Abstract
Although Mycoplasma genitalium is increasingly recognized as a sexually transmitted pathogen, at present there is no defined public health response to this relatively newly identified sexually transmitted infection. Currently available data are insufficient to justify routinely screening any defined population for M. genitalium infection. More effective therapies, data on acceptability of screening and its impact on clinical outcomes, and better information on the natural history of infection will likely be required before the value of potential screening programs can be adequately assessed. Insofar as diagnostic tests are available or become available in the near future, clinicians and public health agencies should consider integrating M. genitalium testing into the management of persons with sexually transmitted infection (STI) syndromes associated with the infection (ie urethritis, cervicitis, and pelvic inflammatory disease) and their sex partners. Antimicrobial-resistant M. genitalium is a significant problem and may require clinicians and public health authorities to reconsider the management of STI syndromes in an effort to prevent the emergence of ever more resistant M. genitalium infections.
Keywords: Mycoplasma genitalium, public health, prevention
Mycoplasma genitalium was originally described as a sexually transmitted infection (STI) in 1981[1] and, over the last 2 decades, has become an infection of increasing concern as mounting evidence has documented the organism’s association with diverse STI syndromes, its potential to cause significant reproductive tract sequelae, and the rapid selection for antimicrobial resistance following treatment [2–4]. In this article, we discuss the public health implications of M. genitalium, including whether a screening program is justified, how M. genitalium laboratory tests might best be used, and the treatment of the infection. We highlight some of the challenges inherent to developing public health policy for STIs caused by an organism that can rapidly develop resistance and for which the natural history of infection remains ill-defined.
SCREENING FOR M. GENITALIUM: CRITERIA FOR INSTITUTING A SCREENING PROGRAM AND ADDRESSING THE NEED FOR M. GENITALIUM SCREENING
Screening is the testing of asymptomatic persons to find those with an infection, disease, or disorder. However, principles of screening are often misunderstood in clinical practice [5]. Criteria for determining if a screening test is warranted include the following: (1) the prevalence of infection is well defined and the severity of the morbidity associated with the infection justifies action; (2) the natural history of the infection is established and earlier diagnosis and treatment prevents symptoms or disease and improves quality of life or survival; (3) patients will adhere to the course of antimicrobials or any other known effective prevention interventions in the absence of symptoms; and (4) the accuracy, predictive values, safety, and cost of the screening test are acceptable [6]. In addition, any screening program should be cost-effective, be accessible to those who would benefit, and be potentially available in healthcare facilities that have the expertise and resources to diagnose and treat. Furthermore, the follow-up after a positive result should be agreeable and acceptable to those screened [7].
The criteria for instituting a screening program are similar to those used in public health to prioritize diseases and conditions of public health importance, establish which diseases should be nationally notifiable by law in the United States, and implement national public health prevention and control programs. In general, public health uses 7 criteria to determine if infections, diseases, or other conditions are of public health importance: frequency or burden, severity in terms of morbidity or mortality, communicability, preventability, disparities or inequities associated with the health event, costs associated with the health event, and public interest. As an example, most recently, these criteria were used to develop the Centers for Disease Control and Prevention (CDC) antibiotic-resistant bacteria threat report [8]. Table 1 summarizes the status of M. genitalium in meeting criteria to establish a screening program of public health importance. Of the 7 criteria listed, current evidence is insufficient for establishing a M. genitalium screening program as only 3 conditions are met: this infection is common, communicable, and has disparate impact on different populations. Further evidence is needed regarding the morbidity, costs, preventability, and public interest associated with M. genitalium infection.
Table 1.
Status of Mycoplasma genitalium in Meeting Criteria to Establish a Screening Program of Public Health Importance
| Criteria | Criteria Met | Some but Insufficient Evidence to Meet Criteria | No Evidence or Evidence Does Not Support Criteria | Comments |
|---|---|---|---|---|
| Infection is common | ✓ [25, 48–50] | |||
| Infection causes significant morbidity | ✓ [3] | Uncertain how often infection causes PID, TFI, or other major sequelae | ||
| Condition is communicable | ✓ [51, 52] | |||
| Morbidity is preventable | ✓ | Treatment not consistently effective | ||
| Infection has disparate impact on different populations | ✓ [25, 50, 53] | Prevalence is higher in African Americans | ||
| Infection is associated with significant costs | ✓ | Few data exist on the potential medical costs and population-level morbidity associated with infection | ||
| Condition associated with significant public interest | ✓ |
Abbreviations: PID, pelvic inflammatory disease; TFI, tubal factor infertility.
