Abstract
West Nile virus (WNV) is an important cause of disease in humans and animals. Risk of WNV infection varies seasonally, with the greatest risk during the warmest parts of the year due in part to the accelerated extrinsic incubation rate of the virus in mosquitoes. Rates of extrinsic incubation have been shown in constant-temperature studies to increase as an approximately linear function of temperature, but for other vector-borne pathogens, such as malaria or dengue virus, nonlinear relationships have been demonstrated under cycling temperatures near the thermal limits of pathogen replication. Using typical daily air temperature profiles from three key periods of WNV amplification in a hyperendemic area of WNV activity in California’s Central Valley, as well as a fourth temperature profile based on exposures that would result from daily mosquito host-seeking and resting behavior, we explored the impacts of cycling temperatures on WNV transmission by Culex tarsalis Coquillett, one of the principal vectors in the western United States. The daily cycling temperature ranges studied were representative of those that occur across much of California, but they did not significantly alter the extrinsic incubation period of WNV compared with estimates from mean temperatures alone. This suggests that within the relatively broad range we studied, WNV incubation rates are a simple function of mean temperature. Realistic daily temperature patterns that reflected mosquitoes’ avoidance of daytime high temperatures during summer reduced transmission over time compared with air temperatures, indicating that adjustment for mosquito exposure temperatures would be prudent for calculating risk.
Keywords: West Nile virus, mosquito-borne disease, vector competence, extrinsic incubation period, Culex tarsalis
Transmission of mosquito-borne West Nile virus (WNV) is affected strongly by temperature (Hartley et al. 2012). All of the parameters that determine vectorial capacity, a summary metric for the entomological components of transmission (Garrett-Jones 1964), are driven, in part, by temperature. These include mosquito abundance (Reisen and Reeves 1990, Pecoraro et al. 2007, Reisen et al. 2008, Chuang et al. 2011), the biting rate (Reisen et al. 1992b), the daily probability of survival (Reeves et al. 1994, Reisen 1995), and the extrinsic incubation period (EIP), defined as the time elapsed from a mosquito’s initial viremic bloodmeal to when it becomes capable of transmission. The EIP is strongly temperature dependent, shortening at warm temperatures and ranging from about 1 wk to 1 mo or more over the range of temperatures that occur at temperate latitudes (Dohm et al. 2002, Reisen et al. 2006, Kilpatrick et al. 2008, Danforth et al. 2015). There is evidence that EIP is based primarily on the thermodynamics of viral replication, as the length of the EIP is indistinguishable in four important vectors of WNV in California, including Culex tarsalis Coquillett, at 26°C (Goddard et al. 2002). Estimates for EIP as a function of temperature and the limiting zero-transmission threshold (Reisen et al. 2006) have been used in the California Mosquito-Borne Virus Surveillance and Response Plan to assess the temperature-related risk of WNV transmission (California Department of Public Health [CDPH et al.] 2015).
Daily temperature fluctuations typical of those that occur in nature also have been shown to alter infection and transmission of several pathogens within mosquitoes when compared with expectations based on mean temperature alone (Paaijmans et al. 2009, Lambrechts et al. 2011, Carrington et al. 2013). The effects of large daily temperature ranges (DTRs) can differ between the cool and the warm ends of the range of naturally occurring temperatures, presumably reflecting nonlinearities in the relationship between extrinsic incubation rates and temperature that become evident when portions of the day are beyond the virus’ thermal limits. Incubation of dengue virus in Aedes aegypti (L.), for example, was accelerated by a large DTR at low mean temperature (20°C; Carrington et al. 2013) but was slowed by the same DTR at higher temperature (26°C; Lambrechts et al. 2011) compared with constant temperatures at the same means. However, there was no impact of a smaller DTR on the EIP at 26°C (Carrington et al. 2013). Similar effects have been observed for Plasmodium falciparum, where typical daily temperature cycling typical of the Kenyan Highlands increased the rate of transmission at mean temperatures <21°C, whereas the same fluctuations around mean temperatures >21°C decreased pathogen transmission (Paaijmans et al. 2009). On the other hand, much earlier studies on yellow fever and eastern equine encephalitis viruses showed that varying daily temperature led to no changes in the duration of the EIP when compared with estimates based on the mean temperature alone (Bates and Roca-García 1946, Chamberlain and Sudia 1955). Interestingly, cool temperatures were associated with increased susceptibility to infection with chikungunya and yellow fever viruses in Ae. aegypti (Adelman et al. 2013) and to Western equine encephalomyelitis virus in Cx. tarsalis (Reisen et al. 1996). With WNV, there is evidence that the virus’s incubation rate is not a simple linear function of time and temperature (Kilpatrick et al. 2008).
