Abstract
Multiple candidate vaccines against Staphylococcus aureus infections have failed in clinical trials. Analysis of a recent prematurely halted vaccine trial revealed increased mortality rates among vaccine recipients in whom postsurgical S. aureus infection developed, emphasizing the potential for induction of detrimental immune responses and the need to better understand the requirements for protective immunity against S. aureus. These failures of single-antigen vaccines have prompted ongoing development of multicomponent vaccines to target the multitude of S. aureus virulence factors. In the current study, we used lethally irradiated S. aureus as a model multicomponent vaccine and showed that vaccination of mice decreased survival in a bacteremia challenge model. These deleterious effects were due to a CD4 T-cell–dependent interferon γ response and could be prevented by inhibiting development of this response during vaccination. Our results identify the potential for vaccination to induce pathological immune responses, and they have implications for recent vaccine failures and the design of future staphylococcal vaccines.
Keywords: Staphylococcus aureus, vaccination, systemic infection, Th1 cells, IFN-γ;, immunopathology.
Staphylococcus aureus is a major human pathogen and leading cause of infectious morbid effects and death in the industrialized world [1]. Infections caused by S. aureus range from superficial skin and soft-tissue infections to life-threatening diseases such as pneumonia, endocarditis, and disseminated bacteremia. These diverse clinical manifestations can be attributed to expression by S. aureus of a wide array of virulence factors that evade and modulate components of the immune system, as well as to its ability to rapidly acquire resistance mechanisms toward antibiotics [2, 3].
Despite its pathogenic potential, S. aureus is a well-adapted component of the human microbiota and persistently colonizes up to 30% of the population at mucocutaneous sites [4]. Colonization increases the risk of an individual to develop invasive infections, but higher mortality rates for infection in noncolonized than in colonized individuals suggest the development of a partially protective immune response with colonization [5]. However, immune correlates of protection from and susceptibility to staphylococcal infections are incompletely understood, and hence the design of an efficacious vaccine has proved to be a challenge.
Most vaccine candidates against S. aureus to date were selected based on their ability to induce toxin-neutralizing or opsonizing antibodies against specific antigens [6, 7]. The failure of these strategies has prompted the ongoing development of multicomponent vaccines to more broadly target the multiple virulence factors of S. aureus. In recent years, preclinical studies have further indicated a potentially important contribution of T cells to vaccine-mediated protection [8–10]. However, multiple failures of candidate vaccines in clinical trials [11], including the recently reported increase in mortality rates seen after infection in vaccinated individuals [12], highlight the need to understand the requirements for protective vaccination against this important human pathogen. Using killed whole-cell S. aureus as a model multiepitope vaccine, we report here the potential for induction of a deleterious T-cell interferon (IFN) γ response that increased mortality rates in vaccinated mice on bacteremic challenge.
MATERIALS AND METHODS
Bacteria
Staphylococcus aureus strain USA300 (Los Angeles County clone, LAC) and USA300 spa deletion mutant were kindly provided by Michael Otto (National Institutes of Health [NIH]).
Preparation of Inoculum for Infection
For bacterial challenges, 20 mL of brain-heart infusion (BHI) broth (Difco Laboratories) was inoculated with a swab of community-acquired methicillin-resistant S. aureus (MRSA) USA300 (LAC) from a freshly streaked blood agar plate, and culture was grown for 18 hours at 37°C, with shaking at 230 rpm. Culture was centrifuged at 3000 rpm for 10 minutes at room temperature, pellet washed twice with phosphate-buffered saline (PBS), and resuspended in PBS. Aliquots were prepared and stored at −80°C until further use. Aliquots were periodically thawed and plated to confirm the colony-forming unit (CFU) count (per milliliter) after storage.
Preparation of Lethally Irradiated S. aureus Whole-Cell Vaccine
Community-acquired MRSA USA300 (LAC) was grown as described above, and irradiation was performed in air on wet ice at 6 kGy using a Gammacell irradiation unit (484-R-2 Co-60 irradiator; J L Shepherd). The CFU count for the culture was determined before irradiation. Absence of viable MRSA within irradiated samples was ensured by culturing irradiated aliquots in BHI broth and on blood agar plates and incubating up to 72 hours at 37°C and 230 rpm. No viable bacteria were detected.
Animals
Female C57BL/6J and µMT (B6.129S2-lghmtm1Cgn/J) mice were purchased from the Jackson Laboratory; IFN-γ knockout mice (C57BL/6Tac-[KO]IFN gamma N12) were obtained through a supply contract between the National Institute of Allergy and Infectious Diseases (NIAID), NIH, and Taconic Farms or were purchased from the Jackson Laboratory (B6.129S7-Ifngtm1Ts/J). The starting age of mice in each experiment was 6 weeks for active vaccination studies and 12 weeks for adoptive transfer studies. Mice were maintained under pathogen-free conditions and fed laboratory chow and water ad libitum. All animal experiments were conducted in compliance with guidelines approved by the NIAID institutional animal care and use committee.
