The uptake of dissolved inorganic carbon by marine diatoms is facilitated by solute carrier transporters or external carbonic anhydrases. The occurrence of these distinct strategies is unrelated to phylogeny.
Keywords: Bicarbonate transporter, CO2 assimilation, CO2-concentrating mechanism, external carbonic anhydrase, marine phytoplankton, photosynthesis
Abstract
The acquisition of dissolved inorganic carbon (DIC) in CO2-limited seawater is a central issue to understand in marine primary production. We previously demonstrated the occurrence of direct HCO3– uptake by solute carrier (SLC) 4 transporters in a diatom, a major marine primary producer. Homologs of SLC are found in both centric and pennate marine diatoms, suggesting that SLC transporters are generally conserved. Here, the generality of SLC-mediated DIC uptake in diatoms was examined using an SLC inhibitor, diisothiocyano-2,2ʹ-stilbenedisulfonic acid (DIDS), and an inhibitor of external carbonic anhydrase, acetazolamide. DIDS suppressed high-DIC-affinity photosynthesis in the pennate diatom Phaeodactylum tricornutum and the centric diatom Chaetoceros muelleri, but there was no effect on either the pennate Cylindrotheca fusiformis or the centric Thalassiosira pseudonana. Interestingly, the DIC affinity of DIDS-insensitive strains was sensitive to treatment with up to 100 μM acetazolamide, displaying a 2–4-fold increase in K0.5[DIC]. In contrast, acetazolamide did not affect the DIDS-sensitive group. These results indicate the occurrence of two distinct strategies for DIC uptake—one primarily facilitated by SLC and the other being passive CO2 entry facilitated by external carbonic anhydrase. The phylogenetic independence of these strategies suggests that environmental demands drove the evolution of distinct DIC uptake mechanisms in diatoms.
Introduction
Marine diatoms are major primary producers in the ocean and they are estimated to contribute up to 20% of annual global carbon fixation (Falkowski et al., 1998; Smetacek, 1999). Thus, diatom primary production is a major driving force for incorporating organic carbon into the ocean ecosystem and is central to the biological carbon pump from the surface to deep sea (Falkowski et al., 1998; Smetacek, 1999). Despite the ecological importance of marine diatoms, the molecular basis underlying active carbon fixation by marine diatoms is not fully understood. In seawater, the dominant form of dissolved inorganic carbon (DIC) is HCO3– (2 mM), while the concentration of CO2 is low (up to 15 μM under the present atmosphere at >15 °C) (Raven, 1994). The half-saturation constant (Km) values for CO2 of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) in marine diatoms range from 23 to 65 μM at 25 °C, much higher than the CO2 concentration ([CO2]) in seawater (Badger et al., 1998; Young et al., 2016). In addition, owing to the slow rates of dissolved CO2 diffusion and the spontaneous equilibrium between CO2 and HCO3–, CO2-dependent DIC acquisition can become a rate-limiting step of photosynthesis. To avoid such problems, most marine phytoplankton, including diatoms, developed the CO2-concentrating mechanism (CCM) by which [CO2] in the vicinity of Rubisco is elevated by active accumulation of DIC from the surrounding medium, resulting in the establishment of high-DIC-affinity photosynthesis (Badger et al., 1998; Giordano et al., 2005; Kaplan and Reinhold, 1999; Matsuda et al., 2011). A general model of CCM in eukaryotic algae comprises active DIC transport across plasma membranes and chloroplast membranes as well as carbonic anhydrase (CA)-mediated supply of CO2 in the vicinity of Rubisco (Giordano et al., 2005).
Previous physiological studies have demonstrated occurrences of both active CO2 and HCO3– uptake in marine diatoms (Burkhardt et al., 2001; Matsuda et al., 2001; Nimer et al., 1997; Rost et al., 2003). However, the molecular identity of the HCO3– transporter was unknown until Nakajima et al. (2013) demonstrated that the marine pennate diatom Phaeodactylum tricornutum uses solute carrier (SLC) 4 transporters for direct uptake of HCO3– from seawater across the plasma membrane. Transcription of three SLC4 genes in P. tricornutum was highly CO2 responsive and the nature of one of these, SLC4-2, was characterized by generating overexpressing strains. When SLC4-2 was constitutively overexpressed in P. tricornutum under high CO2 conditions in which the endogenous CCM was suppressed (Harada et al., 2006; Hennon et al., 2015; Matsuda et al., 2011; Nakajima et al., 2013; Ohno et al., 2012; Tanaka et al., 2016), cells showed increased affinity for DIC and highly halophilic Na+-dependent HCO3– uptake (Nakajima et al., 2013). To maintain the optimum uptake rate, PtSLC4-2 requires more than 100 mM Na+ (Nakajima et al., 2013), meaning that it is one of the most halophilic Na+-dependent HCO3– transporters so far identified in microalgae and cyanobacteria (Price et al., 2004; Shibata et al., 2002). Such a high Na+ requirement suggests specific adaptation of the diatom CCM to a high-salinity environment.
Apart from the diatom SLC4 system, in a freshwater green alga, Chlamydomonas reinhardtii, transporters other than SLC4, such as high light activated 3 (HLA3) and low CO2-inducible protein 1 (LCI1), are known to facilitate HCO3– uptake across the plasma membrane (Duanmu et al., 2009; Ohnishi et al., 2010; Yamano et al., 2015). The occurrence of independent transporters in different lineages strongly suggests convergent evolution of DIC uptake systems among eukaryotic algae.
The possibility of convergent evolution of DIC uptake has been suggested even within the diatom clade. Although functional characterization of SLC4 has been performed only in P. tricornutum, genomic information indicates the possibility of SLC4-mediated HCO3– transport in Thalassiosira pseudonana (Nakajima et al., 2013), suggesting a common mechanism of HCO3– transport mediated by SLC4s. On the other hand, substantial differences in the subtypes and subcellular localization of CAs in P. tricornutum (Tachibana et al., 2011) and T. pseudonana (Samukawa et al., 2014) indicate that the CCM in these diatom species may be distinct, suggesting different evolutionary trajectories.
Diatoms have multiple CAs, which are classified into different subtypes based on amino acid sequence and localized in different subcellular compartments. These CAs are hypothesized to facilitate the efficient mobilization of DIC to Rubisco and prevent leakage of CO2 (Harada et al., 2005; Kikutani et al., 2012; Samukawa et al., 2014; Tachibana et al., 2011; Tanaka et al., 2005). In P. tricornutum, two pyrenoidal β-CAs are considered to be key components of the CCM; T. pseudonana has neither β-CA nor pyrenoid-localized CA (Samukawa et al., 2014; Tachibana et al., 2011). Another divergence between the species is the presence of a cytosol-localized γ-CA in T. pseudonana; there is no evidence for a cytosolic CA in P. tricornutum. Additionally, T. pseudonana has many δ-CAs and a ζ-CA, while these subtypes are absent in P. tricornutum. In T. pseudonana, one δ-CA and one ζ-CA are localized in the periplasmic space and their transcription is activated under low-CO2 conditions, suggesting the involvement of these external CAs (eCAs) in DIC acquisition. There is currently no molecular evidence for eCAs in P. tricornutum (Samukawa et al., 2014; Tachibana et al., 2011), lending further evidence for the existence of fundamentally different DIC uptake strategies in these diatoms. Recently, a novel CA, named θ-CA, located in the lumen of the pyrenoid-penetrating thylakoid of P. tricornutum has been identified and revealed to have an essential role in efficient CCM and growth (Kikutani et al., 2016), but the localization and role of θ-CA in T. pseudonana has yet to be examined.