In addition, mosquito behavior influences the temperatures to which they are exposed. Current estimates for time to transmission of WNV are based on mean air temperatures (Reisen et al. 2006, Kilpatrick et al. 2008, Danforth et al. 2015). However, Cx. tarsalis is a night-biting mosquito and spends the daytime in underground refugia (Reisen and Reeves 1990). As a result of spending the warmest parts of the day in sheltered habitat, Cx. tarsalis is exposed to cooler mean temperature than expected from ambient conditions alone (Meyer et al. 1990).
Despite the evidence above that fluctuating temperatures could potentially impact transmission rates, current estimates for the temperature-based risk of WNV transmission in California are based solely on constant temperatures (Reisen et al. 2006). Here, we test the hypothesis that daily cycling temperatures in California alter mean-based expectations for the EIP of WNV in Cx. tarsalis, the primary rural vector of WNV. We expected that the EIP with cycling temperatures would be shorter than constant-temperature estimates based on cool daily mean temperature and longer than constant-temperature estimates based on warm daily means. Based on our findings, we developed a model of WNV transmission as a logistic function of time and temperature that was used to quantify the impact of temperatures experienced by nocturnally active Cx. tarsalis as compared with daily air temperature cycles measured at weather-monitoring stations.
Materials and Methods
Virus Strain
For all experiments, we used WNV stock derived from KERN11 (KERN2000-2011, Gen Bank KR348980), a recent isolate from Kern County, which is a hyperendemic area of WNV activity in California’s Central Valley (Reisen et al. 2009). The isolate was obtained from a pool of Cx. tarsalis mosquitoes and was passaged twice in Vero cells before experimentation.
Mosquitoes
Mosquitoes for this project were Cx. tarsalis from the KNWR colony, which was established in 2002 from mosquitoes collected at the Kern National Wildlife Refuge (35.7458° N, 118.6179° W), in Kern County, CA, near the location represented by our temperature treatments and the site where our experimental viral isolate was found. Larvae were fed on ground fish food and adults had constant access to a 10% sucrose-soaked cotton pad. All were reared in an insectary at 24°C with 40–60% relative humidity (RH) and a photoperiod of 14:10 h. For experiments, adults were transferred to a biosafety-level three (BSL-3) containment facility 3–5 d after emergence.
Infection
Once adult Cx. tarsalis were transferred to the BSL-3 facility, their access to sucrose was removed, and they were held at the respective prescribed cycling temperature treatments in programmable incubators for 1 d. After the 24-h adjustment period, mosquitoes were offered a 1:5 mixture of WNV (109 plaque-forming units [pfu] per ml) to heparinized sheep’s blood (Hemostat Laboratories, Dixon, CA) for 1 h via a Hemotek membrane-feeding apparatus (Discovery Workshops, Lancashire, United Kingdom) maintained at 37°C. At the end of the hour, mosquitoes were lightly anesthetized with CO2, transferred to a Petri dish on ice, sorted into blood fed and nonblood fed mosquitoes, and an aliquot of the bloodmeal was stored at −80°C for analysis by plaque assay on Vero cells (Kramer et al. 2002). Groups of 25–28 blood-fed females (47–48 for the March treatment) were placed in half-liter cartons then placed in programmable Binder incubators (model KBF115, Tuttlingen, Germany) with a photoperiod of 12:12 (L:D) h and 50% RH. Incubators were programed to reflect long-term (30-yr) mean daily temperature patterns during 15 March, 15 May, and 15 July at the Bakersfield Airport (National Climatic Data Center [NCDC] 2015) in order to reflect the midpoint of the month, with the addition of a fourth temperature regime based on mosquito behavior patterns during July (“July-Mosquito”), when they spend the nocturnal period host seeking in ambient air temperatures and the diurnal period in natural shelters such as rodent burrows, avoiding the heat of the day (Meyer et al. 1990; Fig. 1 and Supp. Information [online only]). Mean temperatures for the four treatments were 14.2°C, 21.5°C, 29.0°C, and 26.5°C, with DTRs of 11.0°C, 13.5°C, 14.2°C, and 10.1°C, respectively. Because prior research indicated that transmission did not occur until >1 mo postinfection at 14°C (Reisen et al. 2006), the mosquito group sizes were increased for March temperatures in order to ensure sufficient mosquitoes for testing. Each carton had a dried cranberry and water-soaked cotton pad that were changed every 2 d.
