Abstract
Knockdown resistance ( kdr ) in insects resulting from mutation(s) in the voltage-gated sodium channel (VGSC) gene is one of the mechanisms of resistance against DDT and the pyrethroid group of insecticides. Earlier, we reported the presence of two classic kdr mutations, i.e., L1014F and L1014S in Anopheles stephensi Liston, a major Indian malaria vector affecting mainly urban areas. This report presents the distribution of these alleles in different An. stephensi populations. Seven populations of An. stephensi from six states of India were screened for the presence of two alternative kdr mutations L1014F and L1014S using allele-specific polymerase chain reaction assays. We recorded the presence of both kdr mutations in northern Indian populations (Alwar and Gurgaon), with the preponderance of L1014S, whereas only L1014F was present in Raipur (central India) and Chennai (southern India). None of the kdr mutations were found in Ranchi in eastern India and in Mangaluru and Mysuru in southern India. This study provides evidence for a focal pattern of distribution of kdr alleles in India.
Keywords: Anopheles stephensi, voltage-gated sodium channel, knockdown resistance, insecticide resistance
Anopheles stephensi Liston is one of the major vectors of malaria in India, Pakistan, and the Middle East ( Dash et al . 2007 ). Its distribution extends beyond the west of India to Afghanistan, Iran, Iraq, Bahrain, Oman, and Saudi Arabia, and in the east to Bangladesh, South China, and Myanmar ( Rao 1984 ). Recently, this vector has been reported to invade Djibouti located in the horn of Africa, where it was found to harbor the Plasmodium falciparum circumsporozoite protein ( Faulde et al . 2014 ).
Vector control is an essential component of malaria control program, which relies majorly on the use of chemical insecticides. Indoor residual spraying (IRS) is a major vector control strategy for rural areas, and antilarval measures for urban areas. Close to 60% of the high-risk areas are targeted through IRS, in which three insecticide groups are currently being used, i.e., DDT, malathion, and synthetic pyrethroids ( National Vector Borne Disease Control Programme [NVBDCP] 2012 ). For urban malaria control, temephos and fenthion are being used as larvicides. Pyrethroids serve as an alternative to DDT ( World Health Organization [WHO] 1989 ) and are extensively being used for impregnation in long-lasting insecticidal mosquito nets (LLIN), with the government targeting coverage of 80% of population under the risk area ( WHO 2009 , NVBDCP 2012 ).
DDT and pyrethroids act on the insect's voltage-gated sodium channel (VGSC) proteins found in nerve cell membranes by altering the gating kinetics that leads to paralysis and eventual death of the insect. Knockdown resistance ( kdr ) against DDT and pyrethroids is one of the resistance mechanisms in insects including anophelines, which confers reduced neuronal sensitivity against these insecticides, leading to the development of cross-resistance to all synthetic pyrethroids ( Soderlund 2008 ). Knockdown resistance mutations in the VGSC are documented in conferring resistance against DDT and pyrethroids ( Reimer et al . 2008 , Davies et al . 2009 , Ramphul et al . 2009 ), several of which are found occurring across the arthropod phyla ( Davies et al . 2007a , Soderlund 2008 ). In anophelines, several kdr mutations are reported viz. L1014F, L1014S, L1014C, L1014W, N1013S, N1575Y, and V1010L ( Silva et al . 2014 ) of which two classic kdr mutations—L1014F ( Martinez-Torres et al . 1998 ) and L1014S ( Ranson et al . 2000 )—are most prevalent and are known to be associated with DDT and pyrethroid resistance.