In the United States, the United States Preventive Services Task Force (USPSTF) makes evidence-based recommendations for clinical preventive services to be used by the primary care community, including screening along with counseling and preventive medications, which apply to adults and children with no recognized signs or symptoms. Recommendations are based on rigorous review of existing peer-reviewed evidence and evaluation of benefits and harms of each preventive service. While many of the screening program indices above are included in the USPSTF process, the cost of the health event, screening, and intervention are not included.
Based on current US criteria for defining when a screening program is justified, available data do not currently support the institution of widespread M. genitalium screening among young women and/or men or other populations sometimes screened for STI (eg, pregnant women).
Ideally, the decision to institute a M. genitalium screening program would be informed by 1 or more randomized controlled trials evaluating the impact of screening on major reproductive health morbidity in women and associated cost-effectiveness analyses. Prevention of tubal factory infertility (TFI), the most morbid and costly sequelae of bacterial STIs in women [9], would be the ideal outcome, perhaps with population-level trials evaluating the impact of serial rounds of screening on M. genitalium incidence or prevalence and M. genitalium–related TFI. Studies undertaken in the United States and some other nations suggest that chlamydia screening decreases the risk of pelvic inflammatory disease (PID) [10–13] and are the basis for the USPSTF recommendation for annual chlamydia screening of females <26 years of age [14]. However, the impact of chlamydia screening on infertility remains uncertain. This uncertainty has prompted some experts to question the value of chlamydia screening and suggest the need for much higher evidentiary standards to justify prevention programs [15]. However, it is not clear that any STI screening program could be definitively shown to affect an outcome like TFI, and more feasible approaches for defining the potential impact of screening are needed. TFI is a rare event and a randomized trial would need to be extremely large and involve many years of follow-up to demonstrate an effect. If 2.5% of women screened have M. genitalium infection, PID develops in 10% of women with M. genitalium infection, TFI occurs in 10% of women with PID (similar to estimates for Chlamydia trachomatis [16]; ie, 0.25 women per 1000 women screened TFI from M. genitalium), and screening averts 50% of cases of TFI, one would need >400000 persons in a randomized trial to demonstrate a benefit. Such a trial is not realistic. A M. genitalium screening trial with a PID outcome similar to the previously conducted chlamydia studies would be more feasible and could provide valuable information on the impact of M. genitalium screening. At the same time, such a trial would be subject to a number of limitations as the clinical diagnosis of PID is relatively subjective, the histologic diagnosis of endometritis is difficult to make and not consistently associated with disease in the fallopian tubes [17, 18], and biologic markers of STI-related TFI are not available. In addition, a trial with a PID outcome would leave unanswered the question of how often M. genitalium causes TFI or what proportion of TFI cases screening could avert.
The difficulties inherent in trying to establish the value of screening for M. genitalium infection requires careful consideration of how much evidence is needed to advocate for screening, a topic that affects a wide array of medical problems, not just M. genitalium or STIs. A first step related to screening for M. genitalium might be to define under what circumstances screening would be cost-effective. This would require defining a range of parameters related to the natural history of M. genitalium, the effectiveness of screening, and the costs associated with TFI and other sequelae of PID. Results of such a study could help inform decisions on whether a trial looking at a PID outcome would be worthwhile. If efficacy in averting PID was cost-effective in preventing infertility across a wide range of assumptions regarding how often PID results in TFI, a trial with a PID outcome might be sufficient to justify widespread screening.
In considering a way to determine the cost-effectiveness of a M. genitalium screening program, the subject of how to value infertility merits particular attention. Studies assessing how much infertility affects health have produced widely variable estimates [19, 20]. Health economists typically express the value of an intervention in terms of cost per quality-adjusted life year (QALY) gained. The number of QALYs associated with infertility (or the number gained by averting infertility) depends on factors such as the time horizon used and discount rates; QALYs lost per case of TFI vary from 1 to approximately 6.5 (Thomas Gift, January 2017). From a population perspective, the impact of screening is further influenced by whether one assumes that infertility has a cost for all or almost all affected women independent of treatment costs. Many cost-effectiveness analyses of chlamydial screening have focused only on the medical costs associated with infertility treatment among women seeking such treatment, essentially ignoring the morbidity associated with the condition among those not seeking treatment [21]. Better defining the cost of infertility beyond medical treatment will be critical in defining the potential impact of M. genitalium screening, and could also lead to a reappraisal of the cost-effectiveness of other STI screening programs that impact reproductive health.
DIAGNOSTIC TESTING FOR M. GENITALIUM
Although the available data are insufficient to support population-level programs to prevent M. genitalium, clinicians are frequently required to make testing and treatment decisions for patients presenting with M. genitalium–associated syndromes and their sex partners. This need is particularly acute in public health sexually transmitted disease (STD) clinics, which see large numbers of patients with STD syndromes, and which are ideally centers where patients can seek expert care for STIs. The following discussion reviews recent efforts to provide clinical guidelines for the medical management of M. genitalium.
