HIV-infected participants initiating first-line antiretroviral therapy in Kenya were tested for pretreatment drug resistance (PDR) using an oligonucleotide ligation assay. PDR was detected in 9.6% (95% CI, 7.9%–11.6%), including 19.5% (95% CI, 8.8%–34.9%) of all 18–24-year-old women.
Keywords: Pretreatment drug resistance, transmitted drug resistance, HIV, oligonucleotide ligation assay, antiretroviral therapy
Abstract
Background
Pre-antiretroviral-treatment drug resistance (PDR) is a predictor of human immunodeficiency virus (HIV) treatment failure. We determined PDR prevalence and correlates in a Kenyan cohort.
Methods
We conducted a cross-sectional analysis of antiretroviral (ARV) treatment-eligible HIV-infected participants. PDR was defined as ≥2% mutant frequency in a participant’s HIV quasispecies at pol codons K103N, Y181C, G190A, M184 V, or K65R by oligonucleotide ligation assay and Illumina sequencing. PDR prevalence was calculated by demographics and codon, stratifying by prior ARV experience. Poisson regression was used to estimate prevalence ratios.
Results
PDR prevalences (95% confidence interval [CI]) in 815 ARV-naive adults, 136 ARV-experienced adults, and 36 predominantly ARV-naive children were 9.4% (7.5%–11.7%), 12.5% (7.5%–19.3%), and 2.8% (0.1%–14.5%), respectively. Median mutant frequency within an individual’s HIV quasispecies was 67%. PDR prevalence in ARV-naive women 18–24 years old was 21.9% (9.3%–40.0%). Only age in females associated with PDR: A 5-year age decrease was associated with adjusted PDR prevalence ratio 1.20 (95% CI, 1.06–1.36; P = .004).
Conclusions
The high PDR prevalence may warrant resistance testing and/or alternative ARVs in high HIV prevalence settings, with attention to young women, likely to have recent infection and higher rates of resistance.
Clinical Trials Registration
Human immunodeficiency virus type 1 (HIV-1) infection continues to be a serious health threat globally, particularly in low-resource countries. Whereas antiretroviral (ARV) therapy (ART) has significantly improved the health and quality of life of HIV-infected individuals [1, 2], the selection and transmission of drug-resistant HIV have increased with increasing time since ART “roll-out” [3]. Individuals with drug-resistant HIV (DR-HIV) are at increased risk for treatment failure, leading to poor health outcomes and higher risk of transmitting HIV [3–5]. While testing for DR-HIV is performed in resource-rich countries to guide clinicians in the selection of ART, most lower-resource countries do not routinely test for DR-HIV [6–8]. In lower-resource settings, first-line combination therapies containing nonnucleoside transcription inhibitors (NNRTIs) are provided gratis, and those who experience first-line treatment failure are prescribed second-line combination therapies with protease inhibitors. Should treatment failure to second-line combinations occur, third-line combinations are generally available only to individuals with the means to purchase the drugs [9–14]. Thus, understanding the burden and epidemiology of DR-HIV in these populations is important for evaluating the effectiveness of currently dispensed ART and for determining when and how to modify programmatic ART strategies.
The World Health Organization (WHO) defines pre-antiretroviral-treatment drug resistance (PDR) as DR-HIV in adult populations starting ART, and transmitted drug resistance (TDR) as DR-HIV in recently infected populations [15]. TDR is often defined more broadly as DR-HIV infection among those who were previously uninfected with HIV [3]. Generally, PDR includes, but is not limited to, TDR. These terms are distinct from acquired drug resistance (ADR), which refers to likely selection of DR-HIV in ART-experienced individuals, usually after virologic failure [3, 15].
On average, younger HIV-infected adults have been infected for less time than older individuals [16]. Therefore, in communities with increasing prevalence of DR-HIV, it logically follows that those infected with HIV more recently may be more likely to have TDR. Young women, who account for 25% of all new HIV infections in sub-Saharan Africa [17], may also be at a particularly high risk of TDR in the region, as they are more likely to have been infected with HIV via older partners [18, 19] with potentially higher likelihoods of ART use due to longer duration of infection. An additional risk factor for DR-HIV is prior ARV experience, which may occur in women and children with a history of using mono- or dual-therapy ARVs previously recommended for prevention of mother-to-child transmission (PMTCT) [20–24]. Geographic locations may differ in the risk of PDR. For example, persons in urban centers may have a higher DR-HIV prevalence compared with rural communities due to earlier ART scale-up [12].
To address an objective of the WHO 2016–2021 Global Action Plan For HIV Drug Resistance, and generate evidence to inform policy [3], we report our analysis of risk factors associated with PDR in a large cohort of HIV-infected individuals initiating ART at 3 sites, 1 rural and 2 urban clinics, in Kenya between May 2013 and November 2014, including among women of childbearing age, who may be at especially high risk of PDR. Understanding the burden of PDR is crucial to ensuring that pregnant and breastfeeding women receive effective HIV treatment for PMTCT.
METHODS
Study Setting and Design
We conducted a cross-sectional analysis of PDR using pre-ART baseline data collected from individuals initiating ART at the Coptic Hope Center for Infectious Diseases, a network of HIV treatment clinics that administer care based on Kenya national guidelines [25]. In 2013–2014, Hope Center patients who were 2 years of age and older and eligible to initiate ART were invited to enroll into a randomized clinical trial (RCT) examining the use of a sensitive point mutation assay to detect and manage PDR (RCT name: Oligonucleotide Ligation Assay (OLA) Resistance Study; ClinicalTrials.gov identifier: NCT01898754). This RCT was implemented at 3 Coptic Hope Center clinic sites, 2 located in urban Nairobi and the third in rural Maseno in Western Kenya. Participants with a history of prior use of ARV administered from another clinic, for PMTCT, or postexposure prophylaxis, were not excluded. At enrollment, prior to clinicians prescribing ART, blood samples were collected along with sociodemographic information, medical history, and risk behaviors. For this analysis, information regarding prior ARV use was obtained from the study enrollment form and the Hope Center medical records. All participants provided written informed consent prior to study enrollment as approved by Human Subjects’ Committees at Seattle Children’s Hospital in Seattle, Washington, and Kenyatta National Hospital in Nairobi, Kenya.
