Abstract
Background.
The seasonality of influenza is thought to vary according to environmental factors and human behavior. During winter holidays, potential disease-causing contact and travel deviate from typical patterns. We aim to understand these changes on age-specific and spatial influenza transmission.
Methods.
We characterized the changes to transmission and epidemic trajectories among children and adults in a spatial context before, during, and after the winter holidays among aggregated physician medical claims in the United States from 2001 to 2009 and among synthetic data simulated from a deterministic, age-specific spatial metapopulation model.
Results.
Winter holidays reduced influenza transmission and delayed the trajectory of influenza season epidemics. The holiday period was marked by a shift in the relative risk of disease from children toward adults. Model results indicated that holidays delayed epidemic peaks and synchronized incidence across locations, and that contact reductions from school closures, rather than age-specific mixing and travel, produced these observed holiday influenza dynamics.
Conclusions.
Winter holidays delay seasonal influenza epidemic peaks and shift disease risk toward adults because of changes in contact patterns. These findings may inform targeted influenza information and vaccination campaigns during holiday periods.
Keywords: influenza, age patterns, travel patterns, epidemiology, winter holidays, United States.
(See the editorial commentary by Hadler on pages 671–2.)
Influenza epidemics are characterized by large variation in disease burden across seasons and across locations within a given season [1]. While we do not fully understand what drives this variation, contact and travel patterns have been observed to influence local and global influenza transmission [2–5]. Winter holidays alter typical contact and travel patterns through school closures and holiday travel and occur early in typical influenza seasons in temperate climates, yet the measurement and the subsequent effects of these temporary behavioral changes on seasonal influenza remain poorly understood.
Consideration of age patterns is an important component to understanding the transmission and relative disease burden of influenza. Empirical contact surveys have illustrated that individuals tend most to associate with others in a similar age group and that school-aged children tend to have the greatest number of potential disease-causing contacts [6, 7]. Additionally, modeling studies have demonstrated that social mixing by age is sufficient to capture much of the heterogeneity in contact across populations [8]. In large population settings, school-aged children are thought to drive local influenza transmission because of their large number of contacts, while adults are thought to seed influenza in different locations owing to their global mobility [3, 4, 9].
Since school-aged children are of particular importance for influenza transmission, temporary school closures are a commonly considered reactive intervention in pandemic and severe influenza seasons [10, 11]. However, results of empirical studies of the effect of these interventions vary, with some reporting no significant effect on influenza transmission and others showing a 29% reduction in transmission among children alone [12–14]. Owing to these mixed results, the impact of tempory school closures on children and their trickle-down effects on other age groups remain unclear. While school holidays have similarities to closures, they occur at predetermined times and induce changes to both contact and travel patterns. In the United States, the Christmas break occurs in late December, and epidemics typically start in December and peak in February; changes in influenza transmission during the winter holidays could crucially affect the resulting influenza epidemic [15]. The number of contacts among children decreases because children are out of school for the holiday [16], and mixed results suggest that winter school holidays reduce or delay the risk of influenza among school-aged children by 33%–42% [17, 18] and that periods around the holidays experience high variability in influenza-like illness (ILI) across seasons [19].
Local and global travel are mechanisms by which respiratory pathogens are commonly thought to spread, and travel patterns have long been studied to understand the spatial spread of diseases. Winter holidays are characterized by increases in the frequency of visits to friends and family, which deviate from typical travel patterns. Additionally, in the United States, there are notable increases in travel among children and in the volume of long-distance travel [20, 21]. Human movement is tied to the phylogeographic spread of influenza viruses [22], and substantial evidence suggests that travel can influence the spatial spread and timing of influenza epidemics [3, 4, 23–25]. However, travel restrictions have been shown to produce little to no effect on the spread of pandemic influenza [26–28].
Our work aims to determine the impact of changing contact and travel patterns during winter holidays on influenza transmission and the resulting epidemic trajectories. With US medical claims data, we examine changes in influenza transmission during and after the holidays and characterize common patterns in the rates of ILI among school-aged children and working-aged adults during the holiday period across multiple influenza seasons. To understand the mechanisms behind these empirical patterns, we develop a parsimonious model with features that enable the isolated study of interactions between child and adult populations and the importance of travel in spatial spread. Using the model, we examine two hypotheses that may work independently or in concert: (1) holiday changes to travel patterns spatially synchronize influenza epidemics [29, 30] and (2) holiday changes in contact patterns due to school closures dampen transmission [17, 18]. Using both empirical and theoretical approaches, our work highlights the significant role of the holidays on shaping influenza seasonal dynamics and has implications for influenza control through vaccination prioritization and school closures.