There are currently no laboratory tests for M. genitalium approved by the US Food and Drug Administration (FDA), and it is uncertain when an FDA-approved test will be available. However, commercial assays are available outside the United States (including a test that includes detection of macrolide resistance), some laboratories in the United States offer locally developed tests, and analyte-specific M. genitalium reagents are available in the United States that laboratories can use for clinical testing following internal validation of the test’s performance [22]. The expanding availability of non-FDA-approved tests and potential future availability of FDA-approved tests should prompt the development of guidance on the indications for M. genitalium testing. Specific guidance on the use of these tests should reflect an effort to minimize the morbidity associated with M. genitalium, preserve the reservoir of antimicrobial susceptibility, and limit cost. Such an approach will be particularly important in health departments or medical facilities that operate STD specialty clinics, venues that should ideally be early adopters of M. genitalium testing, and where antimicrobial resistance monitoring and research can be conducted, similar to current prevention efforts for Neisseria gonorrhoeae.
Table 2 summarizes recently developed European guidelines and provisional local Public Health–Seattle & King County (PHSKC) guidelines for M. genitalium testing [23]. Development of these guidelines was prompted by the recognition that M. genitalium is a relatively common cause of urethritis, and that azithromycin therapy is not consistently effective in treating M. genitalium and contributes to the development of antimicrobial resistance [2, 24]. In the absence of a consistently effective treatment and stronger evidence supporting routine screening, use of M. genitalium assays should initially be used for diagnostic testing only in certain clinical situations. A limited role for a new assay might include testing persons with persistent or recurrent urethritis, PID, or cervicitis following receipt of recommended therapy, and perhaps testing the sex partners of persons with diagnosed M. genitalium infections. (Persistent urethritis does not resolve following recommended therapy, while recurrent urethritis recurs following initial improvement in the absence of likely reexposure to infection.) This approach would confine testing to persons who would likely benefit most and minimize costs, but would delay patients’ M. genitalium microbiologic diagnosis and result in some infected persons not being optimally treated if they did not return for reevaluation.
Table 2.
Potential Indications for Mycoplasma genitalium Testinga
| Indication | European Guidelines [23] | Provisional PHSKC Guidelines | Comment |
|---|---|---|---|
| Persons failing treatment for urethritis, PID, or cervicitis | Recommended | Recommended | - Diagnosis helps inform counseling and treatment - Affects small numbers of persons |
| Persons presenting with urethritis, PID, cervicitis, or epididymitis-orchitis | Recommended | Recommended | - Allows earlier diagnosis (ie, does not require treatment failure) - Might be useful as part of effort to use doxycycline (not azithromycin) for NGU, PID, and cervicitis, and limit azithromycin to treatment of M. genitalium known to be susceptible |
| Sex partners of persons diagnosed with M. genitalium | Recommended | Recommended | - Help avert reinfection and clinically inapparent morbidity |
| Women presenting with vaginal discharge | Recommended | Not recommended | - Uncertain how often M. genitalium causes vaginal discharge in the absence of MPC |
| Women being screening for chlamydial infection | Not recommended | Not recommended | - Insufficient evidence to justify screening |
| MSM being screening for STI | Not recommended | Not recommended | - Insufficient evidence to justify screening |
Abbreviations: MPC, mucupurulent cervicitis; MSM, men who have sex with men; NGU, nongonococcal urethritis; PHSKC, Public Health–Seattle & King County; PID, pelvic inflammatory disease; STI, sexually transmitted infection.
aTesting ideally includes testing for macrolide resistance.
An alternative approach would be to perform testing on all men with nongonococcal urethritis (NGU) (or urethritis if Gram stain testing is not performed), and women with PID or cervicitis. This may increase costs, but would identify a larger number of M. genitalium–infected persons and might avoid some clinical visits as those persons with persistent symptoms would have test results available to guide treatment decisions prior to seeking reevaluation. In addition, if coupled with a decision to adopt doxycycline, not azithromycin, as the standard therapy (or part of standard therapy) for NGU, PID, and cervicitis, such an approach might decrease antibiotic pressure that promotes the selection and dissemination of azithromycin-resistant M. genitalium [24], though recent data suggest that azithromycin resistance is already very widespread in many areas [25, 26]. European guidelines also suggest testing women for M. genitalium with vaginal discharge if they have risk factors for STI [23]. While some women with vaginal discharge have cervical infections, a proportion of which are due to M. genitalium, vaginal discharge has not been consistently associated with M. genitalium [27, 28], and most women presenting for evaluation of vaginal discharge have vaginitis, not cervicitis. Because of this and until additional data are available on the association of vaginal discharge with M. genitalium in the United States, testing all women with vaginal discharge is a lower priority than testing persons with STI syndromes more closely linked to M. genitalium infection.