Pre-ART peripheral blood mononuclear cells (PBMC) underwent nested polymerase chain reaction (PCR) of HIV pol to generate amplicons for oligonucleotide ligation assays (OLA) to detect point mutations K103N, Y181C, G190A, M184V, and K65R. Our and others previous studies have shown that virologic failure during ART with NNRTIs nevirapine or efavirenz is associated with mutations K103N, Y181C, and G190A, to the NRTIs lamivudine and emtricitabine by M184V, and to the NRTI tenofovir disoproxil fumarate by K65R [4, 26–28]. PBMC were used because we have found that OLA detection of DR-HIV is similar to plasma among untreated individuals, and testing of PBMC is less costly compared to testing plasma. For OLA, the proportion of mutant in each participant’s HIV quasispecies was estimated by comparing optical densities to standards containing 0%, 2%, 5%, 10%, 25%, 50%, 75%, or 100% mutant, with PDR defined by ≥2% mutant [4, 28, 29]. In addition, Illumina sequencing was performed to confirm PDR for low-level mutations (<25%) and for selected specimens with high frequency mutations detected via OLA for validation purposes. For Illumina sequencing, 2 approximately 330-bp regions of HIV pol encoding reverse transcriptase were amplified from approximately 1000 HIV DNA copies, quantified by an in-house qPCR assay targeting the long terminal repeat region. First-round PCR amplicons were generated as previously described [4], except for using a high-fidelity PCR enzyme (FastStart, Roche Diagnostics, Mannheim, Germany). Second-round PCR primers targeting the regions of interest were synthesized with forward and reverse adapter sequences allowing subsequent tagging of PCR product from distinct study participants with a unique combination of index sequences. Indexed amplicons were then pooled together and approximately 300 bp were bidirectionally sequenced on an Illumina Miseq. Trimming at the 5′ and 3′ ends of raw sequence reads used a sliding window of 9 base pairs and an average Phred quality score of less than 30. Trimmed reads <75 bp in length or containing fully degenerate bases were filtered from the final data set. High-quality filtered reads were aligned to the HXB2 reference HIV sequence using the Burrows–Wheeler algorithm [30]. Nucleotide variants (single-nucleotide polymorphisms and insertion-deletions) were called at relevant codons associated with resistance and filtered for statistical significance using the open-source LoFreq* [31] and FreeBayes [32] software. Remaining variants were annotated using SNPEff [33], indicating their frequency within the overall viral sequence population and their effects on encoded amino acids. PCR and sequencing error rates at each nucleotide generated from a plasmid DNA control were assessed by a perl script developed in house to allow more accurate estimation of genuine PDR populations. The average mismatch error rate for this PCR and sequencing method ranged from 0.6% to 0.65%, so a conservative cut-off of 1% mutant detected by Illumina was used as a minimum frequency to confirm the presence of mutant via OLA. Mutations detected by OLA but not confirmed via Illumina were defined as wild type.
Statistical Analyses
PDR prevalence and 95% binomial exact confidence intervals (CIs) by age, gender, clinic location, prior ARV experience, and specific pol codon mutations were calculated. Age was defined as a continuous variable (years) and, to identify associations by age groups, categorized into 5 age groups (children <18, and adults 18–24, 25–35, 35–50, and over 50 years of age). Clinic locations were defined as urban (Ngong Road and Industrial Area) or rural (Maseno). “ARV experienced” was defined as any ARV use prior to study as reported by the participant at enrollment and/or by review of the Coptic Hope Center clinic and pharmacy records, and analyzed as a binary variable. Socioeconomic, health care access, and sexual risk behavior variables were evaluated to investigate other potential correlations with PDR in exploratory analyses. Poisson regression with robust standard errors was used to conduct univariable and multivariable regression analyses to estimate the prevalence ratios of PDR (≥2% mutant virus at any pol codon) by potential correlates (alpha = 0.05). The unadjusted prevalence ratio was estimated for all variables. For regression analyses, age was defined as a continuous variable, and location was defined both as a categorical variable (3 clinic sites) and as a binary variable (rural vs 2 urban clinics combined) in separate analyses. Multivariable regression analyses were conducted to estimate the prevalence ratio of PDR by gender, age, and location adjusted for potential confounding variables, and to explore other potential correlates of PDR. An interaction term between age and gender was included in separate regression analyses to investigate differences in the effect of age by gender. Gender-stratified analyses were conducted to further investigate our hypothesis that younger women have a higher prevalence of PDR. In addition to investigating the full cohort, all analyses were also stratified by prior ARV experience to potentially differentiate between ADR and TDR. Children (<18 years old) were excluded from regression analyses due to a paucity of socioeconomic and demographic data. Participants ≥16 years of age were included in sensitivity analyses to investigate the impact of older adolescents on our results.
RESULTS
Enrollment and Eligibility
Between 25 May 2013 and 5 November 2014, 1198 HIV-infected Hope Center patients over 2 years of age, who qualified to initiate first-line ART by Kenya national guidelines, were screened for eligibility into the parent RCT. Of these, 991 (82.7%) enrolled. OLA testing was successful on the entry specimen from 987 (99.6%) participants. Of the 987 individuals included in analyses, prior ARV use was identified in 138 (14.0%) participants either by self-report or through Hope Center clinic and/or pharmacy records, including 2 children. Overall, 815 of 951 (85.7%) adults and 34 of 36 (94.4%) children were defined as ARV naive at enrollment.
Participant Characteristics
Of participants with a successful OLA test, the median age was 37 years (interquartile range [IQR], 31–45), 642 (65.1%) were female, and 849 (86.0%) were ARV naive. Of the ARV-naive and ARV-experienced participants, 519/849 (61.1%) and 123/138 (89.1%) were women, respectively. ARV-experienced were slightly younger than ARV-naive participants (median 34 vs 38 years). Most participants (76.7%) were enrolled at the urban study sites in Nairobi (67.5% from Ngong Road, 9.2% from Industrial Area). Table 1 presents descriptive statistics by ARV experience and PDR for adults. Of the 36 children, 20 (55.6%) were female and the median age was 10 years (IQR, 7–15 years). Unlike adults, children were enrolled in similar proportions from study sites in urban Nairobi (38.9% Ngong Road, 11.1% Industrial Area) and rural Maseno (50.0%).
Table 1.