Vaccination and Infection
For active immunization studies, mice were vaccinated intramuscularly into the hind limb 3 times at 2-week intervals with lethally irradiated USA300 in PBS or mock-vaccinated with PBS alone. For passive serum transfer studies, serum samples from mice previously vaccinated with lethally irradiated USA300 or serum samples from mock-vaccinated mice were pooled and administered intraperitoneally to naive C57BL/6 mice 24 hours before bacterial challenge. For depletion studies, CD4-depleting GK1.5 or isotype control antibodies (BioXCell) were injected intraperitoneally (0.5 mg per mouse) 48 hours before bacterial challenge. Depletion was repeated with anti-CD4 antibodies (0.5 mg per mouse) 72 hours after infection.
In select experiments, IFN-γ–neutralizing XMG1.2, interleukin 17 (IL-17)–neutralizing 17F3, tumor necrosis factor (TNF) α–neutralizing XT3.11 antibodies, or the isotype control antibodies (BioXCell) were injected intraperitoneally (0.5 mg per mouse) 24 hours before bacterial challenge. Neutralization of IFN-γ, IL-17, and TNF-α was repeated with of anti–IFN-γ, anti–IL-17, anti–TNF-α, or isotype control antibodies (0.5 mg per mouse) every other day, for a total of 6 treatments after infection. To inhibit T-helper (Th) 1 differentiation, mIL-12p40–neutralizing antibody (C17.8) or isotype control antibody (BioXCell) was injected intraperitoneally (0.5 mg per mouse) 24 hours before, at the time of, and 24 hours after vaccination. Mice were infected intravenously via the lateral tail vein, with approximately 1 × 107 CFUs of USA300 in a 200-μL volume of PBS, 2 weeks after the last active vaccination or 24 hours after passive transfer of serum samples. Animals were monitored for survival and morbid effects (weight loss, hunched posture, lethargy, and ruffled fur) for up to 15 days after infection. Mock-vaccinated mice served as controls.
Serum Antibody Titers
Blood samples collected before first and 7 days after each vaccination were centrifuged in serum separator tubes, and serum samples were stored at −80°C until further use in enzyme-linked immunosorbent assays. Briefly, 96-well plates were coated with 1 × 107 CFUs of γ-irradiated USA300 spa deletion mutant overnight at 4°C. Plates were blocked with 10% fetal bovine serum in PBS for 2 hours at room temperature. Serum samples were prepared in semilog dilutions, using 1% fetal bovine serum in PBS as diluent. Plates were washed 3 times, and sample dilutions were applied in a 100-μL volume per well. Plates were incubated for 1 hour at room temperature and washed 3 times before application of the conjugate, goat anti-mouse immunoglobulin G (heavy and light chains)–horseradish peroxidase in diluent. Plates were incubated for 1 hour at room temperature, washed as described above, and incubated with TMB (3,39,5,59-tetramethylbenzidine) to detect horseradish peroxidase for 20 minutes. Optical density at 450 nm was measured using a Beckman Coulter DTX 880 plate reader. Data analysis for full-dilution curves was performed using the GraphPad Prism software program, version 6.
Bacterial Load of Blood and Organs
Organs were homogenized in 2-mL round-bottomed tubes (Eppendorf) containing 500 μL of PBS and a single 5-mm stainless steel bead (Qiagen), using the Qiagen TissueLyser LT set at 50 oscillations per second. Blood samples and organ homogenates were streaked out on BHI agar (Becton Dickinson), either undiluted or in serial dilutions in PBS, and CFUs were counted after an overnight incubation at 37°C.
Cell Isolation and In Vitro Restimulation
Single-cell suspensions of spleens were prepared by mechanical disruption and dispersion through 40-μm pore-size cell strainers. Red blood cells were lysed with ACK lysis buffer (Lonza), washed and once more filtered through 40-μm pore-size cell strainers. Cells were counted, and 2 × 106 cells were seeded per well into a 96-well plate and stained for surface antigens. Cells were then taken up in Fix/Perm buffer (eBioscience) for permeabilization and fixation overnight before intracellular staining was performed the next day. For cytokine staining, cells were resuspended in Dulbecco’s minimum essential medium (Life Technologies), at 2 × 106 cells per well in a 96-well plate, and incubated in the presence or absence of 1 × 107 CFUs of lethally irradiated USA300 or anti-CDC3/CD28 at 37°C, 5% carbon dioxide for 4 hours in the presence of brefeldin A. Cells were then surface stained and fixed/permeabilized overnight, and intracellular staining was performed the next day.