Fig. 1.
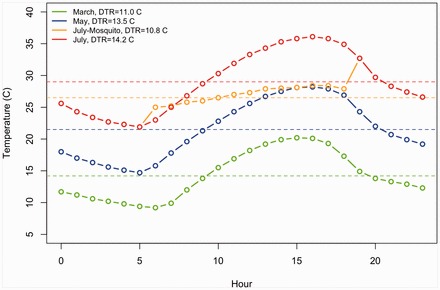
Daily temperature profiles for each monthly setting. Daily means for treatments are indicated in matched colors (dashed lines).
Sample Collection
Four or five time points were selected for transmission attempts for each treatment to reflect the range of transmission found in recent constant-temperature studies (Reisen et al. 2006, Danforth et al. 2015; Table 1). At each prescribed time, a single group of mosquitoes was anesthetized with triethylamine, and individual mosquitoes then had their expectorant collected via the capillary tube method (Aitken 1977), in which their proboscis was inserted into a capillary tube filled with a 1:1 mixture by volume of fetal bovine serum (Gibco, Grand Island, NY) and 10% sucrose solution for 20 min. Expectorant samples and bodies then were placed in separate 1.5-ml centrifuge tubes prefilled with 300 µl of viral transport media (Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum by volume, 50 mg/ml gentamicin, 10,000 units/ml penicillin, 10 mg/ml streptomycin, and 5 mg/ml mycostatin), and stored at −80°C before testing.
Table 1.
Summary statistics for infection and transmission
| Setting | Time point 1 |
Time point 2 |
Time point 3 |
Time point 4 |
Time point 5 |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DPF | N | Inf | Trans | DPF | N | Inf | Trans | DPF | N | Inf | Trans | DPF | N | Inf | Trans | DPF | N | Inf | Trans | |
| March | 20 | 18 | 100 (18) | 0 (0) | 24 | 12 | 100 (12) | 0 (0) | 28 | 9 | 100 (9) | 0 (0) | 32 | 18 | 38.9 (7) | 0 (0) | 36 | 12 | 91.7 (11) | 8.3 (1) |
| May | 10 | 19 | 89.5 (17) | 0 (0) | 12 | 19 | 69.4 (13) | 5.3 (1) | 14 | 14 | 92.9 (13) | 14.3 (1) | 16 | 16 | 100 (16) | 25 (4) | 18 | 14 | 64.3 (9) | 21.4 (3) |
| July | 2 | 19 | 94.7 (18) | 0 (0) | 5 | 22 | 90.9 (20) | 4.5 (1) | 7 | 22 | 100 (22) | 45.5 (10) | 10 | 21 | 100 (21) | 66.7 (14) | N/A | N/A | N/A | N/A |
| July-Mosquitoa | 6 | 14 | 100 (14) | 0 (0) | 9 | 13 | 100 (13) | 23.1 (3) | 11 | 16 | 93.8 (15) | 18.8 (3) | 14 | 17 | 94.1 (16) | 64.7 (11) | N/A | N/A | N/A | N/A |
DPF—days postfeeding, N—number of mosquitoes per group, Inf–percent infected out of total mosquitoes (number), Trans—percent transmitting out of total mosquitoes (number). Days on which mosquitoes were tested differed by treatment, depending on expected EIPs.
aMosquito exposure treatment based on daily mosquito behavior patterns (see text)
Sample Analysis
All samples were thawed on wet ice before processing. Two 5-mm glass beads were placed in tubes containing bodies, and the bodies were homogenized in a Retsch Tissuelyser (model 85210; Haan, Germany) at 30 Hz for 2 min, whereas expectorant samples were vortexed for 3–5 s. Next, both body and expectorant samples were centrifuged at 14,000 rpm for 5 min. Viral RNA was extracted from the supernatant using a 5× MagMAX-96 well Viral RNA Isolation Kit (ABI Life Technology, Waltham, MA) on a MagMAX Express-96 Deep Well Magnetic Particle Processor (ABI Life Technology). Finally, samples were analyzed via singleplex quantitative reverse-transcriptase PCR using published primers (Lanciotti et al. 2000) on a ViiA 7 platform (ABI Life Technology). All samples that exceeded the amplification threshold within 40 cycles (Ct score < 40) were considered positive.