It has been suggested that eCAs facilitate DIC uptake across the plasma membrane in many microalgae (Aizawa and Miyachi, 1986). When cytosolic [CO2] is lower than that in the external media, CO2 enters the cytosol by diffusion. eCA helps to maintain diffusive CO2 entry across the plasma membrane by facilitating a constant supply of CO2 from HCO3– at the periplasmic space (Aizawa and Miyachi 1986; Hopkinson et al., 2011; Matsuda et al., 2017). While alternative roles of eCA, such as pH regulation, have been proposed, eCA activity primarily responded to the CO2 concentration in medium, rather than pH, in the marine-centric diatoms T. pseudonana and Thalassiosira weissflogii (Hopkinson et al., 2013), supporting the idea that eCA enhances CO2 supply in these marine diatoms. eCA activity has been detected in many marine diatoms other than these two species (Burkhardt et al., 2001; Hopkinson et al., 2013; Martin and Tortell, 2008; Nimer et al., 1997; Samukawa et al., 2014), suggesting that eCA-mediated CO2 uptake is an alternative way to increase DIC availability besides direct HCO3– uptake.
While the molecular basis of CCM in marine diatoms has been studied mainly in P. tricornutum, careful examination is needed to generalize the model of CCM to other marine diatoms. Diatoms comprise more than 105 species (Mann and Droop, 1996) and there is extensive diversity in their morphological and physiological properties (Admiraal et al., 1986; Kooistra et al., 2007). This diversity is one of the reasons explaining their wide distribution in the world’s oceans, from polar to tropical regions; such diversity in living environment may also be reflected in the diversity of the CCM. As mentioned earlier, significant differences in the subtypes and locations of CAs between P. tricornutum and T. pseudonana suggest that they employ different CCM strategies, which may have differentiated during the diversification of the pennate and centric groups or may have arisen as a result of convergent evolution, utilizing a set of components obtained from partially (or totally) independent origins.
In the present study, a comparative physiological analysis was carried out to investigate the spectrum of CCM strategies among four marine diatom strains—two raphid pennate diatoms (P. tricornutum and Cylindrotheca fusiformis) and two multipolar centric diatoms (T. pseudonana and Chaetoceros muelleri). By examining the effect of specific SLC4 and eCA inhibitors on photosynthetic affinity for DIC and maximum photosynthetic rate, we demonstrate the occurrence of two distinct strategies for the initial uptake of DIC from the surrounding seawater.
Materials and methods
Strains and culture conditions
Organisms used in this study were P. tricornutum UTEX642, C. fusiformis NEPCC417, T. pseudonana CCMP1335, and C. muelleri CCMP1316. All diatoms were grown in F/2 artificial seawater (F/2ASW) (Guillard and Ryther, 1962; Harrison et al., 1980) supplemented with 10 nM sodium selenite. Diatoms except T. pseudonana were grown under continuous illumination at 80 μmol photons m–2 s–2 and T. pseudonana was grown at 20 μmol photons m–2 s–2. All cells were grown under constant bubbling with atmospheric air at 20 °C. Growth was monitored by measuring optical density at 730 nm (OD730nm) and cells at logarithmic growth phase (OD730nm = 0.2–0.4) were used for all experiments. Chlorophyll concentration was determined according to Jeffrey and Humphrey (1975).
Measurement of photosynthetic parameters
Photosynthetic DIC affinity and maximum rate were determined by using a Clark-type oxygen electrode (Hansatech Instruments Ltd, Norfolk, UK) according to Nakajima et al. (2013). For measurement, all diatoms were suspended in DIC-free F/2ASW (pH8.2, buffered with 10 mM Tris-HCl) at 10 μg Chl a ml–1. Cells were maintained in a sealed water-jacketed acrylic chamber and illuminated to allow cells to reach the CO2 compensation point (CP). Then, the DIC concentration ([DIC]) at the CP was determined by gas chromatography (GC-8A, Shimadzu Co., Kyoto, Japan) equipped with a methanizer and flame ionization detector (GC-FID) (Birmingham and Colman 1979). The O2 evolution rate at various [DIC] was measured by injecting a known amount of NaHCO3 as a substrate. Maximum photosynthetic O2 evolution rate (Pmax) and half-saturation constant for DIC (K0.5[DIC]) were determined by rectangular-hyperbola fitting to data with non-linear least squares regression. Apparent photosynthetic conductance (APC), which is a coefficient representing changes in photosynthetic rate as the [DIC] tends toward zero, was calculated from the initial slope of the O2 evolution rate versus [DIC] curve (Johnston et al., 1992). Light intensity used for measurements was as follows: 300 μmol photons m–2 s–2 for P. tricornutum, C. fusiformis, and C. muelleri, and 200 μmol photons m–2 s–1 for T. pseudonana. Light intensity was set at about saturation point and no inhibitory effects were observed throughout the experiment (data not shown). 4,4ʹ-Diisothiocyano-2,2ʹ-stilbenedisulfonic acid (DIDS), an inhibitor of SLC4, and acetazolamide (AZA), a weakly permeable inhibitor of CA (Moroney et al., 1985), were dissolved in DMSO and ethanol, respectively. As mock treatment, 1% (v/v, final concentration) DMSO or 1% (v/v, final concentration) ethanol was added.
Determination of DIC uptake rate
DIC uptake and efflux of CO2 were measured according to Nakajima et al. (2013) with modification. Cells in logarithmic growth phase were harvested and resuspended in DIC-free F/2ASW at a concentration of 20 μg Chl a ml–1. Cells were then transferred to a 1.5 ml microtube equipped with a filter cartridge (Nanosep MF GHP, pore size 0.45 μm, Pall Corp., NY, USA) and pre-incubated under illumination for 30 s. The reaction was initiated by addition of NaHCO3 at a final concentration of 100 μM (for P. tricornutum and C. muelleri) or 200 μM (for C. fusiformis and T. pseudonana). After the various incubation periods, the filter cartridge containing the cell suspension was centrifuged to allow separation of medium and cells. Then, the remaining DIC in the medium at each time point was immediately measured by GC-FID. The initial depletion rate of DIC during the first 240 s of the light period was calculated as the net DIC uptake rate (NDIC). The efflux rate of DIC (EC) was calculated from the CO2 evolution rate in the dark immediately after 240 s of light exposure. Gross DIC uptake rate (GDIC) was calculated from NDIC and EC using the DIC flux model presented by Nakajima et al. (2013).
To examine the energy source of DIC uptake, we used 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU) as an inhibitor of linear electron transport (LET). Additionally, carbonyl cyanide-m-chlorophenyl hydrazone (CCCP) was used to inhibit proton gradient and membrane potential-coupled ATP synthesis. These inhibitors were dissolved in DMSO and then added to the cell suspension immediately before the pre-incubation period. The final concentration of DCMU and CCCP was 2 μM and 10 μM, respectively. In control experiments, 1% (v/v) DMSO was added as a mock treatment. In C. fusiformis and T. pseudonana, initial [DIC] was set higher (200 μM) than for the other two diatoms (100 μM), since C. fusiformis and T. pseudonana have lower affinity for DIC and showed only weak DIC uptake activity at 100 μM DIC.