DDT resistance was first reported in An . stephensi larvae in 1955 in Erode, a town in Tamil Nadu, south of India ( Rajagopalan et al . 1956 ). It was subsequently detected in adult mosquitoes (WHO. 1986) and confirmed in other parts of the country ( Kumari et al . 1998 ). Studies on knockdown resistance in Indian Anopheles populations by Singh et al. (2011) revealed the presence of two classic kdr mutations L1014F and L1014S in An. stephensi ( Singh et al . 2011 ) and Anophelesculicifacies Giles ( Singh et al. 2009 , 2010 ), although at low frequencies ( Dykes et al . 2015 ), and L1014F in Anopheles subpictus Grassi ( Singh et al. 2015 ). The spread of such kdr alleles is deleterious to the expanding use of DDT and pyrethroids in public health and agricultural sectors across the country. It is therefore important to try and curb their spread before they become fixed in the population. Knowledge of the prevalence and distribution of kdr alleles in this An. stephensi is important for insecticide resistance management. This study provides an insight to the existing kdr alleles, their level of presence in the Indian An. stephensi population, and the focal pattern of distribution exhibited by them.
Materials and Methods
Study Sites
Anopheles stephensi were collected from Gurgaon city (32° 42′ N, 75° 9′ E) of Haryana state, villages Kishangarh and Umren (27° 26′–29′ N and 76° 31′–35′ E) of Alwar district (Rajasthan), Mangaluru city (12.87° N, 74.88° E) of Karnataka, Mysuru city (12.26° N, 76.6° E) of Karnataka, Raipur city (21.25° N, 81.63° E) of Chhattisgarh, villages Karsidih and Giridih (23.35° N, 85.33° E) of Ranchi district (Jharkhand), and Chennai city (13° 5′ N, 80° 16′ E) of Tamil Nadu. The years of collection have been shown in Table 1 .
Table 1.
Distribution of kdr alleles in different field populations of An. stephensi
| Locality | Year | n |
Genotypes (frequency)
|
Allelic frequencies
|
HWE parameters
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L/L | L/F | L/S | F/S | F/F | S/S | L1014 | L1014F | L1014S | H O | H E | p | |||
| Alwar (Rajasthan) | 2012 | 217 | 133 (0.613) | 11 (0.051) | 87 (0.401) | 6 (0.028) | 0 | 22 (0.101) | 0.703 | 0.033 | 0.264 | 0.401 | 0.436 | 0.410 |
| Gurgaon (Haryana) | 2011 | 32 | 17 (0.53) | 4 (0.12) | 1 (0.03) | 0 | 0 | 10 (0.31) | 0.61 | 0.06 | 0.33 | 0.156 | 0.525 | 0.000 |
| Raipur (Chhattisgarh) | 2012 | 44 | 17 (0.386) | 25 (0.568) | 0 | 0 | 2 (0.045) | 0 | 0.670 | 0.329 | 0.000 | 0.568 | 0.446 | 0.092 |
| Ranchi (Jharkhand) | 2012 | 84 | 84 (1.0) | 0 | 0 | 0 | 0 | 0 | 1.000 | 0.000 | 0.000 | 0.000 | 0.000 | 1.000 |
| Mangaluru (Karnataka) | 2012 | 129 | 129 (1.000) | 0 | 0 | 0 | 0 | 0 | 1.000 | 0.000 | 0.000 | 0.000 | 0.000 | 1.000 |
| Mysuru (Karnataka) | 2011 | 33 | 33 (1.000) | 0 | 0 | 0 | 0 | 0 | 1.000 | 0.000 | 0.000 | 0.000 | 0.000 | 1.000 |
| Chennai (Tamil Nadu) | 2012 | 101 | 101 (1.000) | 0 | 0 | 0 | 0 | 0 | 1.000 | 0.000 | 0.000 | 0.000 | 0.000 | 1.000 |
| 2015 | 99 | 91 (0.919) | 8 (0.081) | 0 | 0 | 1 (0.010) | 0 | 0.949 | 0.051 | 0.000 | 0.081 | 0.964 | 0.213 | |
Mosquito Collection
Adult female An. stephensi were collected from human dwellings and cattle sheds in rural areas (Ranchi and Alwar) during 06:00–08:00 a.m. using an aspirator and flash torch. From urban settings (Gurgaon, Raipur, Mangaluru, Mysuru, and Chennai) immatures (larvae and pupae) were collected from breeding sites such as cemented tanks, fountain tanks, building construction sites, cisterns etc., with the help of a dipper. At least 50 breeding sites were checked. Immatures were brought to the laboratory and reared till their emergence into adults. Rearing of larvae was done in enamel trays containing water, and supplemented with fish-food flakes and yeast powder as food. Upon pupation, pupae were transferred to a plastic cup containing water and kept inside a cloth cage measuring 30 by 30 by 30 cm 3 for their emergence into adults. The emergent mosquitoes were given access to water-soaked resin and wet cotton pad. The temperature and relative humidity (RH) of insectary was maintained at 27 ± 2°C and 70–75%, respectively. Adult mosquitoes were identified morphologically using Christophers’ key ( Christopher 1933 ) and were preserved individually in a micro centrifuge tube containing silica gel.