Statistical Analysis
Data were analyzed using R software version 3.0.2 (R Core Team 2015). The outcome was each mosquito’s transmission status, equal to one or zero for positive or negative saliva, respectively. Candidate explanatory variables included temperature treatment as a categorical variable, time in days postfeeding (dpf) as a continuous variable, and mean temperature in degrees Celsius, as continuous variables. All mosquitoes, regardless of their infection status, were included in analyses for transmission.
For our initial analysis to estimate the EIP under cycling conditions, we compared the results from each temperature setting that represented the full range of daily air temperatures for March, May, and July. We calculated the proportion of blood-fed mosquitoes that were transmitting WNV for each temperature setting at each time point. Using those measures, we estimated the range when transmission was probable under each condition.
To characterize the effects of cycling as compared with constant temperatures, we then compared theoretical expectations for the median extrinsic incubation rate (EIP−1) expected for each daily mean temperature based on our constant-temperature studies (Danforth et al. 2015) with the median extrinsic incubation rate estimated from the current cycling-temperature studies. Due to low expectation for the proportion transmitting WNV in the March temperature treatment, we only included results from the May and July infections. After calculating the median extrinsic incubation rate of transmission in mosquitoes incubated at constant temperatures, using a model based on time, temperature, and their interaction, we applied that rate to the hourly settings for each temperature regime to calculate hourly extrinsic incubation rates and then took their mean to find the mean daily extrinsic incubation rate. If a temperature point was below the zero-transmission threshold, it was assigned an extrinsic incubation rate of zero.
After comparing transmission under cycling-temperature conditions to constant-temperature conditions, we developed a model to estimate transmission of WNV in Cx. tarsalis as a function of time and temperature and their interaction, using logistic regression.
Using the coefficients from this final logistic regression model, we quantified the effects of typical mosquito behavior patterns on the EIP by comparing mosquitoes from the two July treatment groups. From the logistic equation, we calculated the probability density function for the EIP in terms of temperature, which shows the distribution of EIPs expected for a population of mosquitoes at a particular temperature.
Results
Infection and Transmission
In total, 295 female Cx. tarsalis imbibed an infectious bloodmeal. Mosquitoes in different temperature treatments imbibed bloodmeals with similar WNV titers: 7.60 log10 pfu/ml for the March treatment, 7.54 log10 pfu/ml for the May treatment, and 7.88 and 7.91 log10 pfu/ml for July and July-Mosquito treatments, respectively. Of the mosquitoes that took an infectious bloodmeal, 262 became infected for an overall infection rate of 88.8% (Table 1). Mosquitoes at March temperatures had an infection rate of 82.6%, the May treatment had an infection rate of 81.7%, the July treatment had an infection rate of 95.2%, and the July-Mosquito treatment had an infection rate of 96.7%. March infection rates were significantly lower than those of July (P = 0.02) and July-Mosquito (P = 0.02), as were May infection rates (vs. July P = 0.01, vs. July-Mosquito P = 0.02), but there were no significant differences in infection rates between March and May (P = 0.89) or July and July-Mosquito (P = 0.67) treatments, all based on contrasts between logistic regression coefficients. When predicting the probability of infection of each temperature setting as a function of time, the only category that had a significant change in the proportion of infected mosquitoes over time was March, where time had a negative association with infection status (P = 0.01). Generally, there were no trends in mosquito body Ct values over time, except in the July experiment, which saw significant decreases in Ct scores, which indicates virus levels increased over the course of the experiment when analyzed by linear regression (P = 0.01). When examining only the mosquitoes with positive expectorant samples, all came from individuals that tested positive for infection. Of those mosquitoes that had virus in their expectorant, there were no significant trends within temperature settings for Ct values over time. All temperature settings had significantly different transmission patterns, with mosquitoes completing the EIP earliest under July conditions, then July-Mosquito, May, and March, when analyzed as a categorical variable by logistic regression.
Extrinsic Incubation Periods for Cycling Temperatures
Under March conditions, transmission was not observed until 36 dpf, the final time point, and only one of twelve (8.3%) mosquitoes was capable of transmission (Fig. 2). For mosquitoes reared under May temperatures, we generally observed increases in transmission over time, with the transmission beginning 12 dpf, and reaching the observed maximum of percent transmitting at 16 dpf, with a slight decrease at 20 dpf. Transmission was first observed at 4 dpf under July cycling conditions, which then experienced a consistent and statistically significant increase in transmission over time, reaching 50% transmitting between 7 and 10 dpf.
