Abstract
Chlamydia trachomatis elementary body enzyme-linked immunosorbent assay (ELISA) was used to investigate serum anti-CT immunoglobulin G1 (IgG1; long-lived response) and immunoglobulin G3 (IgG3; short-lived response indicating more recent infection) from treatment (enrollment) and 6-month follow-up visits in 77 women previously classified as having spontaneous resolution of chlamydia. Of these women, 71.4% were IgG1+IgG3+, consistent with more recent chlamydia resolution. 15.6% were IgG3− at both visits, suggesting absence of recent chlamydia. Using elementary body ELISA, we demonstrated approximately 1 in 6 women classified as having spontaneous resolution of chlamydia might have been exposed to C. trachomatis but not infected. Further, we classified their possible infection stage.
Keywords: chlamydia, immunoglobulin, antibody, IgG1, IgG3, resolution, clearance, elementary body enzyme-linked immunosorbent assay (EB ELISA).
Persisting genital Chlamydia trachomatis (CT) infection in women can lead to chronic inflammation, which may result in pelvic inflammatory disease and tubal factor infertility. Studying the natural clearance of CT infection in humans is ethically challenging because CT detection obligates treatment to eradicate infection and limit complications. Sparse studies that tested stored specimens suggest that approximately 50% of CT infections spontaneously resolve (without treatment) within 1 year, presumably through immune-mediated clearance [1, 2]. Most studies on spontaneous resolution of CT infection have reported the resolution frequency between the time of CT screening (typically with a nucleic acid amplification test [NAAT]) and return for follow-up, usually for treatment of a positive test. Those with a positive CT screening test but negative CT test at follow-up are classified as having spontaneous resolution, which studies report occurs in 11%−44% of individuals within a few weeks to several months of a positive screening CT NAAT [3]. We found a spontaneous resolution frequency of approximately 20% in subjects studied at a sexually transmitted disease (STD) clinic in Birmingham, Alabama [4].
The potential clinical significance of spontaneous resolution is that patients who clear CT infection before treatment have a lower reinfection risk than those with persisting infection [5]. It has been suggested this may be because patients whose infections spontaneously resolve develop protective immunity, in contrast with those with persisting infection having “arrested” immunity if treated too early in their infection [6]. However, some patients classified as spontaneous resolution based on a repeat NAAT being negative may have been misclassified. A NAAT detects nucleic acids and cannot distinguish viable from nonviable organisms. Thus, a NAAT cannot differentiate established infection from exposure (ie, from CT in a partner’s secretions) that does not lead to infection. In contrast, culture only detects viable organisms and was used to define spontaneous resolution in sparse studies [7, 8], but culture is less sensitive than NAAT and not widely available. Neither NAAT nor culture, if positive, provides information on potential timing/duration of the recently resolved CT infection; some infections could be acute (early stage of infection) or primary (an individual’s first CT infection). Neither test can identify a remote infection (in the distant past; likely years ago).
We previously used a CT elementary body (EB) enzyme-linked immunosorbent assay (ELISA) to characterize CT-specific immunoglobulin (Ig) responses in individuals with a positive CT NAAT and found that immunoglobulin G1 (IgG1) and immunoglobulin G3 (IgG3) comprised the predominant serum anti-CT Ig response [9]. Among immunoglobulin G (IgG) subclasses, IgG1 has the longest half-life and is most abundant [10], whereas IgG3 is of lower abundance with a shorter half-life. IgG3, however, is the first IgG subclass to increase following infection and is associated with effector functions, including antibody-dependent cell-mediated cytotoxicity and neutralization [10]. In this study, we used EB ELISA to measure anti-CT IgG1 and IgG3 responses in women previously classified as having spontaneous resolution of CT infection to address the following objectives: (1) distinguish true spontaneous resolution from CT exposure without established infection, and (2) delineate differences in timing/duration of CT infection by measuring differences in duration of anti-CT IgG1 and IgG3 responses [10], including measurements at a 6-month follow-up visit to assess for seroconversion after treatment.
