Abstract
Background.
Patients with active visceral leishmaniasis are important reservoirs in the anthroponotic transmission cycle of Leishmania donovani. The role of the blood or skin as a source of infection to sand flies remains unclear, and the possible effect of multiple exposures to fly bites on transmissibility has not been addressed.
Methods.
L. donovani–infected hamsters underwent xenodiagnoses with Lutzomyia longipalpis on the same or different sites on the abdomen on 2 consecutive days or by artificial feeding on the skin or blood.
Results.
The transmission of L. donovani from sick hamsters to flies was surprisingly low (mean, 24% of fed flies). New flies fed on the same site acquired significantly more infections (mean, 61%; P < .0001). By artificial feeding, flies could acquire infection from blood and skin. However, only artificial feeding on blood produced infections that correlated with the natural feeding (R = 0.792; P < .0001). Infections acquired from blood increased dramatically for blood obtained after exposure to bites, as did the parasitemia level and the number of monocytes in the circulation.
Conclusions.
The bites of uninfected sand flies favor the transmissibility of L. donovani by infected hosts, owing to a systemic effect that exposure to bites has on the parasitemia.
Patients with active visceral leishmaniasis are important reservoirs in the anthroponotic transmission cycle of Leishmania donovani. Using the hamster model of visceral disease, we demonstrate that prior exposure to bites of uninfected sand flies potentiates their ability to transmit infection to the vector.
Keywords: Visceral leishmaniasis, sand flies, Leishmania donovani, saliva, skin.
Visceral leishmaniasis (VL), also known as “kala-azar,” is the fatal form of leishmaniasis characterized by fever, weight loss, enlargement of the spleen and liver, and anemia. VL is caused by 2 closely related Leishmania species: L. infantum, in the Mediterranean basin, North Africa, and Central and South America, and L. donovani, in the Indian subcontinent and East Africa. While the infection reservoirs for VL due to L. infantum are animals, mainly dogs [1], L. donovani is considered to have an anthroponotic transmission cycle in which patients with active disease are considered to be important reservoirs, based on the clustering of cases around households with a history of VL [2–6]. A study from Ethiopia that modeled the infectiousness of asymptomatic and symptomatic individuals to the natural vector, Phlebotomus orientalis, predicted that the symptomatic individuals with the heaviest parasite loads in the blood were responsible for 62% of the transmissions to flies [7]. On the other hand, the ability of L. donovani to colonize the skin of infected individuals is evident from cases of post–kala azar dermal leishmaniasis (PKDL), who are thought to play an important role in transmission, based on the high rates of sand fly infections following their exposure to nodular PKDL-associated lesions [8]. Whether L. donovani might colonize the skin of patients with VL who do not present with dermal lesions is unknown. Thus, the importance of the blood or skin as a source of parasites for transmission back to the vector is still not clear. Furthermore, since, in regions of endemicity, infected individuals may be routinely exposed to sand fly bites, it is not known whether multiple exposures might influence transmissibility, such as by provoking an inflammatory reaction that results in higher numbers of infected cells infiltrating localized bite sites in the skin.
Because it mimics key clinicopathologic features of human disease, including hepatosplenomegaly and hematological defects associated with fatal outcome, the hamster model of VL has been extensively used for studies of disease pathogenesis and to test the efficacy of drugs and vaccines [9]. In the present studies, we have used the model to address questions pertaining to the efficiency with which sick hamsters transmit L. donovani to sand flies, the tissue source of infection for flies, and the effect of multiple exposures to sand fly bites on transmissibility.
METHODS
Parasites
Promastigotes of the L. donovani Indian strain Mongi (MHOM/IN/83/Mongi-142) and the L. donovani strain 1S from Sudan (MHOM/SD/00/1S-2D) were grown from frozen stocks of tissue amastigotes at 26°C in medium 199 supplemented with 20% heat-inactivated fetal calf serum, 100 U/mL penicillin, 100 mg/mL streptomycin, 2 mM L-glutamine, 40 mM Hepes, 0.1 mM adenine (in 50 mM Hepes), 5 mg/mL hemin (in 50% triethanolamine), and 1 mg/mL 6-biotin (M199/C). After 5 days in culture, metacyclic promastigotes were obtained by centrifugation through a Ficoll gradient [10].
Hamsters
Male Syrian hamsters 3–4 weeks old were purchased from Harlan Laboratories (Indianapolis, IN). All animals were maintained under pathogen-free conditions in the animal care facility of the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH). All hamster studies were performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the NIH. The protocol was approved by the Animal Care and Use Committee of the NIAID (protocol LPD 68E). Hamsters were anesthetized by intraperitoneal injection of ketamine (100 mg/kg) and xylazine (10 mg/kg). Animals were infected intracardially under anesthesia with 5 × 107–5 × 108 of Ficoll-purified metacyclic promastigotes and monitored weekly for weight loss.