Fig. 2.
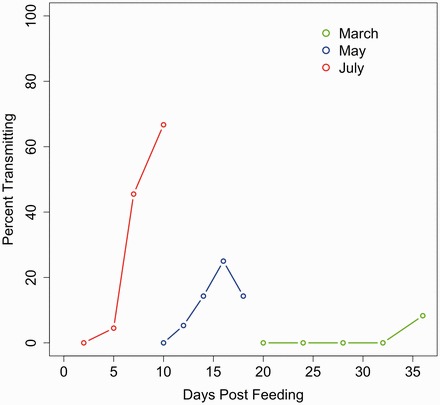
Percent of mosquitoes transmitting WNV on each day of observation by temperature setting.
Cycling Versus Constant Temperature Extrinsic Incubation Rate
When we developed a logistic regression model of time, temperature, and their interaction based solely on our prior constant-temperature results (using all blood-fed mosquitoes rather than only infected females), we found the following estimates for our coefficients: intercept = −9.75 (P < 0.01), dpf = −0.32 (P < 0.01), temperature = 0.19 (P < 0.01), and dpf × temperature = 0.03 (P < 0.01). From those coefficients, we estimated an effective zero-transmission threshold of 11.0°C. Using the constant temperature-based extrinsic incubation rate model, our new zero-transmission threshold, and the hourly temperatures for each temperature regime, we were able to calculate the expected extrinsic incubation rate for each setting. When we compared the estimated median EIP from our treatments with the values expected based on mean temperatures in the constant-temperature regression model, we found that under May cycling conditions, 22 dpf (95% CI: 4, 500) were estimated to reach the median EIP compared with 18 d expected under constant conditions, while the median EIP for July was 9 dpf (95% CI: 4, 21) compared with an expectation of 8 dpf.
Model of Time and Temperature to Transmission
As there was no significant difference between estimates for time to transmission under cycling conditions when compared with our earlier constant-temperature experiments, we were able to build a model for transmission that was not based on a simple linear relation with time and mean temperature. Using the fitted model, we derived the following equation for estimating the median EIP:
Based on this equation, the EIP exceeds one month below 16.5°C, and the rate of extrinsic incubation approaches zero around 9°C.
Effects of Diurnal Shelter
Using the above coefficients for predicting transmission probability as a function of time and mean temperature, we were able to quantify the impact of the temperatures to which mosquitoes are actually exposed based on their diurnal behavior, as opposed to estimates that assumed that mosquitoes are exposed to the full daily range of air temperatures. We calculated the probability density function of the EIP for mosquitoes reared under July ambient conditions and compared it with temperature conditions that mimic mosquito behavior during that time of year (Fig. 3). For July air temperatures, we estimated Cx. tarsalis typically would require 3, 9, and 15 dpf for 5, 50, and 95% of females to complete the EIP, respectively. However, cooler temperatures created by Cx. tarsalis diurnal resting behavior delayed transmission by 2–3 d to 5, 12, and 18 dpf, respectively, for the same percentages of mosquitoes to complete the EIP.
Fig. 3.
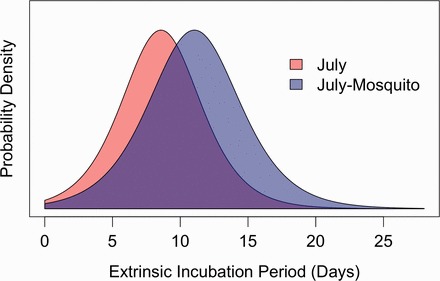
Distribution of EIPs for mosquitoes reared under typical July air temperatures, in red, versus those reared under the same July conditions accounting for daytime mosquito resting in sheltered habitats, in blue.
Discussion
Under the conditions simulated in this experiment, the typical daily cycling temperatures in Kern County did not impact the rate of extrinsic incubation of WNV by Cx. tarsalis as compared with estimates from the same constant mean temperatures. Using this information, we developed a nonlinear model of the probability of transmission as a function of time and mean temperature that can be used to estimate time to transmission at different temperatures. Finally, we showed that the daily behavior of Cx. tarsalis can delay the EIP compared with values expected from ambient air temperatures.