Characteristics of Participants ≥18 Years of Age Initiating First-Line ARTa by History of ARV Use and PDR Detected by OLAb
| Variables c | Total (n = 951) | ARV naive (n = 815) | ARV exposed (n = 136) | |||
|---|---|---|---|---|---|---|
|
No PDR
(n = 857, 90%) |
PDR
(n = 94, 10%) |
No PDR
(n = 738, 91%) |
PDR
(n = 77, 9%) |
No PDR
(n = 119, 88%) |
PDR
(n = 17, 12%) |
|
| ARV-exposure status | ||||||
| ARV naive | 738 (90.6%) | 77 (9.4%) | – | – | – | – |
| ARV exposed | 119 (87.5%) | 17(12.5%) | ||||
| Demographic | ||||||
| Age in years | 38 (32, 46) | 34 (29, 42) | 39 (32, 47) | 35 (30, 44) | 34 (30, 40) | 32 (27, 36) |
| 18–24 | 40 (83.3%) | 8 (16.7%) | 32 (82.1%) | 7 (17.9%) | 8 (88.9%) | 1 (11.1%) |
| 25–34 | 269 (87.1%) | 40 (12.9%) | 217 (87.9%) | 30 (12.1%) | 52 (83.9%) | 10 (16.1%) |
| 35–49 | 410 (92.6%) | 33 (7.5%) | 359 (93.0%) | 27 (7.0%) | 51 (89.5%) | 6 (10.5%) |
| 50+ | 138 (91.4%) | 13 (8.6%) | 130 (90.9%) | 13 (9.1%) | 8 (100%) | 0 (0.0%) |
| Female | 551 (88.6%) | 71 (11.4%) | 444 (89.0%) | 55 (11.0%) | 107 (87.0%) | 16 (13.0%) |
| Male | 306 (93.0%) | 23 (7.0%) | 294 (93.0%) | 22 (7.0%) | 12 (92.3%) | 1 (7.7%) |
| Location | ||||||
| Study Site | ||||||
| Ngong Rd Clinic in Nairobi, | 594 (91.1%) | 58 (8.9%) | 526 (91.8%) | 47 (8.2%) | 68 (86.1%) | 11 (13.9%) |
| Industrial Area Clinic in Nairobi | 76 (87.4%) | 11 (12.6%) | 62 (84.9%) | 11 (15.1%) | 14 (100%) | 0 (0.0%) |
| Maseno Clinic in rural Nyanza | 187 (88.2%) | 25 (11.8%) | 150 (88.8%) | 19 (11.2%) | 37 (86.1%) | 6 (14.0%) |
| Socioeconomic | ||||||
| Married/steady partner | 521 (60.8%) | 59 (62.8%) | 442 (59.9%) | 46 (59.7%) | 79 (66.4%) | 13 (76.5%) |
| Education in years | 11 (8, 13) | 10 (8, 12) | 11 (8, 13) | 10 (8, 12) | 12 (8, 14) | 11 (8, 15) |
| Unemployed | 157 (18.3%) | 27 (28.7%) | 130 (17.6%) | 19 (24.7%) | 27 (22.7%) | 8 (47.1%) |
| Monthly rent in US$d | 22 (0, 66) | 22 (0, 66) | 28 (0, 66) | 22 (0, 66) | 22 (0, 66) | 28 (0, 56) |
| Flush toiletd | 404 (47.2%) | 41 (43.6%) | 352 (47.8%) | 33 (42.9%) | 52 (43.7%) | 8 (47.1%) |
| Persons living in house | 4 (2, 5) | 4 (3, 5) | 3 (2, 5) | 3 (2, 5) | 4 (3, 5) | 4 (4, 5) |
| Access to care | ||||||
| Cost of travel ≥US$2d | 402 (47.6%) | 45 (48.9%) | 336 (46.3%) | 36 (47.4%) | 66 (55.9%) | 9 (56.3%) |
| Travel time to clinic in hrs | 1 (0.5, 2) | 1 (0.5, 1.5) | 1 (0.5, 2) | 1 (0.5, 1.5) | 1 (0.5, 2) | 1 (1, 2) |
| Sexual risk behavior | ||||||
| Age of sexual debut in yearsd | 18 (16, 20) | 18 (16, 20) | 18 (16, 20) | 18 (16, 20) | 18 (16, 20) | 17 (15, 18) |
| Lifetime sexual partnersd | 3 (2, 5) | 4 (2, 5) | 3 (2, 5) | 4 (2, 5) | 3 (2, 5) | 4 (2, 5) |
| Ever exchange money for sex | 46 (5.4%) | 4 (4.3%) | 42 (5.7%) | 3 (3.9%) | 4 (3.4%) | 1 (5.9%) |
| Sexual risk in 18–24-year-old girls | n = 31 (3.7%) | n = 7 (8.6%) | n = 23 (2.9%) | n = 6 (8.4%) | n = 8 (7.1%) | n = 1 (4.6%) |
| Age of sexual debut in yearsd | 18 (15, 19) | 18 (15, 19) | 17 (15, 19) | 18 (15, 19) | 19 (18, 20) | 15 |
| Lifetime sexual partnersd | 2 (1, 3) | 1 (1, 4) | 2 (1, 3) | 2 (1, 4) | 2 (1, 3) | 1 |
| Ever exchange money for sex | 1 (3.0%) | 0 (0%) | 1 (4.0%) | 0 (0%) | 0 (0.0%) | 0 (0.0%) |
| Laboratory | ||||||
| Median CD4 cells/uLd | 230 (116, 311) | 224 (73, 296) | 211 (103, 306) | 155 (69, 270) | 277 (227, 332) | 308 (257, 400) |
Abbreviations: ART, antiretroviral therapy; ARV, antiretroviral; PDR, pretreatment drug resistance; OLA, oligonucleotide ligation assay.
aFirst-line ART indicates nonnucleoside reverse transcriptase inhibitor-based antiretroviral treatment.
bOligonucleotide ligation assay is a point mutation test designed to detect K103N, Y181C, M184V, G190A, and K65R.
cFor continuous variables, the median (interquartile range) are presented. For ARV-exposure status, age category, gender, and study site, the n (%) with and without PDR is shown. For other categorical variables, the n (%) within that category is shown.
dData are complete for all variables except the following: Monthly rent (n = 927, 834 PDR−, 93 PDR+); Type of toilet (n = 950, 856 PDR−, 94 PDR+); Cost of travel ≥$2.00 (n = 936, 844 PDR−, 92 PDR+); Age of sexual debut (n = 891, 803 PDR−, 88 PDR+); Lifetime sexual partners (n = 893, 807 PDR−, 86 PDR+); CD4 count (n = 948, 855 PDR−, 93 PDR+).
Illumina Confirmation of OLA
Samples from 67 participants with mutations detected via OLA were retested via Illumina, and 49 (73%) were confirmed at a frequency >1%. Of these, 14 had mutations detected via OLA at <10% mutant frequency, 18 between 10% and 24%, and 17 between 25% and 100%. Across a total of 71 mutant codons for these 49 samples, the median difference in mutant frequency between OLA and Illumina was 4.5% (IQR, 1.4%–13.8%). Of the 18 (27%) samples that were not confirmed via Illumina, the median mutant frequency detected via OLA was 2% (range 2%–6%). These 18 were considered “wild type” in all analyses.