Direct Feeding on Infected Hamsters
Hamsters were exposed to sand fly bites at approximately 12 weeks after infection, after they had lost >20% of their maximum body weight. Lutzomyia longipalpis females aged 2–4 days were obtained from a colony initiated from field specimens collected in Jacobina, Brazil. One day before feeding, their diet of 30% sucrose was removed, and on the day of transmission, 25–30 flies were transferred to small plastic vials (volume, 11.9 cm2; height, 3 cm; and diameter, 1.8 cm) covered at one end with a 0.25-mm nylon mesh. The vials were attached to the shaved abdomens of anesthetized hamsters with tape on specific areas demarcated by indelible ink. A new group of flies was used 24 hours after the first exposure on the same and/or different spots on the belly.
Artificial Feeding on Skin or Blood Specimens From Infected Hamsters
Flies were exposed by artificial feeding through a chicken skin membrane on heparinized whole blood obtained from retro-orbital blood specimens collected from infected, anesthetized hamsters. Flies were also artificially fed through the skin of infected hamsters on heparinized blood obtained from normal BALB/c mice. Skin was prepared by excision of a 20-mm diameter skin segment from the belly of an infected, euthanized hamster. The excised tissue was washed twice in a Petri dish containing phosphate-buffered saline, and the subcutaneous fat was removed by scraping with a scalpel. In some experiments, skin from adjacent areas on the belly were obtained before and immediately following perfusion of the whole hamster with 500 mL of saline sodium citrate buffer through the heart, using an automatic pipetting machine that was repurposed as a perfusion pump. Fifty flies were allowed to feed for 1–2 hours in the dark. Following natural or artificial feeds, blood-engorged flies were separated and maintained at 26°C and 75% humidity and were provided 30% sucrose ad libitum. Two days after the feeding, the blood-fed flies were washed and dissected, and each midgut was macerated individually with a pestle (Kimble Chase, Vineland, NJ) in an Eppendorf tube containing 100 µL of M199/C. The homogenized midguts were cultured in 96-well plates and evaluated over 2 weeks for the presence of promastigotes. The results are expressed as the percentage of blood-fed flies positive for infection [11].
Quantification of Parasites in Blood or Skin Specimens by Titration Culture
Fifty microliters of heparinized whole blood from an infected hamster was added to 150 µL in the first well of a 96-well flat-bottomed microtiter plate (quadruplicate). A serial 2-fold dilution to extinction was made in M199/C, the culture plate was sealed, and incubation at 27°C was performed for 10–14 days for determination of wells positive for promastigotes. A 4-mm skin biopsy specimen obtained from the hamster immediately after it was euthanized was incubated for digestion in Dulbecco’s modified Eagle’s medium containing 100 U/mL penicillin, 100 µg/mL streptomycin, and 0.2 mg/mL Liberase CI purified enzyme blend (Roche Diagnostics) at 37°C. After 1 hour, the skin tissue was forced through a 70-µm cell strainer (Falcon Products), washed in M199 (at 3000 x g for 15 minutes at 4°C), and resuspended in 100 µL of M199/C for serial 2-fold dilutions.
Quantification of Parasites in Skin or Whole-Blood Specimens by Quantitative Polymerase Chain Reaction (qPCR)
DNA from a 4-mm skin tissue specimen or a 0.2-mL whole-blood specimen was extracted using a QIAamp DNA mini-kit (Qiagen, Hilden, Germany) in accordance with the manufacturer’s instructions. A standard curve was generated using DNA prepared from a known number of L. donovani promastigotes from culture that were serially diluted in normal skin tissue or in naive hamster blood (from 106 to 5 × 10−1 parasites per well). DNA was stored at –80°C until use. The PCR assay was performed in a final volume of 10 μL, using SYBR Green Master Mix and kinetoplast DNA–specific primers (KDNA5) as previously described [12]. Amplification was conducted using QuantStudio6 real-time PCR software (Applied Biosystem, Carlsbad, CA).
Leukocyte Counting in Whole-Blood Specimens
Total leukocytes were counted manually in Neubauer chambers. Differential cell counts were determined on a thin smear of blood stained with a Romanowsky stain. One hundred leukocytes were counted, and the absolute number and proportion of each cell type were calculated on the basis of the total number of leukocytes per unit volume.
Statistical Analysis
Statistical tests were performed with Graph Pad 6.0 Prism Software (La Jolla, CA). The distribution normality of samples was determined by the D’Agostino-Pearson Amnibus test. Statistical significance was determined using the 2-tailed Student t test. Longitudinal data from the same hamster were compared using a paired t test. Pearson correlation coefficients (r) were determined to test the strength of the correlation between infections obtained by natural feeding and those obtained by artificial feeding. P values of < .05 were considered statistically significant.