Insecticide Susceptibility Tests
Insecticide susceptibility tests of mosquitoes were carried out using WHO’s standard susceptibility test. Briefly, 4–6-d-old and sugar-fed adult females, which emerged from field-collected larvae (F 0 ) or F 1 progeny from adult collection, were kept in a holding tube (a maximum of 20 mosquitoes per tube) and were transferred to exposure tubes lined with insecticide impregnated and control paper supplied by Vector control and Research unit, Universiti Sains Malaysia. Tests were carried out exposing mosquitoes to 4% DDT-, 0.05% deltamethrin-, and 0.75% permethrin-impregnated papers keeping appropriate controls. On 1 h of exposure to the insecticide papers, mosquitoes were transferred back to holding tubes and allowed for recovery. Mosquitoes were given access to 10% glucose solution soaked in cotton pad during recovery. All the experiments were carried out in a room with a constant temperature of 25 ± 1°C and 75% RH. Mortalities were counted after 24 h of recovery period, and corrected mortalities were calculated using Abbott’s formula.
DNA Isolation
Prior to DNA isolation, the lower one-third of the mosquito’s abdomen was removed to eliminate contamination of DNA originating from sperms stored in spermatheca. Genomic DNA from individual mosquitoes was isolated following Livak’s method ( Livak 1984) , eluted in 200 µl TE (Tris-EDTA) buffer, and stored at 4°C until use.
kdr Genotyping and DNA Sequencing
kdr genotyping of An. stephensi was carried out using polymerase chain reaction (PCR)-based assays as described by Singh et al . (2011) . Two allele-specific PCR assays, i.e., PCR-F and PCR-L/S, were carried out for detection of kdr alleles where the former PCR discriminates 1014F from all other alleles (wild and 1014S) and the latter discriminates 1014S from L1014. The primers used for PCR-F were 0.50 μM of St-PheR (5′-GAT CGG AAA GTA AGT TAC TTA CGg CA-3′), 0.25 μM of St-L/SR and 0.25 μM of St-F (5′-GAT TGT GTT CCG TGT GCT GT-3′), and for PCR-L/S, primers used were 0.50 μM each of St-F, St-L/SR (5′-GCG GGC AGG GCG GCG GGG GCG GGG CCC GAT CGG AAA GTA AGT TAC TTA CGt CT-3′), and St-SerR (5′-CGA TCG GAA AGT AAG TTA CTT ACG AtT G-3′). Each PCR reaction (15 µl) contained 1× buffer, 1.5 mM MgCl 2 , 200 µM of each dNTP, 0.375 units of AmpliTaq Gold taq polymerase (Applied Biosystems), and primers. The thermal cycling conditions for both ASPCR were one cycle at 95°C for 5 min, followed by 35 cycles each at 95°C for 30 s, 55°C for 30 s, and 72°C for 45 s, and a final extension step at 72°C for 7 min. Positive and negative controls were used for all PCR assays.