We designed the experimental conditions in this study to represent typical daily temperature cycling at a location with hyperendemic WNV transmission in California’s Central Valley that also was the source of both the WNV isolate and the mosquito colony used in our experiments. Further studies with additional mosquito colonies or species and viral strains would be needed to confirm the universality of our finding that DTR did not modify the association between daily mean temperatures and extrinsic incubation rates of WNV over the ranges of temperatures we studied. There is some evidence that strains of WNV can differ in their EIP (Moudy et al. 2007, Kilpatrick et al. 2008), although other studies, including one from California that used the same isolate as in this study, found no difference in EIP between strains (Anderson et al. 2012, Danforth et al. 2015). For relevance of our findings to other areas, the DTRs in this area (10.1–14.2°C) are representative of DTRs seen across much of California and also span those typical of more humid regions of the United States (NCDC 2015). This suggests that the EIP of WNV in such areas can be predicted accurately from mean daily temperatures alone.
The lack of impact of DTRs around 11°C on EIPs expected from mean temperature alone has been observed for other arboviruses. In studies of yellow fever and eastern equine encephalitis viruses, DTRs of 10 to 11°C, at the bottom limit of the range we used, also did not impact transmission (Bates and Roca-García 1946, Chamberlain and Sudia 1955). Similarly, for dengue virus transmission by Ae. aegypti, DTRs in that range had only slight impacts on the probability of transmission at temperatures <22°C; however, those same DTRs had a negative impact at warmer temperatures (Lambrechts et al. 2011). In addition, malaria transmission was impacted significantly by DTRs of 12°C, which is within the range used in our study (Paaijmans et al. 2009).
As we were able to determine that mean daily ambient temperature is sufficient to represent environmental conditions, we were able to develop a more accurate model for transmission of WNV as a function of time and mean temperature. By using logistic regression as the basis for the model, we captured the naturally occurring curvilinear relationship between transmission and time and temperature. Prior research took a simpler approach using only the linear portion of the extrinsic incubation rate–temperature curve, which may not fully capture that relationship between those factors (Reisen et al. 2006). Other models accounted for the curved shape by using a sinusoidal function, however, that assumes that at some point in time, transmission will start to decline (Kilpatrick et al. 2008). Although this model allows for transmission at temperatures as low as 9°C, WNV replication does not occur at 10°C, and meaningful transmission has not been observed below 14°C (Reisen et al. 2006, Danforth et al. 2015).
Culex tarsalis is nocturnally active (Reisen et al. 1997) and rests within sheltered habitats such as rodent burrows during the day, which can significantly reduce maximum temperature exposure (Meyer at al. 1990) and therefore the duration of the EIP. The estimate for time to median transmission during the month of July was delayed by 3 d, after accounting for daily mosquito resting behavior. However, Culex females only bite vertebrate hosts when seeking blood for laying a batch of eggs and then require several days for blood digestion, oogenesis, and oviposition before biting again, a process referred to as the gonotrophic cycle. This cycle takes ∼4–5 d in Cx. tarsalis at summer temperatures (Reisen et al. 1983, 1992b), so that the 9 d to median EIP under July ambient conditions requires two gonotrophic cycles, while the sheltering EIP of 12 d shifts transmission to the next bite cycle, 4–5 d later. These estimates do not account for possible delays related to unsuccessful searching for oviposition sites or bloodmeal hosts, which can lengthen the gonotrophic cycle as shown in mark–release–recapture studies in Kern County (Reisen et al. 1992a). As the time required for a mosquito to transmit a virus increases, the probability that it will survive long enough to complete the EIP decreases.
Taken together, our results indicate that the extrinsic incubation of WNV in Cx. tarsalis can be estimated from daily mean temperatures over the range of daily temperature fluctuations that occur in most of California. However, temperature-based estimates of EIP should account for exposure temperatures based on mosquito behavior, not just on commonly used air temperatures from climate-monitoring stations. In addition, risk assessment models for WNV activity based on linear assumptions of the accumulation of time and temperature should be adjusted to account for the more accurate curvilinear models that capture the natural plateauing of the relationships among mosquito transmission, time, and temperature.
Supplementary Material
Acknowledgments
We thank Ying Fang, Sandra Garcia, Sarah Wheeler, and Veronica Armijos (Center for Vectorborne Diseases, Department of Pathology, Microbiology, and Immunology, School of Veterinary Medicine, University of California, Davis) for technical support and Dr. Philip Kass (Department of Population Health and Reproduction, School of Veterinary Medicine, University of California, Davis) for statistical advice and editing. This research was funded by Grant 2013-17 from the Mosquito Research Foundation. CMB acknowledges additional support from the Research and Policy for Infectious Disease Dynamics (RAPIDD) program of the Science and Technology Directorate, Department of Homeland Security and Fogarty International Center, National Institutes of Health.