TREATMENT OF M. GENITALIUM INFECTION AND IMPACT OF M. GENITALIUM ON STI TREATMENT
The recognition of M. genitalium as a sexually transmitted pathogen requires guidance on the optimal treatment for confirmed infection, and has implications for syndromic treatment of NGU, PID, and cervicitis in the absence of microbiologic data. The CDC 2015 STD treatment guidelines do not make specific recommendations related to the treatment of M. genitalium, but suggest clinicians consider M. genitalium as a potential pathogen in those with persistent urethritis, and recommend azithromycin 1 g orally once in men who did not receive azithromycin for initial NGU therapy, and moxifloxacin 400 mg orally daily for 7 days in those who were initially treated with azithromycin [29]. European guidelines recommend M. genitalium testing of persons with STI syndromes (Table 2), including macrolide resistance testing [23, 30], and recommend azithromycin (500 mg orally once, then 250 mg once daily for 4 days) to treat macrolide-susceptible infections and moxifloxacin (400 mg orally once daily for 7–10 days) to treat macrolide- resistant infections.
Both of the above-mentioned approaches are likely to cure many, and in areas where macrolide resistance is rare, most M. genitalium infections. However, approximately 10% of persons initially infected with azithromycin-susceptible organisms develop persistent, azithromycin- resistant infections following azithromycin treatment [4, 24, 26, 31–33]. Some evidence suggests that 5-day courses of azithromycin are less likely to select for resistance than single-dose therapy [24, 34, 35], though this finding has not been consistently observed [36, 37], and recent data suggest that approximately 50% of M. genitalium infections in the United States are already resistant to azithromycin [25]. How often moxifloxacin treatment selects for persistent, resistant infections is not known, but recent data suggest that fluoroquinolone resistance is increasing. Mutations associated with quinolone resistance have been identified in 15%–33% of infections in studies from Australia and Asia [33, 38–41] and occur at least occasionally among infections in Europe [39, 42], though in some instances these mutations may not be sufficient to cause treatment failure.
Developing treatment recommendations for M. genitalium presents several dilemmas. First, to what extent should treatment regimens incorporate considerations related to antimicrobial resistance, particularly in the absence of data demonstrating a differential impact of different regimens on the development of resistance? Second, should treatment recommendations incorporate efforts to contain the development of resistance at the population level and what, if any, risks is it reasonable for individual patients to assume as part of an effort to contain population-level antimicrobial resistance? Finally, how much evidence do we need to develop guidelines that incorporate an effort to prevent antimicrobial resistance at both the individual and population levels?
Evidence that M. genitalium is responsible for 15%–20% of cases of NGU, that azithromycin selects for macrolide- resistant M. genitalium, and that this selective pressure could play an important role in fostering M. genitalium resistance at the population level has led European authorities and at least 1 US health department (PHSKC) to change standard treatment recommendations for NGU to move away from the use of azithromycin and toward routine use of doxycycline 100 mg orally twice daily for 7 days [29, 43]. Multiple observational studies suggest that doxycycline may be more effective than azithromycin in the treatment of rectal chlamydial infection, and some evidence exists that doxycycline may be slightly more effective than azithromycin in treating chlamydia-associated NGU [44, 45]. Findings from these studies, in addition to concerns related to M. genitalium resistance, may justify some reconsideration of standard NGU and chlamydia treatment recommendations.
The more difficult problem relates to treatment of urethritis diagnosed without the aid of a urethral Gram stain. In many settings in the United States, clinicians do not perform Gram stains and treat urethritis syndromically with ceftriaxone and azithromycin [29]. This approach is designed to treat chlamydial infection and ensure that gonorrhea therapy includes 2 active antimicrobial agents, a strategy thought to be effective in diminishing the emergence of antimicrobial-resistant gonorrhea. However, dual therapy for urethritis that includes azithromycin may help foster the selection and dissemination of macrolide-resistant M. genitalium. How clinicians should balance the potentially competing treatment issues related to gonorrhea, chlamydia, and M. genitalium infection when empirically treating men with urethritis presents a number of dilemmas that may also merit reconsideration of how urethritis is managed.
Given the risk of resistance, one could argue for treating M. genitalium with combination therapy. The rationale for this approach, like the rationale for using combination therapy to treat gonorrhea, is that dual therapy might raise the genetic barrier to resistance by limiting treatment failures to organisms with resistance to 2 classes of drug with separate mechanisms of action. While there is no direct evidence supporting this approach to mitigate emerging M. genitalium or gonorrhea resistance, the paucity of treatment options for both infections necessitates measures to preserve the efficacy of the drugs we currently have.