Results
Effects of inhibitors of SLC and extracellular CA on pennate diatoms
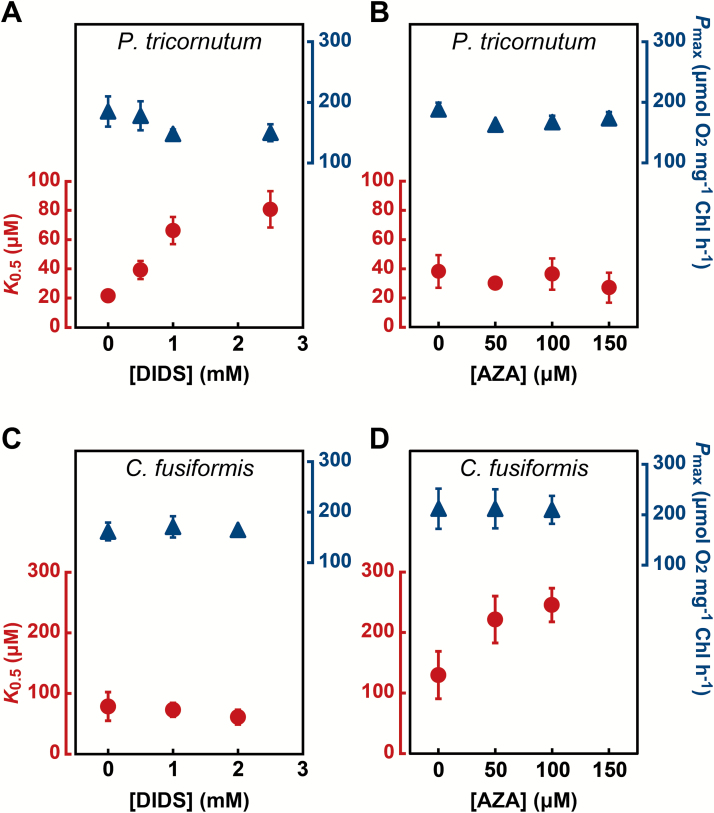
To examine the contribution of SLC4 transporters to DIC acquisition in pennate and centric diatoms, we tested the effect on K0.5[DIC] and Pmax of the commonly used SLC4 HCO3– uptake inhibitor DIDS and the weakly permeable CA inhibitor AZA relative to the 1% (v/v) DMSO or 1% (v/v) ethanol-treated mock cells with four diatom species: two raphid pennates, P. tricornutum and C. fusiformis, and two multipolar centrics, T. pseudonana and C. muelleri. DMSO and ethanol had no effect on K0.5[DIC] and Pmax (data not shown). P. tricornutum displayed a significant sensitivity to DIDS. The K0.5[DIC] increased with increasing concentrations of DIDS, reaching a 4-fold increase at 2.5 mM DIDS relative to the non-treated cells (Fig. 1A and Supplementary Table S1 at JXB online). Changes in APC, an index of photosynthetic DIC affinity calculated from the initial slope of the photosynthetic O2 evolution versus [DIC] curve (Johnston et al., 1992), and [DIC] at the CO2 CP, are listed in Table 1. In P. tricornutum, changes in [DIC] at the CO2 CP were not significant, but APC was decreased 4-fold in the presence of 2.5 mM DIDS (Table 1). In contrast, when the role of eCA was examined by treatment with AZA, K0.5[DIC] and APC were unaffected in P. tricornutum (Fig. 1B, Table 1, Supplementary Tables S1 and S2), consistent with a previous report showing no eCA in this diatom (Satoh et al., 2001). These results suggest that DIC uptake of P. tricornutum is primarily supported by DIDS-sensitive active transport of HCO3–, most probably by SLC4 as indicated by Nakajima et al., 2013.
Fig. 1.
Effects of DIDS and AZA on pennate diatoms. Photosynthetic parameters (K0.5[DIC] and Pmax) in air-grown P. tricornutum (A, B) and C. fusiformis (C, D) were determined in the presence of various concentrations of DIDS (A, C) or AZA (B, D). Circles, K0.5; triangles, Pmax. Values represent means ± SD of three or four biological replicates. Panel A was generated from data in Nakajima et al. (2013).
Table 1.
Effects of inhibitors on apparent photosynthetic conductance (APC) and [DIC] at the CO2 compensation point (CO2 CP) in pennate diatoms
| Species | Inhibitor | Concentration of inhibitor | APC [μmol O 2 mg –1 Chl h –1 (μM DIC) –1 ] | [DIC] at CO 2 CP (μM)a |
|---|---|---|---|---|
| P. tricornutum | DIDSb | 0 mM | 4.80 ± 0.62 | 9.1 ± 4.3 |
| 0.5 mM | 2.83 ± 0.93 | 9.4 ± 5.4 | ||
| 1 mM | 1.47 ± 0.31 | 7.6 ± 4.6 | ||
| 2.5 mM | 1.17 ± 0.12 | 11.9 ± 3.1 | ||
| AZA | 0 μM | 3.13 ± 0.38 | nd | |
| 50 μM | 3.07 ± 0.80 | nd | ||
| 100 μM | 2.70 ± 0.18 | nd | ||
| 150 μM | 2.73 ± 0.54 | nd | ||
| C. fusiformis | DIDS | 0 mM | 1.46 ± 0.54 | 5.3 ± 3.5 |
| 1 mM | 1.47 ± 0.16 | 5.4 ± 0.7 | ||
| 2 mM | 1.58 ± 0.26 | 6.4 ± 2.8 | ||
| AZA | 0 μM | 1.08 ± 0.51 | nd | |
| 50 μM | 0.73 ± 0.2 | nd | ||
| 100 μM | 0.62 ± 0.13 | nd |
nd, not determined. Values represent means ± SD of three or four replicates.
a[DIC] in the presence of AZA could not be determined because AZA was dissolved in ethanol, which interferes with the detection of DIC by GC-FID.
bValues of DIDS experiments in P. tricornutum were calculated from data in Nakajima et al. (2013).
The response of another raphid pennate diatom, C. fusiformis, to DIDS and AZA treatment was opposite to that observed in P. tricornutum. In C. fusiformis, values for K0.5[DIC], APC, and [DIC] at CO2 CP were not affected by treatment with DIDS at concentrations up to 2 mM (Fig. 1C, Table 1, Supplementary Tables S1 and S2). In contrast to the effect of DIDS, treatment with100 μM AZA doubled the K0.5[DIC] value and decreased the APC value to 50% compared with corresponding values in non-treated cells (Fig. 1D, Table 1). These data strongly suggest that eCA-mediated DIC uptake is the major process in the DIDS-insensitive pennate C. fusiformis, as an alternative strategy to direct HCO3– uptake. Neither DIDS nor AZA had a significant effect on the Pmax of either diatom (Fig. 1A–D), indicating that the effect is specific to the CCM, and there was no impact of these agents on the central machinery of the photosystem or the Calvin cycle.