References Cited
- Adelman Z. N., Anderson M. A., Wiley M. R., Murreddu M. G., Samuel G. H., Morazzani E. M., Myles K. M. 2013. Cooler temperatures destabilize RNA interference and increase susceptibility of disease vector mosquitoes to viral infection. PLoS Negl. Trop. Dis. 7: e2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitken T. 1977. An in vitro feeding technique for artificially demonstrating virus transmission by mosquito. Mosq. News 37: 130–133. [Google Scholar]
- Anderson J. F., Main A. J., Cheng G., Ferrandino F. J., Fikrig E. 2012. Horizontal and vertical transmission of West Nile virus genotype NY99 by Culex salinarius and genotypes NY99 and WN02 by Culex tarsalis. Am. J. Trop. Med. Hyg. 86: 134–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates M., Roca-García M. 1946. The development of the virus of yellow fever in Haemagogus mosquitoes. Am. J. Trop. Med. Hyg. 1: 585–605. [DOI] [PubMed] [Google Scholar]
- (CDPH) California Department of Public Health, Mosquito and Vector Control Association of California, and University of California. 2015. California Mosquito-Borne Virus Surveillance & Response Plan. (http://westnile.ca.gov/resources.php) [Google Scholar]
- Carrington L. B., Armijos M. V., Lambrechts L., Scott T. W. 2013. Fluctuations at a low mean temperature accelerate dengue virus transmission by Aedes aegypti. PLoS Negl. Trop. Dis. 7: e2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain R. W., Sudia W. D. 1955. The effects of temperature upon the extrinsic incubation of eastern equine encephalitis in mosquitoes. Am. J. Epidemiol. 62: 295–305. [DOI] [PubMed] [Google Scholar]
- Chuang T. W., Hildreth M. B., Vanroekel D. L., Wimberly M. C. 2011. Weather and land cover influences on mosquito populations in Sioux Falls, South Dakota. J. Med. Entomol. 48: 669–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danforth M. E., Reisen W. K., Barker C. M. 2015. Extrinsic incubation rate is not accelerated in recent California strains of West Nile virus in Culex tarsalis (Diptera: Culicidae). J. Med. Entomol. 52: 1083–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohm D. J., O’Guinn M., Turell M. J. 2002. Effect of environmental temperature on the ability of Culex pipiens (Diptera: Culicidae) to transmit West Nile virus. J. Med. Entomol. 39: 221–225. [DOI] [PubMed] [Google Scholar]
- Garrett-Jones C. 1964. The human blood index of malaria vectors in relation to epidemiological assessment. Bull. World Health Org. 30: 241–261. [PMC free article] [PubMed] [Google Scholar]
- Goddard L. B., Roth A. E., Reisen W. K., Scott T. W. 2002. Vector competence of California mosquitoes for West Nile virus. Emerg. Infect. Dis. 8: 1385–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley D. M., Barker C. M., Le Menach A., Niu T., Gaff H. D., Reisen W. K. 2012. Effects of temperature on emergence and seasonality of West Nile virus in California. Am. J. Trop. Med. Hyg. 86: 884–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick A. M., Meola M. A., Moudy R. M., Kramer L. D. 2008. Temperature, viral genetics, and the transmission of West Nile virus by Culex pipiens mosquitoes. PLoS Pathogens 4: e1000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer L. D., Wolfe T. M., Green E. N., Chiles R. E., Fallah H., Fang Y., Reisen W. K. 2002. Detection of encephalitis viruses in mosquitoes (Diptera: Culicidae) and avian tissues. J. Med. Entomol. 39: 312–323. [DOI] [PubMed] [Google Scholar]
- Lambrechts L., Paaijmans K. P., Fansiri T., Carrington L. B., Kramer L. D., Thomas M. B., Scott T. W. 2011. Impact of daily temperature fluctuations on dengue virus transmission by Aedes aegypti. Proc. Natl. Acad. Sci. 108: 7460–7465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciotti R. S., Kerst A. J., Nasci R. S., Godsey M. S., Mitchell C. J., Savage H. M., Komar N., Panella N. A., Allen B. C., Volpe K. E., et al. 2000. Rapid detection of West Nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J. Clin. Microbiol. 38: 4066–4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer R. P., Hardy J. L., Reisen W. K. 1990. Diel changes in adult mosquito microhabitat temperatures and their relationship to the extrinsic incubation of arboviruses in mosquitoes in Kern County, California. J. Med. Entomol. 27: 607–614. [DOI] [PubMed] [Google Scholar]