Pre-antiretroviral-Treatment Drug Resistance
PDR was detected by OLA in 94 adults (9.9%; 95% CI, 8.1%–12.0%) with 130 total mutant codons, and only 1 child (2.8%; 95% CI, 0.1%–14.5%), a 16-year-old ARV-naive male (Table 2). Among those with PDR, the median frequency of mutant virus within an individual’s HIV quasispecies was 67% by OLA (range 2%–100%; IQR, 14%–92%). Of the 95 participants with PDR, 30 (32%) had a mutant frequency <20% (11 women and 3 men, all >24 years old) and 16 (17%) had <10% (all women >24 years old) detected via OLA. The majority (96.8%) with PDR had at least 1 NNRTI mutation, and relatively fewer had NRTI mutations (16.8% with M184V and 9.5% with K65R). Among the 815 ARV-naive adults, OLA detected mutants in 77 (9.4%; 95% CI, 7.5%–11.7%) participants with a total of 111 mutant codons and a median mutant frequency in their quasispecies of 80% (range 2%–100%; IQR, 15%–92%); and in the child, Y181C was detected at 24%. PDR was 2.9% more prevalent among the 136 ARV-experienced versus ARV-naive adults, with 17 (12.5%; 95% CI, 7.5%–19.3%) participants harboring 19 mutant codons, though this was not statistically significant (Χ2P value = .270). The median mutant frequency among ARV-experienced adults was 30% (range 4%–100%; IQR, 10%–83%), 50% lower than the ARV-naive adult median, though this was not statistically significant (Wilcoxon rank-sum P value = .244).
Table 2.
Frequency of PDR Detected by Oligonucleotide Ligation Assaya in Participants Qualifying for First-Line ARTb by History of ARV Use (n = 987)
| Mutant Codon c |
ARV naive
(n = 849)d |
ARV experienced
(n = 138) |
Total
(n = 987) |
|---|---|---|---|
| K103N | 38 (4.5%) | 11 (8.0%) | 49 (5.0%) |
| Y181C | 5 (0.6%) | 2 (1.5%) | 7 (0.7%) |
| G190A | 5 (0.6%) | 2 (1.5%) | 7 (0.7%) |
| M184V | 1 (0.1%) | 0 (0.0%) | 1 (0.1%) |
| K65R | 2 (0.2%) | 0 (0.0%) | 2 (0.2%) |
| K103N + Y181C | 4 (0.5%) | 2 (1.5%) | 6 (0.6%) |
| K103N + G190A | 5 (0.6%) | 0 (0.0%) | 5 (0.5%) |
| K103N + M184V | 8 (0.9%) | 0 (0.0%) | 8 (0.8%) |
| Y181C + K65R | 2 (0.2%) | 0 (0.0%) | 2 (0.2%) |
| G190A + M184V | 2 (0.2%) | 0 (0.0%) | 2 (0.2%) |
| K103N + Y181C + M184V | 1 (0.1%) | 0 (0.0%) | 1 (0.1%) |
| K103N + M184V + K65R | 1 (0.1%) | 0 (0.0%) | 1 (0.1%) |
| Y181C + M184V + K65R | 2 (0.2%) | 0 (0.0%) | 2 (0.2%) |
| G190A + M184V + K65R | 1 (0.1%) | 0 (0.0%) | 1 (0.1%) |
| K103N + Y181C + G190A + K65R | 1 (0.1%) | 0 (0.0%) | 1 (0.1%) |
| Total cases of PDR | 78 (9.2%) | 17 (12.3%) | 95 (9.6%) |
Abbreviations: PDR, pretreatment drug resistance; OLA, oligonucleotide ligation assay; ART, antiretroviral therapy; ARV, antiretroviral.
aOLA is a point mutation test designed to detect K103N, Y181C, M184V, G190A, and K65R.
bFirst-line ART indicates nonnucleoside reverse transcriptase inhibitor-based antiretroviral treatment.
c1 ARV-naive child had PDR detected at Y181C. All other mutations were detected in adults.
dARV naive includes 815/951 adults and 34/36 children.
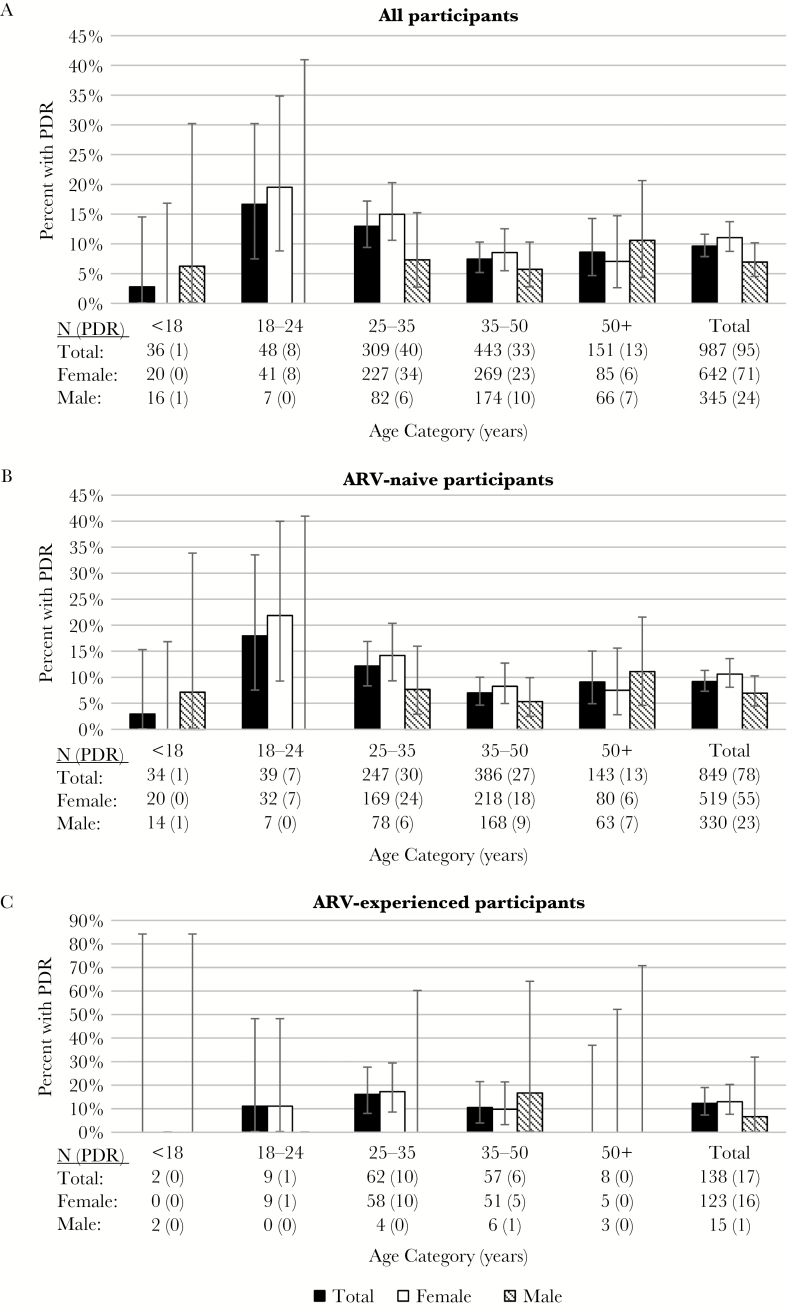
Women 18–24 years of age (n = 41) had the highest PDR prevalence (19.5%; 95% CI, 8.8%–34.9%), with similar prevalence among ARV-naive 18–24-year-old women (n = 32) (21.9%; 95% CI, 9.3%–40.0%). Figure 1 illustrates PDR prevalence by gender, age category, and ARV experience, and shows a declining trend in PDR prevalence with increasing age among women, specifically in the ARV-naive group who were the majority cohort of women in this study.