At least 10 DNA isolates from each locality, which were negative for L1014F and L1014S alleles, were sequenced for the partial S4–S6 segment of the domain 2 of the VGSC for verification of results and to screen the presence of any other existing alternative mutation. PCR products were amplified using primers VGS1F (5′-CTG AAT TTA CTC ATT TCC ATC-3′) and VGS1R (5′-CGA AAT TGG ACA AAA GCA AGG-3′) following the PCR conditions described by Singh et al. (2011) . The PCR product was sequenced using primer VGS1F and internal primers VGS2R (5′-GAT ATG GTG CGA GCG AAT TT-3′) and VGS2F (5′-ATC CGT TTG CCC AAA CTA CA-3′). Sequence chromatograms were analyzed and edited using software Finch TV and aligned using ClustalW implemented in Mega 5 ( Tamura et al. 2013 ).
Genetic Analysis
The observed and expected heterozygotes of kdr alleles and test of significance were calculated using Arlequin ver 3.5 software ( Excoffier et al . 2005 ).
Results
Insecticide susceptibility tests carried out on three populations viz. Alwar, Ranchi, and Chennai showed that the populations are susceptible to synthetic pyrethroids but resistant to DDT. Results are shown in Table 2 .
Table 2.
Susceptibility status of An. stephensi against DDT and pyrethroids
| Locality |
DDT 4%
|
PER 0.75%
|
DEL 0.05%
|
|||
|---|---|---|---|---|---|---|
| N | Corrected mortality | N | Corrected mortality | N | Corrected mortality | |
| Alwar | 40 | 10% | 70 | 98.7% | 50 | 100% |
| Ranchi | 40 | 20% | 40 | 92.5% | 40 | 100% |
| Chennai | 39 | 66.7% | ND | 52 | 100% | |
Abbreviations used: PER—permethrin, DEL—deltamethrin, ND—not done.
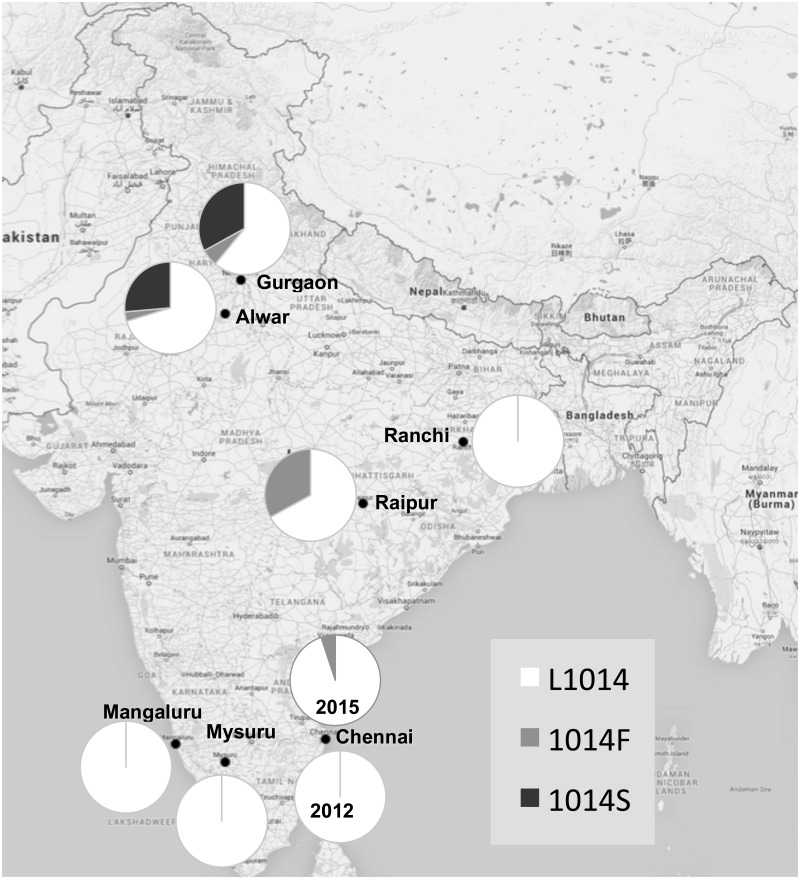
The result of kdr genotyping of mosquitoes for different kdr alleles for the seven field populations, their allelic frequencies, and Hardy–Weinberg equilibrium (HWE) parameters are provided in Table 1 . It was observed that of the seven field populations studied, kdr allele 1014F was present in Gurgaon, Alwar, Raipur, and Chennai with allelic frequencies 0.06, 0.033, 0.329, and 0.051, respectively. No mutations were found in Chennai during 2012; however, we recorded a low frequency of 1014F mutation in 2015. The 1014F allele was absent in the remaining field populations. The kdr allele 1014S was present in Alwar and Gurgaon populations with relatively high frequencies (0.264 and 0.33, respectively) and in association with very low frequency of 1014F mutation (0.033 and 0.06, respectively). Genotypes in all populations were in HWE except for Gurgaon, which may be due to its small sample size. The compliance of HWE in other populations indicates random mating and rules out any possibility of genotyping error and presence of null allele. Distribution of allelic frequencies of different L1014 alleles in the field populations is presented in Fig. 1 .
Fig. 1.
Distribution of kdr alleles L1014, 1014F, and 1014S in Indian An. stephensi populations.
DNA sequencing of samples negative for kdr allele through PCR confirmed the absence of mutation.
Discussion