Flow Cytometry
Antibodies against mouse surface and intracellular antigens and cytokines were purchased from eBioscience, Biolegend, and BD Biosciences and used in 10-color flow cytometry, either biotinylated or directly conjugated. The antibodies used were directed against CD4 (clone RM4-5), CD8 (clone 52–6.7), CD44 (clone IM7), Foxp3 (clone FJK-16s), Tbet (clone 4B10), RORγt (clone B2D), GATA-3 (clone TWAJ), CD45 (clone 30-F11), TNF-α (clone MP6-XT22), IL-10 (clone JESS-16E3), IFN-γ (clone XMG1.2), IL-17A (clone 17B7), IL-22 (clone IL22JOP), Ki67 (clone B56), IL-3 (clone 13A), and IL-5 (clone TRFK5). Biotinylated antibodies were detected with streptavidin- conjugated BV605 or BV510 (eBioscience). Near-infrared fixable live-dead cell stain was purchased from Molecular Probes–Invitrogen and used in accordance with the manufacturer’s protocol. All samples were acquired on a LSRII flow cytometer (BD Biosciences) and analyzed with FlowJo software (Tree Star) version 10.1r5.
Histology
Kidneys and livers of mice were harvested on day 5 after infection for histological evaluation. Representative tissue samples from the aforementioned organs were resected from the formalin-fixed (10% neutral buffered formalin) tissues for each animal and placed into histopathology cassettes. Processing of the samples was completed by Histoserv. Briefly, tissue was embedded in paraffin, sectioned at 5 µm, and stained with hematoxylin-eosin for routine histopathology. Sections were examined by means of light microscopy using an Olympus BX51 microscope, and photomicrographs were taken using an Olympus DP73 camera. Histopathological scoring was determined in a blinded fashion, as described in (Table 1).
Table 1.
Kidney Histological Scoringa
| Group | Mouse Identifier | Neutrophil Infiltration | Tissue Destruction and Necrosis | Bacterial Aggregates | Total Score, Mean |
|---|---|---|---|---|---|
| Mock-vaccination | A | 0 | 0 | 0 | 3.3 |
| B | 3 | 3 | 0 | ||
| C | 2 | 2 | 0 | ||
| Vaccination plus isotype | A | 2 | 2 | 0 | 8.3 |
| B | 4 | 4 | 4 | ||
| C | 3 | 2 | 4 | ||
| Vaccination plus anti–IL-17 | A | 3 | 4 | 4 | 8.7 |
| B | 2 | 2 | 2 | ||
| C | 2 | 3 | 4 | ||
| Vaccination plus anti–IFN-γ | A | 4 | 1 | 0 | 6.7 |
| B | 3 | 1 | 0 | ||
| C | 4 | 4 | 3 |
Abbreviations: IFN, interferon; IL-17, interleukin 17.
aKidney sections from each mouse from the indicated groups (3 mice per group) were individually evaluated in a blinded fashion for the presence of neutrophil infiltration, tissue destruction and necrosis, and bacterial aggregates. Scores within each category were ranked by severity according to the following scale: 0, absent; 1, minimal; 2, mild; 3, moderate; and 4, severe/abundant. Within each group, the sum of scores across each pathological category was averaged for the 3 mice per group to obtain the mean total score.
Statistical Analysis
Statistically significant differences in mortality rates between groups in bacterial challenge studies were analyzed by means of log-rank (Mantel-Cox) test using GraphPad Prism software, version 6. Mann–Whitney test using 2-tailed P values and 1-way analysis of variance followed by Bonferroni multiple-comparison test were used to determine significant differences between groups, as indicated in figure legends. Nonparametric tests were chosen to avoid assumption of normal distribution. Individual data points were displayed to show variation within each group of data.
RESULTS
Decreased Survival After Bacterial Challenge in Mice Vaccinated With Lethally Irradiated MRSA
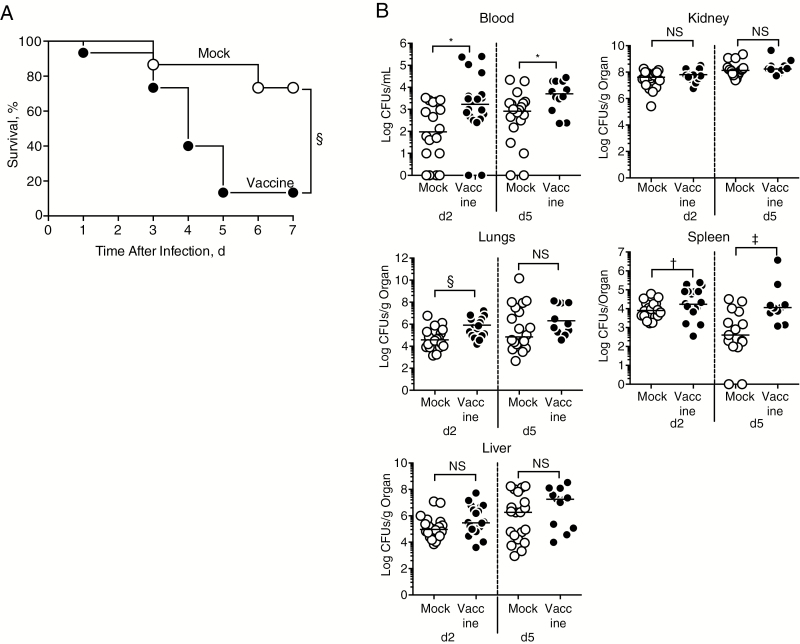
We have previously shown that exposure of bacteria to lethal γ-irradiation preserves immunogenic epitopes that elicit protective immunity in models of Listeria, anthrax, and cutaneous S. aureus infection [13–15]. In this current study, we vaccinated mice with lethally irradiated MRSA (strain USA300 LAC) as a tool to investigate the immune response induced against the multiple epitopes provided by this whole-cell vaccine. Vaccination did not elicit protection when mice were challenged intravenously with a lethal dose of live MRSA (data not shown), and challenge with a sublethal dose of 1 × 107 CFUs of live MRSA revealed increased mortality rates in vaccinated compared with mock-vaccinated mice (Figure 1A). Vaccination failed to control bacterial growth in blood and multiple organs on days 2 and 5 after infection and actually led to increased bacterial burdens in lungs, spleen, and blood (Figure 1B).