METHODS
Study Participants and Clinical Procedures
Our study evaluated sera and clinical data previously collected from women returning to the Jefferson County Department of Health (JCDH) STD clinic in Birmingham, Alabama, for treatment of a recent positive screening CT NAAT who were enrolled into a CT natural history study. Investigations focus on women classified as having spontaneous resolution of CT infection based on a negative repeat CT NAAT at enrollment, at which time women were interviewed regarding their medical and sexual history, underwent phlebotomy, had a cervical swab collected for repeat CT NAAT (Aptima Combo 2 [AC2]; Hologic, Marlborough, MA), and were treated with 1 g of azithromycin. Participants had a 6-month follow-up visit scheduled. Written informed consent was obtained from patients before enrollment. The study was approved by the University of Alabama at Birmingham Institutional Review Board (IRB) and JCDH. The Centers for Disease Control and Prevention (CDC) determined that CDC involvement did not constitute engagement in human subjects research, and CDC IRB review was therefore not required.
Chlamydia trachomatis Elementary Body Enzyme-Linked Immunosorbent Assay
CT–specific IgG1 and IgG3 responses were measured by EB ELISA as described previously [9, 11, 12]. Briefly, ELISA was performed using formalin-fixed CT EBs pooled from serovars D, F, and J. IgG1 and IgG3 responses were detected using alkaline phosphatase–labeled mouse antihuman IgG1 (a pool of clones 4E3, Southern Biotech, Birmingham, AL; and HP6069, Cal Biochem, San Diego, CA) and mouse antihuman IgG3 (clone HP6050; Southern Biotech, Birmingham, AL) at an optical density of 405 nm (OD405). The cutoff OD405 values for positive IgG1 and IgG3 anti-CT responses were >0.35 and >0.1, respectively. Each subject’s serum was run in triplicate at a 1:32 dilution.
Statistical Analysis
Differences in EB ELISA IgG1 and IgG3 OD405 readings from treatment (ie, enrollment) to follow-up visits for women categorized into different CT infection groups (described in the Results) were analyzed with SAS 9.3 (SAS Institute, Cary, NC) using the Wilcoxon signed rank test. Differences in OD405 readings between groups were analyzed using the Wilcoxon rank sum test. Only groups with >5 women were analyzed.
RESULTS
Frequency of Immunoglobulin G1 and Immunoglobulin G3 Seropositivity in Women Identified as Having Spontaneous Resolution of Chlamydia trachomatis Infection
Of 108 women identified with spontaneous resolution of CT infection, 77 had serum for serological testing from both treatment and follow-up visits. The median age was 24 years (range = 16–41), and 94.0% were non-Hispanic black women. The median interval between CT screening and treatment visits was 13 days (range = 4–45) and between treatment and follow-up visits was 184 days (range = 38–315). At the treatment visit, 74 (96.1%) women were seropositive for IgG1, 65 (84.4%) women were seropositive for IgG3, and 74 (96.1%) women were seropositive for IgG1 and/or IgG3. At the follow-up visit, 71 (92.2%) women were seropositive for IgG1, 61 (79.2%) women were seropositive for IgG3, and 73 (94.8%) women were seropositive for IgG1 and/or IgG3.
Using Anti–Chlamydia trachomatis Immunoglobulin G3 Seropositivity Status to More Accurately Classify Spontaneous Resolution of Chlamydia trachomatis Infection
Immunoglobulin G3 is the first IgG subclass to increase upon infection [10]. Therefore, it would be expected that most CT-infected individuals would be IgG3 seropositive. However, a small proportion of women could be initially IgG3 seronegative if the infection was acute, and, in that instance, they would be expected to seroconvert within a few weeks. Of 77 subjects classified as having spontaneous resolution, 15 (19.5%) were IgG3 seronegative at the treatment visit; 3 seroconverted IgG3 at follow-up, whereas the other 12 remained IgG3 seronegative. Thus, the positive CT NAAT at the screening visit in 12 (15.6%) subjects classified as having spontaneous resolution of CT infection may reflect an exposure to CT without an established CT infection.