Figure 1.
Pretreatment HIV drug resistance (PDR) by age, gender, and antiretroviral (ARV) experience. Bar charts illustrate the prevalence of PDR and 95% confidence intervals by 5 age categories (<18, 18–24, 25–35, 35–50, and 50 years and older). Data are shown for all 987 study participants (A), 849 ARV-naive participants (B), and 138 ARV-experienced participants (C). In each panel, data are shown stratified by gender.
In univariable and multivariable regression (Table 3), the PDR prevalence among all adult women was 1.63 times greater than men (95% CI, 1.04–2.56; P value = .033). ARV-experienced adults had a PDR prevalence 1.32 times greater than ARV-naive adults, though this was not statistically significant (95% CI, 0.81–2.17; P value = .226). A 5-year decrease in age was associated with a 1.14-fold greater PDR prevalence (95% CI, 1.02–1.27; P = .033) among all adults, a 1.11-fold greater PDR prevalence (95% CI, 0.99–1.25; P value = .082) among ARV-naive adults, and a 1.35-fold greater PDR prevalence (95% CI, 1.00–1.83; P value = .054) among ARV-experienced adults (Table 3). The interaction term between age and gender was statistically significant among all adults (P value = .026) and among ARV-naive (P value = .039), though not for ARV-experienced (P value = .571).
Table 3.
Prevalence Ratios of PDR in Participants ≥18 Years of Age by History of Prior ARV Use Using Poisson Regression With Robust Standard Errorsa
| Variables | Total (n = 951) | ARV Naive (n = 815) | ARV Experienced (n = 136) | |||
|---|---|---|---|---|---|---|
|
Univariable
Regression |
Multivariable
Regression b |
Univariable
Regression |
Multivariable
Regression b |
Univariable
Regression |
Multivariable
Regression b |
|
| PR (95% CI); P value | PR (95% CI); P value | PR (95% CI); P value | PR (95% CI); P value | PR (95% CI); P value | PR (95% CI); P value | |
| Demographic | ||||||
| Gender (female vs male) | 1.63 (1.04–2.56); .033 | 1.48 (0.95–2.32); .086 | 1.58 (0.98–2.54); .058 | 1.48 (0.93–2.37); .100 | 1.69 (0.24–11.83); .597 | 1.19 (0.17–8.51); .864 |
| Age (5-year decrease) | ||||||
| Total | 1.14 (1.02–1.27); .022 | 1.12 (1.01–1.24); .037 | 1.11 (0.99–1.25); .082 | 1.10 (0.98–1.22); .110 | 1.35 (1.00–1.83); .054 | 1.34 (0.99–1.80); .056 |
| Males | 0.93 (0.78–1.12); .465 | 0.94 (0.79–1.13); .522 | 0.92 (0.77–1.12); .418 | 0.94 (0.78–1.12); .475 | 1.20 (0.89–1.61); .234 | 1.20 (0.82–1.75); .339 |
| Females | 1.20 (1.06–1.36); .004 | 1.20 (1.06–1.36); .004 | 1.18 (1.03–1.35); .016 | 1.18 (1.03–1.35); .016 | 1.36 (0.97–1.91); .076 | 1.35 (0.97–1.87); .072 |
| Location | ||||||
| Study Site (clinic) | ||||||
| Ngong Rd (Nairobi, urban) | Ref | Ref | ||||
| Industrial Area (Nairobi, urban) | 1.42 (0.78–2.60); .255 | – | 1.83 (1.00–3.38); .051 | – | – | – |
| Maseno (Nyanza, rural) | 1.32 (0.85–2.06); .212 | 1.37 (0.83–2.27); .221 | ||||
| Study Site (clinic location) c | ||||||
| Nairobi (Ngong Rd + Industrial) | Ref | Ref | Ref | Ref | Ref | Ref |
| Maseno | 1.26 (0.82–1.94); .289 | 1.22 (0.79–1.89); .364 | 1.25 (0.77–2.04); .368 | 1.23 (0.75–2.02); .405 | 1.18 (0.47–2.99); .728 | 1.10 (0.45–2.70); .831 |
| Socioeconomic | ||||||
| Married/steady partner vs single | 1.08 (0.72–1.60); .710 | – | 0.99 (0.64–1.53); .979 | – | 1.55 (0.53–4.51); .417 | – |
| Education (1 years increase) | 0.96 (0.92–1.01); .118 | – | 0.95 (0.91–1.01); .082 | – | 1.00 (0.89–1.11); .934 | – |
| Unemployed vs employed | 1.68 (1.11–2.55); .015 | – | 1.46 (0.90–2.38); .125 | – | 2.56 (1.07–6.15); .035 | – |
| Monthly rent ($1 increase) | 1.00 (1.00–1.00); .889 | – | 1.00 (1.00–1.00); .909 | – | 1.00 (1.00–1.00); .975 | – |
| Flush toilet vs pit latrine | 0.88 (0.60–1.30); .516 | – | 0.84 (0.54–1.29); .420 | – | 1.12 (0.46–2.75); .795 | – |
| Persons living in house | 1.03 (0.94–1.12); .537 | – | 0.99 (0.90–1.09); .907 | – | 1.18 (1.00–1.40); .045 | – |
| Access to Care | ||||||
| Cost of travel ≥$2.00 vs <$2.00 | 1.05 (0.71–1.54); .815 | – | 1.04 (0.68–1.60); .857 | – | 1.01 (0.40–2.56); .981 | – |
| Travel time to clinic (1 h increase) | 0.92 (0.78–1.10); .365 | – | 0.89 (0.72–1.11); .321 | – | 1.05 (0.72–1.53); .814 | – |
| Sexual risk behavior | ||||||
| Age of sexual debut (1 year increase) | 0.97 (0.92–1.04); .421 | – | 0.99 (0.93–1.06); .825 | – | 0.88 (0.72–1.07); .188 | – |
| Lifetime sex partners (1 increase) | 1.00 (1.00–1.00); .400 | – | 1.00 (1.00–1.00); .285 | – | 0.99 (0.98–1.01); .436 | – |
| Ever exchange money for sex | 0.80 (0.31–2.09); .651 | – | 0.69 (0.23–2.12); .520 | – | 1.64 (0.27–10.1); .595 | – |
Abbreviations: PDR, pretreatment drug resistance; ARV, antiretroviral; PR, prevalence ratio; CI, confidence interval; Ref, reference category
aPRs with P value < .05 are shown in bold.