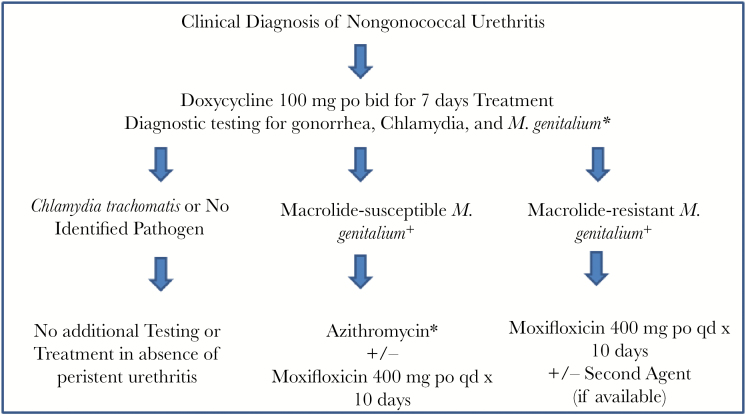
Figure 1 presents a suggested approach to testing and treatment of NGU developed, but not yet implemented, for use in the PHSKC STD clinic. Men with NGU will initially be treated with doxycycline while being tested for M. genitalium using an assay that can detect macrolide resistance. Subsequent treatment is then restricted to persons with diagnosed M. genitalium infections. European guidelines suggest that men with macrolide-susceptible infections be treated with azithromycin and those men with macrolide-resistant infections be treated with moxifloxacin. The theoretical rationale for this approach, which involves either sequential or partially concurrent 2-drug therapy depending on laboratory result turnaround time, is that doxycycline, though not a highly effective treatment for M. genitalium, may decrease the M. genitalium bacterial load, thereby facilitating the success of treatment with azithromycin or moxifloxacin. An alternative approach, presented in the figure, would involve concurrent dual therapy. Potential regimens might include azithromycin plus moxifloxacin in persons with macrolide-susceptible infections, and moxifloxacin plus a second drug in persons with macrolide-resistant infections. Unfortunately, at present, in the United States there is no other drug available that is consistently effective in the treatment of M. genitalium. This alternative approach always includes moxifloxacin, which is more costly than azithromycin alone and involves a 0.08%–0.2% risk of tendon injury, though this risk may be lower in patients with M. genitalium–related sexually transmitted syndromes, which involve a relatively short course of therapy among persons who typically lack risk factors for tendinopathy (older age, corticosteroid use, and comorbid conditions) [46]. The rationale for treating with combination therapy following initial doxycycline treatment reflects uncertainty about the benefit associated with doxycycline given its poor efficacy as a single therapy for M. genitalium, and an effort to more aggressively avoid promoting resistance. It should be noted that much of the approach outlined here is how one clinic proposes to integrate M. genitalium testing into routine clinical management of NGU. It will require rigorous evaluation and perhaps modifications based on experience.
Figure 1.
Proposed Public Health–Seattle & King County testing and treatment algorithm for nongonococcal urethritis. Abbreviations: bid, twice daily; po, oral; qd, once daily.
PARTNER NOTIFICATION AND TREATMENT
At present, there are very few data related to the role of partner notification for M. genitalium, and the appropriate contact period for defining at-risk partners is undefined. Given that uncertainty, medical providers performing M. genitalium testing could consider adopting an approach that is somewhat similar to that employed for chlamydia and gonorrhea; sex partners from the 60 days prior to diagnosis (or last partner) of persons diagnosed with M. genitalium infection should be notified, clinically evaluated, and tested for infection, with partner treatment guided by the results of testing. In the absence of M. genitalium testing, some clinicians may empirically treat the sex partners of persons with persistent or recurrent NGU for M. genitalium. However, only 19%–41% of men with persistent or recurrent urethritis have M. genitalium infection [47], highlighting the desirability of microbiologic testing when possible. The ideal approach for managing the sex partners of persons with M. genitalium infection merits future study.
SURVEILLANCE
Unlike gonorrhea or chlamydial infection, M. genitalium is not legally reportable in any area of the United States, and it seems unlikely that many health departments will make it reportable in the absence of a larger control program. At the same time, insofar as specialized STD clinics begin testing for M. genitalium, sentinel surveillance in such sites could be useful in defining the prevalence of infection and, if possible, levels of antimicrobial resistance to macrolides and quinolones. Such surveillance, coupled with rigorous program evaluation, could also be useful in refining clinical management algorithms.