RESULTS
Increase in Transmissibility Following Previous Exposure to Sand Fly Bites
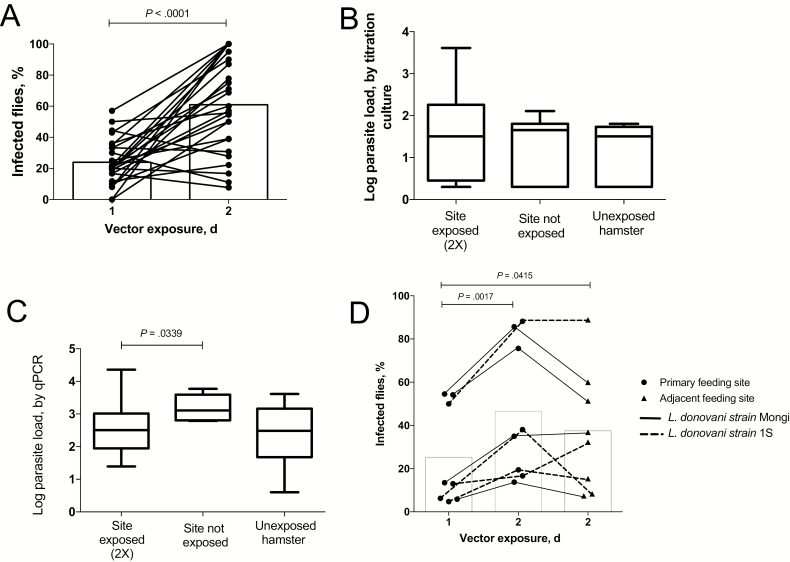
Hamsters were inoculated intracardially by using a high dose of L. donovani metacyclic promastigotes to reduce the time to patency for development of visceral disease. When the hamsters had lost >20% of their peak body weight (10–12 weeks; Supplementary Figure 1), their shaved abdomens were exposed on specific sites to the bites of L. longipalpis sand flies. These colonized flies have been shown previously to support the full development and transmission of L. donovani [11]. The transmission from the hamsters to the flies in the initial feeding varied from 0% to 57% of fed flies positive for parasites (mean, 24%; Figure 1A). To address the possible effect of sand fly preexposure, the xenodiagnoses were performed on 2 consecutive days on the same sites. Following the second feeding, a significant increase in the percentage of flies picking up parasites was observed (8%–100%; mean, 61%; P < .0001), with 21 of 26 reexposed sites presenting higher rates of transmission. Combining the results of the 2 sites, we found that 9 of 11 hamsters showed a significant increase in the transmission on the second day of exposure.
Figure 1.
Increase in transmissibility following reexposure to sand fly bites. A, Transmission of Leishmania donovani strain Mongi from sick hamsters to Lutzomyia longipalpis sand flies in the initial feeds on 2 sites on the abdomen and to a new set of flies exposed to the same sites 24 hour later. Nine hamsters were exposed on 2 sites, and 2 hamsters were exposed on 4 sites. Data are percentages of blood-fed flies fed on individual sites positive for parasites in the first and second feeds, with mean values denoted by bars (data are for 26 sites and 11 hamsters). B, Parasite loads as determined by titration culture in 4-mm skin biopsy specimens collected from the sites on the abdomen exposed 2 times to sand fly bites (data are for 16 sites and 8 hamsters), from adjacent nonexposed sites on the abdomen (11 sites and 8 hamsters), and from abdominal sites from sick hamsters never exposed to sand fly bites (8 sites and 2 hamsters). Data are mean values ± SD, with maximum and minimum values. C, Parasite loads as determined by quantitative polymerase chain reaction (qPCR) in 4-mm skin biopsy specimens collected from the sites on the abdomen exposed 2 times to sand fly bites (data are for 14 sites and 7 hamsters), from adjacent nonexposed sites on the abdomen (5 sites and 3 hamsters), and from sick hamsters never exposed to sand fly bites (8 sites and 2 hamsters). Data are mean values ± SD, with maximum and minimum values. D, Transmission of L. donovani strain Mongi (solid lines) or L. donovani 1S (dotted lines) from sick hamsters to flies in the initial feeds on 2 sites on the abdomen and to a new set of flies exposed to the same sites or 2 adjacent, previously nonexposed sites 24 hours later. Data are the percentages of the total number of the blood-fed flies fed on the 2 sites in each group that were positive for parasites, with mean values denoted by bars (data are for 8 sites/group and 8 hamsters).
We assessed the parasite load in the skin of the hamsters after 2 consecutive days of sand fly exposures, comparing 4-mm skin biopsy samples from exposed and adjacent, nonexposed sites on the abdomen. In addition, the skin parasite loads in infected hamsters never exposed to sand flies were evaluated. While all of the skin biopsy specimens were positive for parasites, no increase in the parasite loads in skin sampled from the previously exposed sites was found by either titration culture (Figure 1B) or qPCR (Figure 1C), suggesting that the increased transmissibility following sand fly exposure may not be explained by increased numbers of parasitized cells infiltrating the localized bite sites.
To determine whether the increased transmissibility is site specific, we exposed 8 sick hamsters to sand fly bites in 2 different spots on the abdomen on the first day and reexposed the same spots to a new set of flies on the second day. In addition, the second day of fly exposures included 2 adjacent sites on the abdomen that were not previously exposed (Supplementary Figure 2). The data again showed a significant increase in the transmissibility of parasites at the reexposed bite sites (mean, 25% vs 47%; P = .0017), with 7 of 8 hamsters transmitting to a higher proportion of flies on the second day (Figure 1D). The skin sites not previously exposed also yielded significantly better transmission to flies as compared to the adjacent sites on their first day of exposure (mean, 25% vs 38%; P = .0415), although the effect appeared to be less pronounced than that at the reexposed sites.