bThe PDR PR by location was adjusted for both age and sex in multivariable regression. The PDR PR by location stratified by sex and adjusted for age showed similar results and are not presented. Analyses adjusting for additional variables showed similar results to univariate analyses and are not presented.
cIndustrial Area had no ARV-experienced participants with PDR so location was defined Maseno vs Nairobi in multivariable analyses.
When stratifying by gender, a 5-year decrease in age in adult women was associated with a 1.20-fold greater PDR prevalence (95% CI, 1.06–1.36; P value = .004), including a 1.18-fold greater prevalence (95% CI, 1.03–1.35; P value = .016) among ARV-naive women and a 1.36-fold greater prevalence (95% CI, 0.97–1.91; P value = .076) among ARV-experienced women, though the latter was not statistically significant (Table 3). Age was not associated with PDR prevalence among men. Similar results for gender and age associations were found in multivariable regression that included gender, age, and location (Table 3), and in exploratory models that also included marital/partner status, years of education, unemployment status, type of toilet, and age of sexual debut (results not shown). The increased prevalence of PDR by decreasing age remained similar in magnitude and was statistically significant only among females in all analyses.
Among all adult participants, being unemployed was associated with a 1.68-fold greater prevalence of PDR (95% CI, 1.11–2.55; P value = .015), though this was attenuated and not statistically significant in multivariable analyses (results not shown). Among ARV-experienced adults, being unemployed was associated with a 2.56-fold greater PDR prevalence (95% CI, 1.07–6.15; P value = .035), and a 1-person increase in the number of people living with the participant was associated with a 1.18-fold greater PDR prevalence (95% CI, 1.00–1.40; P value = .045). No other statistically significant associations were detected between PDR and clinic location, socioeconomic status, access to care, or sexual risk behavior variables regardless of prior ARV use (Table 3). Sensitivity analyses that additionally included 8 participants ≥16 years old (7 women and 1 man), resulted in a slight attenuation of the associations between PDR and age overall and among women, but did not impact the overall interpretation of the results.
DISCUSSION
In this study, we observed a high prevalence of PDR to currently recommended first-line ARVs [9–14] among individuals initiating ART in Kenya. PDR prevalence was highest among HIV-infected young women, 18–24 years old, particularly among ARV-naive women. This is consistent with younger women accounting disproportionately for incident HIV infections in sub-Saharan Africa [17], and younger adults becoming HIV infected more recently, and thus at a time with greater transmission of HIV-DR, compared with older individuals [16]. The observed prevalence of PDR in young women in our study exceeds the 10% threshold at which the WHO recommends reassessment and modifications to the ART strategy [34]. While a high PDR prevalence was found in the population as a whole (10%), PDR may be especially problematic for young women and their children, as PDR may adversely affect the effectiveness of first-line ART that is recommended for ARV-naive pregnant women for PMTCT.
The statistically significant association between lower age and higher PDR prevalence among women remained after controlling for potential confounders. The absence of an association in men may be due to differences in risk factors for HIV acquisition. The increased PDR prevalence among young women compared with young men could be due to differences in their partners and their likelihood of DR-HIV, with women having greater acquisition of infection from older, potentially ARV-experienced men [18, 19], or due to unreported prior ARV use. Additionally, our study is limited by the small number of younger men (n = 8 between age 16 and 24 years), and further research is needed to investigate PDR prevalence by age and other factors in this group. Very little PDR was observed in children, which may indicate that untreated children were infected by mothers with lower rates of DR-HIV or that resistance had decayed to undetectable levels by the time of evaluation.
As expected, we found a higher PDR prevalence among ARV-experienced compared to ARV-naive individuals. The 2.9% difference we observed is slightly lower than the 6% difference found in Zimbabwe (12% in ARV-experienced participants and 6% in ARV-naive) [20]. The Zimbabwean study was conducted in 2008–2010, with likely less time since ART “roll-out” compared to our study cohort that enrolled more recently, in 2013–2014, which correlates with prevalence of resistance [35]. PDR prevalence among ARV-experienced participants in our cohort was lower than that found in studies of mothers and children who received mono- or dual-ARVs for PMTCT (approximately 36%) [21, 23]. This could be due to greater decay of mutant viruses [36, 37] in our study compared with other cohort studies, resulting from a longer time interval between ARV use and DR testing, although TDR to NNRTI has been shown to persist at high frequencies for years [38].
We found a similar PDR prevalence (9%–13%) across urban and rural sites, which was contrary to our expectation that PDR prevalence would be lower at the rural than the urban clinics. Whereas some previous studies have generally found lower PDR in rural settings [39–49], our more recent results may reflect improved access to ART in the rural regions. Our observed 12% PDR prevalence among 212 adult participants (11% among ARV-naive) at the rural Maseno clinic was slightly higher than the 9% TDR prevalence observed in another recently published study of 87 ARV-naive participants conducted in 2012, also based in Western Kenya [50], providing further evidence of PDR in this rural setting. In addition, the higher HIV incidence and prevalence in Western Kenya suggests a greater proportion of recent infections among these subjects, which may have contributed to the rate of PDR.
Our study has several limitations. The relatively few 18–24-year-old males and few children limit the precision of PDR estimates in these subgroups. Prior ARV experience was based on self-report and clinic and pharmacy records and could be subject to misclassification. Additionally, OLA testing may have underestimated PDR because only 5 codons were examined for mutations as opposed to more comprehensive testing. While our previous studies at these sites did not find that mutations at other codons were associated with virologic failure [4], if mutations were missed, PDR may actually be higher than what was detected. While we found unemployment to be correlated with PDR prevalence in unadjusted analyses, no other socioeconomic status or sexual risk behaviors were identified as potential correlates. Because those we defined as unemployed included women who considered themselves to be housewives, a category for which there is no male equivalent, the association is likely in part due to more unemployed participants being women. Our study may be underpowered to detect associations and larger surveillance cohorts that allow for multiple comparison adjustment may be necessary to identify other risk factors of PDR transmission.