Figure 1.
Vaccination with lethally irradiated methicillin-resistant Staphylococcus aureus (MRSA) decreases survival after live challenge. A, Percentage of survival in mice vaccinated with phosphate-buffered saline (mock) or lethally irradiated MRSA (vaccine) after intravenous infection (1 × 107 colony-forming units [CFUs]). Each group included 15 mice, and results were pooled from 3 independent experiments. Significant differences between groups were determined using log-rank (Mantel-Cox) test. §P <.001. B, Bacterial load in blood and organs of mock-vaccinated and vaccinated mice on days 2 and 5 after infection (d2 and d5); each group included 20 mice, except for the vaccinated group at day 5, with 12 mice; results are pooled from 2 independent experiments. Significant differences in median values were determined using Mann–Whitney test. *P = .01; †P = .048; ‡P = .006; §P <.001.
Role of CD4 T Cells in Detrimental Vaccine Effects
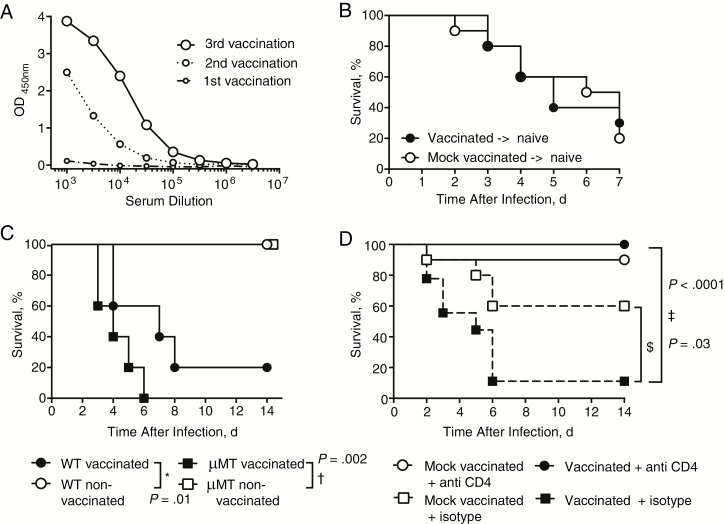
Vaccination induced USA300-specific serum antibodies (Figure 2A). Antibody responses generated against staphylococcal antigens can enhance virulence during infection [16]. To assess the potential contribution of antibodies to vaccine-induced deaths, we transferred serum samples from vaccinated or mock-vaccinated mice into naive mice. Transfer of immune serum samples from vaccinated mice into naive mice did not increase mortality rates on live challenge (Figure 2B). Furthermore, we vaccinated wild-type and B-cell–deficient (μMT) mice. Vaccinated animals of both strains showed similarly increased susceptibility to infection compared with mock-vaccinated controls (Figure 2C). These data indicate that B-cell antibody responses were not responsible for the observed vaccine-induced deaths.
Figure 2.
CD4 T cells, but not B cells or antibodies, drive vaccine-induced deaths. A, Pooled serum samples of vaccinated mice, collected 10 days after the first, second, and third vaccination were tested for USA300-specific antibodies by enzyme-linked immunosorbent assay. Each group included 10 mice; results are representative of 3 independent experiments. B, Percentage survival in mice after intravenous infection with 2 × 107 colony-forming units (CFUs) of live methicillin-resistant Staphylococcus aureus (MRSA) on receipt of serum from naive or previously vaccinated mice. Each group included 10 mice; results are pooled from 2 independent experiments. C, Percent survival of vaccinated or mock-vaccinated wild-type (WT) and B-cell–deficient (μMT) mice after intravenous MRSA infection (1 × 107 CFUs). Each group included 5 mice; results represent a single experiment. D, Percentage survival in vaccinated or mock-vaccinated mice after intravenous MRSA infection (1 × 107 CFUs) and treatment with 500 μg of CD4 T-cell–depleting or isotype control antibodies. Each group included 10 mice; results represent a single experiment. Significant differences between 2 groups were determined using log-rank (Mantel-Cox) test. *P = .01; †P = .002; ‡P < .0001; $P = .03.