Artificial Feeding Suggests That Parasites Can Be Acquired From the Blood and/or Skin
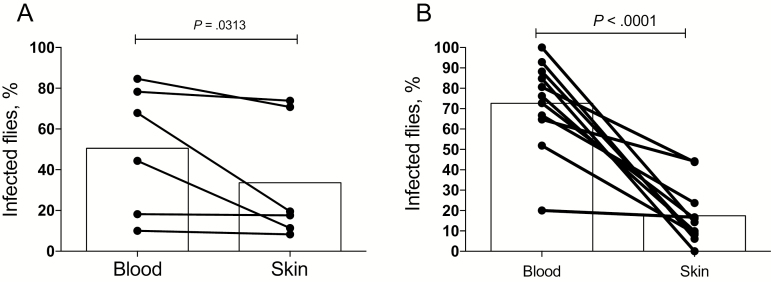
To determine the blood or tissue source of parasites picked up by the flies and to better define the localized versus systemic nature of the effects of exposure to fly bites, we performed artificial feeding experiments involving skin and blood specimens from infected hamsters. Immediately after blood specimen collection, the sick hamsters were euthanized, and a piece of skin large enough for artificial feeding was excised from the shaved abdomen. The flies were fed through the infected hamster skin on uninfected mouse blood or through a normal chicken skin membrane on the infected hamster blood. Only fully engorged flies were analyzed for infection. As shown in Figure 2A, both tissues were a source of parasites acquisition by the vector, with 5 of 5 sick hamsters transmitting to flies from both the skin and the blood. The blood, however, presented the greater potential for transmission (mean, 57%) as compared to the skin (mean, 37%), although this difference was not significant (P = .0625). An identical pattern of transmissibility was observed when artificial feeding was performed on blood and skin specimens from 11 sick hamsters that had been previously exposed to sand fly bites. The hamsters underwent blood specimen collection immediately after the second exposure to sand fly bites, were immediately euthanized, and skin was excised from the area of fly exposure. Both tissue specimens from all but 1 hamster transmitted to the flies (Figure 2B). However, the rate of successful transmission from the blood specimens (20%–100%; mean, 73%) was in this case significantly greater than from the skin specimens (0%–44%; mean, 18%; P < .0001). To address the possibility that the artificial feeds using the naive mouse blood specimens might be inhibitory to parasite development, the flies were fed through a chick membrane on either normal mouse or hamster blood seeded with L. donovani amastigotes. While all flies were positive for parasites at 3 days after infection, the feeds on the infected mouse blood produced a significantly greater level of infection in the flies (Supplementary Figure 3). To address the possibility that the parasites picked up from the skin were present in the blood vasculature, the skin specimens used for artificial feeds were obtained from 2 adjacent sites on the belly excised before and following perfusion of the euthanized hamster. In pair-wise comparisons involving 7 hamsters, no significant reduction was observed in the transmissibility of parasites on the skin following perfusion (Supplementary Figure 4), suggesting that the parasites picked up by the flies were present in the skin.
Figure 2.
Sand flies can acquire infection from both blood and skin. A, Transmission of Leishmania donovani strain Mongi to flies by artificial feeding on the blood or skin of sick hamsters not previously exposed to sand fly bites. Fifty flies were used in each of the artificial feeding conditions. Data are percentages of blood-fed flies positive for parasites, with mean values denoted by bars (data are for 5 hamsters). B, Transmission of L. donovani strain Mongi to flies by artificial feeding on the blood or skin of sick hamsters with an initial exposure to sand fly bites and a second exposure 24 hours later on the same sites. Blood and skin specimens were obtained immediately following the second exposure. Data are percentages of blood-fed flies positive for parasites, with mean values denoted by bars (data are for 11 hamsters).