At present, vector control in India relies mainly on the use of chemical insecticides for IRS in rural areas and antilarval measures and fogging in urban areas besides the use of insecticide-treated mosquito nets. Currently, DDT, malathion, and synthetic pyrethroids are used for IRS, and organophosphates (temephos and fenthion) for larval control. The use of synthetic pyrethroid-treated LLINs is currently being promoted in areas with high API (annual parasite incidence per thousand population). The environmental burden of insecticides is high, as >40 insecticide formulations are currently registered with Central Insecticide Board and Registration Committee ( [CIBRC] 2015 ) for the control of household and agricultural pests, contributing to the development of resistance in malaria vectors. Anopheles stephensi is reported to be resistant to the commonly used insecticides in public health in the past, particularly DDT, dieldrin (Subbarao 1980), malathion ( Tikar et al. 2011 ), and BHC ( Singh et al. 2014 ). However, An. stephensi is still susceptible to pyrethroids, which has emerged as a potential and preferred insecticide in public health and agricultural sectors due to their high knockdown action, low residual effect, and low mammalian toxicity. A prevention or delay in resistance against this precious group of insecticide relies on the rational use of the insecticide and an understanding of insecticide resistance management, which is crucial.
Anopheles stephensi is widely distributed in urban area in India, as it breeds in cemented structures which are abundantly found as overhead tanks, cisterns, fountain tanks, and uncovered water storage cemented underground tanks. Profuse breeding has been recorded in active building construction sites, which has been attributed as a cause of epidemics in a metropolitan city ( Covell 1928 ). The control of this vector in urban areas relies on the use of antilarval methods, legislative measures, and personal protection in the form of mosquito nets or repellents. Pyrethroids are an important group of insecticides that are being used for the impregnation of mosquito nets and in commercially available mosquito-killing devices, such as coils, evaporators, and mats, which are common in urban area for protection against mosquito nuisance, besides space spray and fogging. Therefore, emergence of pyrethroid resistance in the coming years may hamper vector control program, as synthetic pyrethroids would be rendered ineffective.
Anopheles stephensi , although reported to be resistant to DDT, is still susceptible to pyrethroids. We did not detect pyrethroid resistance in Alwar and Chennai even in the presence of kdr mutation, indicating that kdr alone is not sufficient to produce resistance phenotype against discriminating dose. An earlier report on laboratory selection of resistance against deltamethrin has shown selection of 1014F kdr trait ( Gayathri et al. 2006 ), indicating the positive role of kdr mutation against deltamethrin. However, the role of L1014- kdr in conferring resistance against DDT and pyrethroids has been established in a plethora of insect species ( Silva et al. 2014 ) and is manifested in homozygous condition being recessive or incomplete recessive ( Chandre et al. 2000 , Davies et al . 2007b ).