METHODS
We used reports of ILI to explore spatial and age-specific patterns of seasonal influenza activity around the winter holidays in the United States. Here, we introduce the empirical data, the mathematical model structure, and the measures through which we characterized spatial and age-specific epidemiological patterns within the empirical and simulated data (Supplementary Materials).
Medical Claims Data
Weekly visits for ILI and any diagnosis from October 2001 to May 2009 were obtained from a records-level database, managed by IMS Health, of US medical claims. Visits were aggregated to 3-digit US zip code prefixes (zip3s), and cases of ILI were derived from influenza-associated International Classification of Diseases, Ninth Revision codes and were previously validated against ILI surveillance systems for city and region levels and across multiple age groups [31]. In 2009, roughly 60% of all US physicians-reported claims in the medical claims database. Weekly visits for ILI were divided by weekly all-cause visits (ie, a visit was included regardless of its purpose) in the medical claims database and standardized by population size to calculate an ILI incidence ratio (Supplementary Materials and Supplementary Figure 1) [31]. This calculation accounted for temporal variation in healthcare-seeking behavior (eg, physician’s office closures and a lower probability of seeking care for illness during the holidays) under the assumption that holiday-associated dips in ILI-associated visits and all-cause visits were proportional. For age-specific analyses, we calculate ILI incidence ratios for children (age, 5–19 years) and adults (age, 20–69 years). The epidemic period was defined as October through March, and periods before, during, and after the holidays were defined as consecutive 2-week periods, with the period during the holidays starting with the week containing Christmas.
To understand the effect of winter holidays on influenza transmission in the empirical data, we estimated the effective reproductive number (), defined as the mean number of secondary cases generated by each infected individual under the conditions at time , over weekly periods during the 8 influenza seasons from 2001–2002 through 2008–2009 [32]. Details on the calculation of can be found in the Supplementary Materials.
Metapopulation Model
We used an epidemiological model to simulate influenza epidemics excluding and including holiday-associated behavioral changes in contact and travel patterns. Our model was adapted from an age-specific metapopulation model that incorporates contact between children and adults and is spatially divided into metropolitan areas linked through air traffic flows [4, 33]. Infection followed a susceptible-infected-recovered disease progression, and the entire population was assumed susceptible at the start of an outbreak. Each model run was seeded with 1 infected child in 1 metropolitan area. Disease spread deterministically in discrete time steps according to age-specific contact patterns, and infection reached additional metropolitan areas through travel. Displayed model results represent the mean across all possible seeds. Details on parameter sources and initial conditions can be found in the Supplementary Materials.
Experimental Design
To mimic holiday-associated behaviors in the model, we altered age-specific contact and travel parameters during a 14-day holiday period (Supplementary Materials). We identified the holiday period on the basis of the mean number of days between Christmas and the epidemic peak in the national empirical data. The intervals before, during, and after the holidays period were defined as 2-week periods, with the period during the holidays starting with the week of Christmas. We performed sensitivity analyses to compare epidemiological patterns when holiday period contact rates and timing were altered (Supplementary Materials).
In the school closure model, we altered each value of the baseline contact matrix according to empirical survey data that reported age-specific contact rates during school holidays. More specifically, the total contact rate was reduced in both age groups, and the rate of child-to-adult contact increased proportionally to the total number of child contacts [16]. In the travel model, during the holiday period, we altered the travel-based connectivity between metropolitan areas on the basis of air traffic patterns from December 2005 and by increasing the fraction of child travelers, , to 15% [20]. In general, there was a greater volume of travelers that passed through fewer locations during the holidays than during the baseline period (Supplementary Materials). The holiday model combined the changes associated with both the school closure and travel models.
