In a randomized, placebo-controlled, phase 2b clinical trial, an adjuvanted vaccine containing the respiratory syncytial virus (RSV) fusion protein was immunogenic but did not protect older adults from disease caused by RSV.
Keywords: Adjuvant, clinical trial, efficacy, respiratory syncytial virus, subunit, vaccine
Abstract
Background
Respiratory syncytial virus (RSV) is an important cause of illness in older adults. This study assessed efficacy of a vaccine for prevention of RSV-associated acute respiratory illness (ARI), defined by specified symptoms with virologic confirmation.
Methods
This phase 2b study evaluated RSV postfusion F protein (120 µg) with glucopyranosyl lipid adjuvant (5 µg) in 2% stable emulsion. Subjects aged ≥60 years were randomly assigned at a ratio of 1:1 to receive vaccine or placebo (all received inactivated influenza vaccine). Ill subjects recorded symptoms and provided blood and nasal swab samples.
Results
In the per-protocol population (n = 1894), the incidence of RSV-associated ARI occurring ≥14 days after dosing was 1.7% and 1.6% in the vaccine and placebo groups, respectively, for a vaccine efficacy (VE) of –7.1% (90% confidence interval [CI], –106.9%–44.3%). Efficacy was not observed in secondary analyses that included seroresponse to nonvaccine RSV antigens (VE, 8.9%; 90% CI, –28.5%–35.4%) or symptoms combined with seroresponse (VE, 10.0%; 90% CI, –45.4%–44.4%). On day 29, 92.9% of vaccinees had an anti-F immunoglobulin G antibody seroresponse. Overall, 48.5% and 30.9% of RSV vaccine recipients reported local and systemic solicited symptoms, respectively.
Conclusion
The RSV vaccine was immunogenic but did not protect older adults from RSV illness.
Clinical Trials Registration
(See the editorial commentary by Langley, on pages 1334–6.)
The recognition that respiratory syncytial virus (RSV) is an important cause of illness in older adults has driven the development of RSV vaccines for this age group [1]. RSV circulates annually, but protective immunity is short-lived despite multiple exposures [2]. Neutralizing antibodies are important for protection; however, cellular immunity is also thought to play a role, probably in limiting viral spread [3–5]. Although adults maintain neutralizing antibodies as they age, older adults may be deficient in cellular immune responses to RSV, compared with younger adults [6]. Immunosenescence poses a challenge for the development of vaccines for older adults, and inclusion of an adjuvant might improve immune responses in this population [4].
RSV vaccine candidates generally include the fusion (F) protein of the virus, a highly conserved surface protein essential for infection containing multiple neutralizing and CD4+ and CD8+ T-cell epitopes. The F protein exists in pre- and postfusion conformations, with prefusion antibodies significantly contributing to the magnitude of neutralizing antibodies in human sera [7]. The development of vaccines to the prefusion conformation was hindered by manufacturing difficulties, but prefusion vaccine candidates are now being studied [8, 9]. The monoclonal antibody palivizumab binds postfusion F protein and prevents RSV disease in infants; thus, vaccines that stimulate antibody to postfusion F have been considered viable vaccine candidates [9, 10]. The postfusion F used in this vaccine study protected cotton rats and mice from experimental challenge [11].
We hypothesized that an adjuvanted, RSV postfusion F–based vaccine shown to be immunogenic in phase 1 studies would protect older adults from RSV illness [12, 13]. Based on animal studies demonstrating generation of a T-helper type 1–biased immune response, the adjuvant selected was glucopyranosyl lipid adjuvant (GLA), a Toll-like receptor 4 agonist, in a squalene-based oil-in-water stable emulsion (SE) [11, 14, 15]. Phase 1 data demonstrated appropriate safety and immunogenicity and, based on both cellular and humoral responses, provided support for inclusion of the adjuvant [12, 13]. Thus, a phase 2b study was conducted to assess the immunogenicity and efficacy of this investigational RSV vaccine among older adults.
METHODS
The investigational RSV vaccine (MEDI7510) comprised RSV F protein derived from the A2 virus in the postfusion configuration (120 µg, produced in Chinese hamster ovary cells) and GLA (5 µg), a synthetic analogue of monophosphoryl lipid A, in a 2% squalene-based oil-in-water SE. GLA-SE was provided by and licensed from Immune Design (Seattle, WA) pursuant to an existing agreement. The vaccine was mixed on site from lyophilized RSV F protein (diluted in sterile water) and liquid GLA-SE. It was administered as a 0.5-mL intramuscular dose. Blinding was maintained by wrapping syringe barrels if all were a visual match or by use of an unmasked administrator and a visual shield.
The randomized, double-blinded, phase 2b clinical study (clinical trials identifier: NCT02508194) was conducted during the 2015–2016 RSV season primarily in the United States but also in Canada, Eastern Europe, Chile, and South Africa. Subjects were aged ≥60 years, medically stable, and capable of visiting their study site. Exclusion criteria included influenza vaccination within the prior 6 months, history of an autoimmune disorder other than hypothyroidism, immunosuppression, or receipt of immunoglobulins or blood products within the prior 4 months.