This is the first report on the distribution of kdr alleles in Indian An. stephensi populations. Earlier, we reported the presence of two alternative kdr mutations i.e., L1014F and L1014S, in just one population in northern India ( Singh et al . 2011 ). The focal presence of kdr alleles in this study warrants a close monitoring of the spread of such alleles, considering the presence of widespread resistance to DDT in India.
In An. stephensi , regional differences in the distribution pattern of kdr mutations and their allelic frequencies were noticed in the Indian populations in this study. Both kdr mutations, L1014F and L1014S, were found in north Indian populations—Alwar and Gurgaon, with L1014S frequencies of 26 and 33%, respectively. In central Indian population (Raipur), only L1014F was found to be present, with a frequency of 33%. In eastern and southern India, we did not record any kdr mutation except for Chennai where the presence of L1014F mutation is found at low frequency in 2015. Earlier record showed the presence of L1014F in a laboratory colony originating from Chennai, southern India, which was selected for deltamethrin resistance for several generations ( Gayathri et al . 2006 ). The data suggest that kdr is focal in distribution in the case of An. stephensi.
With the kdr trait said to be incompletely recessive ( Chandre et al . 2000 ) or recessive ( Davies et al . 2007b ), it may not be a major insecticide resistance mechanism unless kdr alleles are present in high frequency so as to maintain sufficient kdr- homozygotes. We noted high frequency of 1014S in northern Indian populations and 1014F in a central Indian population (>25%), while very low frequency of kdr mutation or its absence was observed in other populations. Fixation of kdr alleles may play a discrete role in insecticide resistance in the long run as evident from the study in Kenya ( Mathias et al. 2011 ). In considering efforts toward effective monitoring of insecticide resistance, knowledge of the frequency distribution of kdr alleles is essential to prevent their wide spreading and in effecting a delay in the imminent resistance that is gradually expanding against pyrethroids, an invaluable class of insecticide that remains for the fight against Malaria in India.
Acknowledgments
We are grateful to Mr. Uday Prakash for genotyping assistance and to Mr. Bhopal Ram, Mr. Shri Bhagwan, and Mr. N. S. Bhakuni for insectary and field assistance. C.L.D. was supported by Defence Research Development Establishment (DRDE) grant, and S.M. was supported by National Institutes of Health/National Institute of Allergy and Infectious Diseases grant U19AI089676. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of funding agencies.
References Cited
- (CIBRC) Central Insecticide Board and Registration Committee . 2015. . Insecticides approved by the registration committee to control household pests in houses under the insecticides act, 1968. ( www.cibrc.nic.in/insecticides.doc ) (accessed 1 December 2015) . [Google Scholar]
- Chandre F., Darriet F., Duchon S., Finot L., Manguin S., Carnevale P., Guillet P. . 2000. . Modifications of pyrethroid effects associated with kdr mutation in Anopheles gambiae . Med. Vet. Entomol. 14 : 81 – 88 . [DOI] [PubMed] [Google Scholar]
- Christopher S. R. 1933. . The Fauna of British India, Including Ceylon and Burma, Diptera, Volume IV, Family Culicidae, Tribe Anophelini . Taylor and Francis; , Red Lion Court, Fleet Street, London, United Kingdom: . [Google Scholar]
- Covell G. 1928. . Malaria in Bombay . Government Central Press; , Bombay, India: . [Google Scholar]