Evaluating Combinations of Anti–Chlamydia trachomatis Immunoglobulin G1 and Immunoglobulin G3 Serostatus to Predict Timing/Duration of Chlamydia trachomatis Infection
As previously discussed, IgG1 and IgG3 differ in response time and half-life [10]. Thus, we assessed whether different IgG1 and IgG3 seropositive/seronegative combinations from both visits could be used to predict timing/duration of CT infection. We created the following categories (participant groups) of timing/duration of CT infection based on expected differences in IgG1/IgG3 serostatus: recent infection (IgG1+ and IgG3+ at the treatment visit; IgG3+ at the follow-up visit); primary infection (IgG1− at the treatment visit; seroconversion to IgG1+ at follow-up); acute infection (IgG3− at the treatment visit; seroconversion to IgG3+ at follow-up); remote infection (IgG1+ at the treatment visit; IgG3− at follow-up); and no infection (IgG1− and IgG3− at both treatment and follow-up visits). Table 1 summarizes results from our cohort. The majority (n = 57/77; 74.0%) were categorized as resolution of recent infection. Two (2.6%) were categorized as resolving primary CT infection and 2 (2.6%) with resolution of acute infection. Thirteen (16.9%) were categorized as having remote infection, meaning they likely had CT infection years ago but not recently. There were 3 women with no serological evidence of infection, suggesting despite CT antigen exposure (positive screening CT NAAT), they never acquired CT infection. Our data highlight the heterogeneity in IgG1 and IgG3 responses that exists among women classified as having spontaneous resolution of CT infection.
Table 1.
Classification of Chlamydia trachomatis Infection Based on Different Combinations of Anti–Chlamydia trachomatis Immunoglobulin G1 and Immunoglobulin G3 Serostatus From Women Classified as Having Spontaneous Resolution by a Nucleic Acid Amplification Test
| Type of infection | IgG subclass responses | No. of subjects (%) | |||
|---|---|---|---|---|---|
| Treatment visit | Follow-up visit | ||||
| Recent a | IgG1+ | IgG3+ | IgG1− | IgG3+ | 2 (2.60) |
| IgG1+ | IgG3+ | IgG1+ | IgG3+ | 55 (71.40) | |
| Primary b | IgG1− | IgG3+ | IgG1+ | IgG3+ | 1 (1.30) |
| IgG1− | IgG3− | IgG1+ | IgG3+ | 1 (1.30) | |
| Acute c | IgG1+ | IgG3− | IgG1+ | IgG3+ | 2 (2.60) |
| Remote d | IgG1+ | IgG3+ | IgG1− | IgG3− | 1 (1.30) |
| IgG1+ | IgG3+ | IgG1+ | IgG3− | 3 (3.90) | |
| IgG1+ | IgG3− | IgG1+ | IgG3− | 9 (11.70) | |
| No infection | IgG1− | IgG3− | IgG1− | IgG3− | 3 (3.90) |
Abbreviations: IgG, immunoglobulin G; IgG1, immunoglobulin G1; IgG3, immunoglobulin G3.
aRecently resolved Chlamydia trachomatis infection.
bAn individual’s first Chlamydia trachomatis infection.
cEarly stage of infection.
dIndividual was infected in the distant past (likely years ago).
Effect of Predicted Timing/Duration of Chlamydia trachomatis Infection on Changes in Immunoglobulin G1 and Immunoglobulin G3 Responses Between Treatment and Follow-Up Visits
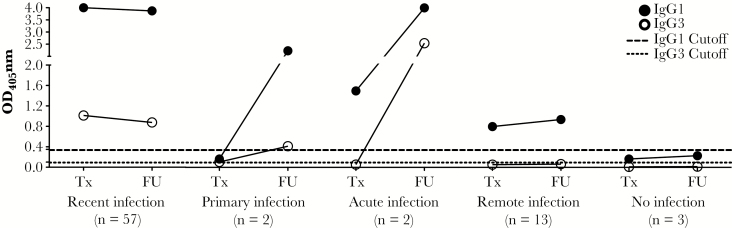
As illustrated in Figure 1, we analyzed the change in median OD405 IgG1 and IgG3 anti-CT responses between treatment and follow-up visits to determine whether the magnitude of the responses correlated with the categories of CT infection shown in Table 1. Women resolving recent infection had a significant decrease in the OD405 for IgG3 from the treatment to follow-up visit (P = .01) but no significant change in OD405 for IgG1. The small number of women that resolved primary or acute CT infection had an increase in OD405 for both IgG1 and IgG3 at the follow-up visit. The remote CT infection group had no significant change in the OD405 for both IgG1 and IgG3 responses between visits, and the CT-exposed women who never had infection also showed no noticeable change in the OD405 for IgG1 and IgG3 between visits. The recent infection group had a significantly higher OD405 for IgG1 and IgG3 compared with those with remote infection (P < .001 for both IgG1 and IgG3), possibly reflecting boosted antibody responses from recent CT exposure. Overall, changes in the median OD405 for IgG1 and IgG3 responses were consistent with those expected for the defined categories.