RESULTS
Characterizing Empirical Influenza Patterns During the Holidays
Here, we report age-specific and spatial patterns of influenza transmission, based on US medical claims data for ILI during the months around Christmas, after having corrected for variation in reporting rates. During the weeks following Christmas, we observed temporary reductions in US influenza activity across 8 seasons, even after accounting for holiday-associated reductions in healthcare-seeking behavior (Figure 1A and Supplementary Figure 1). Influenza transmission, as measured by , decreased by approximately 15% (from 1.1 to 0.9) in most seasons and decreased to <1, the epidemic threshold, immediately following Christmas. Within a few weeks, influenza transmission exceeded the epidemic threshold and rebounded to preholiday levels (Figure 1B). These patterns were observed consistently within our study period, including the notably early 2003–2004 influenza season. This pattern was also observed across smaller spatial regions, specifically across zip codes sharing zip3 values in the United States (Supplementary Figure 2). Analysis of viral surveillance data on influenza-positive laboratory confirmations verified that influenza was indeed circulating around Christmas for each season in our study period, so decreases in ILI incidence and may be attributed to influenza dynamics; similarly, we did not investigate dynamics around the time of Thanksgiving because influenza circulation was limited during this period (Supplementary Figure 3).
Figure 1.
Decreases in transmission are observed following Christmas. A, National influenza-like illness (ILI) incidence ratio (calculated as the number of ILI cases per total number visits per 100 000 population) calculated using weekly ILI medical claims data from the first week in November to the last week in January for influenza seasons 2001–2009. The week of Christmas is marked with the dashed line. B, National daily effective reproductive number (Rt) over time from November to January for influenza seasons from 2001 to 2009. (Rt) was calculated over 7-day windows, using ILI medical claims data adjusted for healthcare facility closures and care-seeking behavior. The date of Christmas is marked with the dashed line.
We also examined weekly ILI medical claims for school-aged children and adults from November through January. Across 8 influenza seasons, both children and adults experienced temporary declines and rebounds in ILI incidence around Christmas (Figure 2A). However, the changes in incidence patterns were not synchronous across age groups, with adults experiencing a reduction only after the holiday. We examined the relative risk of ILI activity between children and adults to understand the relative impacts on these age groups. The risk of disease shifted toward adults during and after the holiday, and these dynamics coincided with the temporary reductions in influenza activity and influenza transmission (Figure 2B). We posit that these patterns may be driven by altered interaction patterns due to children being home from school or to families (rather than business travelers) traveling for the holidays.
Figure 2.
The impact of the holidays varies by age group. A, Age-specific influenza-like illness (ILI) incidence ratio calculated from weekly ILI medical claims data from November to January for influenza seasons 2001–2009 among school-aged children and adults. The week of Christmas is denoted by the dashed line. B, The risk of incident ILI among school-aged children relative to that among adults calculated over time in weeks from November to January, using medical claims data for influenza seasons 2001–2009. The week of Christmas is denoted by the dashed line. A relative risk of >1 indicates a greater risk among children, with a value of <1 indicating greater risk among adults.
To investigate spatial patterns of influenza spread during the holiday period, we characterized the peak timing and synchrony of ILI reports across zip3s in the medical claims. We observed that the timing and variation of seasonal influenza peaks across zip3s in the United States was comparable for most seasons, occurring with a mean of 5 weeks after the holiday period; the early 2003–2004 season was an exception, with the holiday period occurring after the epidemic peak in a majority of locations (Figure 3A). Additionally, the distribution of incidence across zip3 areas showed little variation from the periods before to those after the holidays (Figure 3B). We hypothesize that these patterns may be driven by increased travel that homogenizes and synchronizes influenza risk across the country.
Figure 3.
Peak timing and spatial synchrony in empirical data. A, Distribution of weeks to peak timing of influenza epidemics across all 3-digit US zip code prefixes (zip3s) during the influenza season (ie, from October to March). Distributions are compared across 8 influenza seasons in the study period. The horizontal dashed lines highlight the holiday period. B, Distributions of ILI reports across all zip3s for the mean of the 2-week durations before, during, and after the holiday periods. Distributions are compared across 8 influenza seasons in the study period. A small number of outlying data points are not depicted here.
