positive and CxFV-negative mosquitoes over 120 h ( Fig. 1 ). Both groups of mosquitoes showed a bimodal activity pattern beginning just prior to (∼2200 hours) and continuing through each scotophase (until ∼0600 hours). Average flight activity peaked with the onset of the scotophase and generally decreased over the period of darkness with a second, smaller increase in activity leading into each photophase.
Fig. 1.
Overall circadian activity pattern of CxFV-positive and CxFV-negative Cx. pipiens over 120 h (5 d) in an acoustic flight activity chamber. The average activity, measured in seconds per hour, of 22 CxFV-positive mosquitoes is represented by the solid line and the average activity of 24 CxFV-negative mosquitoes is represented by the dashed line. Shaded areas represent the scotophase (dark). The flight activity of both groups shows a clear circadian pattern, with the majority of activity taking place at the onset and continuing throughout the 8-h scotophase. Overall, the flight activity of the CxFV-positive mosquitoes appears lower than the CxFV-negative mosquitoes, and this difference was significant ( F = 5.18; df = 1, 44; P < 0.05). Consistent with increased activity during scotophase when compared with photophase, the hour by hour flight activity also differed ( F = 29.17; df = 1, 119; P < 0.001). Moreover, there was a significant interaction between infection status and time: the average activity of CxFV-positive mosquitoes decreased more than the average activity of CxFV-negative mosquitoes over time ( F = 6.47; df = 1, 119; P < 0.001).
Discussion
We used an acoustic flight activity chamber to evaluate the effect of natural CxFV infection on the flight activity of Cx. pipiens . Scotophase flight activity of CxFV-positive Cx. pipiens was significantly lower than that of CxFV-negative Cx. pipiens . This trend persisted in a repeated-measures ANOVA which showed that CxFV-positive mosquitoes had lower flight activity than CxFV-negative mosquitoes at any given point in time during scotophase. Additionally, there was a significant interaction between infection status and time point: the average flight activity of CxFV-positive mosquitoes decreased more steeply than that of CxFV-negative mosquitoes. Overall, these results suggest that CxFV infection has a dampening effect on Cx. pipiens flight activity.
The mechanisms underlying the trends we have documented are currently unclear. For example, it is not known whether CxFV infects mosquito flight muscles. The observed decrease in flight activity of CxFV-positive mosquitoes could be associated with pathology; however, CxFV in mosquito cell culture is associated with few to moderate cytopathic effects (CPE) ( Hoshino et al. 2007 ). Further, in Culex tritaeniorhynchus Giles cells persistently infected with CxFV, there is no discernible CPE ( Kuwata et al. 2015 ). CxFV may infect nervous tissue, as studies by Kent at al. (2010) and Saiyasombat et al. (2011) showed evidence of CxFV infection in head tissues of mosquitoes. Given that activity patterns in Diptera are regulated by circadian clock neurons in the brain ( Hall 2003 ), this is a plausible mechanism and has previously been invoked by Lima-Camara et al. (2011) in dengue virus studies. In addition, dengue virus has been shown to extensively invade the mosquito nervous system ( Platt et al. 1997 ), suggesting this could occur with other flaviviruses. The decrease in flight activity of CxFV-positive mosquitoes might also suggest a fitness cost associated with CxFV infection and potentially an increase in energy expenditure. In Drosophila melanogaster infected with an insect-only, vertically transmitted rhabdovirus, D. melanogaster sigma virus (DmelSV), a decrease in fitness is observed compared with uninfected flies ( Longdon et al. 2012 ). Infection with DmelSV is also associated with a decrease in overwinter survival potentially suggesting that energy expenditure is increased in infected flies ( Fleuriet 1981 ).
ISFVs differ from medically important arboviruses in a number of ways. Unlike arboviruses, ISFVs appear to infect only insects and insect-derived cells. This suggests that ISFVs may be similar to the evolutionary precursors of arboviruses ( Hoshino et al. 2007 ). ISFVs are vertically transmitted ( Saiyasombat et al. 2011 , Bolling et al. 2012 ), whereas vertical transmission of arboviruses is infrequent ( Dohm et al. 2002 , Anderson et al. 2012 ). Thus, ISFVs may be more “vector adapted” than their arbovirus counterparts ( Lobo et al. 2009 ). If so, the effects we have documented may reflect an unrecognized negative effect of an insect-specific flavivirus on the host.