CONCLUSIONS
The public health response to M. genitalium is in its infancy. Current evidence is insufficient to justify a M. genitalium screening program. On the other hand, insofar as accurate assays are available or become available, there is at least a limited role for M. genitalium testing in persons with STI syndromes associated with the microorganism and the sex partners of persons with diagnosed M. genitalium infections. Barriers to defining an appropriate public health response to M. genitalium include the absence of a widely available FDA-approved test, the lack of consistently effective treatments, and uncertainty about the natural history of infections in women. Addressing these gaps, along with the development of effective screening and treatment programs that are acceptable and accessible for persons at risk of M. genitalium infection, will be critical to the success of developing an appropriate clinical and public health response to this increasingly recognized STI pathogen.
Supplementary Material
Notes
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Financial support. This work is an outcome of a Mycoplasma genitalium Experts Technical Consultation that was supported by the Division of Microbiology and Infectious Diseases of the National Institute of Allergy and Infectious Diseases (contract number HHSN272201300012I), with the University of Alabama at Birmingham Sexually Transmitted Infections Clinical Trials Group.
Supplement sponsorship. This work is part of a supplement sponsored by the University of Alabama at Birmingham Sexually Transmitted Infections Clinical Trials Unit and the National Institute of Allergy and Infectious Diseases.
Potential conflicts of interest. M. R. G. has received research support from Hologic. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Tully JG, Taylor-Robinson D, Cole RM, Rose DL. A newly discovered mycoplasma in the human urogenital tract. Lancet 1981; 1:1288–91. [DOI] [PubMed] [Google Scholar]
- 2. Taylor-Robinson D, Jensen JS. Mycoplasma genitalium: from Chrysalis to multicolored butterfly. Clin Microbiol Rev 2011; 24:498–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lis R, Rowhani-Rahbar A, Manhart LE. Mycoplasma genitalium infection and female reproductive tract disease: a meta-analysis. Clin Infect Dis 2015; 61:418–26. [DOI] [PubMed] [Google Scholar]
- 4. Jensen JS, Bradshaw C. Management of Mycoplasma genitalium infections—can we hit a moving target? BMC Infect Dis 2015; 15:343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grimes DA, Schulz KF. Uses and abuses of screening tests. Lancet 2002; 359:881–4. [DOI] [PubMed] [Google Scholar]
- 6. Sackett DL, Haynes RB, Guyatt GH, Tugwell P.. Clinical epidemiology: a basic science for clinical medicine. 2nd ed New York: Lippincott Williams & Wilkins, 1991. [Google Scholar]
- 7. Cuckle HS, Wald NJ. Principles of screening. In: Antenatal and neonatal screening. New York, NY: Oxford University Press, 1984. [Google Scholar]
- 8. Centers for Disease Control and Prevention. Antibiotic resistance threat in the United States, 2013 https://www.cdc.gov/drugresistance/pdf/ar-threats-2013–508.pdf. Accessed 14 May 2017.
- 9. Centers for Disease Control and Prevention. National public health action plan for the detection, prevention and management of infertility. https://www.cdc.gov/reproductive health/infertility/pdf/drh_nap_final_508.pdf. Accessed 14 May 2017. [Google Scholar]
- 10. Scholes D, Stergachis A, Heidrich FE, Andrilla H, Holmes KK, Stamm WE. Prevention of pelvic inflammatory disease by screening for cervical chlamydial infection. N Engl J Med 1996; 334:1362–6. [DOI] [PubMed] [Google Scholar]
- 11. Oakeshott P, Kerry S, Aghaizu A et al. Randomised controlled trial of screening for Chlamydia trachomatis to prevent pelvic inflammatory disease: the POPI (prevention of pelvic infection) trial. BMJ 2010; 340:c1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Low N, Redmond S, Uuskula A et al. Screening for genital chlamydia infection. Cochrane Database Syst Rev 2016; 9:CD010866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ostergaard L, Andersen B, Møller JK, Olesen F. Home sampling versus conventional swab sampling for screening of Chlamydia trachomatis in women: a cluster-randomized 1-year follow-up study. Clin Infect Dis 2000; 31:951–7. [DOI] [PubMed] [Google Scholar]
- 14. LeFevre ML; US Preventive Services Task Force Screening for chlamydia and gonorrhea: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014; 161:902–10. [DOI] [PubMed] [Google Scholar]