Exploring the Mechanisms Behind Holiday Influenza Dynamics
Informed by empirical patterns, we sought to identify the impact of specific behavior changes expected to occur during the holiday period. We hypothesized that 2 potential mechanisms influenced the age-specific and spatial patterns of influenza spread around the holidays: school closures and travel. As these mechanisms both lead to changes in individual-level and large-scale contact patterns during the holidays, we studied their impact on holiday influenza dynamics in a controlled manner through the use of a mathematical model. We compared the results from 3 models with holiday-associated behavioral changes to those of a baseline model, in which no behavioral changes were implemented. Our models implemented behavioral changes as follows: (1) an increased fraction of child travelers and changes to travel volume and connectivity, according to data on altered holiday travel patterns (the travel model); (2) a reduced overall number of potential disease-causing contacts for children and adults and a greater proportion of episodes of child-to-adult mixing, corresponding to a holiday school closure informed by data (the school closure model); and (3) both of the above models together (the holiday model; Supplementary Materials).
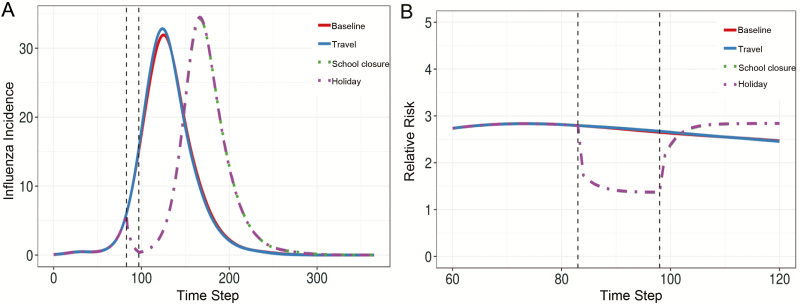
The baseline model results followed an epidemic trajectory with 1 peak, while holiday model epidemics had similar dynamics to the empirical data, illustrating a temporary decrease and rebound of influenza activity during and after the holidays, with a larger subsequent epidemic peak (Figure 4A). The travel model produced results similar to those of the baseline model, while the school closure model results were largely comparable to those of the holiday model. As with the empirical data, children always had a greater risk of influenza than adults, and the risk shifted toward adults with the school closure and holiday models (Figure 4B). No such shift was observed for the baseline and travel simulations. Despite the differences in dynamics, the total epidemic sizes were comparable for all 4 models (Supplementary Table 3).
Figure 4.
Changes to contact patterns appear to drive holiday-associated dynamics in model simulations. A, The total influenza incidence per 10 000 population over time, where the mean was taken across all model runs. B, The risk of disease among children relative to that among adults across all locations, where the mean was taken across all model runs. Epidemic trajectories for the baseline (no changes during the holiday period), travel only, school closure only, and full holiday (travel and school closure changes) models are compared, and the holiday period is demarcated by the dashed black lines. Solid lines represent the baseline and travel simulations, while dot-dashed lines represent the school closure and holiday simulations.
To characterize the spatiotemporal dynamics in the model, we considered peak timing and synchrony in model epidemic results. Peak timing of influenza epidemics across spatial areas was shown to be early in the baseline and travel models and shifted to a later time in the school closure and holiday models (Figure 5A). The baseline model highlighted spatial variability in peak timing across metro areas, with lower variability in the holiday and school closure models. We then compared the distribution of influenza incidence before, during, and after the holiday periods to characterize the trajectory of the model epidemics. The baseline model showed increasing disease incidence from before to after the holiday period, because the baseline epidemic was uninterrupted by the holiday. The baseline and travel models also appeared to show spatial asynchrony, illustrated by the large variance in the distribution of influenza incidence across locations, compared with the relatively low variance (spatial synchrony) in this distribution in the school closure and holiday models (Figure 5B).
Figure 5.
Holiday-associated behavioral changes delay peak timing and increase the synchrony of epidemics across locations in model simulations. A, Distribution of time steps (days) to peak timing of influenza epidemics across all metropolitan areas, where the mean was taken across all model runs. Distributions across metropolitan areas are compared for the baseline, travel only, contact only, and full holiday models, and the holiday period is demarcated by the horizontal black lines. B, Distributions of influenza incidence across all metropolitan areas for the mean of for the 2-week durations before, during, and after the holiday periods, where the mean was taken across all model runs (for each simulation, left, middle, and right, respectively). Distributions are compared for the baseline, travel only, school closure only, and full holiday models.