Subjects received locally approved unadjuvanted, standard-dose inactivated influenza vaccine (IIV) provided by the sponsor (Fluzone Quadrivalent vaccine or Vaxigrip vaccine, a trivalent vaccine) and were randomly assigned a ratio of 1:1 to receive saline placebo or RSV vaccine in the other arm; randomization was stratified by age (≤75 and >75 years) and geographic region (Europe, North America, and southern hemisphere). If subjects experienced 1 day of any respiratory symptom (runny/stuffy nose, sore throat, earache or pain, new/worsening cough, new/worsening sputum, new/worsening subjective wheezing, dyspnea, or exacerbation of chronic pulmonary disease), they recorded symptoms daily for 21 days in a paper workbook modified from Flu-PRO, an existing patient-reported-outcomes instrument [16, 17]. Subjects self-swabbed both nares, using 2 mid-turbinate swabs (Copan Diagnostics, Murrieta, CA), on days 2–4 of illness and returned to the site on day 4 for site staff to collect mid-turbinate swabs and a sputum specimen if the subject was able to expectorate. Subjects were contacted once per week during peak RSV season and otherwise every other week to remind them of illness procedures.
Blood specimens were obtained at baseline (before vaccination on day 1), day 29, at the end of the RSV season (30 April in the northern hemisphere; 31 August in the southern hemisphere), and on days 4 and 22 of illness to test for antibodies to RSV F (anti-F immunoglobulin G [IgG]), glycoprotein G from antigenic groups A and B viruses (Ga and Gb, respectively), and the RSV nucleoprotein (N) [18]. Ga, Gb, and N are not components of the vaccine. Hemagglutination-inhibiting (HAI) antibodies (to influenza virus) were determined by Focus Diagnostics (Cypress, CA) in samples obtained on days 1 and 29 in the first 910 subjects enrolled in North America; seroresponse was defined as a ≥4-fold increase in titer. RSV A2–neutralizing and palivizumab-competitive antibodies [12, 15] were assessed in subjects who met the primary end point of RSV-associated acute respiratory illness (ARI) or were selected to match them (in a 1:6 ratio) or to match the sample size of a group that received the same formulation in a phase 1b study (clinical trials identifier: NCT02289820) through random sampling stratified by age and sex and controlled over baseline anti-F IgG levels, region, and comorbidity status. Cell-mediated response to RSV was measured by RSV interferon γ enzyme-linked immunospot (ELISPOT) assay [19] in subjects who met the primary end point or who were randomly selected on the basis of sample availability (samples were obtained only from sites in the United States capable of appropriate processing and freezing).
RSV was detected using the Quidel Lyra RSV plus human metapneumovirus (hMPV) multiplex real-time polymerase chain reaction (RT-PCR) assay (Quidel, San Diego, CA). RSV genotyping (A or B) was performed by G gene sequencing at Eurofins Viracor-IBT Laboratories (Lee’s Summit, MO).
The clinical protocol was approved by relevant institutional review boards or ethics committees. All subjects provided written informed consent. This study was conducted in accordance with the Declaration of Helsinki and good clinical practice guidelines.
Statistical Analysis
The primary study objective was to assess efficacy for the prevention of RSV-associated ARI in adults aged ≥60 years. RSV-associated ARI was defined on the basis of symptoms (Table 1) with detection of RSV. A sample size of 1900 subjects randomly assigned in a 1:1 ratio to receive vaccine or placebo provided 88% power (by simulation) to demonstrate a lower bound of the 90% confidence interval (CI) of vaccine efficacy (VE) of >0%. VE was calculated as [1 – relative risk] × 100%, where relative risk is calculated as [(rate of RSV-associated ARI in the vaccine group) ÷ (rate of RSV-associated ARI in the placebo group)]. The sample size calculation was based on these assumptions: a VE of 70%, an incidence of RSV-associated ARI in placebo recipients of 2.5% [1, 20], a dropout rate of 10%, a 2-sided α of 0.1, and a superiority margin 0%.
Table 1.
Symptom Locations and Definitions of Respiratory Syncytial Virus (RSV)–Associated Acute Respiratory Illness (ARI) and Lower Respiratory Tract Illness (LRTI)
| Variable | Symptom or Definition |
|---|---|
| Location | |
| Upper respiratory tract | Nasal congestion/rhinorrhea (runny or stuffy nose), sore throat, and earache or ear pain |
| Lower respiratory tract | Cough, dyspnea (shortness of breath), sputum (coughing up sputum or phlegm), and wheezing by self-report |
| Systemic | Myalgias or arthralgias (overall body aches), fatigue (tiredness), headache, decreased appetite, and feverishness |
| Definition | |
| ARI (primary end point) | Detection of RSV in at least 1 respiratory sample at the time of illness plus ≥1 symptom from any 2 of 3 locations |
| LRTI (secondary end point) | Detection of RSV in at least 1 respiratory sample at the time of illness plus ≥2 lower respiratory tract symptoms |
The primary analysis was conducted in the per-protocol population, defined as all subjects dosed and analyzed by product received who were followed for symptoms until their first RSV-associated ARI or the end of the surveillance period. The intention-to-treat (ITT) population, analyzed by randomized treatment group, included all subjects who were randomly assigned to a study group and dosed. A high-risk subgroup was determined before unmasking by selecting terms preferred by the Medical Dictionary for Regulatory Activities, version 19, for medical history that placed the subject in a risk category for severe influenza [21, 22].