- Dash A. P., Adak T., Raghavendra K., Singh O. P. . 2007. . The biology and control of malaria vectors in India . Curr. Sci. 92 : 1571 – 1578 . [Google Scholar]
- Davies T. G., Field L. M., Usherwood P. N., Williamson M. S. . 2007a. . A comparative study of voltage-gated sodium channels in the Insecta: implications for pyrethroid resistance in Anopheline and other Neopteran species . Insect Mol. Biol. 16 : 361 – 375 . [DOI] [PubMed] [Google Scholar]
- Davies T. G., Field L. M., Usherwood P. N., Williamson M. S. . 2007b. . DDT, pyrethrins, pyrethroids and insect sodium channels . IUBMB Life 59 : 151 – 162 . [DOI] [PubMed] [Google Scholar]
- Davies T.G.E., Williamson M. S. . 2009. . Interactions of pyrethroids with the voltage-gated sodium channel . Bayer Crop Sci. J. 62 : 159 – 178 . [Google Scholar]
- Dykes C. L., Kushwah R.B.S., Das M. K., Sharma S. N., Bhatt R. M., Veer V., Agrawal O. P., Adak T., Singh O. P. . 2015. . Knockdown resistance ( kdr ) mutations in Indian Anopheles culicifacies populations . Parasit. Vectors 8 : 333 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Excoffier L., Laval G., Schneider S. . 2005. . Arlequin ver. 3.0: An integrated software package for population genetics data analysis . Evol. Bioinfo. 1 : 47 – 50 . [PMC free article] [PubMed] [Google Scholar]
- Faulde M. K., Rueda L. M., Khaireh B. A. . 2014. . First record of the Asian malaria vector Anopheles stephensi and its possible role in the resurgence of malaria in Djibouti, Horn of Africa . Acta Trop. 139 : 39 – 43 . [DOI] [PubMed] [Google Scholar]
- Gayathri V., Murthy P. B. . 2006. . Reduced susceptibility to deltamethrin and kdr mutation in Anopheles stephensi Liston, a malaria vector in India . J. Am. Mosq. Control Assoc. 22 : 678 – 688 . [DOI] [PubMed] [Google Scholar]
- Kumari R., Thapar B. R., Das Gupta R. K., Kaul S. M., Lal S. . 1998. . Susceptibility status of malaria vectors to insecticides in India . J. Commun. Dis. 30 : 179 – 185 . [PubMed] [Google Scholar]
- Livak K. J. 1984. . Organization and mapping of a sequence on the Drosophila melanogaster X and Y chromosomes that is transcribed during spermatogenesis . Genetics 107 : 611 – 634 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Torres D., Chandre F., Williamson M. S., Darriet F., Bergé J. B., Devonshire A. L., Guillet P., Pasteur N., Pauron D. . 1998. . Molecular characterization of pyrethroid knockdown resistance ( kdr ) in the major malaria vector Anopheles gambiae s.s . Insect Mol. Biol. 7 : 179 – 184 . [DOI] [PubMed] [Google Scholar]
- Mathias D. K., Ochomo E., Atieli F., Ombok M., Bayoh M. N., Olang G., Muhia D., Kamau L., Vulule J. M., Hamel M. J., et al. . 2011. . Spatial and temporal variation in the kdr allele L1014S in Anopheles gambiae s.s. and phenotypic variability in susceptibility to insecticides in Western Kenya . Malar. J. 10 : 10 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- (NVBDCP) National Vector Borne Disease Control Programme . 2012. . Strategic Action Plan for Malaria in India. 2007–2012. Directorate of National Vector Borne Disease Control Programme (NVBDCP), 22, Sham Nath Marg, Delhi-110054, India . [Google Scholar]
- Rajagopalan N., Vedamanickam J. C., Ramani S. R. . 1956. . A preliminary note on the development of resistance to DDT in larvae of Anopheles stephensi in Urban Erode . South India. Bull. Nat. Soc. Mal. Mos. Dis. 4 : 126 – 128 . [Google Scholar]
- Ramphul U., Boase T., Bass C., Okedi L. M., Donnelly M. J., Muller P. . 2009. . Insecticide resistance and its association with target-site mutations in natural populations of Anopheles gambiae from eastern Uganda . Trans. R. Soc. Trop. Med. Hyg. 103 : 1121 – 1126 . [DOI] [PubMed] [Google Scholar]