- Moudy R. M., Meola M. A., Morin L.-L.L., Ebel G. D., Kramer L. D. 2007. A newly emergent genotype of West Nile virus is transmitted earlier and more efficiently by Culex mosquitoes. Am. J. Trop. Med. Hyg. 77: 365–370. [PubMed] [Google Scholar]
- (NCDC) National Climatic Data Center. 2015. Data Tools: 1981–2010 Normals, CA US Daily Normals. (https://www.ncdc.noaa.gov/cdo-web/datatools/normals) [Google Scholar]
- Paaijmans K. P., Read A. F., Thomas M. B. 2009. Understanding the link between malaria risk and climate. Proc. Natl. Acad. Sci. 106: 13844–13849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecoraro H. L., Day H. L., Reineke R., Stevens N., Withey J. C., Marzluff J. M., Meschke J. S. 2007. Climatic and landscape correlates for potential West Nile virus mosquito vectors in the Seattle region. J. Vector Ecol. 32: 22–28. [DOI] [PubMed] [Google Scholar]
- R Core Team. 2015. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ( http://www.R-project.org/) [Google Scholar]
- Reeves W. C., Hardy J. L., Reisen W. K., Milby M. M. 1994. Potential effect of global warming on mosquito-borne arboviruses. J. Med. Entomol. 31: 323–332. [DOI] [PubMed] [Google Scholar]
- Reisen W. K. 1995. Effect of temperature on Culex tarsalis (Diptera: Culicidae) from the Coachella and San Joaquin Valleys of California. J. Med. Entomol. 32: 636–645. [DOI] [PubMed] [Google Scholar]
- Reisen W. K., Reeves W. C. 1990. Bionomics and ecology of Culex tarsalis and other potential mosquito vector species, pp. 254–330. InReeves W. C. (ed.), Epidemiology and control of mosquito-borne arboviruses in California, 1943–1987. California Mosquito and Vector Control Associates, CA. [Google Scholar]
- Reisen W. K., Fang Y., Martinez V. M. 2006. Effects of temperature on the transmission of West Nile virus by Culex tarsalis (Diptera: Culicidae). J. Med. Entomol. 43: 309–317. [DOI] [PubMed] [Google Scholar]
- Reisen W. K., Milby M. M., Meyer R. P. 1992a. Population dynamics of adult Culex mosquitoes (Diptera: Culicidae) along the Kern River, Kern County, California, 1990. J. Med. Entomol. 29: 531–543. [DOI] [PubMed] [Google Scholar]
- Reisen W. K., Milby M. M., Presser S. B., Hardy J. L. 1992b. Ecology of mosquitoes and St. Louis encephalitis virus in the Los Angeles Basin of California, 1987–1990. J. Med. Entomol. 29: 582–598. [DOI] [PubMed] [Google Scholar]
- Reisen W. K., Hardy J. L., Presser S. B., Chiles R. E. 1996. Seasonal variation in the vector competence of Culex tarsalis (Diptera: Culicidae) from the Coachella Valley of California for western equine encephalomyelitis and St. Louis encephalitis viruses. J. Med. Entomol. 33: 433–437. [DOI] [PubMed] [Google Scholar]
- Reisen W. K., Lothrop H. D., Meyer R. P. 1997. Time of host-seeking by Culex tarsalis (Diptera: Culicidae) in California. J. Med. Entomol. 34: 430–437. [DOI] [PubMed] [Google Scholar]
- Reisen W. K., Milby M. M., Reeves W. C., Meyer R. P., Bock M. E. 1983. Population ecology of Culex tarsalis (Diptera: Culicidae) in a foothill environment of Kern County, California: Temporal changes in female relative abundance, reproductive status, and survivorship. Ann. Entomol. Soc. Am. 76: 800–808. [Google Scholar]
- Reisen W. K., Cayan D., Tyree M., Barker C. M., Eldridge B., Dettinger M. 2008. Impact of climate variation on mosquito abundance in California. J. Vector Ecol. 33: 89–98. [DOI] [PubMed] [Google Scholar]
- Reisen W. K., Carroll B. D., Takahashi R., Fang Y., Garcia S., Martinez V. M., Quiring R. 2009. Repeated West Nile virus epidemic transmission in Kern County, California, 2004–2007. J. Med. Entomol. 46: 139–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