CD4 T cells have been reported to contribute to vaccine-induced protection against both cutaneous and systemic models of S. aureus infection [8–10, 17]. However, reports on induction of CD4 T-cell–mediated immunopathological effects by viral vaccines [18, 19] prompted us to evaluate a potential detrimental role of vaccine-induced CD4 T cells. Vaccinated and mock- vaccinated mice received CD4 T-cell–depleting or isotype control antibodies before infection. The efficacy of CD4 T-cell depletion in blood of vaccinated and nonvaccinated mice was confirmed 2 days after treatment with CD4 T-cell–depleting or isotype control antibodies (Supplemental Figure 1). Depletion of CD4 T cells completely reversed the lethal phenotype of vaccination and resulted in 100% survival (Figure 2D). Depletion of CD4 T cells in mock-vaccinated mice had no significant effect on survival after infection. Taken together, these data indicate that CD4 T cells primed during vaccination with lethally irradiated MRSA conferred the deleterious effects seen during subsequent infection.
Characterization of CD4 T Cells Induced by Vaccination
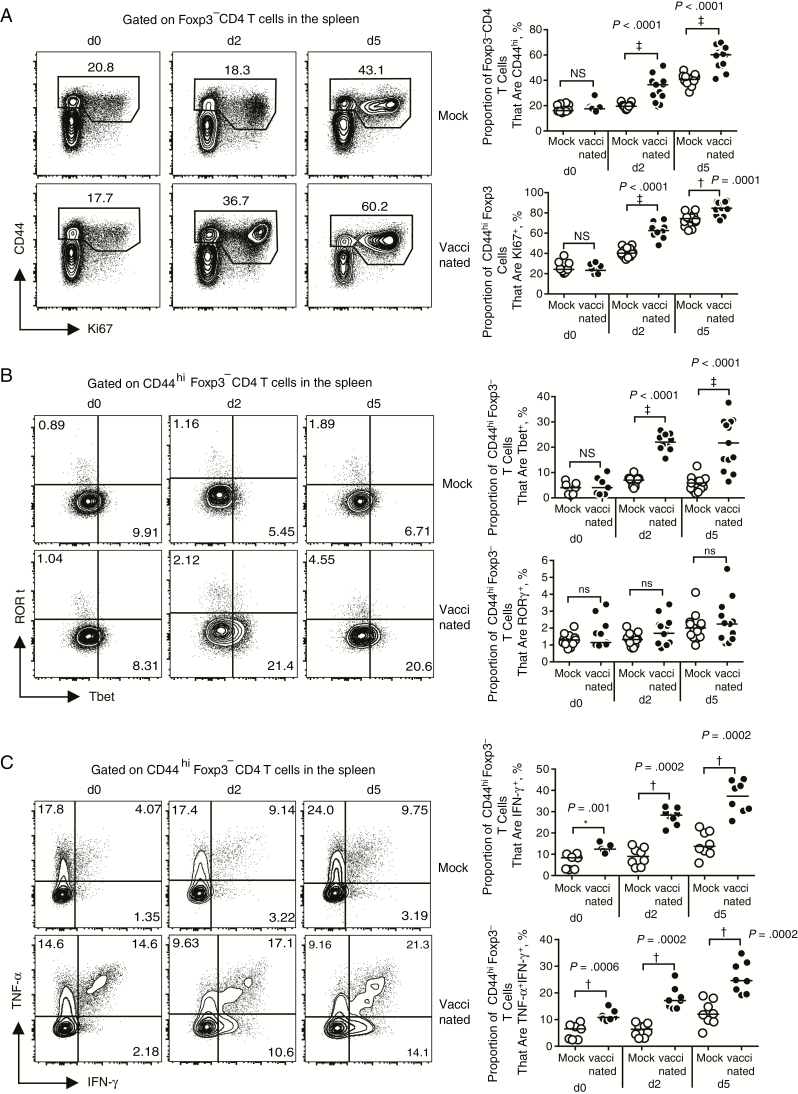
We next analyzed vaccine-generated CD4 T cells for their phenotypic and functional properties by using the gating strategy shown in Supplemental Figure 2. Infection with live MRSA revealed a significant increase in effector memory CD4 T cells (CD44hi) that were highly proliferative (Ki67+) in vaccinated compared with mock-vaccinated mice (Figure 3A). Further analysis of these vaccine-induced CD4 T cells showed that vaccination elicited a Th1 (Tbet+) effector T-cell response when compared with mock-vaccinated mice (Figure 3B). In contrast, there was no significant induction by vaccination of Th17 (RORγt+; Figure 3B) or Th2 (GATA3+; not shown) cells.
Figure 3.