- Ranson H., Jensen B., Vulule J. M., Wang X., Hemingway J., Collins F. H. . 2000. . Identification of a point mutation in the voltage-gated sodium channel gene of Kenyan Anopheles gambiae associated with resistance to DDT and pyrethroids . Insect Mol. Biol. 9 : 491 – 497 . [DOI] [PubMed] [Google Scholar]
- Rao T. R. 1984. . The Anophelines of India . Malaria Research Centre (ICMR) , Delhi, India: , p. 596 . [Google Scholar]
- Reimer L., Fondjo E., Patchoke S., Diallo B., Lee Y., Ng A., Ndjemai H. M., Atangana J., Traore S. F., Lanzaro G., et al. . 2008. . Relationship between kdr mutation and resistance to pyrethroid and DDT insecticides in natural populations of Anopheles gambiae . J. Med. Entomol. 45 : 260 – 266 . [DOI] [PubMed] [Google Scholar]
- Silva A. P., Santos J. M., Martins A. J. . 2014. . Mutations in the voltage-gated sodium channel gene of anophelines and their association with resistance to pyrethroids – a review . Parasit. Vectors 7 : 450 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh O. P., Bali P., Hemingway J., Subbarao S. K., Das A. P., Adak T. . 2009. . PCR-based methods for the detection of L1014 kdr mutation in Anopheles culicifacies sensu lato . Malar. J. 8 : 154 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh O. P., Dykes C. L., Das M. K., Pradhan S., Bhatt R. M., Agrawal O. P., Adak T. . 2010. . Presence of two alternative kdr -like mutations, L1014F and L1014S, and a novel mutation, V1010L, in the voltage gated Na + channel of Anopheles culicifacies from Orissa, India . Malar. J. 9 : 146 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh O. P., Dykes C. L., Lather M., Agrawal O. P., Adak T. . 2011. . Knockdown resistance ( kdr )-like mutations in the voltage-gated sodium channel of a malaria vector Anopheles stephensi and PCR assays for their detection . Malar. J. 10 : 59 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R. K., Kumar G., Kumar P., Mittal P. K. . 2014. . Insecticide susceptibility status of malaria vectors in India: A review . Int. J. Mosq. Res. 1 : 5 – 9 . [Google Scholar]
- Singh O. P., Dykes C. L., Sharma G., Das M. K. . 2015. . L1014F- kdr Mutation in Indian Anopheles subpictus (Diptera: Culicidae) arising from two alternative transversions in the voltage-gated sodium channel and a single PIRA-PCR for their detection . J. Med. Entomol. 52 : 24 – 27 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderlund D. M. 2008. . Pyrethroids, knockdown resistance and sodium channels . Pest. Manag. Sci. 64 : 610 – 616 . [DOI] [PubMed] [Google Scholar]
- Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. . 2013. . MEGA6: Molecular Evolutionary Genetics Analysis version 6.0 . Mol. Biol. Evol. 30 : 2725 – 2729 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikar S. N., Mendki M. J., Sharma A. K., Sukumaran D., Veer V., Prakash S., Parashar B. D. . 2011. . Resistance status of the malaria vector mosquitoes, Anopheles stephensi and Anopheles subpictus towards adulticides and larvicides in arid and semi-arid areas of India . J. Insect. Sc. 11 : 85 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- (WHO) World Health Organization . 1989. . The use of insecticide-impregnated bednets and other materials for vector borne disease control. WHO/VBC/89.981 rev.1 . World Health Organization; , Geneva: . [Google Scholar]
- (WHO) World Health Organization . 2009. . Policies, strategies and targets for malaria control . In“World Malaria Report 2009” . World Health Organization; , Geneva: , pp. 3 – 8 . [Google Scholar]