Increased Parasite Transmissibility in Blood and Parasitemia Level After Exposure to Sand Fly Bites
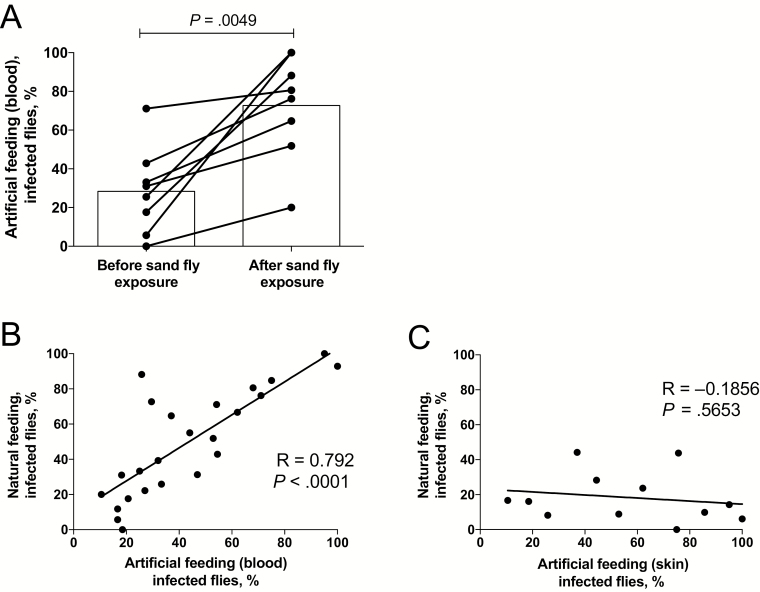
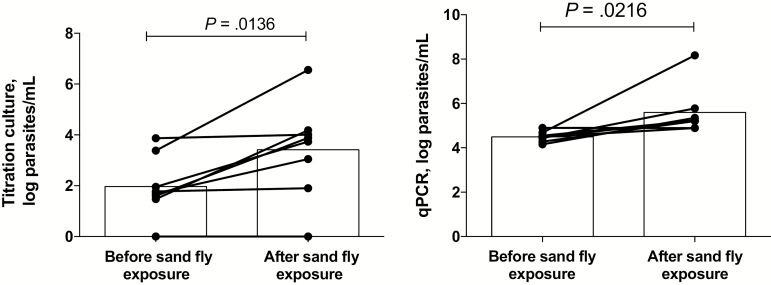
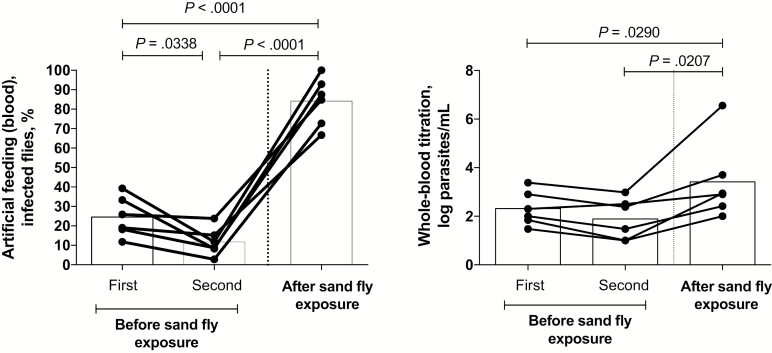
We next investigated whether the effects of prior exposure to sand fly bites on promoting L. donovani transmission could be reproduced by artificial feeding on blood. Eight sick hamsters underwent blood specimen collection just before their initial exposure to flies and then again immediately after the second day of feeding on the same site. The transmission of parasites by blood obtained before fly exposure (0%–71%; mean, 28%) increased in all 8 hamsters, using blood specimens obtained following exposure (20%–100%; mean, 73%; P = .0049; Figure 3A). There was a strong correlation between the frequencies of transmissions to flies by direct feeding and artificial feeding on blood (R = 0.792; P < .0001; Figure 3B), while no correlation was found between the direct feeding and artificial feeding on skin (Figure 3C). Of note, the artificial feeding on skin was confined to hamsters euthanized immediately following their second exposure to flies. To determine the effect of exposure to sand fly bites on the parasitemia level, viable organisms in blood used for artificial feeding were quantified by titration culture, and parasite equivalents were determined by qPCR. Culture of blood specimens obtained after sand fly exposure showed a significant increase in parasitemia levels (mean, 2606 parasites/mL), compared with blood specimens obtained before exposure (mean, 93 parasites/mL; n = 8; P = .0136; Figure 4A). The qPCR results also revealed a significant increase in parasite numbers in blood following fly bites (mean, 18617914 parasites/mL) as compared to before fly bites (mean, 35757 parasites/mL; n = 8; P = .0216; Figure 4B). In each case, the qPCR-based parasitemia estimates were substantially greater than the titration culture results, owing to the inefficiency of the whole blood culture and/or, as has been recently suggested [13], the presence of parasite DNA from other sites.
Figure 3.
Increase in blood transmissibility after sand fly exposures. Sick hamsters underwent blood specimen collection immediately before exposure to sand fly bites and again 24 hours later just before reexposure on the same sites to a new set of flies. Hamsters were euthanized immediately following the second exposure, and skin specimens from the exposed sites were excised for artificial feeding. A, Transmission of Leishmania donovani strain Mongi to flies by artificial feeding on the blood from sick hamsters obtained before or 24 hours after exposure to sand fly bites. Data are percentages of blood-fed flies positive for parasites, with mean values denoted by bars (data are for 8 hamsters). B, Correlation (Pearson r) between transmission of L. donovani strain Mongi to flies by direct feeding on sick hamsters and artificial feeding on the blood obtained from the same hamsters. Results from both before and after exposure to sand fly bites are included. C, Correlation between transmission of L. donovani strain Mongi to flies by direct feeding on sick hamsters and artificial feeding on the skin obtained from the same hamsters. Only results of postfeeding exposures are shown.
Figure 4.
Increase in blood parasitemia level following exposure to sand fly bites. A, Parasite loads in whole blood specimens obtained from hamsters infected with Leishmania donovani strain Mongi as determined by titration culture using blood specimens obtained before and after sand fly exposure. Data are log parasite counts per milliliter of blood, with geometric means denote by bars (data are for 8 mice). B, Parasite loads in whole blood specimens as determined by quantitative polymerase chain reaction (qPCR), using blood obtained before and after sand fly exposure. Data are log parasite counts per milliliter of blood, with geometric means denoted by bars.