Effects of inhibitors of SLC and extracellular CA on centric diatoms
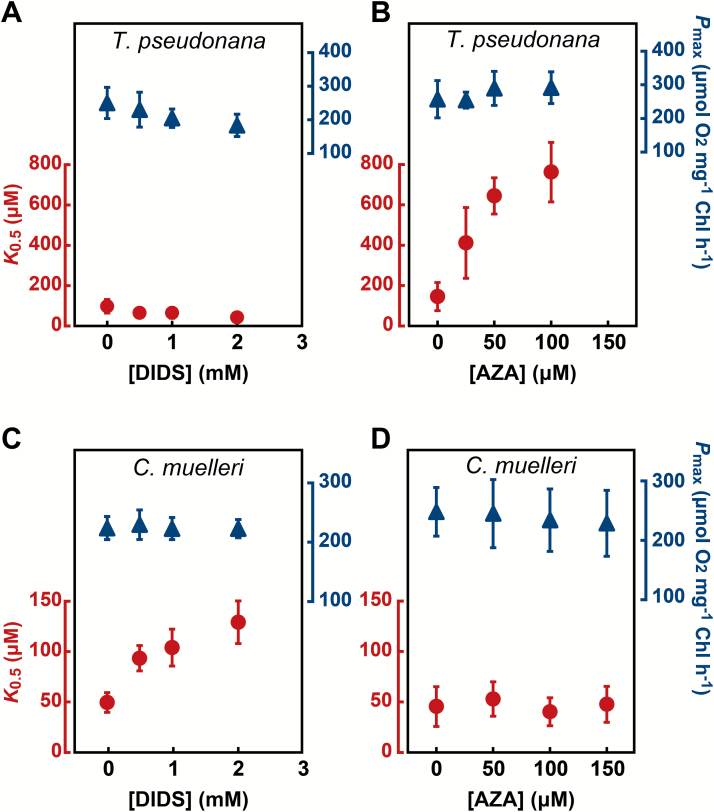
To further explore the evolutionary relationship between DIC uptake strategies, the same treatment and analysis were carried out on two multipolar centric marine diatoms, T. pseudonana and C. muelleri. For T. pseudonana, DIDS treatment at concentrations up to 2 mM did not alter K0.5[DIC] (Fig. 2A, Supplementary Tables S1 and S2); the parameters APC and [DIC] at CO2 CP were similarly not affected by DIDS (Table 2). Similar to the results in C. fusiformis (Fig. 1C), this suggested that HCO3– uptake by SLC4 is not the dominant DIC uptake strategy in T. pseudonana. In sharp contrast, AZA treatment with concentrations up to 100 μM increased K0.5[DIC] more than 5-fold relative to the value in non-treated cells in T. pseudonana (Fig. 2B, Supplementary Tables S1 and S2), again resembling the DIC characteristics in C. fusiformis (Fig. 1D). At concentrations lower than 100 μM AZA, APC also decreased to 25% relative to the non-treated cells in T. pseudonana (Table 2), clearly indicating that the initial DIC uptake strategy in T. pseudonana is highly dependent on eCA and the subsequent permeation of CO2 across the plasma membrane.
Fig. 2.
Effects of DIDS and AZA on centric diatoms. Photosynthetic parameters (K0.5[DIC] and Pmax) in T. pseudonana (A, B) and C. muelleri (C, D) were determined in the presence of various concentrations of DIDS (A, C) or AZA (B, D). Circles, K0.5; triangles, Pmax. Values represent means ± SD of three or four biological replicates.
Table 2.
Effects of inhibitors on apparent photosynthetic conductance (APC) and [DIC] at the CO2 compensation point (CO2 CP) in centric diatoms
| Species | Inhibitor | Concentration of inhibitor | APC [μmol O 2 mg –1 Chl h –1 (μM DIC) –1 ] | [DIC] at CO 2 CP (μM)a |
|---|---|---|---|---|
| T. pseudonana | DIDS | 0 mM | 1.41 ± 0.38 | 3.7 ± 0.8 |
| 0.5 mM | 1.84 ± 0.53 | 4.2 ± 0.9 | ||
| 1 mM | 1.57 ± 0.29 | 4.4 ± 1.8 | ||
| 2 mM | 1.91 ± 0.13 | 3.9 ± 2.0 | ||
| AZA | 0 μM | 1.11 ± 0.27 | nd | |
| 25 μM | 0.40 ± 0.24 | nd | ||
| 50 μM | 0.29 ± 0.04 | nd | ||
| 100 μM | 0.26 ± 0.12 | nd | ||
| C. muelleri | DIDS | 0 mM | 2.36 ± 0.34 | 1.6 ± 0.8 |
| 0.5 mM | 1.53 ± 0.28 | 1.7 ± 0.5 | ||
| 1 mM | 1.14 ± 0.28 | 1.8 ± 0.2 | ||
| 2 mM | 1.02 ± 0.16 | 3.2 ± 0.8 | ||
| AZA | 0 μM | 3.33 ± 1.04 | nd | |
| 50 μM | 3.15 ± 0.84 | nd | ||
| 100 μM | 3.60 ± 0.91 | nd | ||
| 150 μM | 3.31 ± 0.67 | nd |
nd, not determined. Values represent means ± SD of three or four replicates.
a[DIC] in the presence of AZA could not be determined because AZA was dissolved in ethanol, which interferes with the detection of DIC by GC-FID.
In a second centric diatom species, C. muelleri, the responses to the two inhibitors were the opposite of those observed in T. pseudonana, and resembled the results seen in the pennate species P. tricornutum (Fig. 1A, B). K0.5[DIC] increased 2.6-fold after treatment with 2 mM DIDS (Fig. 2C, Supplementary Table S1) but AZA did not affect K0.5[DIC] in C. muelleri at concentrations up to 150 μM (Fig. 2D, Supplementary Table S1). APC decreased 2.5-fold and [DIC] at CO2 CP doubled in the presence of 2 mM DIDS relative to the corresponding values in non-treated cells (Table 2), strongly suggesting that C. muelleri utilizes a direct HCO3– uptake strategy for the initial acquisition of environmental DIC, similar to P. tricornutum. In both centric diatoms, Pmax was fairly stable under all concentrations of DIDS and AZA, indicating no impact of either agent on the central photosynthesis machinery. In addition, the inhibitory effects of DIDS and AZA on K0.5[DIC] were concentration-dependent and near-saturated at the highest concentration used (2.5 mM for DIDS and 100 μM for AZA). These concentrations of DIDS and AZA have been commonly used in previous studies (Huertas et al., 2000; Moroney et al., 1985; Nakajima et al., 2013; Satoh et al., 2001). Furthermore, Nakajima et al. (2013) showed that P. tricornutum engineered for high DIC uptake conferred by constitutive expression of PtSLC4-2 was efficiently inhibited by DIDS at the concentrations used here, indicating that this agent specifically targets SLCs. Given these considerations, the decreased K0.5[DIC] values in response to DIDS and AZA treatments (Fig. 1 and Fig. 2) were considered to be the result of specific inhibition of SLC4 and eCA rather than ‘off-target’ side effects.
Investigation of the energy source for DIC uptake
To study the energetics of DIC uptake, we tested the effects of DCMU and CCCP on DIC uptake. DCMU inhibits LET from the Quinone A socket in photosystem II to plastoquinone (PQ), suppressing NADPH biosynthesis but allowing ATP synthesis via cyclic electron flow (CEF). CCCP is an uncoupler of ATP synthase from the proton motive force, inhibiting ATP synthesis in both the mitochondria and chloroplast while not affecting electron transport chains.