- 15. Low N, Bender N, Nartey L, Shang A, Stephenson JM. Effectiveness of chlamydia screening: systematic review. Int J Epidemiol 2009; 38:435–48. [DOI] [PubMed] [Google Scholar]
- 16. de Wit GA, Over EA, Schmid BV et al. Chlamydia screening is not cost-effective at low participation rates: evidence from a repeated register-based implementation study in the Netherlands. Sex Transm Infect 2015; 91:423–9. [DOI] [PubMed] [Google Scholar]
- 17. Achilles SL, Amortegui AJ, Wiesenfeld HC. Endometrial plasma cells: do they indicate subclinical pelvic inflammatory disease? Sex Transm Dis 2005; 32:185–8. [DOI] [PubMed] [Google Scholar]
- 18. Vicetti Miguel RD, Chivukula M, Krishnamurti U et al. Limitations of the criteria used to diagnose histologic endometritis in epidemiologic pelvic inflammatory disease research. Pathol Res Pract 2011; 207:680–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Smith KJ, Tsevat J, Ness RB, Wiesenfeld HC, Roberts MS. Quality of life utilities for pelvic inflammatory disease health states. Sex Transm Dis 2008; 35:307–11. [DOI] [PubMed] [Google Scholar]
- 20. Stratton KR, Durch JS, Lawrence RS. Vaccines for the 21st century—a tool for decision making. Washington, DC: Institute of Medicine, 1999. [Google Scholar]
- 21. Land JA, Van Bergen JE, Morré SA, Postma MJ. Epidemiology of Chlamydia trachomatis infection in women and the cost-effectiveness of screening. Hum Reprod Update 2010; 16:189–204. [DOI] [PubMed] [Google Scholar]
- 22. Gaydos C. Mycoplasma genitalium: accurate diagnosis is necessary to adequately treat it. J Infect Dis 2017; 216(Suppl 2):S406–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jensen JS, Cusini M, Gomberg M, Moi H. 2016 European guideline on Mycoplasma genitalium infections. J Eur Acad Dermatol Venereol 2016; 30:1650–6. [DOI] [PubMed] [Google Scholar]
- 24. Anagrius C, Loré B, Jensen JS. Treatment of Mycoplasma genitalium. Observations from a Swedish STD clinic. PLoS One 2013; 8:e61481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Getman D, Jiang A, O’Donnell M, Cohen S. Mycoplasma genitalium prevalence, coinfection, and macrolide antibiotic resistance frequency in a multicenter clinical study cohort in the United States. J Clin Microbiol 2016; 54:2278–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bissessor M, Tabrizi SN, Twin J et al. Macrolide resistance and azithromycin failure in a Mycoplasma genitalium-infected cohort and response of azithromycin failures to alternative antibiotic regimens. Clin Infect Dis 2015; 60:1228–36. [DOI] [PubMed] [Google Scholar]
- 27. McGowin CL, Anderson-Smits C. Mycoplasma genitalium: an emerging cause of sexually transmitted disease in women. PLoS Pathog 2011; 7:e1001324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rahman S, Garland S, Currie M et al. Prevalence of Mycoplasma genitalium in health clinic attendees complaining of vaginal discharge in Bangladesh. Int J STD AIDS 2008; 19:772–4. [DOI] [PubMed] [Google Scholar]
- 29. Workowski KA, Bolan GA; Centers for Disease Control and Prevention Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64:1–137. [PMC free article] [PubMed] [Google Scholar]
- 30. Horner PJ, Blee K, Falk L, van der Meijden W, Moi H. 2016 European guideline on the management of non-gonococcal urethritis. Int J STD AIDS 2016; 27:928–37. [DOI] [PubMed] [Google Scholar]
- 31. Walker J, Fairley CK, Bradshaw CS et al. Mycoplasma genitalium incidence, organism load, and treatment failure in a cohort of young Australian women. Clin Infect Dis 2013; 56:1094–100. [DOI] [PubMed] [Google Scholar]
- 32. Twin J, Jensen JS, Bradshaw CS et al. Transmission and selection of macrolide resistant Mycoplasma genitalium infections detected by rapid high resolution melt analysis. PLoS One 2012; 7:e35593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Couldwell DL, Tagg KA, Jeoffreys NJ, Gilbert GL. Failure of moxifloxacin treatment in Mycoplasma genitalium infections due to macrolide and fluoroquinolone resistance. Int J STD AIDS 2013; 24:822–8. [DOI] [PubMed] [Google Scholar]
- 34. Björnelius E, Anagrius C, Bojs G et al. Antibiotic treatment of symptomatic Mycoplasma genitalium infection in Scandinavia: a controlled clinical trial. Sex Transm Infect 2008; 84:72–6. [DOI] [PubMed] [Google Scholar]