Whole-cell vaccination induces a robust Th1 response. A, Representative fluorescence-activated cell sorting (FACS) plots (left) and summary data (right) of splenocytes from mock-vaccinated (mock) or vaccinated mice before (day 0 [d0]) and on days 2 and 5 [d2 and d5] after infection. Isolated cells were analyzed by flow cytometry for the frequency of CD4 T effector memory cells (CD44hi) and proliferation (Ki67+). Groups included 10 (day 0) or 13 (days 2 and 5) mice. ‡P < .0001; NS, not significant. B, Representative FACS plots (left) and summary data (right) for frequency of CD44hi Foxp3− CD4 T cells from mock-vaccinated or vaccinated mice that are Tbet+ or RORγt+ before infection (day 0) and on days 2 and 5 after infection. Groups included 7–11 (day 0) or 10–13 (days 2 and 5) mice. ‡P < .0001. C, Representative FACS plots (left) and summary data (right) for frequency of CD44hi Foxp3− CD4 T cells that produce interferon (IFN) γ or tumor necrosis factor (TNF) α on restimulation with anti-CD3/CD28. Each group included 8 mice. †P = .0002; *P < .01. Results are pooled from 3 (A, B) or 2 (C) independent experiments. Significant differences of median values were determined using the Mann–Whitney test.
Analysis of splenic effector CD4 T cells after restimulation with anti-CD3/CD28 revealed that vaccination increased the frequency of IFN-γ+ and TNF-α+IFN-γ+ cells (Figure 3C), consistent with the induction of Th1 cells. Their frequency was amplified after infection and was highest by day 5 (Figure 3C). There was no significant increase in TNF-α single-positive cells, nor a difference in IL-17 production between vaccinated and mock-vaccinated groups (Supplemental Figure 3A). Antigen-specific restimulation with lethally irradiated MRSA revealed a similar pattern of Th1 cytokine response but also increased the frequency of TNF-α single-positive cells in vaccinated groups compared with mock-treated groups (Supplementary Figure 3B). Restimulation did not induce detectable Th17 (IL-17 and interleukin 22) or Th2 (interleukin 5 and 13) cytokines (not shown). The data above suggest that the Th1 response induced by vaccination was responsible for the observed deaths after infection.
Role of IFN-γ in Detrimental Vaccination Effects
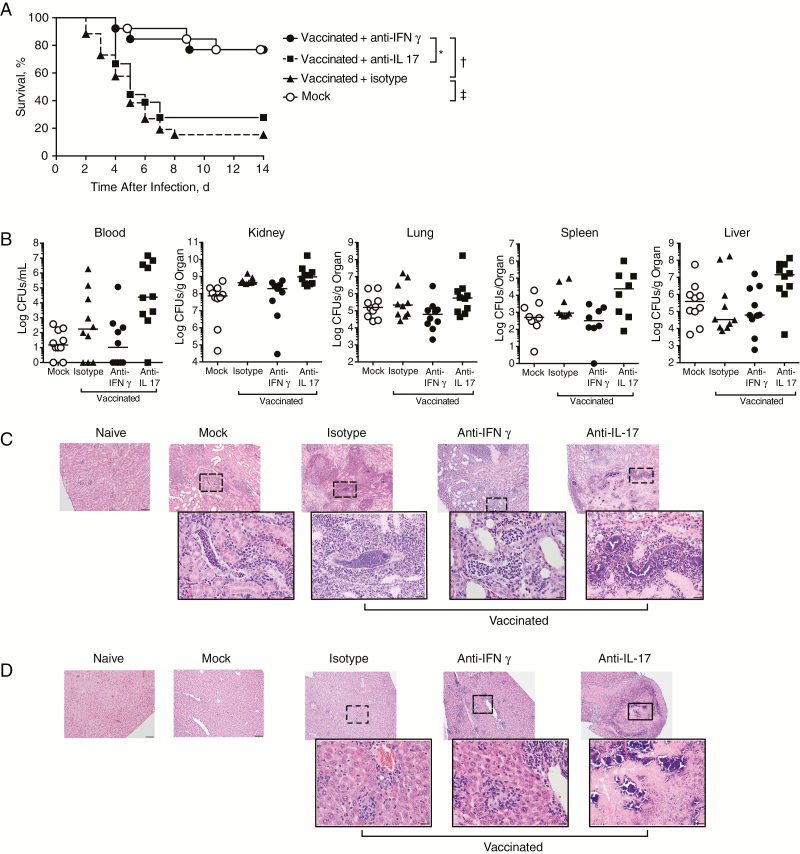
To test the hypothesis that vaccine-induced deaths were driven by the Th1-defining cytokine IFN-γ, we neutralized IFN-γ during infection. Anti–IFN-γ reversed the increased mortality rates seen in vaccinated mice, whereas isotype control antibody, neutralization of IL-17, or neutralization of TNF-α did not (Figure 4A and Supplemental Figure 4). Neutralization of IFN-γ did not alter bacterial loads in blood and organs compared with control groups (Figure 4B), indicating that organ CFU count was not solely driving the observed effects on survival.
Figure 4.