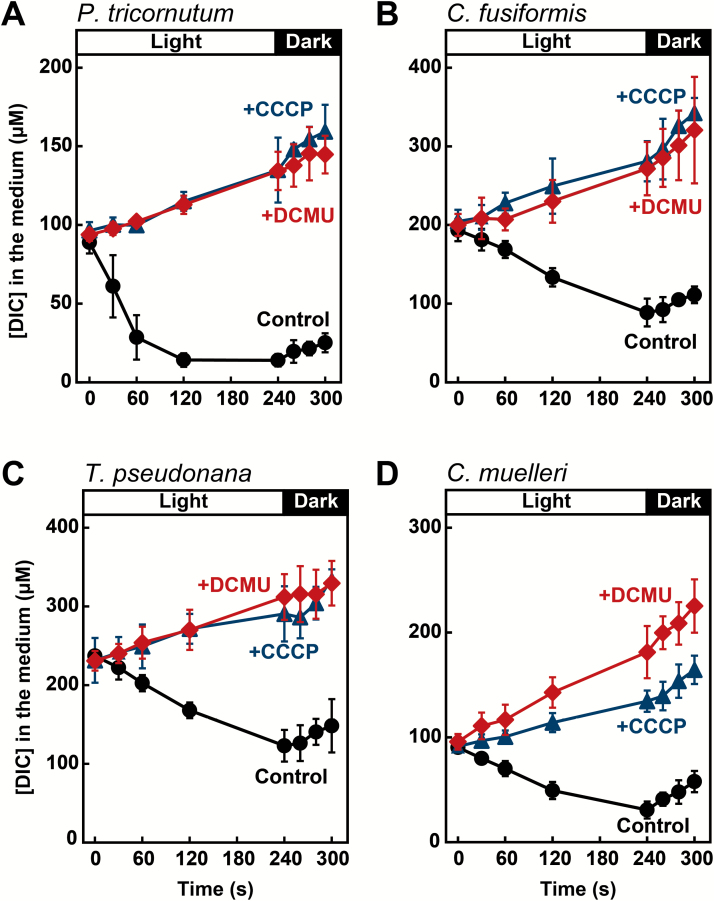
In many phytoplankton, including diatoms, cells actively take up DIC and simultaneously release CO2 during photosynthesis (Hopkinson et al., 2011; Huertas et al., 2000; Nakajima et al., 2013; Tchernov et al., 1997, 2001). Given the occurrence of this massive carbon cycling across the plasma membrane, we measured the rate of net DIC uptake (NDIC) and CO2 efflux (EC) to calculate the gross DIC uptake rate (GDIC) according to the model presented by Nakajima et al. (2013). In this experiment, we measured net uptake of DIC during 240 s of photosynthesis at an initial [DIC] of 100 or 200 μM under light, and then measured CO2 efflux immediately after turning off the light. As shown in Fig. 3, net uptake of DIC was completely stopped by treatment with 2 μM DCMU or 10 μM CCCP. Values for NDIC, EC, and GDIC are summarized in Table 3. In all four diatoms, NDIC, calculated from the initial slope of DIC depletion, was completely inhibited by treatment with DCMU and CCCP (Table 3, Fig. 3). Given the low CEF activity in diatoms (Bailleul et al., 2015), most ATP would be produced by LET during photosynthesis. Inhibition of DIC uptake by CCCP treatment indicated that ATP generated by photophosphorylation might be the major energy source for DIC uptake. On the other hand, EC was insensitive to these inhibitors, suggesting that efflux of CO2 from cells occurs independent of photosynthesis. As a result, GDIC, calculated as the sum of NDIC and EC, was decreased by DCMU and CCCP treatment in all four diatoms.
Fig. 3.
Effects of DCMU and CCCP on DIC uptake by the diatoms P. tricornutum (A), C. fusiformis (B), T. pseudonana (C), and C. muelleri (D). [DIC] in the medium was determined during 240 s of photosynthesis under light and the subsequent 60 s under dark conditions. Cells were pre-incubated with inhibitors for 30 s under light conditions and then photosynthesis was started by the addition of NaHCO3 at 0 s. Circles, control experiments; triangles, +CCCP (10 μM, final concentration); diamonds, +DCMU (2 μM, final concentration). Values represent means ± SD of three biological replicates.
Table 3.
Effect of CCCP and DCMU on DIC uptake and efflux in four marine diatoms
| Species | Parameter | Rate of uptake or efflux (μmol mg Chl–1 min-1) | ||
|---|---|---|---|---|
| Control | +CCCP | +DCMU | ||
| P. tricornutum | N DIC | 6.99 ± 2.38 | –0.54 ± 0.30 | –0.53 ± 0.15 |
| E C | 0.65 ± 0.61 | 0.79 ± 0.91 | 0.75 ± 0.03 | |
| G DIC | 7.64 ± 1.77 | 0.24 ± 1.19 | 0.22 ± 0.17 | |
| C. fusiformis | N DIC | 1.16 ± 0.06 | –0.67 ± 0.14 | –0.60 ± 0.22 |
| E C | 0.77 ± 0.33 | 2.16 ± 0.26 | 1.49 ± 0.71 | |
| G DIC | 1.93 ± 0.39 | 1.49 ± 0.40 | 0.94 ± 0.70 | |
| T. pseudonana | N DIC | 2.18 ± 0.37 | –0.72 ± 0.20 | –1.03 ± 0.28 |
| E C | 2.80 ± 0.88 | 2.17 ± 0.11 | 0.82 ± 0.94 | |
| G DIC | 4.97 ± 1.21 | 1.46 ± 0.22 | –0.21 ± 1.01 | |
| C. muelleri | N DIC | 1.70 ± 0.64 | –0.57 ± 0.10 | –1.07 ± 0.21 |
| E C | 1.48 ± 0.20 | 1.63 ± 0.09 | 2.15 ± 0.21 | |
| G DIC | 3.18 ± 0.47 | 1.06 ± 0.14 | 1.08 ± 0.22 | |
CCCP was added at a final concentration of 10 μM and DCMU at a final concentration of 2μM. NDIC, net DIC uptake rate; EC, CO2 efflux rate, GDIC, gross DIC uptake rate. Values represent means ± SD of three replicates.
Discussion
Phylogeny-independent strategies of DIC uptake in marine diatoms
In the oceanic environment, where HCO3– is the predominant DIC species, utilization of HCO3– can be advantageous, since [CO2] is much lower than the Km [CO2] of Rubisco. Many diatoms can take up HCO3– directly from the surrounding water to overcome the limitations of low CO2 for photosynthesis (Matsuda et al., 2011, , 2017). SLC4 has previously been shown to transport HCO3– across the plasma membrane in the pennate diatom P. tricornutum. Conservation of SLC4 homologs in the distantly related centric species T. pseudonana suggests that HCO3– uptake by SLC4 is a common mechanism across diverse diatoms. However, our study, using a specific inhibitor for SLC4, did not support this idea. Photosynthetic affinity for DIC in two evolutionarily distinct diatoms, C. fusiformis and T. pseudonana, was unaffected by DIDS treatment (Fig. 1C, Fig. 2A, Table S2) but decreased by AZA treatment (Fig. 1D, Fig. 2B, Table S2), suggesting that eCA-mediated indirect uptake is a major route facilitating DIC uptake. Conversely, P. tricornutum and C. muelleri, which are also evolutionarily distinct species, were sensitive to DIDS treatment but unaffected by AZA, suggesting SLC4 to be a major path for DIC uptake in these species. Interestingly, all diatoms displayed sensitivity to only DIDS or AZA, with no examples of dual sensitivity or insensitivity to both agents. These results strongly suggest that marine diatoms take at least two different, independent strategies, supported mainly by SLC4 or eCA. Additionally, there was no evidence of cooperativity in one cell. Interestingly, the occurrence of these two strategies in diatom CCM is not related to their phylogenetic position. For instance, C. fusiformis and T. pseudonana were shown to be eCA-dependent, but these strains are phylogenetically very distant pennate and centric diatoms, respectively; the same holds true for the SLC4-dependent diatoms, P. tricornutum and C. muelleri. These results support the idea that strategies for DIC uptake have been established independently as diatoms adapt to their living environment, rather than by a process of phylogenetic diversification. While there is no molecular evidence of the occurrence of eCA in P. tricornutum, it has been reported by physiological measurement that eCA activity occurs in some strains of P. tricornutum, suggesting that environmental adaptation of the CCM strategy could occur even within the same species. At present, it is difficult to draw conclusions on the exact environmental factors that directed the evolution of the SLC4- or eCA-dependent strategy. One notable feature of SLC4 is a high requirement for Na+ in HCO3– transport, possibly due to the Na+-HCO3– co-transport mechanism (Nakajima et al. 2013). On the other hand, since eCA-based DIC uptake is likely to be independent of Na+, eCA-dependent species may possibly be adapted to a wide range of Na+ concentrations, or fluctuating Na+ conditions, such as those of estuary areas. Therefore, Na+ concentration might be an environmental factor that influenced the evolution of CCM.