Although CxFV-positive and CxFV-negative mosquitoes differed in flight activity, there was extensive within-group variation. This may reflect small sample size or geographic and genetic variation in mosquito populations. There is evidence that WNV and dengue virus vector competence may vary between regions and mosquito populations ( Bennett et al. 2002 , Vaidyanathan et al. 2007 ). Indeed, a pilot experiment conducted with mosquitoes from Wisconsin and Chicago showed preliminary evidence of such variation (C. M. N., unpublished data).
C x . pipiens from Chicago are active during the crepuscular and nighttime hours. In this region, large aggregations of avian hosts for WNV form at dusk and persist until early morning ( Krebs et al. 2014 ). Because these are the same times that Cx. pipiens engage in host-seeking behaviors ( Mitchell 1982 , Savage et al. 2008 ), our findings raise the possibility that CxFV infection may influence WNV transmission. We previously observed a positive association between CxFV and WNV in mosquito pools collected during 2006 ( Newman et al. 2011 ). These data suggest that CxFV and WNV may interact, perhaps indirectly, in nature. If CxFV infection decreases the overall flight activity of a mosquito, this could indirectly decrease contact with arbovirus amplification hosts. We speculate that such an effect may explain the geographically clustered pattern of WNV infection observed in some regions on a fine scale, in addition to factors such as vector and host abundance, land cover, and climate ( Centers for Disease Control and Prevention 2010 , Crowder et al. 2013 ). Although we did not examine the effects of WNV and coinfection on mosquito behavior, such experiments will be necessary to develop an understanding of the potential for viruses such as CxFV to modify WNV transmission. Understanding ISFVs remains an important avenue of future research, particularly in systems where ISFVs and other arbovirus cocirculate.
Acknowledgments
We thank the village of Alsip, IL, and homeowners in Madison, WI, for allowing mosquito collection for this research. We also thank S. Paskewitz and B. Christensen for providing advice and insectary facilities, B. Krebs and G. Hamer for comments on the manuscript and analyses, and M. O. Ruiz, J. Brawn, E. Walker, U. Kitron, B. Krebs, and A. Gardner for field support and analytical advice. This work was funded by the National Science Foundation/National Institutes of Health Ecology of Infectious Diseases program (0840403).
References Cited
- Anderson J. F., Main A. J., Cheng G., Ferrandino F. J., Fikrig E. . 2012. . Horizontal and vertical transmission of West Nile virus genotype NY99 by Culex salinarius and genotypes NY99 and WN02 by Culex tarsalis . Am. J. Trop. Med. Hyg. 86 : 134 – 139 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D., Maechler M., Bolker B., Walker S. . 2014. . lme4: Linear mixed effects models using Eigen and S4. R package version 1.1-7 . ( http://CRAN.R-project.org/package=lme4 ) (accessed 6 October 2015) . [Google Scholar]
- Bennett K. E., Olson K. E., de Lourdes Muñoz M., Fernandez-Salas I., Farfan-Ale J. A., Higgs S., Black W. C., Beaty B. J. . 2002. . Variation in vector competence for dengue 2 virus among 24 collections of Aedes aegypti from Mexico and the United States . Am. J. Trop. Med. Hyg. 67 : 85 – 92 . [DOI] [PubMed] [Google Scholar]
- Berry W. J., Rowley W. A., Christensen B. M. . 1986. . Influence of developing Brugia pahangi on spontaneous flight activity of Aedes aegypti (Diptera: Culicidae) . J. Med. Entomol. 23 : 441 – 445 . [DOI] [PubMed] [Google Scholar]
- Berry W. J., Rowley W. A., Clarke J. L., III, Swack N. S., Hausler W. J., Jr . 1987. . Spontaneous flight activity of Aedes trivittatus (Diptera: Culicidae) infected with Trivittatus virus (Bunyaviridae: California serogroup) . J. Med. Entomol. 24 : 286 – 289 . [DOI] [PubMed] [Google Scholar]