Figure 1.
Effect of timing/duration of Chlamydia trachomatis (CT) infection on the change in the magnitude of anti-CT immunoglobulin G1 (IgG1) and immunoglobulin G3 (IgG3) responses between treatment and follow-up visits. Line graph depicting the changes in the median optical density of 405 nm (OD405) of anti-CT IgG1 and IgG3 responses detected by CT elementary body (EB) enzyme-linked immunosorbent assay (ELISA) from a CT treatment visit (Tx) to a follow-up visit (FU) (on average approximately 6 months after treatment). Closed and open circles represent median OD405 value for IgG1 and IgG3 responses, respectively. The different infection timing/duration categories that were defined based upon seropositivity of IgG1 and IgG3 at Tx and FU visits are indicated on the x-axis. Dashed and dotted lines indicate the EB ELISA OD405 cutoff for IgG1 (0.35) and IgG3 (0.1), respectively. In the recent infection group, the magnitude of the IgG3 response significantly declined between the Tx and FU visits (median OD405 = 1.012 vs 0.876; P = .01); however, there was no significant change in the IgG1 response (median OD405 = 4.000 vs 3.872; P = .44). In the remote infection group, there was no significant change in the magnitude of the IgG1 or IgG3 response between Tx and FU visits (IgG1: median OD405 = 0.798 vs 0.936, P = .74; IgG3: median OD405 = 0.053 vs 0.063, P = .73). The magnitude of the IgG1 and IgG3 responses were significantly higher at both the Tx and FU visits for the recent infection group versus the remote infection group (all P values < .001). Due to the small sample size in the acute, primary, and no infection groups (n < 5 in each group), statistical analyses were only performed for the recent and remote infection groups. Abbreviations: FU, follow-up visit; IgG1, immunoglobulin G1; IgG3, immunoglobulin G3; OD405, optical density of 405 nm; Tx, treatment visit.
DISCUSSION
Our investigation of CT-specific IgG1 and IgG3 responses in women classified as having spontaneous resolution of CT infection contributed 2 main findings that advance our understanding of the natural history of CT infection: (1) some women classified as having spontaneous resolution based on CT NAAT results may have been misclassified and actually were exposed to CT but never infected; and (2) among women who spontaneously resolved infection, there exists heterogeneity with respect to infection timing/duration.
Persisting CT infection generally elicits CT-specific antibody responses. Failure to detect anti-CT antibody responses after sufficient follow-up in those with a positive genital CT NAAT suggests either they lack the ability to generate antibody responses or were not infected, rather only exposed. We found 12 of 77 (15.6%) women classified as spontaneous resolution never elicited an IgG3 response at the treatment and follow-up visits: 9 of 12 were IgG1-positive, likely reflecting a remote CT infection and confirming their ability to mount anti-CT antibodies. However, 3 of 12 were never seropositive for either IgG1 or IgG3 at the treatment or follow-up visits, most likely because they were never infected and their positive screening CT NAAT may have reflected a CT exposure, although we cannot rule out infection with absence of antibody responses. The minimal change in IgG1 and IgG3 OD405 readings between treatment and follow-up visits in these 12 women classified as having spontaneous resolution further supports absence of CT infection. This suggests 1 in 6 women were misclassified as having spontaneously resolved a CT infection. Women categorized in the recent infection group showed no change in IgG1 and a decline in IgG3 (Figure 1), which is in line with our published findings showing IgG3 responses, with a shorter half-life than IgG1, start to decline within 6 months after infection eradication [9]. In contrast, women categorized with either primary or acute infection showed an increase in IgG1 and IgG3, reflecting detection of their infection at an earlier stage. With the lack of behavioral data in our study, we cannot exclude the possibility that subjects had re-exposures to CT that could have affected magnitude of antibody responses at follow-up.