While this study presents the occurrence of at least two different strategies in marine diatoms, our results do not exclude the possibility that diatoms employ additional DIDS- or AZA-insensitive DIC acquisition strategies. Redundancy of DIC transporters has been documented in cyanobacteria (Kaplan and Reinhold, 1999; Ogawa and Kaplan, 2003) and green algae (Duanmu et al., 2009; Ohnishi et al., 2010; Wang et al., 2015; Yamano et al., 2015), and therefore it is possible that diatoms also have other DIC uptake mechanisms.
Possible role of SLC4 as a chloroplast DIC transporter in DIDS-insensitive species
Our primary interest is the generality of SLC4-dependent HCO3– uptake among marine diatoms. The results of DIDS treatment showed that direct uptake by SLC4 is not universal among marine diatoms, although SLC4 homologs are widely conserved across pennate and centric diatoms. One possible function of putative SLC4 in diatoms dependent on eCA (i.e. those insensitive to DIDS) is that SLC4 is located on chloroplast membranes to facilitate DIC transport into the stroma. DIDS is a membrane-impermeable inhibitor that reacts with a specific Lys residue of SLC exposed to the outside of cells (Romero et al., 2013; Spiro and Spiro, 1985). Therefore, DIDS cannot inhibit SLC4 on intracellular membranes such as the chloroplast envelope. Diatoms have a red-algal-derived chloroplast, which is surrounded by two chloroplast envelopes and an additional outer two membranes termed the chloroplastic endoplasmic reticulum. SLC4s that play a role at the chloroplast envelope and/or chloroplastic endoplasmic reticulum should be insensitive to DIDS treatment of living cells. T. pseudonana has putative SLC4s containing chloroplast-targeting signal (Supplementary Table S3), but the localization of these putative SLCs in eCA-dependent diatoms has yet to be determined. Unlike diatoms, the green alga C. reinhardtii possesses a two-layered chloroplast, and functional cooperation between plasma-membrane-localized HLA3 and chloroplast-envelope-localized LCIA in uptake of HCO3– has been reported (Duanmu et al., 2009; Wang et al., 2015; Yamano et al., 2015). Such cooperation between the plasma membrane and plastid system has yet to be elucidated in any secondary symbiotic alga, but the significant complexity of the four-layered chloroplastic envelope in diatoms probably includes many regulatory points in the transport of DIC from the surrounding media to the pyrenoid compartment in the chloroplast.
Photosynthetic ATP production drives DIC uptake in marine diatoms
To reveal the energetics of DIC uptake, we tested the effects of DCMU and CCCP. DCMU inhibits photosynthetic LET in photosystem II but does not inhibit CEF around photosystem I. CCCP does not inhibit LET but dissipates both membrane potential and proton gradients, resulting in the disruption of ATP synthesis in both the chloroplast and mitochondrion (Heytler and Prichard, 1962; Terada 1981). In our experiments, both DCMU and CCCP completely inhibited NDIC in all diatoms examined (Fig. 3). It has recently been reported that, unlike higher plants and green algae, diatoms may modulate the ATP/NADPH ratio mainly by bypassing the photochemical electron flow through a mitochondrial respiratory pathway instead of CEF (Bailleul et al., 2015). Considering the very low CEF activity in diatoms reported by Bailleul et al. (2015), ATP synthesis in the chloroplast is mostly dependent on LET. Therefore, it is probable that DCMU completely inhibited photophosphorylation, with little contribution of a negligible amount of ATP produced by CEF. As indicated by the inhibition of NDIC by CCCP or under dark conditions, ATP generated by photophosphorylation is a major energy source driving active HCO3– uptake in the DIDS-sensitive group. This feature is different from green algae and cyanobacteria, in which involvement of CEF is suggested to drive DIC uptake (Sültemeyer et al., 1991; Tchernov et al., 2001). Negligible activity of CEF has been demonstrated in both pennate and centric diatoms and is thus considered to be a general feature (Bailleul et al., 2015). Nonetheless, a recent study showed high levels of CEF in a polar diatom, Fragilariopsis cylindrus (Goldman et al., 2015), suggesting the possibility of involvement of CEF in the CCM of some psychrophilic diatoms and lending further support toward the hypothesis of environment-specific adaptations for DIC uptake.
While further studies are needed to reveal a coupling of ATP hydrolysis and DIC uptake, ATP is presumably utilized to maintain a Na+ gradient across the plasma membrane in SLC4-dependent diatoms, since SLC4 is a secondary transport system that is dependent on Na+ for HCO3– uptake, most likely co-transporting HCO3– and Na+. In eCA-dependent diatoms, eCA-mediated CO2 entry is most probably passive (Hopkinson et al., 2013); thus, it is unlikely that ATP hydrolysis operates in this process. However, active DIC transport across chloroplast membranes is proposed to drive the CCM of diatoms (Hopkinson, 2014), and if this is the case, ATP should be consumed to drive an active uptake of HCO3–into the chloroplast; possibly in an SLC4-dependent manner.
Conclusion
While conservation of SLC4 in representative pennate and centric diatoms suggested generalizability of SLC4-mediated DIC uptake in the diatom CCM, here we demonstrated SLC4-dependent and eCA-dependent strategies of the diatom CCM. These two strategies are unrelated to phylogenetic position, suggesting that each marine diatom independently developed different DIC uptake mechanisms to adapt to its living environment. While molecular studies of diatom CCM are most advanced in P. tricornutum, the present study indicates the necessity of detailed studies on eCA-dependent marine diatoms such as T. pseudonana to fully understand the evolutionary adaptation of marine diatoms to different environments.
Supplementary data
Supplementary data are available at JXB online.
Table S1. Effects of DIDS and AZA on photosynthetic parameters of four diatoms.
Table S2. Qualitative summary of effects of inhibitors on photosynthetic parameters.
Table S3. Predicted localization of putative SLC4s in T. pseudonana.
Supplementary Material
Acknowledgements
We thank Jared Broddrick of UCSD for critical reading of this manuscript, Nobuko Higashiuchi for her technical assistance, and Miyabi Inoue for her skillful secretarial help. This work was supported by JSPS KAKENHI grant numbers 24310015 (to YM), 16H06557 (to YM), and 15K16156 (to YT), by the MEXT-Supported program for the Strategic Research Foundation for the Advancement of Environmental Protection Technology and for Development of Intelligent Self-Organized Biomaterials (to YM), and by the Science Research Promotion Fund of the Promotion and Mutual Aid Corporation for Private Schools of Japan (to YM). We also thank Beasiswa Unggulan, Ministry of Education, Indonesia, for scholarship support (to AM).