- Blitvich B. J., Lin M., Dorman K. S., Soto V., Hovav E., Tucker B. J., Staley M., Platt K. B., Bartholomay L. C. . 2009. . Genomic sequence and phylogenetic analysis of Culex flavivirus, an insect-specific flavivirus isolated from Culex pipiens (Diptera: Culicidae) in Iowa . J. Med. Entomol. 46 : 934 – 941 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolling B. G., Eisen L., Moore C. G., Blair C. D. . 2011. . Insect-specific flaviviruses from Culex mosquitoes in Colorado, with evidence of vertical transmission . Am. J. Trop. Med. Hyg. 85 : 169 – 177 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolling B. G., Olea-Popelka F. J., Eisen L., Moore C. G., Blair C. D. . 2012. . Transmission dynamics of an insect-specific flavivirus in a naturally infected Culex pipiens laboratory colony and effects of co-infection on vector competence for West Nile virus . Virology 427 : 90 – 97 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackney D. E., Scott J. C., Sagawa F., Woodward J. E., Miller N. A., Schilkey F. D., Mudge J., Wilusz J., Olson K. E., Blair C. D., et al. . 2010. . C6/36 Aedes albopictus cells have a dysfunctional antiviral RNA interference response . PLoS Neglect. Trop. Dis. 4 : e856.(accessed 17 October 2015) . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . 2010. . Surveillance for human West Nile virus disease-United States, 1999–2008 . MMWR Morb. Mortal. Wkly. Rep. 59 : 1 – 17 . [PubMed] [Google Scholar]
- Colpitts T. M., Conway M. J., Montgomery R. R., Fikrig E. . 2012. . West Nile virus: Biology, transmission, and human infection . Clin. Microbiol. Rev. 25 : 635 – 648 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook S., Moureau G., Harbach R. E., Mukwaya L., Goodger K., Ssenfuka F., Gould E., Holmes E. C., de Lamballerie X. . 2009. . Isolation of a novel species of flavivirus and a new strain of Culex flavivirus (Flaviviridae) from a natural mosquito population in Uganda . J. Gen. Virol. 90 : 2669 – 2678 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowder D. W., Dykstra E. A., Brauner J. M., Duffy A., Reed C., Martin E., Peterson W. . 2013. . West Nile virus prevalence across landscapes is mediated by local effects of agriculture on vector and host communities . PLoS ONE 8 : e55006.(accessed 17 October 2015) . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohm D. J., Sardelis M. R., Turell M. J. . 2002. . Experimental vertical transmission of West Nile virus by Culex pipiens (Diptera: Culicidae) . J. Med. Entomol. 39 : 640 – 644 . [DOI] [PubMed] [Google Scholar]
- Fleuriet A. 1981. . Effect of overwintering on the frequency of flies infected with Rhabdovirus sigma in experimental populations of Drosophila melanogaster . Arch. Virol. 69 : 253 – 260 . [DOI] [PubMed] [Google Scholar]
- Hall J. C. 2003. . Genetics and molecular biology of rhythms in Drosophila and other insects . Adv. Genet. 48 : 1 – 280 . [DOI] [PubMed] [Google Scholar]
- Hoshino K., Isawa H., Tsuda Y., Yano K., Sasaki T., Yuda M., Takasaki T., Kobayashi M., Sawabe K. . 2007. . Genetic characterization of a new insect flavivirus isolated from Culex pipiens mosquito in Japan . Virology 359 : 405 – 414 . [DOI] [PubMed] [Google Scholar]
- Keating J. A., Bhattacharya D., Rund S. S., Dasgupta R., Lee S. J., Duffield G., Striker R. . 2013. . Mosquito protein kinase G phosphorylates flavivirus NS5 and alters flight behavior in Aedes aegypti . Vector Borne Zoonotic Dis. 13 : 590 – 600 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent R. J., Crabtree M. B., Miller B. R. . 2010. . Transmission of West Nile virus by Culex quinquefasciatus Say infected with Culex flavivirus Izabal . PLoS Neglect. Trop. Dis. 4 : e671.(accessed 17 October 2015) . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent-Crockett R. J., Burkhalter K., Mead D., Kelly R., Brown J., Varnado W., Roy A., Horiuchi K., Biggerstaff B. J., Miller B., et al. . 2012. . Culex flavivirus and West Nile virus in Culex quinquefasciatus populations in the southeastern United States . J. Med. Entomol. 49 : 165 – 174 . [DOI] [PubMed] [Google Scholar]