The primary end point, the incidence of the first RSV-associated ARI episode occurring ≥14 days after vaccination and within the RSV surveillance period, was evaluated by constructing a 2-sided 90% CI for VE in the vaccine group as compared to the placebo group. A period of 14 days was selected to eliminate the risk of including subjects with incubating disease and to permit an immune response to vaccination. The CI was estimated by an exact conditional method dependent on the total number of cases [23, 24]. If the lower bound of the 90% CI was >0%, VE would be demonstrated. A modified Poisson regression with robust error variance was conducted to adjust for duration of follow-up, and a multiple imputation analysis was conducted to address the impact of missing data, both in the ITT population. To evaluate the effect of RSV vaccine on the immune response to IIV, 910 subjects had predose and day 29 postdose HAI antibodies assessed after receipt of Fluzone Quadrivalent, for a 90% power to demonstrate a 1.5-fold noninferiority margin for the postdose HAI antibody geometric mean titer (GMT) ratios (calculated as the GMT of HAI antibody in the placebo group divided by that of the vaccine group) for each of 4 strains. The power calculation was based on a 2-sample t test, assuming log-normal distribution, a 1-sided α of 0.025, a standard deviation for the HAI GMT of 1.48 in natural log transformation, an attrition rate of 10%, and no true difference between both groups.
RESULTS
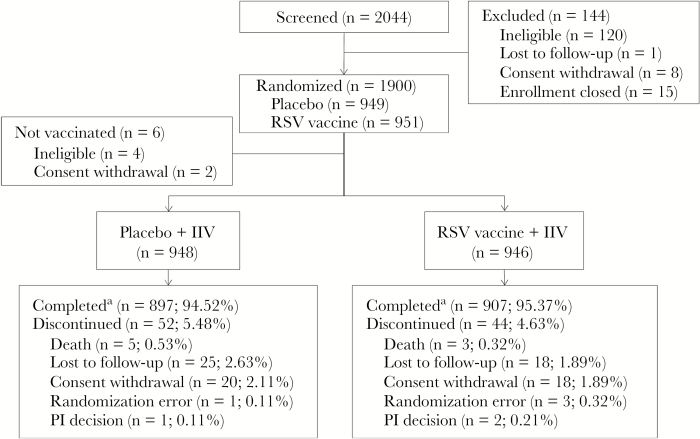
As planned, 1900 subjects were enrolled. Dosing occurred from 30 September–24 November 2015 and from 15 to 29 April 2016, in the northern and southern hemispheres, respectively. Median follow-up duration was 368 and 370 days for RSV vaccine and placebo groups, respectively, and efficacy follow-up was high (Figure 1). Groups were demographically comparable (Supplementary Table 1) except that more placebo recipients were female. The mean age was 67 years, with 14.1% of subjects aged >75 years. Similar proportions of subjects had high-risk comorbidities at baseline.
Figure 1.
Disposition of subjects (as treated; 1 subject who was randomly assigned to receive respiratory syncytial virus (RSV) vaccine received placebo in error). IIV, inactivated influenza vaccine; PI, principal investigator. aCompleted efficacy follow-up.
Overall, 539 subjects (28.9%) had an illness episode; 25.8% in the RSV vaccine group and 28.6% in the placebo group had ≥1 event that met RSV-associated ARI symptom criteria. At least 1 swab specimens was self-collected by 93.1% of subjects with an illness episode, and 96.5% had a site-collected swab; the optional sputum sample was collected from 37.1% of subjects. RSV was detected in a respiratory sample during 33 illnesses (6.1%). Of 30 viruses sequenced, 15 each were type A (following RSV vaccination in 7 subjects and following placebo receipt in 8) and type B (in 8 and 7, respectively). The genotypes were consistent with that of ON1 for RSV A and BAIX for RSV B [25, 26]. In the RSV vaccine and placebo groups, 96.6% and 98.9% of subjects, respectively, provided data on at least 70% of days, for a median of 21 days (range, 5–21 days) of data.
VE
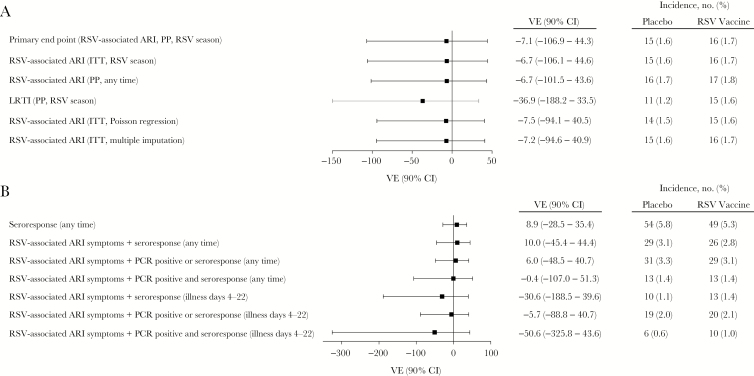
The incidence of the RSV-associated ARI end point occurring ≥14 days after vaccination was 1.7% in the RSV vaccine arm and 1.6% in the placebo arm, for an estimated VE of –7.1% (90% CI, –106.9%–44.3%; Figure 2). All subjects with RSV detected in a respiratory specimen who provided any symptom data met the RSV-associated ARI end point; when a more stringent definition (2 lower respiratory tract illness symptoms) was used, efficacy again was not observed. Efficacy was not observed in subset analyses by age, sex, race, ethnicity, or region (data not shown).