To explore the sensitivity of holiday dynamics to contact pattern changes, we considered models in which the overall number of contacts was reduced for children and adults (to match that of the school closure model), but the proportion of contact within and between age groups was not altered. We found that this new model (the partial school closure model) produced epidemic dynamics and spatial synchrony patterns matching those of the school closure model (Supplementary Figure 6). We also explored the sensitivity of our results to holiday period timing and found that later holidays did not impact the overall dynamics, age-specific patterns, or spatial synchrony outcomes reported above (Supplementary Figures 8, 10, and 11), but they did result in multiple epidemic peaks owing to the resetting of the epidemic trajectory.
DISCUSSION
In this study, we have considered the impact of winter holiday periods in the United States on influenza transmission. We have observed that these periods are associated with temporary reductions in rates of ILI, especially among children, at both national and local scales, after controlling for temporal variation in reporting. These observations corroborate those from previous empirical studies that reported reduced influenza transmission during the winter holidays in Argentina [17] and Arizona [18]. Additionally, we observed that the holidays may alter the synchrony of ILI incidence across locations, even for seasons marked by early peaks (occurring before the holiday period).
These empirical patterns are descriptive of the dynamics of influenza during the holiday period, but questions about the mechanisms driving these patterns remain. We used an age-specific spatial metapopulation model to compare outcomes among models including and excluding temporary, holiday-associated changes in contact and travel. This model framework allowed us to determine which behavioral change drives observed holiday epidemic dynamics and the impact of holiday timing on epidemic outcomes. We found that holiday-associated changes to age-specific contact patterns reproduced many of the patterns observed in our empirical data. In the model, holiday contact patterns were responsible for causing temporary reductions in influenza activity during the holiday period, shifting the risk of disease from children to adults, pushing the epidemic peak later in time, and increasing the synchrony in influenza incidence during the holiday period. However, total epidemic sizes were not affected (in contrast to the findings by Eames [34]). Our analysis also illustrated that the holiday-induced age shift in burden and spatial synchronization was insensitive to the timing of the holiday period during the epidemic but that later holiday periods resulted in multiple peaks (as experienced in the 2009 influenza A[H1N1] pandemic [34]). On the other hand, the empirical patterns from the 2003–2004 early influenza season highlighted that a holiday period arriving well after an epidemic peak had minimal impact on epidemic dynamics.
In comparing the effects of 2 purported mechanisms driving holiday dynamics—school closure and increased travel—we were surprised to find that changes due to school closure explained nearly all of the delay in peak timing and increase in spatial synchrony in our model. Travel restrictions have been shown to produce little to no effect on the spread of pandemic influenza [26–28], and our study adds to this literature on the minimal effect of holiday-related travel rerouting on spatial influenza transmission. While holiday travel is more commonly linked with seeding and synchronizing influenza in multiple locations [23], we found that school closures and, more specifically, reductions in the mean number of holiday contacts, rather than changes in mixing among age groups, could create a dampening and synchronizing spatial effect (Supplementary Materials). Thus, our study is consistent with other model-based studies supporting the idea that school closures may be successful when timed early during the epidemic [10].
In both empirical and modeling analyses, evidence suggested that children and adults have staggered, temporary dips in reported ILI after the winter holidays; the reduction was later and smaller in magnitude for adults, supporting results that holiday effects are delayed by 1 week among other age groups, relative to children [17]; that children are the primary drivers of household transmission [35, 36]; and that children experience the greatest disease burden when populations are naive to new strains of influenza, whereas adults are more affected in subsequent seasons or epidemic waves [37, 38]. Nevertheless, the timing of the overall epidemic peak was shifted equally in the holiday model for both children and adults; this suggests that, while children may experience greater influenza burden and local transmission owing to their high contact rates [6, 8, 39], they do not necessarily lead the epidemic wave [5, 40]. Finally, we observed that holiday-associated behavioral changes consistently increased the risk of disease among adults relative to that among children. Our previous work leveraged the consistency of this temporary change to detect early warning signals of seasonal influenza severity [1], and future work could examine how this early influenza testbed might signal other actionable epidemiological information about the influenza season.
The findings from our study may be used to inform influenza control strategies during the holiday period. In response to our finding that the holidays shift the disease risk from children to adults, public health organizations may ramp up vaccination and social-distancing campaigns in workplaces and public transit stations to target adults during the holiday period. For populations that are concerned about exceeding their healthcare surge capacity during a severe epidemic or pandemic, schools may consider coordinating to stagger their winter holiday periods to shift influenza season peaks among subpopulations, thus reducing the magnitude of the epidemic peak in the broader population.