Glossary
Abbreviations:
- APC
apparent photosynthetic conductance
- AZA
acetazolamide
- CA
carbonic anhydrase
- CCM
CO2-concentrating mechanism
- CCCP
carbonyl cyanide-m-chlorophenyl hydrazone
- CEF
cyclic electron flow
- [CO2]
CO2 concentration
- CP
compensation point
- DCMU
3-(3,4-dichlorophenyl)-1,1-dimethylurea
- DIC
dissolved inorganic carbon
- [DIC]
DIC concentration
- DIDS
diisothiocyano-2,2ʹ-stilbenedisulfonic acid
- EC
rate of CO2 efflux
- eCA
external CA
- F/2ASW
F/2 artificial seawater
- GC-FID
gas chromatography with flame ionization detector
- GDIC
rate of gross DIC uptake
- K0.5[DIC]
half-saturation constant for DIC
- Km
half-saturation constant
- LET
linear electron transport
- NDIC
rate of net DIC uptake
- Pmax
maximum photosynthetic O2 evolution rate
- SLC
solute carrier.
References
- Admiraal W, Peletier H, Laane RWPM. 1986. Nitrogen metabolism of marine planktonic diatoms; excretion, assimilation and cellular pools of free amino acids in seven species with different cell size. Journal of Experimental Marine Biology and Ecology 98, 241–263. [Google Scholar]
- Aizawa K, Miyachi S. 1986. Carbonic anhydrase and CO2 concentrating mechanisms in microalgae and cyanobacteria. FEMS Microbiology Letters 39, 215–233. [Google Scholar]
- Badger MR, Andrews TJ, Whitney SM, Ludwig M, Yellowlees DC, Leggat W, Price GD. 1998. The diversity and coevolution of Rubisco, plastids, pyrenoids, and chloroplast-based CO2-concentrating mechanisms in algae. Canadian Journal of Botany 76, 1052–1071. [Google Scholar]
- Bailleul B, Berne N, Murik O, et al. 2015. Energetic coupling between plastids and mitochondria drives CO2 assimilation in diatoms. Nature 524, 366–369. [DOI] [PubMed] [Google Scholar]
- Birmingham B, Colman B. 1979. Measurement of carbon dioxide compensation points of freshwater algae. Plant Physiology 64, 892–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt S, Amoroso G, Riebesell U. 2001. CO2 and HCO3– uptake in marine diatoms acclimated to different CO2 concentrations. Limnology and Oceanography 46, 1378–1391. [Google Scholar]
- Duanmu D, Miller AR, Horken KM, Weeks DP, Spalding MH. 2009. Knockdown of limiting-CO2-induced gene HLA3 decreases HCO3– transport and photosynthetic Ci affinity in Chlamydomonas reinhardtii. Proceedings of the National Academy of Sciences of the United States of America 106, 5990–5995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkowski PG, Barber RT, Smetacek V. 1998. Biogeochemical controls and feedbacks on ocean primary production. Science 281, 200–206. [DOI] [PubMed] [Google Scholar]
- Giordano M, Beardall J, Raven JA. 2005. CO2 concentrating mechanisms in algae: mechanisms, environmental modulation, and evolution. Annual Review of Plant Biology 56, 99–131. [DOI] [PubMed] [Google Scholar]
- Goldman JA, Kranz SA, Young JN, Tortell PD, Stanley RH, Bender ML, Morel FM. 2015. Gross and net production during the spring bloom along the Western Antarctic Peninsula. New Phytologist 205, 182–191. [DOI] [PubMed] [Google Scholar]
- Guillard RR, Ryther JH. 1962. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt, and Detonula confervacea (cleve) Gran. Canadian Journal of Microbiology 8, 229–239. [DOI] [PubMed] [Google Scholar]
- Harada H, Nakatsuma D, Ishida M, Matsuda Y. 2005. Regulation of the expression of intracellular beta-carbonic anhydrase in response to CO2 and light in the marine diatom Phaeodactylum tricornutum. Plant Physiology 139, 1041–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada H, Nakajima K, Sakaue K, Matsuda Y. 2006. CO2 sensing at ocean surface mediated by cAMP in a marine diatom. Plant Physiology 142, 1318–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PJ, Waters RE, Taylor FJR. 1980. A broad spectrum artificial seawater medium for coastal and open ocean phytoplankton. Journal of Phycology 16, 28–35. [Google Scholar]
- Hennon GMM, Ashworth J, Groussman RD, Berthiaume C, Morales RL, Baliga NS, Orellana MV, Armbrust EV. 2015. Diatom acclimation to elevated CO2 via cAMP signalling and coordinated gene expression. Nature Climate Change 5, 761–765. [Google Scholar]
- Heytler PG, Prichard WW. 1962. A new class of uncoupling agents–carbonyl cyanide phenylhydrazones. Biochemical and Biophysical Research Communications 7, 272–275. [DOI] [PubMed] [Google Scholar]
- Hopkinson BM. 2014. A chloroplast pump model for the CO2 concentrating mechanism in the diatom Phaeodactylum tricornutum. Photosynthesis Research 121, 223–233. [DOI] [PubMed] [Google Scholar]
- Hopkinson BM, Dupont CL, Allen AE, Morel FMM. 2011. Efficiency of the CO2-concentrating mechanism of diatoms. Proceedings of the National Academy of Sciences of the United States of America 108, 3830–3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkinson BM, Meile C, Shen C. 2013. Quantification of extracellular carbonic anhydrase activity in two marine diatoms and investigation of its role. Plant Physiology 162, 1142–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huertas IE, Espie GS, Colman B, Lubian LM. 2000. Light-dependent bicarbonate uptake and CO2 efflux in the marine microalga Nannochloropsis gaditana. Planta 211, 43–49. [DOI] [PubMed] [Google Scholar]
- Jeffrey SW, Humphrey GF. 1975. New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochemie und Physiologie der Pflanzen 167, 191–194. [Google Scholar]
- Johnston AM, Marberly SC, Raven JA. 1992. The acquisition of inorganic carbon by four red macroalgae. Ocealogica 92, 317–326. [DOI] [PubMed] [Google Scholar]
- Kaplan A, Reinhold L. 1999. CO2 concentrating mechanisms in photosynthetic microorganisms. Annual Review of Plant Physiology and Plant Molecular Biology 50, 539–570. [DOI] [PubMed] [Google Scholar]
- Kikutani S, Nakajima K, Nagasato C, Tsuji Y, Miyatake A, Matsuda Y. 2016. Thylakoid luminal θ-carbonic anhydrase critical for growth and photosynthesis in the marine diatom Phaeodactylum tricornutum. Proceedings of the National Academy of Sciences of the United States of America 113, 9828–9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikutani S, Tanaka R, Yamazaki Y, Hara S, Hisabori T, Kroth PG, Matsuda Y. 2012. Redox regulation of carbonic anhydrases via thioredoxin in chloroplast of the marine diatom Phaeodactylum tricornutum. Journal of Biological Chemistry 287, 20689–20700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooistra WHCF, Gersonde R, Medlin LK, Mann DG. 2007. The origin and evolution of the diatoms: their adaptation to a planktonic existence. In: Falkowski PG, Knoll AH, eds. Evolution of primary producers in the sea. Heidelberg: Elsevier, 207–249. [Google Scholar]
- Mann DG, Droop SJM. 1996. Biodiversity, biogeography and conservation of diatoms. Hydrobiologia 336, 19–32. [Google Scholar]