To address the possibility that the increase in blood transmission and parasitemia level observed in the artificial feeding experiments might have been due to the trauma associated with the collection of blood specimens itself, we collected blood specimens from a new set of 6 hamsters on 2 successive days before their exposure to sand fly bites, along with a third blood specimen 1 day after their exposure. No increase in transmission was observed by artificial feeding on the samples of blood obtained before sand fly exposure. In fact, a slight but significant reduction was observed in comparing the first and second blood specimens obtained before exposure (mean, 25% vs 12%; P = .0338). By contrast, a strong and highly significant increase in transmission as compared to that for both specimens obtained before exposure was observed using the blood specimen obtained after exposure (mean, 84%; P < .001; Figure 5A). This was again associated with a significant increase in the parasitemia level, as determined by titration culture, in blood specimens obtained following sand fly exposure, compared with either blood specimen obtained before exposure (mean, 208 log parasites/mL in the first specimen obtained before exposure, 78 log parasites/mL in the second specimen obtained before exposure, and 2630 log parasites/mL in the specimen obtained after exposure; n = 6; P = .0207; Figure 5B).
Figure 5.
Bleeding does not alter transmissibility or parasitemia level. Retro-orbital blood specimens from sick hamsters infected with Leishmania donovani strain Mongi were obtained on 2 successive days before and 24 hours after exposure to sand fly bites . A, Transmission success by artificial feeding on blood. Data are percentages of blood-fed flies positive for parasites, with mean values denoted by bars (data are for 6 hamsters). B, Parasite loads as determined by titration culture of whole blood. Data are for individual blood samples, with mean values denoted by bars (data are for 6 hamsters).
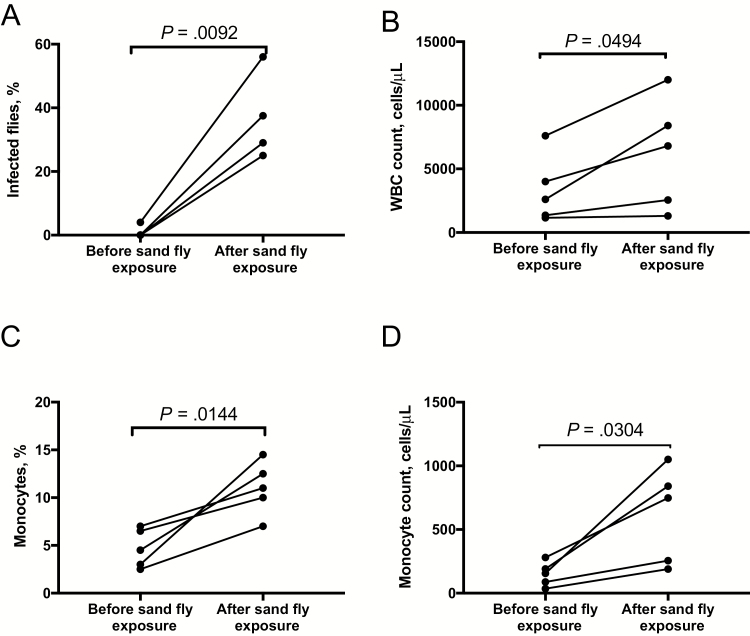
The increase in transmission and parasitemia level following sand fly exposure suggested that infected cells are mobilized into the peripheral blood in response to fly bites. Blood specimens were obtained from sick hamsters before and after exposure to sand fly biting, for artificial feeding, and total and differential leukocyte counts were compared. A striking increase in L. donovani transmissibility to flies was again observed in the postexposure blood specimen (Figure 6A) that was associated with a significant increase in the total number of leukocytes in the peripheral blood (Figure 6B). This change included an increase in the total number of monocytes and a >2-fold increase in the percentage of monocytes in the postexposure blood specimen (mean, 4.7% vs 11%; P = .0144; Figure 6C). There was also a significant increase in the total number of lymphocytes but no change in the proportion or total number of neutrophils in the postexposure blood specimen (Supplementary Figure 5).
Figure 6.
Monocyte mobilization in the peripheral blood following exposure to sand fly bites. Blood specimens were obtained from sick hamsters before and after exposure to sand fly bites for artificial feeding and underwent comparisons of total and differential leukocyte counts. A, Transmission of Leishmania donovani strain Mongi to flies by artificial feeding on blood obtained before or 24 hours after exposure to sand fly bites. B and C, Total white blood cell (WBC) counts per microliter of whole blood (B) and monocytes expressed as a percentage of total leukocytes and as the total number of cells per microliter of whole blood obtained before and after exposure to sand fly bites (C).