Our study contributes uniquely to understanding holiday influenza dynamics because it combines large-scale epidemiological data with mathematical modeling to generate mechanistic understanding; however, some limitations exist. While our parameters were indeed based on empirical data, we have limited knowledge about holiday-associated behavioral changes to contact and travel patterns at a population scale [20, 21, 41]. It is difficult to isolate counterfactual empirical scenarios in which holidays do not exist, but future studies could compare seasonal influenza dynamics across locations with different holiday timings (eg, outside the United States) or very late influenza seasons. The average holiday travel changes in our model neither captured nonroutine travel and local travel patterns that have been shown previously to synchronize and spread local epidemics [2–4] nor considered the individual identities of travelers, which additionally slows the speed of epidemic waves [42]. The effects of travel may also have been affected by the deterministic nature of the model, which spread disease across metropolitan areas throughout the simulation course. Additionally, influenza transmission and ILI reporting were conflated in the model, as the effects of holiday model interventions were observed immediately. Future work may incorporate delays between holiday-associated transmission and reporting (eg, the influenza incubation period and the time delay before seeking care for ILI) through the use of a revised model or empirical data at a finer temporal scale. Seasonal fluctuations in factors such as temperature and humidity, both of which are hypothesized to modulate influenza transmission and survival, were also not considered here [15, 43, 44]. If winter holidays are indeed pushing US epidemics later into the winter, environmental conditions may be more favorable to influenza transmission. Future work should extend the examination of the link between environmental factors and holiday-induced shifts in peak timing [45, 46]. Additionally, we acknowledge limitations of medical claims data, which may have limited specificity for laboratory-confirmed influenza and poorly capture uninsured or impoverished populations that likely engage in different holiday behaviors than other populations [47–49]. Future studies with US medical claims data may better represent the entire population, as the Patient Protection and Affordable Care Act has substantially expanded insurance coverage among adults [50].
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online and model code may be accessed freely at https://github.com/bansallab/holidayflu. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. We thank IMS Health for kindly sharing the medical claims data with us and Vittoria Colizza for her insightful comments on an earlier draft of this work.
Author contributions. A. E. parsed the medical claims data, assembled the demographic and contact data, performed analyses, interpreted the results, and wrote the first draft of the manuscript. E. C. L. assembled all other data and performed analyses. C. V. interpreted the results and edited the manuscript. S. B. and E. C. L. jointly conceived and designed the study, guided the analysis, interpreted the results, and edited the manuscript. All authors read and approved the final manuscript.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily reflect the official views of JKTG, the National Institutes of Health, or IMS Health.
Financial support. This work was supported by the Jayne Koskinas Ted Giovanis Foundation for Health and Policy (dissertation support grant to E. C. L.); the Reseach and Policy for Infectious Disease Dynamics program, Science and Technology Directorate, Department of Homeland Security; and the Fogarty International Center, National Institutes of Health.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Lee EC, Viboud C, Simonsen L, Khan F, Bansal S. Detecting signals of seasonal influenza severity through age dynamics. BMC Infect Dis 2015; 15:587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stark JH, Cummings DA, Ermentrout B, et al. Local variations in spatial synchrony of influenza epidemics. PLoS One 2012; 7:e43528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Viboud C, Bjørnstad ON, Smith DL, Simonsen L, Miller MA, Grenfell BT. Synchrony, waves, and spatial hierarchies in the spread of influenza. Science 2006; 312:447–51. [DOI] [PubMed] [Google Scholar]