Interferon (IFN) γ neutralization reverses vaccine-induced reduction in survival. A, Survival in mock-vaccinated and vaccinated mice after intravenous infection on repeated treatment with 500 μg of anti–IFN-γ, anti–interleukin 17 (IL-17), or isotype control antibodies. Groups included 13 (vaccination plus anti–IFN-γ or mock-vaccination) or 25 (vaccination plus isotype) mice; results were pooled from 2 independent experiments. Significant differences between 2 groups were determined using log-rank (Mantel-Cox) test. *P = .007; †P < .001. B, Bacterial load in blood and organs assessed on day 5 after infection, with results pooled from 2 independent experiments. Data were analyzed using 1-way analysis of variance, followed by Bonferroni multiple-comparison test. No significant differences were detected between groups. C, D, Hematoxylin-eosin staining of representative kidney (C) and liver (D) sections from uninfected (naive) mice or on day 5 after infection of mock-vaccinated or vaccinated mice treated with the indicated antibodies (original magnification, ×10 for all photomicrographs and ×40 for insets). Each group included 3 mice, with 1 representative photomicrograph shown per group.
Because bacterial burden did not absolutely correlate with mortality in the above studies, we next histologically assessed cytokine-mediated effects of vaccination on organ pathology (Figure 4C and Table 1). The majority of infected mice displayed variable degrees of tubulointerstitial glomerulonephritits within the renal cortex. This was characterized by variably abundant neutrophilic infiltrates and adjacent renal tubules that exhibited histological signs of degeneration and necrosis, evidenced by renal tubular epithelial cells with diffusely hypereosinophilic cytoplasm and nuclei that exhibited coarsely clumped and disorganized chromatin. In addition to the presence of neutrophils, within some groups the renal tissues contained multiple areas of bacterial aggregation.
Compared with mock-vaccinated mice, vaccinated mice responded to infection with pronounced increases in bacterial aggregates, neutrophilic infiltration, and microabscesses accompanied by focal regions of coagulative tubular necrosis. IFN-γ neutralization during infection of vaccinated mice resulted in an overall attenuation of pathological changes with increased neutrophil infiltration that was associated with decreased bacterial aggregates and reduced tissue destruction. The most severe histopathological changes were observed after neutralization of IL-17, which resulted in renal tissues that contained myriads of bacteria intimately associated with focally extensive areas of coagulative tubular necrosis that displayed complete loss of normal tissue architecture. The effects of cytokine neutralization on liver histological findings followed a similar pattern to that seen in the kidneys, with pathological changes reduced by IFN-γ neutralization and exacerbated by IL-17 neutralization (Figure 4D).
Inhibition of Th1 Polarization During Vaccination
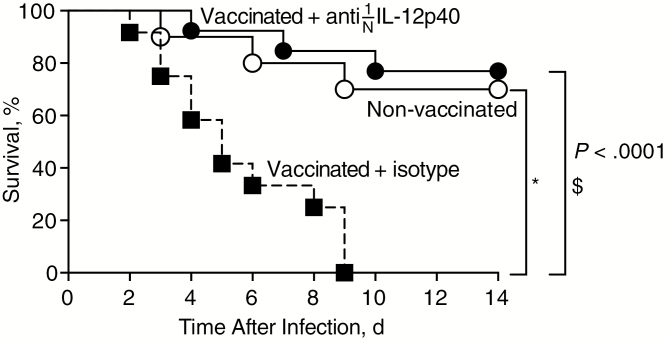
Based on our observations that the vaccine-induced Th1 response was detrimental, we hypothesized that preventing development of this response during vaccination would improve outcomes. Because polarization of Th1 cells is driven by IL-12 [20, 21], we neutralized IL-12 during vaccination to inhibit development of the Th1 response. Mice were vaccinated either in the presence of IL-12p40–neutralizing antibody or isotype control antibody. Neutralization of IL-12p40 at the time of vaccination significantly decreased the frequency of Th1 (Tbet+) cells and had no effect on the frequency of Th17 (RORγt+) cells measured 2 days after infection (Supplemental Figure 5). Neutralization of IL-12p40 reversed vaccine-related mortality during subsequent infection, supporting a deleterious role of vaccine-induced Th1 cells in this model (Figure 5).
Figure 5.
Inhibition of T-helper 1 development reverses vaccine-induced mortality. Mice were mock-vaccinated or vaccinated in the presence of anti– interleukin 12p40 (IL-12p40) or isotype control antibody and monitored for survival after intravenous infection. Groups included 13 (vaccination plus anti–IL 12p40), 12 (vaccination plus isotype), or 10 (mock-vaccination) mice; results were pooled from 2 independent experiments. Significant differences between 2 groups were determined using log-rank (Mantel-Cox) test. *P < .001; $P < .0001.
DISCUSSION
Our findings demonstrate that vaccination with lethally irradiated MRSA primes an antigen-specific Th1 CD4 T-cell response that fails to control subsequent MRSA infection and ultimately increases mortality rates and tissue immunopathological changes. This highlights the potential for induction of deleterious immune responses by vaccination against S. aureus. Our model may also hold implications for susceptibility to natural infection in humans that may be influenced by preexisting S. aureus–specific memory CD4 T cells that develop during prior exposure [22].