- Krebs B. L., Anderson T. K., Goldberg T. L., Hamer G. L., Kitron U. D., Newman C. M., Ruiz M. O., Walker E. D., Brawn J. D. . 2014. . Host group formation decreases exposure to vector-borne disease: A field experiment in a ‘hotspot’ of West Nile virus transmission . Proc. R. Soc. B. 281 : 20141586.(accessed 17 October 2015) . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno G. 2007. . Host range specificity in flaviviruses: correlation with in vitro replication . J. Med. Entomol. 44 : 93 – 101 . [DOI] [PubMed] [Google Scholar]
- Kuwata R., Isawa H., Hoshino K., Sasaki T., Kobayashi M., Maeda K., Sawabe K. . 2015. . Analysis of mosquito-borne Flavivirus superinfection in Culex tritaeniorhynchus (Diptera: Culicidae) cells persistently infected with Culex Flavivirus (Flaviviridae) . J. Med. Entomol. 52 : 222 – 229 . [DOI] [PubMed] [Google Scholar]
- Lee J., Rowley W. A., Platt K. B. . 2000. . Longevity and spontaneous flight activity of Culex tarsalis (Diptera: Culicidae) infected with Western equine encephalomyelitis virus . J. Med. Entomol. 37 : 187 – 193 . [DOI] [PubMed] [Google Scholar]
- Lima-Camara T. N., Bruno R. V., Luz P. M., Castro M. G., Lourenço-de-Oliveira R., Sorgine M.H.F., Peixoto A. A. . 2011. . Dengue infection increases the locomotor activity of Aedes aegypti females . PLoS ONE 6 : e17690.(accessed 17 October 2015) . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo F. P., Mota B. E., Pena S. D., Azevedo V., Macedo A. M., Tauch A., Machado C. R., Franco G. R. . 2009. . Virus-host coevolution: common patterns of nucleotide motif usage in Flaviviridae and their hosts . PLoS ONE 4 : e6282 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longdon B., Wilfert L., Jiggins F. M. . 2012. . The sigma viruses of Drosophila , pp. 117 – 132 . InDietzgen R., Kuzmin I. (eds), Rhabdoviruses: Molecular taxonomy, evolution, genomics, ecology, cytopathology, and control . Caister Academic Press; , Norfolk, England: . [Google Scholar]
- Mitchell L. 1982. . Time-segregated mosquito collections with a CDC miniature light trap . Mosq. News 42 : 12 – 18 . [Google Scholar]
- Morales-Betoulle M. E., Monzón Pineda M. L., Sosa S. M., Panella N., López B.M.R., Cordón-Rosales C., Komar N., Powers A., Johnson B. W. . 2008. . Culex flavivirus isolates from mosquitoes in Guatemala . J. Med. Entomol. 45 : 1187 – 1190 . [DOI] [PubMed] [Google Scholar]
- Newman C. M., Cerutti F., Anderson T. K., Hamer G. L., Walker E. D., Kitron U. D., Ruiz M. O., Brawn J. D., Goldberg T. L. . 2011. . Culex flavivirus and West Nile virus mosquito coinfection and positive ecological association in Chicago, United States . Vector Borne Zoonotic Dis. 11 : 1099 – 1105 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt K. B., Linthicum K. J., Myint K.S.A., Innis B. L., Lerdthusnee K., Vaughn D. W. . 1997. . Impact of dengue virus infection on feeding behavior of Aedes aegypti . Am. J. Trop. Med. Hyg. 57 : 119 – 125 . [DOI] [PubMed] [Google Scholar]
- R Core Team, 2014. . R: A language and environment for statistical computing . R Foundation for Statistical Computing; , Vienna, Austria: . ( http://www.R-project.org ) (accessed 17 October 2015) . [Google Scholar]
- Rowley W. A., Jones M.D.R., Jacobson D. W., Clarke J. L., III . 1987. . A microcomputer-monitored mosquito flight activity system . Ann. Entomol. Soc. Am. 80 : 534 – 538 . [Google Scholar]
- Saiyasombat R., Bolling B. G., Brault A. C., Bartholomay L. C., Blitvich B. J. . 2011. . Evidence of transovarial transmission of Culex flavivirus by Culex pipiens (Diptera: Culicidae) . J. Med. Entomol. 48 : 1031 – 1038 . [DOI] [PubMed] [Google Scholar]
- Savage H. M., Anderson M., Gordon E., Mcmillen L., Colton L., Delorey M., Sutherland G., Aspen S., Charnetzky D., Burkhalter K., et al. . 2008. . Host-seeking heights, host-seeking activity patterns, and West Nile virus infection rates for members of the Culex pipiens complex at different habitat types within the hybrid zone, Shelby County, TN, 2002 (Diptera: Culicidae) . J. Med. Entomol. 45 : 276 – 288 . [DOI] [PubMed] [Google Scholar]
- Vaidyanathan R., Scott T. W. . 2007. . Geographic variation in vector competence for West Nile virus in Culex pipiens (Diptera: Culicidae) complex in California . Vector Borne Zoonotic Dis. 7 : 193 – 198 . [DOI] [PubMed] [Google Scholar]