Figure 2.
Forest plot of vaccine efficacy (VE) for the first episode of acute respiratory syncytial virus (RSV)–associated respiratory illness (ARI) or by seroresponse in the per protocol (PP) population. Assessment was during the surveillance period, starting 14 days after dosing, unless otherwise noted. A, Efficacy according to RSV-associated ARI definition (first episode of RSV-associated ARI symptoms plus RSV detection in respiratory specimen by polymerase chain reaction analysis). B, Efficacy according to seroresponse definition (ie, RSV-associated ARI symptoms plus seroresponse to nonvaccine antigens). CI, confidence interval; ITT, intention to treat; LRTI, lower respiratory tract illness.
A total of 802 patients (406 in the RSV vaccine group and 396 in the placebo group) were considered high risk for ARI at baseline; the VE in this group was 51.2% (90% CI, –33.3%–83.9%), with 1.2% and 2.5% in the RSV vaccine and placebo groups, respectively, meeting the RSV-associated ARI end point. Serious adverse cardiopulmonary events, which could have represented an RSV end point (no subject was tested for RSV while hospitalized), were balanced across study arms (occurring in 1.5% of RSV vaccine recipients and 1.9% of placebo recipients). When defined as 2 lower respiratory tract illness symptoms plus seroresponse, the VE was 10.7% (90% CI, –47.1%–46.0%). There was no subgroup of subjects identifiable by baseline clinical conditions in which efficacy was demonstrable. Among subjects who had RSV-associated ARI, a numerically larger proportion receiving placebo than RSV vaccine had cardiac disorders (31.3% vs 11.8%), type 2 diabetes mellitus (31.3% vs 29.4%), or respiratory disorders (12.5% vs 11.8%). No subject who met the RSV-associated ARI end point in the RSV vaccine group had a history at baseline of cardiac failure, chronic kidney disease, asthma, or chronic obstructive pulmonary disorder. RSV-associated ARI events were not clustered at the end of the RSV season in RSV vaccine recipients (10 of 16 events occurred in months 3 and 4 after dosing).
Using the broadest definition (either a 4-fold increase from baseline at any time during the study or a 3-fold increase between illness days 4 and 22 in response to nonvaccine RSV antigens), 5.4% of all subjects (5.3% and 5.8% of RSV vaccine and placebo recipients, respectively) had an RSV seroresponse (VE, 8.9%; 90% CI, –28.5%–35.4%; Figure 2B). Approximately one half of these subjects (55 of 103) reported illness. Of subjects with ARI symptoms and RSV detected, 83.9% (26 of 31) had a seroresponse.
Analyses intended to compare disease severity across arms, including cycle threshold values from the RT-PCR assay as an estimate of viral load, duration of RSV illness, individual and composite symptom scores from the illness workbook, proportion of subjects treated with antibiotics or nonantibiotic medications, and proportion of subjects with various levels of healthcare professional visits, did not suggest RSV VE (data not shown).
Immunogenicity
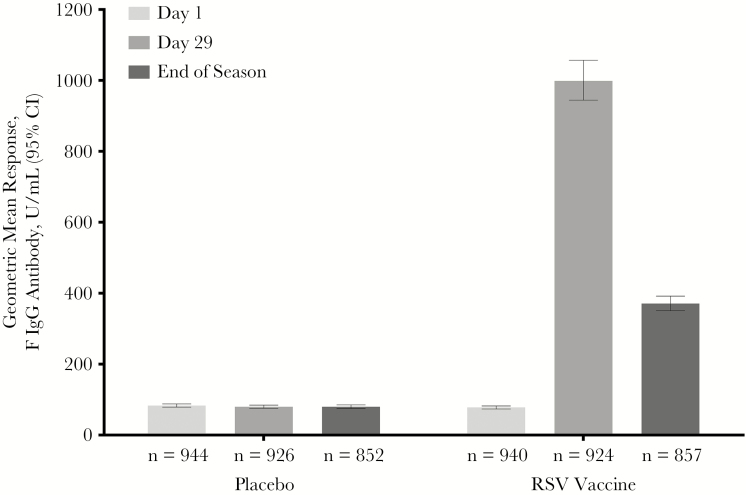
Subjects who received RSV vaccine developed an anti-F IgG immune response that was not observed in placebo recipients (Figure 3). Anti-F IgG levels declined by the end of the RSV season but remained significantly higher than in the placebo group (geometric mean fold rise [GMFR], 4.6 [95% CI, 4.34–4.88] vs 0.94 [95% CI, 0.91–0.96]). In RSV vaccine recipients, anti-F IgG levels on day 29 were 1015.46 (95% CI, 955.73–1078.94) units/mL in subjects aged 60–75 years and 905.05 (95% CI, 778.03–1052.82) units/mL in subjects aged >75 years. As previously demonstrated [12], baseline values affected day 29 values. Those with baseline values below the baseline median values had lower geometric mean responses (GMRs) but higher GMFRs than those with higher baseline values (GMR, 823.81 antibody units/mL [95% CI, 757.25–896.23] vs 1226.2 antibody units/mL [95% CI, 1144.26–1314.00]; GMFR, 20.73 [95% CI, 19.00–22.61] vs 7.64 [95% CI, 7.09–8.24]).