- 35. Horner P, Ingle S, Blee K, Muir P, Moi H. Treatment of Mycoplasma genitalium with azithromycin 1g is less efficacious and associated with induction of macrolide resistance compared to a 5 day regimen. Sex Transm Dis 2015; 91:A10. [Google Scholar]
- 36. Jernberg E, Moghaddam A, Moi H. Azithromycin and moxifloxacin for microbiological cure of Mycoplasma genitalium infection: an open study. Int J STD AIDS 2008; 19:676–9. [DOI] [PubMed] [Google Scholar]
- 37. Read TR, Fairley CK, Tabrizi SN et al. Azithromycin 1.5 g over 5 days compared to 1 g single dose in urethral Mycoplasma genitalium: impact on treatment outcome and resistance. Clin Infect Dis 2017; 64:250–6. [DOI] [PubMed] [Google Scholar]
- 38. Hamasuna R, Takahashi S, Matsumoto M, Sho T, Matsumoto T. Mutations on gyrA or parC genes of Mycoplasma genitalium and efficacies of treatment with floroquinolones against M. genitalium-related urethritis [abstract P3-S7.09]. In: 19th Biennial Conference of the International Society for Sexually Transmitted Disease Research, Quebec City, Canada, 2011. [Google Scholar]
- 39. Dumke R, Thürmer A, Jacobs E. Emergence of Mycoplasma genitalium strains showing mutations associated with macrolide and fluoroquinolone resistance in the region Dresden, Germany. Diagn Microbiol Infect Dis 2016; 86:221–3. [DOI] [PubMed] [Google Scholar]
- 40. Tagg KA, Jeoffreys NJ, Couldwell DL, Donald JA, Gilbert GL. Fluoroquinolone and macrolide resistance-associated mutations in Mycoplasma genitalium. J Clin Microbiol 2013; 51:2245–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kikuchi M, Ito S, Yasuda M et al. Remarkable increase in fluoroquinolone-resistant Mycoplasma genitalium in Japan. J Antimicrob Chemother 2014; 69:2376–82. [DOI] [PubMed] [Google Scholar]
- 42. Shipitsyna E, Rumyantseva T, Golparian D et al. Prevalence of macrolide and fluoroquinolone resistance-mediating mutations in Mycoplasma genitalium in five cities in Russia and Estonia. PLoS One 2017; 12:e0175763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Horner PJ. Editorial commentary: Mycoplasma genitalium and declining treatment efficacy of azithromycin 1 g: what can we do? Clin Infect Dis 2015; 61:1400–2. [DOI] [PubMed] [Google Scholar]
- 44. Kong FY, Tabrizi SN, Fairley CK et al. The efficacy of azithromycin and doxycycline for the treatment of rectal chlamydia infection: a systematic review and meta-analysis. J Antimicrob Chemother 2015; 70:1290–7. [DOI] [PubMed] [Google Scholar]
- 45. Kong FY, Tabrizi SN, Law M et al. Azithromycin versus doxycycline for the treatment of genital chlamydia infection: a meta-analysis of randomized controlled trials. Clin Infect Dis 2014; 59:193–205. [DOI] [PubMed] [Google Scholar]
- 46. Stephenson AL, Wu W, Cortes D, Rochon PA. Tendon injury and fluoroquinolone use: a systematic review. Drug Saf 2013; 36:709–21. [DOI] [PubMed] [Google Scholar]
- 47. Manhart LE, Broad JM, Golden MR. Mycoplasma genitalium: should we treat and how? Clin Infect Dis 2011; 53:S129–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Munson E, Wenten D, Jhansale S et al. Expansion of comprehensive screening of male sexually transmitted infection clinic attendees with Mycoplasma genitalium and Trichomonas vaginalis molecular assessment: a retrospective analysis. J Clin Microbiol 2017; 55:321–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Napierala M, Munson E, Wenten D et al. Detection of Mycoplasma genitalium from male primary urine specimens: an epidemiologic dichotomy with Trichomonas vaginalis. Diagn Microbiol Infect Dis 2015; 82:194–8. [DOI] [PubMed] [Google Scholar]
- 50. Manhart LE, Holmes KK, Hughes JP, Houston LS, Totten PA. Mycoplasma genitalium among young adults in the United States: an emerging sexually transmitted infection. Am J Public Health 2007; 97:1118–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hjorth SV, Björnelius E, Lidbrink P et al. Sequence-based typing of Mycoplasma genitalium reveals sexual transmission. J Clin Microbiol 2006; 44:2078–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ma L, Taylor S, Jensen JS, Myers L, Lillis R, Martin DH. Short tandem repeat sequences in the Mycoplasma genitalium genome and their use in a multilocus genotyping system. BMC Microbiol 2008; 8:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sonnenberg P, Ison CA, Clifton S et al. Epidemiology of Mycoplasma genitalium in British men and women aged 16–44 years: evidence from the third National Survey of Sexual Attitudes and Lifestyles (NATSAL-3). Int J Epidemiol 2015; 44:1982–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.