- 4. Apolloni A, Poletto C, Colizza V. Age-specific contacts and travel patterns in the spatial spread of 2009 H1N1 influenza pandemic. BMC Infect Dis 2013; 13:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schanzer D, Vachon J, Pelletier L. Age-specific differences in influenza A epidemic curves: do children drive the spread of influenza epidemics? Am J Epidemiol 2011; 174:109–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wallinga J, Teunis P, Kretzschmar M. Using data on social contacts to estimate age-specific transmission parameters for respiratory-spread infectious agents. Am J Epidemiol 2006; 164:936–44. [DOI] [PubMed] [Google Scholar]
- 7. Mossong J, Hens N, Jit M, et al. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Med 2008; 5:e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kucharski AJ, Kwok KO, Wei VW, et al. The contribution of social behaviour to the transmission of influenza A in a human population. PLoS Pathog 2014; 10:e1004206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bedford T, Riley S, Barr IG, et al. Global circulation patterns of seasonal influenza viruses vary with antigenic drift. Nature 2015; 523:217–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jackson C, Mangtani P, Hawker J, Olowokure B, Vynnycky E. The effects of school closures on influenza outbreaks and pandemics: systematic review of simulation studies. PLoS One 2014; 9:e97297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cauchemez S, Ferguson NM, Wachtel C, et al. Closure of schools during an influenza pandemic. Lancet Infect Dis 2009; 9:473–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cowling BJ, Lau EH, Lam CL, et al. Effects of school closures, 2008 winter influenza season, Hong Kong. Emerg Infect Dis 2008; 14:1660–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cauchemez S, Bhattarai A, Marchbanks TL, et al. Role of social networks in shaping disease transmission during a community outbreak of 2009 H1N1 pandemic influenza. Proc Natl Acad Sci U S A 2011; 108:2825–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cauchemez S, Valleron AJ, Boëlle PY, Flahault A, Ferguson NM. Estimating the impact of school closure on influenza transmission from Sentinel data. Nature 2008; 452:750–4. [DOI] [PubMed] [Google Scholar]
- 15. Lofgren E, Fefferman NH, Naumov YN, Gorski J, Naumova EN. Influenza seasonality: underlying causes and modeling theories. J Virol 2007; 81:5429–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eames KTD, Tilston NL, Brooks-Pollock E, John Edmunds W. Measured dynamic social contact patterns explain the spread of H1N1v influenza. PLoS Comput Biol 2012; 8:e1002425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Garza RC, Basurto-dávila R, Ortega-sanchez IR, et al. Effect of Winter School Breaks on Influenza-like Illness, Argentina, 2005–2008. Emerg Infect Dis 2013; 19:2005–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wheeler CC, Erhart LM, Jehn ML. Effect of school closure on the incidence of influenza among school-age children in Arizona. Public Health Rep 2010; 125:851–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gao H, Wong KK, Zheteyeva Y, Shi J, Uzicanin A, Rainey JJ. Comparing observed with predicted weekly influenza-like illness rates during the winter holiday break, United States, 2004–2013. PLoS One 2015; 10:2004–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kucharski AJ, Conlan AJ, Eames KT. School’s out: seasonal variation in the movement patterns of school children. PLoS One 2015; 10:e0128070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bureau of Transportation Statistics, US Department of Transportation. America on the go: findings from the National Household Travel Survey. Technical report. Washington, DC: Bureau of Transportation Statistics, US Department of Transportation, 2003. [Google Scholar]
- 22. Lemey P, Rambaut A, Bedford T, et al. Unifying viral genetics and human transportation data to predict the global transmission dynamics of human influenza H3N2. PLoS Pathog 2014; 10:e1003932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brownstein JS, Wolfe CJ, Mandl KD. Empirical evidence for the effect of airline travel on inter-regional influenza spread in the United States. PLoS Med 2006; 3:e401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grais RF, Ellis JH, Glass GE. Assessing the impact of airline travel on the geographic spread of pandemic influenza. Eur J Epidemiol 2003; 18:1065–72. [DOI] [PubMed] [Google Scholar]
- 25. Rvachev LA, Longini IM., Jr A mathematical model for the global spread of influenza. Math Biosci 1985; 75:3–22. [Google Scholar]
- 26. Hollingsworth TD, Ferguson NM, Anderson RM. Will travel restrictions control the international spread of pandemic influenza? Nat Med 2006; 12:497–9. [DOI] [PubMed] [Google Scholar]