Figure 3.
Anti–respiratory syncytial virus (RSV) fusion protein immunoglobulin G (IgG) antibody results at baseline, day 29, and the end of the RSV infection season. CI, confidence interval.
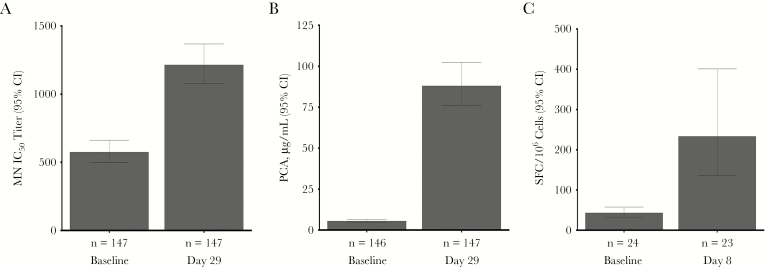
In immunogenicity subset analyses, microneutralizing and palivizumab-competitive antibodies and ELISPOT results were significantly higher after dosing than at baseline (Figure 4).
Figure 4.
Geometric mean respiratory syncytial virus (RSV) microneutralizing (MN) antibody titer (A) and palivizumab-competitive antibody (PCA) values (B) at baseline and on day 29 after dosing in subjects who met the primary end point or were selected to match them (in a 1:6 ratio) or to match the sample size of a group that received the same formulation in a phase 1b study (clinical trials registration: NCT02289820) through random sampling stratified by age and sex and controlled over baseline anti–RSV fusion protein immunoglobulin G levels, region, and comorbidity status. C, Cell-mediated response to RSV, as measured by RSV interferon γ enzyme-linked immunospot assay, in subjects who met the primary end point or who were randomly selected on the basis of sample availability. CI, confidence interval; IC50, inhibitory concentration at 50%; SFC, spot-forming cells.
The ratio of the HAI antibody GMT in the placebo group to that in the vaccine group was 1.04 to 1.82 among the 4 influenza virus strains, with upper bounds of the 95% CIs of 1.190 to 1.266, meeting the preestablished boundary of 1.5. The corresponding ratios of GMFRs were 1.07 to 1.13, with the highest upper bound being 1.321.
Using a definition of hMPV illness that required RSV-associated ARI symptoms and detection of hMPV by RT-PCR, 0.86% (8 of 931) and 1.82% (17 of 935) RSV vaccine and placebo recipients, respectively, had hMPV illness (VE, 52.7%; 90% CI, –1.9–79.4).
Safety
Local reactogenicity (Table 2) was greater after RSV vaccine receipt than after placebo or IIV receipt, but grade 3 events were uncommon (0.5% each for RSV vaccine and IIV). More subjects who received RSV vaccine plus IIV experienced systemic events of fatigue and muscle aches than those who received placebo plus IIV (Supplementary Table 2). Adverse events through the day 29 visit were balanced (Supplementary Table 3). One adverse event of special interest (autoimmune thyroiditis; Supplementary Tables 4 and 5) was considered by the investigator to be caused by RSV vaccine; in this case, chronic lymphocytic thyroiditis was observed in a specimen obtained during surgery for papillary thyroid carcinoma. Eight subjects died (3 RSV vaccine recipients and 5 placebo recipients); no death was considered to be related to study dosing (Supplementary Table 4).
Table 2.
Proportion of Subjects With Local Solicited Symptoms During Days 1–7 After Receipt of Placebo, Inactivated Influenza Vaccine (IIV), and Respiratory Syncytial Virus (RSV) Vaccine, by Study Group
| Symptom | Placebo and IIV Group, Recipients, No. (%) (n = 948) |
RSV Vaccine and IIV Group, Recipients, No. (%) (n = 946) | All IIV Recipients, No. (%)a (n = 1894) | ||
|---|---|---|---|---|---|
| Placebo | IIV | RSV Vaccine | IIV | ||
| Any | 199 (21.0) | 429 (45.3) | 459 (48.5) | 393 (41.5) | 822 (43.4) |
| Pain at injection site | 126 (13.3) | 261 (27.5) | 299 (31.6) | 266 (28.1) | 527 (27.8) |
| Tenderness/soreness at injection site | 147 (15.5) | 360 (38.0) | 383 (40.5) | 336 (35.5) | 696 (36.7) |
| Redness at injection site | 7 (0.7) | 50 (5.3) | 65 (6.9) | 40 (4.2) | 90 (4.8) |
| Swelling at injection site | 4 (0.4) | 50 (5.3) | 49 (5.2) | 32 (3.4) | 82 (4.3) |
Abbreviations: IIV, inactivated influenza vaccine; RSV, respiratory syncytial virus.
aData are for all subjects who received IIV.