Strengths of this study include its relatively large, representative cohort of HIV-infected Kenyans who initiated ART in 2013–2014, and testing for PDR by a sensitive method, with results available from 99.7% of participants. This study provides recent evidence of a high prevalence of PDR in Kenyan patients. The especially high prevalence in young women could compromise the outcomes of ART programs for women’s health and interventions given for PMTCT. ART outcome data are needed to determine whether testing for PDR and/or if scale up of alternative ARV combinations without NNRTI cross-resistance should be implemented throughout Kenya or within PMTCT programs.
Notes
Acknowledgments. We thank the study participants and their families who are committed to advancing HIV care, and the research personnel, clinic, and laboratory staff, and data management teams in Nairobi and Seattle for their efforts as well as the Coptic Hope Center for Infectious Diseases and its patients.
Financial support. This work was supported by grants from the National Institutes of Health (R01-AI058723 and R01-AI100037), including an American Recovery and Reinvestment Act supplement (R01-AI058723). Support of the study was provided by the University of Washington Center for AIDS Research (P30AI027757). The Coptic Hope Center for Infectious Diseases is supported by the President’s Emergency Plan for AIDS Relief through a cooperative agreement (U62/CCU024512) from the Centers for Disease Control and Prevention.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in parts: These data were presented at the 2015 International Workshop on HIV Drug Resistance, held February 21–22, 2015 in Seattle, Washington (Abstract/Poster Number: 86).
References
- 1. World Health Organization (WHO). Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. Geneva, Switzerland: WHO, 2015. [PubMed] [Google Scholar]
- 2. Mutevedzi PC, Newell ML. Review: [corrected] The changing face of the HIV epidemic in sub-Saharan Africa. Trop Med Int Health 2014; 19:1015–28. [DOI] [PubMed] [Google Scholar]
- 3. World Health Organization (WHO). Global action plan for HIV drug resistance 2016–2021. Geneva, Switzerland: WHO, 2016. [Google Scholar]
- 4. Chung MH, Beck IA, Dross S et al. . Oligonucleotide ligation assay detects HIV drug resistance associated with virologic failure among antiretroviral-naive adults in Kenya. J Acquir Immune Defic Syndr 2014; 67:246–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kuritzkes DR, Lalama CM, Ribaudo HJ et al. . Preexisting resistance to nonnucleoside reverse-transcriptase inhibitors predicts virologic failure of an efavirenz-based regimen in treatment-naive HIV-1-infected subjects. J Infect Dis 2008; 197:867–70. [DOI] [PubMed] [Google Scholar]
- 6. Gupta RK, Jordan MR, Sultan BJ et al. . Global trends in antiretroviral resistance in treatment-naive individuals with HIV after rollout of antiretroviral treatment in resource-limited settings: a global collaborative study and meta-regression analysis. Lancet 2012; 380:1250–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hosseinipour MC, Gupta RK, Van Zyl G, Eron JJ, Nachega JB. Emergence of HIV drug resistance during first- and second-line antiretroviral therapy in resource-limited settings. J Infect Dis 2013; 207(suppl 2):S49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van Zyl GU, Frenkel LM, Chung MH, Preiser W, Mellors JW, Nachega JB. Emerging antiretroviral drug resistance in sub-Saharan Africa: novel affordable technologies are needed to provide resistance testing for individual and public health benefits. AIDS 2014; 28:2643–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ministry of Health, National AIDS and STI Control Programme (NASCOP). Guidelines on use of antiretroviral drugs for treating and preventing HIV infections in Kenya. Nairobi, Kenya: NASCOP, 2016. [Google Scholar]
- 10. Ministry of Health, National AIDS and STI Control Programme (NASCOP). Guidelines for antiretroviral drug therapy in Kenya. Rapid advice booklet. Nairobi, Kenya: NASCOP, 2014. [Google Scholar]
- 11. Ministry of Health, National AIDS and STI Control Programme (NASCOP). Guidelines for antiretroviral drug therapy in Kenya. 4th ed Nairobi, Kenya: NASCOP, 2011. [Google Scholar]
- 12. Ministry of Health, National AIDS and STI Control Programme (NASCOP). Guidelines for antiretroviral drug therapy in Kenya. 3rd ed Nairobi, Kenya: NASCOP, 2005. [Google Scholar]
- 13. World Health Organization (WHO). Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: Recommendations for a public health approach. 2nd ed Geneva, Switzerland: WHO, 2016. [PubMed] [Google Scholar]
- 14. World Health Organization (WHO). Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: Recommendations for a public health approach. Geneva, Switzerland: WHO, 2013. [PubMed] [Google Scholar]
- 15. World Health Organization (WHO). HIV drug resistance surveillance guidance—2015 update. Geneva, Switzerland: WHO, 2015. [Google Scholar]
- 16. Sanders EJ, Wahome E, Powers KA et al. . Targeted screening of at-risk adults for acute HIV-1 infection in sub-Saharan Africa. AIDS 2015; 29(suppl 3):S221–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. UN Joint program on HIV/AIDS (UNAIDS). The gap report, 2014. http://www.refworld.org/docid/53f1e1604.html, accessed 16 October 2017.