DISCUSSION
We have used the hamster model of VL to address a number of outstanding questions pertaining to the infectiousness of diseased hosts to vector sand flies. Using direct and artificial sand fly feeding techniques, these studies provide the first direct demonstration that flies can pick up parasites from both the blood and the skin. The efficiency of transmission from sick hamsters to flies was nonetheless relatively low but could be dramatically improved by prior exposure of the hamsters to sand fly bites. This phenomenon could be explained by a systemic effect that exposure to sand fly bites has on increasing the number of parasites present in the peripheral blood. Overall, the correlation comparing pick up of parasites by direct feeding on the sick hamsters and artificial feeding on their blood was extremely strong (R = 0.792; P < .0001), while there was no correlation with artificial feeding on skin, suggesting that the blood is the most likely source of parasites for cyclical transmission.
Apart from the work in the 1920s that first identified Phlebotomus argentipes as the probable vector of Indian kala-azar [14, 15], the only xenodiagnostic study involving cases of active VL in the Indian subcontinent reported very low rates of infections in fed flies (5.3%) [16]. A similar low level of infectiousness was observed in a xenodiagnostic trial in Brazil, in which only 11 of 44 patients with VL transmitted L. infantum to L. longipalpis and to only 2.5% of the fed flies [17]. In the only human xenodiagnostic study to show high rates of parasite pick up from active cases, the patients were coinfected with human immunodeficiency virus, and the infection rates in flies negatively correlated to the CD4+ T lymphocyte counts that may have predisposed these cases to high parasitemia levels [18]. The present studies suggest that xenodiagnostic trials involving active VL cases that do not take into account their recent exposure history to sand fly bites may greatly underestimate their reservoir potential.
The remarkable effect that a prior exposure to sand fly bites has on increasing the number of parasites in the peripheral blood represents another example of how Leishmania can exploit the host response to sand fly bites to promote its cyclical transmission. Up to now, these studies have focused on the sand fly– and parasite-derived factors that are coinoculated by infected flies, such as saliva, PSG, and exosomes, that can enhance the early establishment of Leishmania infection in the skin [19–22]. The current studies are the first to describe how bites from uninfected flies enhance the transmissibility of Leishmania in already infected hosts, an effect that is likely secondary to the increase in the number of leukocytes, as well as the total and proportionate number of monocytes, that were observed. Blood-sucking arthropods, especially sand flies, are known to present different salivary components during the bite that confer systemic physiologic effects, even in low concentrations, inhibiting hemostasis and immunity [19, 23]. Components in sand fly saliva have been described that are chemoattractive for monocytes/macrophages and that promote Leishmania survival inside these cells [24–26]. Compartmental reservoirs of mature monocytes are found in the bone marrow and spleen, from which monocytes can be rapidly mobilized in response to infection or tissue injury [27, 28]. In hamsters with VL, these reservoirs likely include parasitized cells that are released into the circulation in response to sand fly bites.
Our findings implicate the bites of uninfected sand flies as an important variable that contributes to the maintenance of the L. donovani transmission cycle in regions of endemicity. Human xenodiagnostic studies will be needed to address how meaningful these exposures are to influencing the reservoir potential of active VL cases and, possibly, asymptomatic cases, for whom boosting the blood parasitemia level may be especially critical to achieving the threshold required for transmission to flies. Vaccines targeting vector saliva that are currently being developed to protect against disease following exposure to infected flies [19] might also be used as transmission-blocking vaccines in reservoir hosts.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. We thank Kimberly Webber for assistance with the hamster experiments.
Financial support. This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Healthr; the Bill and Melinda Gates Foundation (grant OPP1067714); the Conselho Nacional de Desenvolvimento Científico e Técnológico (postdoctoral fellowship to J. G. V.), and the European Union Seventh Framework Program (Marie Curie Fellowship to M. L.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Alvar J, Cañavate C, Molina R, Moreno J, Nieto J. Canine leishmaniasis. Adv Parasitol 2004; 57:1–88. [DOI] [PubMed] [Google Scholar]
- 2. Barnett PG, Singh SP, Bern C, Hightower AW, Sundar S. Virgin soil: the spread of visceral leishmaniasis into Uttar Pradesh, India. Am J Trop Med Hyg 2005; 73:720–5. [PubMed] [Google Scholar]
- 3. Bern C, Haque R, Chowdhury R, et al. The epidemiology of visceral leishmaniasis and asymptomatic leishmanial infection in a highly endemic Bangladeshi village. Am J Trop Med Hyg 2007; 76:909–14. [PubMed] [Google Scholar]