DISCUSSION
This phase 2b study demonstrated that an investigational GLA-SE–adjuvanted vaccine based on the RSV F protein in postfusion configuration, although immunogenic, did not prevent RSV disease in older adults. There was no clinically identifiable patient population that could potentially derive benefit from the vaccine.
There were no identifiable flaws in study execution that would have led to these results. Our older adult population was highly compliant, despite limitations of age and health for some, and data quality was good. Although the study was underpowered for the observed incidence, the absence of a trend toward efficacy indicates that a much larger study would not be expected to reach a different conclusion. For example, if 3500 subjects had been enrolled, and, hypothetically, the VE was 70% in the additional 1600 subjects, the overall study VE would be 29% (90% CI, –20%–58%). The study end point, RSV-associated ARI, performed well, and use of a more stringent definition did not result in observed efficacy. The F antigen used in this study was from the same lot used in the phase 1 program. The adjuvant was from a new lot manufactured by the previously used process, and release specifications were met. The study appears to have failed because the vaccine did not generate adequate protective antibodies, either because antibodies to postfusion F were not qualitatively appropriately protective or because sufficiently high titers of anti–postfusion F neutralizing antibodies were not generated.
The vaccine induced a significant immune response, which declined during the RSV season, as has been observed after wild-type RSV infection [27]. However, RSV-associated ARI end points were not clustered in the end of the season, suggesting that the decline in immunogenicity did not lead to low efficacy. Although immunogenicity was lower than that observed in the phase 1b study (data not shown), this finding could have been due to population differences, and responses confirmed correct randomization and excluded significant loss of potency. Neutralizing antibody titers and cellular responses were not collected for all subjects in this study and RSV B neutralizing antibodies were not assessed; however, in a previous study, neutralizing antibodies to a type B strain were demonstrated after immunization with this vaccine. Because similar numbers of types A and B viruses were detected, it is unlikely that failure to prevent type B RSV infection was an important cause of the lack of efficacy. The observation that responses were greater in the F IgG assay than in the microneutralization assay suggests that many antibodies generated were nonneutralizing and therefore ineffective.
Although neutralizing antibody to the F protein is known to be protective in infants and to correlate with protection in older adults, this adjuvanted F protein–based RSV vaccine joins other F-based vaccines in failing to prevent RSV disease in adults [3, 5, 28]. Most recently, after a successful phase 2 study, the Novavax RSV F–based vaccine failed to prevent RSV disease in a phase 3 study, with outcomes very similar to those reported here (available at: http://novavax.com/presentation.show). The F protein used in current study, in the postfusion conformation, contains neutralizing epitopes including antigenic sites I, II, and IV on the F1 subunit [29]. Recently, antibodies to the prefusion conformation were shown to have greater neutralizing activity, including in adults, than antibodies to postfusion F; in particular, antibodies to site Ø, which are not induced by postfusion F, are far more important than those to site II (the palivizumab epitope), which are induced by both prefusion and postfusion F [7, 30, 31]. Thus, it is possible that a postfusion F–based vaccine may not generate appropriate neutralizing antibodies to prevent RSV disease in older adults; however, the efficacies of palivizumab and motavizumab suggest that, at least in young children, there is a concentration of antibodies to site II that can protect against RSV disease [32].
The observed incidence of RSV disease in this study did not reflect the epidemiologic incidence of disease in older adults. Dosing in this study occurred during the upslope of the RSV season so that part of the epidemic curve was excluded [33]. More RSV disease occurred in the southern hemisphere (2.7% in the placebo group), and the start of dosing was more timely for that inverted seasonality (but the sample size was small). Although most study subjects had underlying diseases, the oldest and most frail elderly subjects (who might have the highest RSV disease rates) were underrepresented. End points were probably underreported. Only about 29% of subjects had an illness visit, which is low frequency as compared to other studies [34]; in addition, we know that subjects with respiratory diseases such as pneumonia or exacerbation of respiratory illness often did not consider these to be potential RSV illnesses despite being asked to do so. Also, no patient was tested for RSV during a hospitalization. The RSV incidence assumption for sample size calculation was 2.5%, based primarily on the report from McClure et al [20], with adjustment for lack of requirement of medical attendance in the current study. In healthy adults aged ≥65 years, the incidence of RSV infection (not necessarily medically attended) diagnosed on the basis of culture, RT-PCR, or serologic test results was 3%–7% annually [1]. In our study, diagnosis on the basis of serologic test results was 5.3%–5.8%. The 2015–2016 season, based on MedImmune internal data from laboratory surveillance (primarily based on pediatric respiratory samples), was typical of recent RSV seasons (data not shown).
The trend toward efficacy against hMPV is an intriguing finding. Although this study was not designed to evaluate VE against hMPV, the findings may be meaningful and deserve consideration. Monoclonal antibodies with efficacy against both hMPV and RSV have been generated, and cross-neutralization is hypothesized to occur, based on a conserved region of the F protein [35, 36]. Anti-hMPV efficacy demonstrates biologic activity and suggests that the lack of efficacy was RSV specific.