- 27. Colizza V, Barrat A, Barthelemy M, Valleron AJ, Vespignani A. Modeling the worldwide spread of pandemic influenza: baseline case and containment interventions. PLoS Med 2007; 4:e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cooper BS, Pitman RJ, Edmunds WJ, Gay NJ. Delaying the international spread of pandemic influenza. PLoS Med 2006; 3:e212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Balcan D, Colizza V, Gonçalves B, Hu H, Ramasco JJ, Vespignani A. Multiscale mobility networks and the spatial spreading of infectious diseases. Proc Natl Acad Sci U S A 2009; 106:21484–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dey S, Joshi A. Stability via asynchrony in drosophila metapopulations with low migration rates. Science 2006; 312:434–6. [DOI] [PubMed] [Google Scholar]
- 31. Viboud C, Charu V, Olson D, et al. Demonstrating the use of high-volume electronic medical claims data to monitor local and regional influenza activity in the US. PLoS One 2014; 9:e102429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cori A, Ferguson NM, Fraser C, Cauchemez S. A new framework and software to estimate time-varying reproduction numbers during epidemics. Am J Epidemiol 2013; 178:1505–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Apolloni A, Poletto C, Ramasco JJ, Jensen P, Colizza V. Metapopulation epidemic models with heterogeneous mixing and travel behaviour. Theor Biol Med Model 2014; 11:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Eames KTD. The influence of school holiday timing on epidemic impact. Epidemiol Infect 2014; 142:1963–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Longini IM, Jr, Koopman JS, Monto AS, Fox JP. Estimating household and community transmission parameters for influenza. Am J Epidemiol 1982; 115:736–51. [DOI] [PubMed] [Google Scholar]
- 36. Viboud C, Boëlle PY, Cauchemez S, et al. Risk factors of influenza transmission in households. Br J Gen Pract 2004; 54:684–9. [PMC free article] [PubMed] [Google Scholar]
- 37. Bansal S, Pourbohloul B, Hupert N, Grenfell B, Meyers LA. The shifting demographic landscape of pandemic influenza. PLoS One 2010; 5:e9360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Simonsen L, Clarke MJ, Schonberger LB, Arden NH, Cox NJ, Fukuda K. Pandemic versus epidemic influenza mortality: a pattern of changing age distribution. J Infect Dis 1998; 178:53–60. [DOI] [PubMed] [Google Scholar]
- 39. Frost WH. Statistics of influenza morbidity: with special reference to certain factors in case incidence and case fatality. Public Health Rep 1920; 35:584–97. [Google Scholar]
- 40. Sebastian R, Skowronski D, Chong M, Dhaliwal J, Brownstein JS. Age-related trends in the timeliness and prediction of medical visits, hospitalizations and deaths due to pneumonia and influenza, British Columbia, Canada, 1998 - 2004. Vaccine 2008; 26:1397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Eames KTD, Tilston NL, John Edmunds W. The impact of school holidays on the social mixing patterns of school children. Epidemics 2011; 3:103–8. [DOI] [PubMed] [Google Scholar]
- 42. Keeling MJ, Danon L, Vernon MC, House TA. Individual identity and movement networks for disease metapopulations. Proc Natl Acad Sci U S A 2010; 107:8866–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lowen AC, Mubareka S, Steel J, Palese P. Influenza virus transmission is dependent on relative humidity and temperature. PLoS Pathog 2007; 3:1470–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shaman J, Kohn M. Absolute humidity modulates influenza survival, transmission, and seasonality. Proc Natl Acad Sci U S A 2009; 106:3243–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Earn DJD, He D, Loeb MB, Fonseca K, Lee BE, Dushoff J. Effects of school closure on incidence of pandemic influenza in alberta, Canada. Ann Intern Med 2012; 156:173–81. [DOI] [PubMed] [Google Scholar]
- 46. Flasche S, Hens N, Boëlle PY, et al. Different transmission patterns in the early stages of the influenza A(H1N1)v pandemic: a comparative analysis of 12 European countries. Epidemics 2011; 3:125–33. [DOI] [PubMed] [Google Scholar]
- 47. Cadieux G, Tamblyn R. Accuracy of physician billing claims for identifying acute respiratory infections in primary care. Health Serv Res 2008; 43:2223–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lowcock EC, Rosella LC, Foisy J, McGeer A, Crowcroft N. The social determinants of health and pandemic H1N1 2009 influenza severity. Am J Public Health 2012; 102:51–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. American travel survey: home for the holidays. Technical report. Washington, DC: Bureau of Transportation Statistics, US Department of Transportation, 1997. [Google Scholar]
- 50. Sommers BD, Musco T, Finegold K, Gunja MZ, Burke A, McDowell AM. Health reform and changes in health insurance coverage in 2014. N Engl J Med 2014; 371:867–74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.