- 4. Bern C, Hightower AW, Chowdhury R, et al. Risk factors for kala-azar in Bangladesh. Emerg Infect Dis 2005; 11:655–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Das VN, Pandey RN, Siddiqui NA, et al. Longitudinal study of transmission in households with visceral leishmaniasis, asymptomatic infections and PKDL in highly endemic villages in Bihar, India. PLoS Negl Trop Dis 2016; 10:e0005196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dhiman RC, Sen AB. Epidemiology of kala-azar in rural Bihar (India) using village as a component unit of study. Indian J Med Res 1991; 93:155–60. [PubMed] [Google Scholar]
- 7. Miller E, Warburg A, Novikov I, et al. Quantifying the contribution of hosts with different parasite concentrations to the transmission of visceral leishmaniasis in Ethiopia. PLoS Negl Trop Dis 2014; 8:e3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Addy M, Nandy A. Ten years of kala-azar in west Bengal, Part I. Did post-kala-azar dermal leishmaniasis initiate the outbreak in 24-Parganas? Bull World Health Organ 1992; 70:341–6. [PMC free article] [PubMed] [Google Scholar]
- 9. Sacks DL, Melby PC. Animal models for the analysis of immune responses to leishmaniasis. Curr Protoc Immunol 2015; 108:1–24. [DOI] [PubMed] [Google Scholar]
- 10. Späth GF, Beverley SM. A lipophosphoglycan-independent method for isolation of infective Leishmania metacyclic promastigotes by density gradient centrifugation. Exp Parasitol 2001; 99:97–103. [DOI] [PubMed] [Google Scholar]
- 11. Aslan H, Dey R, Meneses C, et al. A new model of progressive visceral leishmaniasis in hamsters by natural transmission via bites of vector sand flies. J Infect Dis 2013; 207:1328–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weirather JL, Jeronimo SM, Gautam S, et al. Serial quantitative PCR assay for detection, species discrimination, and quantification of Leishmania spp. in human samples. J Clin Microbiol 2011; 49:3892–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Silva JC, Zacarias DA, Silva VC, Rolão N, Costa DL, Costa CH. Comparison of optical microscopy and quantitative polymerase chain reaction for estimating parasitaemia in patients with kala-azar and modelling infectiousness to the vector Lutzomyia longipalpis. Mem Inst Oswaldo Cruz 2016; 111:517–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Knowles R, Napier LE, Smith ROA. On a Herpetomonas found in the gut of the sandfly, Phlebotomus argentipes, fed on kala-azar patients. A preliminary note. Indian Med Gaz 1924; 59:593–7. [PMC free article] [PubMed] [Google Scholar]
- 15. Christophers SR, Shortt HE, Barraud JP. The development of the parasite of Indian kala-azar in the sandfly Phlebotomus argentipes. Indian J Med Res 1925; 122:605–7. [Google Scholar]
- 16. Mukhopadhyay AK, Mishra RN. Development of Leishmania donovani in Phlebotomus argentipes & Ph. papatasi fed on kala-azar patients in Bihar. Indian J Med Res 1991; 93:152–4. [PubMed] [Google Scholar]
- 17. Costa CH, Gomes RB, Silva MR, et al. Competence of the human host as a reservoir for Leishmania chagasi. J Infect Dis 2000; 182:997–1000. [DOI] [PubMed] [Google Scholar]
- 18. Molina R, Lohse JM, Pulido F, Laguna F, López-Vélez R, Alvar J. Infection of sand flies by humans coinfected with Leishmania infantum and human immunodeficiency virus. Am J Trop Med Hyg 1999; 60:51–3. [DOI] [PubMed] [Google Scholar]
- 19. Abdeladhim M, Kamhawi S, Valenzuela JG. What’s behind a sand fly bite? The profound effect of sand fly saliva on host hemostasis, inflammation and immunity. Infect Genet Evol 2014; 28:691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Atayde VD, Aslan H, Townsend S, Hassani K, Kamhawi S, Olivier M. Exosome secretion by the parasitic protozoan leishmania within the sand fly midgut. Cell Rep 2015; 13:957–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Belkaid Y, Kamhawi S, Modi G, et al. Development of a natural model of cutaneous leishmaniasis: powerful effects of vector saliva and saliva preexposure on the long-term outcome of Leishmania major infection in the mouse ear dermis. J Exp Med 1998; 188:1941–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rogers ME, Ilg T, Nikolaev AV, Ferguson MA, Bates PA. Transmission of cutaneous leishmaniasis by sand flies is enhanced by regurgitation of fPPG. Nature 2004; 430:463–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ribeiro JM, Francischetti IM. Role of arthropod saliva in blood feeding: sialome and post-sialome perspectives. Annu Rev Entomol 2003; 48:73–88. [DOI] [PubMed] [Google Scholar]
- 24. Teixeira CR, Teixeira MJ, Gomes RB, et al. Saliva from Lutzomyia longipalpis induces CC chemokine ligand 2/monocyte chemoattractant protein-1 expression and macrophage recruitment. J Immunol 2005; 175:8346–53. [DOI] [PubMed] [Google Scholar]
- 25. Zer R, Yaroslavski I, Rosen L, Warburg A. Effect of sand fly saliva on Leishmania uptake by murine macrophages. Int J Parasitol 2001; 31:810–4. [DOI] [PubMed] [Google Scholar]
- 26. Araújo-Santos T, Prates DB, Andrade BB, et al. Lutzomyia longipalpis saliva triggers lipid body formation and prostaglandin E₂ production in murine macrophages. PLoS Negl Trop Dis 2010; 4:e873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hettinger J, Richards DM, Hansson J, et al. Origin of monocytes and macrophages in a committed progenitor. Nat Immunol 2013; 14:821–30. [DOI] [PubMed] [Google Scholar]
- 28. Swirski FK, Nahrendorf M, Etzrodt M, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 2009; 325:612–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.