Overall, our findings demonstrate the importance of including anti-CT immunoglobulin measures with CT NAAT results for a more accurate study classification of spontaneous resolution of CT infection. Our findings pave the path for future studies related to the longevity of CT-specific immune responses in humans, which were previously not possible because of lack of sufficient knowledge about timing/duration of CT infection. Delineating primary versus acute infection and remote versus recent infection will be important in future studies investigating immune responses in individuals classified as having spontaneous resolution because presence versus absence of recent infection and timing/duration of infection could affect immune response measures.
Notes
Acknowledgments. The authors would like to thank the study participants, JCDH staff, and UAB clinicians Cyndi Poore and Hanne Harbison for their contributions.
Disclaimers. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the National Institutes of Health or Centers for Disease Control and Prevention.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (awards K23AI069505 and R01AI093692 to W. M. G.) and the Centers for Disease Control and Prevention (UAB Center for Community Health Prevention Research Center [PI, Max Michael], award U48DP001915 to W. M. G.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Molano M, Meijer CJ, Weiderpass E, et al. The natural course of Chlamydia trachomatis infection in asymptomatic Colombian women: a 5-year follow-up study. J Infect Dis 2005; 191:907–16. [DOI] [PubMed] [Google Scholar]
- 2. Morré SA, van den Brule AJ, Rozendaal L, et al. The natural course of asymptomatic Chlamydia trachomatis infections: 45% clearance and no development of clinical PID after one-year follow-up. Int J STD AIDS 2002; 13:12–8. [DOI] [PubMed] [Google Scholar]
- 3. Geisler WM. Duration of untreated, uncomplicated Chlamydia trachomatis genital infection and factors associated with chlamydia resolution: a review of human studies. J Infect Dis 2010; 201:S104–13. [DOI] [PubMed] [Google Scholar]
- 4. Geisler WM, Wang C, Morrison SG, Black CM, Bandea CI, Hook EW., 3rd The natural history of untreated Chlamydia trachomatis infection in the interval between screening and returning for treatment. Sex Transm Dis 2008; 35:119–23. [DOI] [PubMed] [Google Scholar]
- 5. Geisler WM, Lensing SY, Press CG, Hook EW., 3rd Spontaneous resolution of genital Chlamydia trachomatis infection in women and protection from reinfection. J Infect Dis 2013; 207:1850–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brunham RC, Rekart ML. The arrested immunity hypothesis and the epidemiology of chlamydia control. Sex Transm Dis 2008; 35:53–4. [DOI] [PubMed] [Google Scholar]
- 7. Parks KS, Dixon PB, Richey CM, Hook EW., 3rd Spontaneous clearance of Chlamydia trachomatis infection in untreated patients. Sex Transm Dis 1997; 24:229–35. [DOI] [PubMed] [Google Scholar]
- 8. Geisler WM, Black CM, Bandea CI, Morrison SG. Chlamydia trachomatis OmpA genotyping as a tool for studying the natural history of genital chlamydial infection. Sex Transm Infect 2008; 84:541–4; discussion 4–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Geisler WM, Morrison SG, Doemland ML, et al. Immunoglobulin-specific responses to chlamydia elementary bodies in individuals with and at risk for genital chlamydial infection. J Infect Dis 2012; 206:1836–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vidarsson G, Dekkers G, Rispens T. IgG subclasses and allotypes: from structure to effector functions. Front Immunol 2014; 5:520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morrison RP, Feilzer K, Tumas DB. Gene knockout mice establish a primary protective role for major histocompatibility complex class II–restricted responses in Chlamydia trachomatis genital tract infection. Infect Immun 1995; 63:4661–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Steiner AZ, Diamond MP, Legro RS, et al. ; Reproductive Medicine Network. Chlamydia trachomatis immunoglobulin G3 seropositivity is a predictor of reproductive outcomes in infertile women with patent fallopian tubes. Fertil Steril 2015; 104:1522–6. [DOI] [PMC free article] [PubMed] [Google Scholar]