The failure of this vaccine has broad implications for the field. Our vaccine contained a potent Toll-like receptor 4 agonist in a SE and a large quantity of antigen (close to the maximum that could feasibly be included in a vaccine), which suggests creating an improved postfusion F protein–based vaccine will be difficult. Inclusion of an F protein in the prefusion configuration may improve immunogenicity and efficacy. It is also possible that antibodies to the RSV G protein are protective; however, the need to match the highly variable G protein to circulating strains might entail periodic reformulation and inclusion of >1 genotype, which would be a significant barrier to a preventive strategy [9]. We plan to continue to investigate the immune responses to this RSV vaccine to better understand protective immunity against RSV.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Author Contribution. J. F., M. T. E., T. V., F. D., T. T., M. J. L., and A. R. F. contributed to study design and data analysis and interpretation. J. Y. and L. Y. contributed to data analysis and interpretation. M. J. L. and A. R. F., as lead investigators, enrolled and followed subjects in the study and contributed to data interpretation. All authors had access to all data, contributed to manuscript writing and provided final approval of the manuscript.
Acknowledgments. We thank the site investigators and their staff, the study programmers Ekaterina Batkhan and Anna Soloviov, Stacie Lambert of MedImmune’s translational medicine group, Amanda Kuehn and Dave Vallo from MedImmune’s clinical operations group, and MedImmune clinical scientist Nancy Mueller, for their contributions; and Disha Patel, PhD, of inScience Communications, Springer Healthcare (Philadelphia, PA, USA), for figure formatting and text editing before submission of the manuscript.
Financial support. This work was supported by MedImmune, a subsidiary of AstraZeneca.
Potential conflicts of interest. J. F., J. Y., M. T. E., T. V., L. Y., F. D., and T. T. are all employees of MedImmune, a wholly owned subsidiary of AstraZeneca, and may hold AstraZeneca stock or stock options. M. J. L. and A. R. F. disclose receipt of research grants from MedImmune for the conduct of this study. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med 2005; 352:1749–59. [DOI] [PubMed] [Google Scholar]
- 2. Hall CB, Walsh EE, Long CE, Schnabel KC. Immunity to and frequency of reinfection with respiratory syncytial virus. J Infect Dis 1991; 163:693–8. [DOI] [PubMed] [Google Scholar]
- 3. Falsey AR, Walsh EE. Relationship of serum antibody to risk of respiratory syncytial virus infection in elderly adults. J Infect Dis 1998; 177:463–6. [DOI] [PubMed] [Google Scholar]
- 4. Malloy AMW, Falsey AR, Ruckwardt TJ. Consequences of immature and senescent immune responses for infection with respiratory syncytial virus. In: Anderson LJ, Graham BS, eds. Challenges and opportunities for respiratory syncytial virus vaccines. Berlin, Heidelberg: Springer Berlin Heidelberg, 2013:211–231. [DOI] [PubMed] [Google Scholar]
- 5. Walsh EE, Peterson DR, Falsey AR. Risk factors for severe respiratory syncytial virus infection in elderly persons. J Infect Dis 2004; 189:233–8. [DOI] [PubMed] [Google Scholar]
- 6. Cherukuri A, Patton K, Gasser RA Jr et al. Adults 65 years old and older have reduced numbers of functional memory T cells to respiratory syncytial virus fusion protein. Clin Vaccine Immunol 2013; 20:239–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Graham BS, Modjarrad K, McLellan JS. Novel antigens for RSV vaccines. Curr Opin Immunol 2015; 35:30–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Langley JM, Aggarwal N, Toma A et al. A randomized, controlled, observer-blinded phase 1 study of the safety and immunogenicity of a respiratory syncytial virus vaccine with or without alum adjuvant. J Infect Dis 2017; 215:24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Melero JA, Mas V, McLellan JS. Structural, antigenic and immunogenic features of respiratory syncytial virus glycoproteins relevant for vaccine development. Vaccine 2017; 35:461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Collins PL, Melero JA. Progress in understanding and controlling respiratory syncytial virus: still crazy after all these years. Virus Res 2011; 162:80–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lambert SL, Yang CF, Liu Z et al. Molecular and cellular response profiles induced by the TLR4 agonist-based adjuvant glucopyranosyl lipid A. PLoS One 2012; 7:e51618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Falloon J, Ji F, Curtis C et al. A phase 1a, first-in-human, randomized study of a respiratory syncytial virus F protein vaccine with and without a toll-like receptor-4 agonist and stable emulsion adjuvant. Vaccine 2016; 34:2847–54. [DOI] [PubMed] [Google Scholar]
- 13. Falloon J, Talbot K, Curtis C et al. Dose selection for an adjuvanted respiratory syncytial virus F protein vaccine for older adults based on humoral and cellular immune responses. Clin Vaccine Immunol 2017. In press. doi: 10.1128/CVI.00157-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lambert SL, Aslam S, Stillman E et al. A novel respiratory syncytial virus (RSV) F subunit vaccine adjuvanted with GLA-SE elicits robust protective TH1-type humoral and cellular immunity in rodent models. PLoS One 2015; 10:e0119509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Patton K, Aslam S, Shambaugh C et al. Enhanced immunogenicity of a respiratory syncytial virus (RSV) F subunit vaccine formulated with the adjuvant GLA-SE in cynomolgus macaques. Vaccine 2015; 33:4472–8. [DOI] [PubMed] [Google Scholar]
- 16. Powers JH, Guerrero ML, Leidy NK et al. Development of the Flu-PRO: a patient-reported outcome (PRO) instrument to evaluate symptoms of influenza. BMC Infect Dis 2016; 16:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Powers JH, Stringer S, Clifford S et al. Development of the patient-reported outcome (PRO) instrument FLU-PRO to standardize and quantify symptoms of influenza. Presented at: 2nd Joint Meeting of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society, IDWeek 2013: Advancing Science, Improving Care, San Francisco, CA, 2–6 October 2013. [Google Scholar]
- 18. Maifeld SV, Ro B, Mok H et al. Development of electrochemiluminescent serology assays to measure the humoral response to antigens of respiratory syncytial virus. PLoS One 2016; 11:e0153019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Patton K, Aslam S, Lin J et al. Enzyme-linked immunospot assay for detection of human respiratory syncytial virus f protein-specific gamma interferon-producing T cells. Clin Vaccine Immunol 2014; 21:628–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McClure DL, Kieke BA, Sundaram ME et al. Seasonal incidence of medically attended respiratory syncytial virus infection in a community cohort of adults ≥50 years old. PLoS One 2014; 9:e102586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Centers for Disease Control and Prevention (CDC). People at high risk of developing flu-related complications http://www.cdc.gov/flu/about/disease/high_risk.htm. Accessed 5 August 2016.