- Martin CL, Tortell PD. 2008. Bicarbonate transport and extracellular carbonic anhydrase in marine diatoms. Physiologia Plantarum 133, 106–116. [DOI] [PubMed] [Google Scholar]
- Matsuda Y, Hara T, Colman B. 2001. Regulation of the induction of bicarbonate uptake by dissolved CO2 in the marine diatom, Phaeodactylum tricornutum. Plant, Cell & Environment 24, 611–620. [Google Scholar]
- Matsuda Y, Nakajima K, Tachibana M. 2011. Recent progresses on the genetic basis of the regulation of CO2 acquisition systems in response to CO2 concentration. Photosynthesis Research 109, 191–203. [DOI] [PubMed] [Google Scholar]
- Matsuda Y, Hopkinson BM, Nakajima K, Dupont CL, Tsuji Y. 2017. Mechanisms of carbon dioxide acquisition and CO2 sensing in marine diatoms—A gateway to carbon metabolism. Philosophical Transactions of the Royal Society B: Biological Sciences in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroney JV, Husic HD, Tolbert NE. 1985. Effect of carbonic anhydrase inhibitors on inorganic carbon accumulation by Chlamydomonas reinhardtii. Plant Physiology 79, 177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K, Tanaka A, Matsuda Y. 2013. SLC4 family transporters in a marine diatom directly pump bicarbonate from seawater. Proceedings of the National Academy of Sciences of the United States of America 110, 1767–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimer NA, Iglesias-Rodriguez MD, Merrett MJ. 1997. Bicarbonate utilization by marine phytoplankton species. Journal of Phycology 33, 625–631. [Google Scholar]
- Ohnishi N, Mukherjee B, Tsujikawa T, Yanase M, Nakano H, Moroney JV, Fukuzawa H. 2010. Expression of a low CO2-inducible protein, LCI1, increases inorganic carbon uptake in the green alga Chlamydomonas reinhardtii. The Plant Cell 22, 3105–3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno N, Inoue T, Yamashiki R, Nakajima K, Kitahara Y, Ishibashi M, Matsuda Y. 2012. CO2-cAMP-responsive cis-elements targeted by a transcription factor with CREB/ATF-like basic zipper domain in the marine diatom Phaeodactylum tricornutum. Plant Physiology 158, 499–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T, Kaplan A. 2003. Inorganic carbon acquisition systems in cyanobacteria. Photosynthesis Research 77, 105–115. [DOI] [PubMed] [Google Scholar]
- Price GD, Woodger FJ, Badger MR, Howitt SM, Tucker L. 2004. Identification of a SulP-type bicarbonate transporter in marine cyanobacteria. Proceedings of the National Academy of Sciences of the United States of America 101, 18228–18233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven JA. 1994. Carbon fixation and carbon availability in marine phytoplankton. Photosynthesis Research 39, 259–273. [DOI] [PubMed] [Google Scholar]
- Romero MF, Chen AP, Parker MD, Boron WF. 2013. The SLC4 family of bicarbonate (HCO3⁻) transporters. Molecular Aspects of Medicine 34, 159–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rost B, Burkhardt S, Sültemeyer D, Riebesell U, Burkhardt S, Sültemeyer D. 2003. Carbon acquisition of bloom-forming marine phytoplankton. Limnology and Oceanography 48, 55–67. [Google Scholar]
- Samukawa M, Shen C, Hopkinson BM, Matsuda Y. 2014. Localization of putative carbonic anhydrases in the marine diatom, Thalassiosira pseudonana. Photosynthesis Research 121, 235–249. [DOI] [PubMed] [Google Scholar]
- Satoh D, Hiraoka Y, Colman B, Matsuda Y. 2001. Physiological and molecular biological characterization of intracellular carbonic anhydrase from the marine diatom Phaeodactylum tricornutum. Plant Physiology 126, 1459–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata M, Katoh H, Sonoda M, Ohkawa H, Shimoyama M, Fukuzawa H, Kaplan A, Ogawa T. 2002. Genes essential to sodium-dependent bicarbonate transport in cyanobacteria: function and phylogenetic analysis. Journal of Biological Chemistry 277, 18658–18664. [DOI] [PubMed] [Google Scholar]
- Smetacek V. 1999. Diatoms and the ocean carbon cycle. Protist 150, 25–32. [DOI] [PubMed] [Google Scholar]
- Spiro MJ, Spiro RG. 1985. Effect of anion-specific inhibitors on the utilization of sugar nucleotides for N-linked carbohydrate unit assembly by thyroid endoplasmic reticulum vesicles. Journal of Biological Chemistry 260, 5808–5815. [PubMed] [Google Scholar]
- Sültemeyer DF, Fock HP, Canvin DT. 1991. Active uptake of inorganic carbon by Chlamydomonas reinhardtii: evidence for simultaneous transport of HCO3– and CO2 and characterization of active CO2 transport. Canadian Journal of Botany 69, 995–1002. [Google Scholar]
- Tachibana M, Allen AE, Kikutani S, Endo Y, Bowler C, Matsuda Y. 2011. Localization of putative carbonic anhydrases in two marine diatoms, Phaeodactylum tricornutum and Thalassiosira pseudonana. Photosynthesis Research 109, 205–221. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Nakatsuma D, Harada H, Ishida M, Matsuda Y. 2005. Localization of soluble beta-carbonic anhydrase in the marine diatom Phaeodactylum tricornutum. Sorting to the chloroplast and cluster formation on the girdle lamellae. Plant Physiology 138, 207–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A, Ohno N, Nakajima K, Matsuda Y. 2016. Light and CO2/cAMP signal cross talk on the promoter elements of chloroplastic β-carbonic anhydrase genes in the marine diatom Phaeodactylum tricornutum. Plant Physiology 170, 1105–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchernov D, Hassidim M, Luz B, Sukenik A, Reinhold L, Kaplan A. 1997. Sustained net CO2 evolution during photosynthesis by marine microorganisms. Current Biology 7, 723–728. [DOI] [PubMed] [Google Scholar]
- Tchernov D, Helman Y, Keren N, Luz B, Ohad I, Reinhold L, Ogawa T, Kaplan A. 2001. Passive entry of CO2 and its energy-dependent intracellular conversion to HCO3– in cyanobacteria are driven by a photosystem I-generated ΔμH+. Journal of Biological Chemistry 276, 23450–23455. [DOI] [PubMed] [Google Scholar]
- Terada H. 1981. The interaction of highly active uncouplers with mitochondria. Biochimica et Biophysica Acta 639, 225–242. [DOI] [PubMed] [Google Scholar]
- Wang Y, Stessman DJ, Spalding MH. 2015. The CO2 concentrating mechanism and photosynthetic carbon assimilation in limiting CO2: how Chlamydomonas works against the gradient. The Plant Journal 82, 429–448. [DOI] [PubMed] [Google Scholar]
- Yamano T, Sato E, Iguchi H, Fukuda Y, Fukuzawa H. 2015. Characterization of cooperative bicarbonate uptake into chloroplast stroma in the green alga Chlamydomonas reinhardtii. Proceedings of the National Academy of Sciences of the United States of America 112, 7315–7320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JN, Heureux AM, Sharwood RE, Rickaby RE, Morel FM, Whitney SM. 2016. Large variation in the Rubisco kinetics of diatoms reveals diversity among their carbon-concentrating mechanisms. Journal of Experimental Botany 67, 3445–3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.