- 18. Leclerc-Madlala S. Age-disparate and intergenerational sex in southern Africa: the dynamics of hypervulnerability. AIDS 2008; 22(suppl 4):S17–25. [DOI] [PubMed] [Google Scholar]
- 19. Kenyon C, Colebunders R. Birds of a feather: homophily and sexual network structure in sub-Saharan Africa. Int J STD AIDS 2013; 24:211–5. [DOI] [PubMed] [Google Scholar]
- 20. Mungati M, Mhangara M, Gonese E et al. . Pre-treatment drug resistance among patients initiating antiretroviral therapy (ART) in Zimbabwe: 2008–2010. BMC Res Notes 2016; 9:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kityo C, Sigaloff KC, Sonia Boender T et al. . HIV drug resistance among children initiating first-line antiretroviral treatment in Uganda. AIDS Res Hum Retroviruses 2016; 32:628–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Salou M, Butel C, Konou AA et al. . High rates of drug resistance among newly diagnosed HIV-infected children in the national prevention of mother-to-child transmission program in Togo. Pediatr Infect Dis J 2016; 35:879–85. [DOI] [PubMed] [Google Scholar]
- 23. Arrivé E, Newell ML, Ekouevi DK et al. ; Ghent Group on HIV in Women and Children Prevalence of resistance to nevirapine in mothers and children after single-dose exposure to prevent vertical transmission of HIV-1: a meta-analysis. Int J Epidemiol 2007; 36:1009–21. [DOI] [PubMed] [Google Scholar]
- 24. Lyons FE, Coughlan S, Byrne CM, Hopkins SM, Hall WW, Mulcahy FM. Emergence of antiretroviral resistance in HIV-positive women receiving combination antiretroviral therapy in pregnancy. AIDS 2005; 19:63–7. [DOI] [PubMed] [Google Scholar]
- 25. Chung MH, Drake AL, Richardson BA et al. . Impact of prior HAART use on clinical outcomes in a large Kenyan HIV treatment program. Curr HIV Res 2009; 7:441–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rhee SY, Gonzales MJ, Kantor R, Betts BJ, Ravela J, Shafer RW. Human immunodeficiency virus reverse transcriptase and protease sequence database. Nucleic Acids Res 2003; 31:298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shafer RW. Rationale and uses of a public HIV drug-resistance database. J Infect Dis 2006; 194(suppl 1):S51–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Beck IA, Deng W, Payant R et al. . Validation of an oligonucleotide ligation assay for quantification of human immunodeficiency virus type 1 drug-resistant mutants by use of massively parallel sequencing. J Clin Microbiol 2014; 52:2320–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Beck IA, Mahalanabis M, Pepper G et al. . Rapid and sensitive oligonucleotide ligation assay for detection of mutations in human immunodeficiency virus type 1 associated with high-level resistance to protease inhibitors. J Clin Microbiol 2002; 40:1413–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li H, Durbin R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 2010; 26:589–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wilm A, Aw PP, Bertrand D et al. . LoFreq: a sequence-quality aware, ultra-sensitive variant caller for uncovering cell-population heterogeneity from high-throughput sequencing datasets. Nucleic Acids Res 2012; 40:11189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Garrison E, Marth G. Haplotype-based variant detection from short-read sequencing. arXiv 2012; 1207.3907v2 [q-bio.GN]. [Google Scholar]
- 33. Cingolani P, Platts A, Wang le L et al. . A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly (Austin) 2012; 6:80–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. World Health Organization (WHO). Guidelines on the public health response to pretreatment HIV drug resistance. Geneva, Switzerland: WHO, 2017. [Google Scholar]
- 35. Chung MH, Silverman R, Beck IA et al. . Increasing HIV-1 pre-treatment drug resistance among antiretroviral-naive adults initiating treatment between 2006 and 2014 in Nairobi, Kenya. AIDS 2016; 30:1680–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yang WL, Kouyos RD, Böni J et al. ; Swiss HIV Cohort Study (SHCS) Persistence of transmitted HIV-1 drug resistance mutations associated with fitness costs and viral genetic backgrounds. PLoS Pathog 2015; 11:e1004722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jain V, Sucupira MC, Bacchetti P et al. . Differential persistence of transmitted HIV-1 drug resistance mutation classes. J Infect Dis 2011; 203:1174–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Little SJ, Frost SD, Wong JK et al. . Persistence of transmitted drug resistance among subjects with primary human immunodeficiency virus infection. J Virol 2008; 82:5510–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zeh C, Inzaule SC, Ondoa P et al. . Molecular epidemiology and transmission dynamics of recent and long-term HIV-1 infections in rural Western Kenya. PLoS One 2016; 11:e0147436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Onsongo S, Abidi SH, Khamadi S et al. . Prevalence of transmitted drug resistance mutations in HIV-1-infected drug-naive patients from urban and suburban regions of Kenya. AIDS Res Hum Retroviruses 2016; 32:220–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cheriro W, Kiptoo M, Kikuvi G, Mining S, Emonyi W, Songok E. High prevalence of HIV low abundance drug-resistant variants in a treatment-naive population in North Rift Kenya. AIDS Res Hum Retroviruses 2015; 31:1274–7. [DOI] [PubMed] [Google Scholar]
- 42. Budambula V, Musumba FO, Webale MK et al. . HIV-1 protease inhibitor drug resistance in Kenyan antiretroviral treatment-naive and -experienced injection drug users and non-drug users. AIDS Res Ther 2015; 12:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kantor R, DeLong A, Balamane M et al. . HIV diversity and drug resistance from plasma and non-plasma analytes in a large treatment programme in western Kenya. J Int AIDS Soc 2014; 17:19262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kiptoo M, Brooks J, Lihana RW et al. . HIV-1 drug resistance-associated mutations among HIV-1 infected drug-naïve antenatal clinic attendees in rural Kenya. BMC Infect Dis 2013; 13:517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hassan AS, Mwaringa SM, Obonyo CA et al. . Low prevalence of transmitted HIV type 1 drug resistance among antiretroviral-naive adults in a rural HIV clinic in Kenya. AIDS Res Hum Retroviruses 2013; 29:129–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sigaloff KC, Mandaliya K, Hamers RL et al. . Short communication: High prevalence of transmitted antiretroviral drug resistance among newly HIV type 1 diagnosed adults in Mombasa, Kenya. AIDS Res Hum Retroviruses 2012; 28:1033–7. [DOI] [PubMed] [Google Scholar]
- 47. Price MA, Wallis CL, Lakhi S et al. ; IAVI Early Infection Cohort Study Group Transmitted HIV type 1 drug resistance among individuals with recent HIV infection in East and Southern Africa. AIDS Res Hum Retroviruses 2011; 27:5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hamers RL, Wallis CL, Kityo C et al. ; PharmAccess African Studies to Evaluate Resistance (PASER) HIV-1 drug resistance in antiretroviral-naive individuals in sub-Saharan Africa after rollout of antiretroviral therapy: a multicentre observational study. Lancet Infect Dis 2011; 11:750–9. [DOI] [PubMed] [Google Scholar]
- 49. Lihana RW, Khamadi SA, Lubano K et al. . HIV type 1 subtype diversity and drug resistance among HIV type 1-infected Kenyan patients initiating antiretroviral therapy. AIDS Res Hum Retroviruses 2009; 25:1211–7. [DOI] [PubMed] [Google Scholar]
- 50. Onywera H, Maman D, Inzaule S et al. . Surveillance of HIV-1 pol transmitted drug resistance in acutely and recently infected antiretroviral drug-naïve persons in rural western Kenya. PLoS One 2017; 12:e0171124. [DOI] [PMC free article] [PubMed] [Google Scholar]