- 22. Grohskopf LA, Shay DK, Shimabukuro TT et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2013–2014. MMWR Morb Mortal Wkly Rep 2014; 63:691–7. [PMC free article] [PubMed] [Google Scholar]
- 23. Breslow NE, Day NE. Statistical methods in cancer research. Volume II–the design and analysis of cohort studies. IARC Sci Publ 1987; 82:1–406. [PubMed] [Google Scholar]
- 24. Chan ISF, Bohidar NR. Exact power and sample size for vaccine efficacy studies. Commun Stat Theory Methods 1998; 27:1305–22. [Google Scholar]
- 25. Duvvuri VR, Granados A, Rosenfeld P, Bahl J, Eshaghi A, Gubbay JB. Genetic diversity and evolutionary insights of respiratory syncytial virus A ON1 genotype: global and local transmission dynamics. Sci Rep 2015; 5:14268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Trento A, Casas I, Calderón A et al. Ten years of global evolution of the human respiratory syncytial virus BA genotype with a 60-nucleotide duplication in the G protein gene. J Virol 2010; 84:7500–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Falsey AR, Singh HK, Walsh EE. Serum antibody decay in adults following natural respiratory syncytial virus infection. J Med Virol 2006; 78:1493–7. [DOI] [PubMed] [Google Scholar]
- 28. Walsh EE, Falsey AR. Humoral and mucosal immunity in protection from natural respiratory syncytial virus infection in adults. J Infect Dis 2004; 190:373–8. [DOI] [PubMed] [Google Scholar]
- 29. McLellan JS, Yang Y, Graham BS, Kwong PD. Structure of respiratory syncytial virus fusion glycoprotein in the postfusion conformation reveals preservation of neutralizing epitopes. J Virol 2011; 85:7788–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fuentes S, Coyle EM, Beeler J, Golding H, Khurana S. Antigenic fingerprinting following primary RSV infection in young children identifies novel antigenic sites and reveals unlinked evolution of human antibody repertoires to fusion and attachment glycoproteins. PLoS Pathog 2016; 12:e1005554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ngwuta JO, Chen M, Modjarrad K et al. Prefusion F–specific antibodies determine the magnitude of RSV neutralizing activity in human sera. Sci Transl Med 2015; 7:309ra162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. O’Brien KL, Chandran A, Weatherholtz R et al. ; Respiratory Syncytial Virus (RSV) Prevention study group Efficacy of motavizumab for the prevention of respiratory syncytial virus disease in healthy Native American infants: a phase 3 randomised double-blind placebo-controlled trial. Lancet Infect Dis 2015; 15:1398–408. [DOI] [PubMed] [Google Scholar]
- 33. Bloom-Feshbach K, Alonso WJ, Charu V et al. Latitudinal variations in seasonal activity of influenza and respiratory syncytial virus (RSV): a global comparative review. PLoS One 2013; 8:e54445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Falsey AR, Walsh EE, Capellan J et al. Comparison of the safety and immunogenicity of 2 respiratory syncytial virus (RSV) vaccines–nonadjuvanted vaccine or vaccine adjuvanted with alum–given concomitantly with influenza vaccine to high-risk elderly individuals. J Infect Dis 2008; 198:1317–26. [DOI] [PubMed] [Google Scholar]
- 35. Schuster JE, Cox RG, Hastings AK et al. A broadly neutralizing human monoclonal antibody exhibits in vivo efficacy against both human metapneumovirus and respiratory syncytial virus. J Infect Dis 2015; 211:216–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wen X, Mousa JJ, Bates JT, Lamb RA, Crowe JE Jr, Jardetzky TS. Structural basis for antibody cross-neutralization of respiratory syncytial virus and human metapneumovirus. Nat Microbiol 2017; 2:16272. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.