Although comparisons between our whole-cell vaccine and single-protein vaccines are limited, our findings are reminiscent of the vaccine-induced mortality seen in a recent human clinical trial with the Merck V710 vaccine targeting IsdB, a S. aureus cell surface protein [12]. The immunological basis for mortality in that vaccine trial is not clear, but the lower vaccine- induced IL-17 response seen in subjects who later succumbed to infection [23] suggested a protective role for IL-17 that is consistent with our IL-17 neutralization results. Preclinical vaccination studies with IsdB showed protective efficacy in mice that was dependent on Th17 but not Th1 responses [9]. Of note, the murine studies used an alum-adjuvanted IsdB vaccine, whereas the human V710 vaccine was unadjuvanted, perhaps contributing to the discrepancy in outcome between the mouse and human studies and the low Th17 response seen in the clinical trial.
Other single-antigen vaccine studies in mice have also identified Th17-dependent protection against subsequent systemic S. aureus challenge, and some have identified a protective contribution of Th1 cells [9, 10, 17, 24–26]. The discrepancy in the role of Th1 cells between those findings and our current findings probably reflects the magnitude of the Th1 response and lack of Th17 response induced by our whole-cell vaccine compared with these single antigens, but it may also be influenced by bacterial strain, site of infection, and other experimental parameters. Future experiments in strains of mice that are less prone to develop Th1 responses than C57BL/6 will be needed to determine whether underlying host immune bias influences vaccine outcome. Furthermore, there may be tissue-specific determinants of protective immunity, as evidenced by previously published work from our group showing that vaccination with a similar whole-cell S. aureus preparation successfully induced an adjuvant-dependent Th17 response that protected against a model of cutaneous infection in which IFN-γ seemed to not play a significant role [8].
Importantly, our studies show that manipulation of the cytokine milieu by neutralization of IL-12p40 during vaccination can influence the subsequent response to infection. Because vaccination did not induce a Th17 response in our studies, neutralization of IL-12p40 probably exerted its effects through the inhibition of its role in Th1 polarization. However, it should be noted that the IL-12p40 subunit also heterodimerizes with p19 to form interleukin 23, which is important for stabilization of Th17 cells that may develop during other vaccination circumstances [27]. Taken in context of the prior studies cited above, our data suggest that a critical balance of Th1 and Th17 response is needed to confer vaccine-mediated protection against S. aureus and identify the detrimental capacity of an overly exuberant Th1 response directed against a multiepitope vaccine.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. H.K designed and carried out all of the experiments, interpreted the results, analyzed the data, and wrote the manuscript; C.C.H carried out experiments and contributed to data analysis and to the manuscript; I.N.M performed histological analysis and pathology scoring and contributed to the manuscript; M. M contributed to execution of experiments and to data analysis; D.L.B contributed to the experimental design and data interpretation; S.K.D contributed to the experimental design and to the interpretation of results and wrote the manuscript.
Financial support. This work was supported by the Intramural Research Program of the NIAID, NIH.
Potential conflicts of interest. All authors; No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG., Jr Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev 2015; 28:603–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chambers HF, Deleo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol 2009; 7:629–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nizet V. Understanding how leading bacterial pathogens subvert innate immunity to reveal novel therapeutic targets. J Allergy Clin Immunol 2007; 120:13–22. [DOI] [PubMed] [Google Scholar]
- 4. Verhoeven PO, Gagnaire J, Botelho-Nevers E, et al. Detection and clinical relevance of Staphylococcus aureus nasal carriage: an update. Expert Rev Anti Infect Ther 2014; 12:75–89. [DOI] [PubMed] [Google Scholar]
- 5. Wertheim HF, Vos MC, Ott A, et al. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet 2004; 364:703–5. [DOI] [PubMed] [Google Scholar]
- 6. Fattom A, Schneerson R, Szu SC, et al. Synthesis and immunologic properties in mice of vaccines composed of Staphylococcus aureus type 5 and type 8 capsular polysaccharides conjugated to Pseudomonas aeruginosa exotoxin A. Infect Immun 1990; 58:2367–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pozzi C, Wilk K, Lee JC, Gening M, Nifantiev N, Pier GB. Opsonic and protective properties of antibodies raised to conjugate vaccines targeting six Staphylococcus aureus antigens. PLoS One 2012; 7:e46648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gaidamakova EK, Myles IA, McDaniel DP, et al. Preserving immunogenicity of lethally irradiated viral and bacterial vaccine epitopes using a radioprotective Mn2+-peptide complex from Deinococcus. Cell Host Microbe 2012; 12:117–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Joshi A, Pancari G, Cope L, et al. Immunization with Staphylococcus aureus iron regulated surface determinant B (IsdB) confers protection via Th17/IL17 pathway in a murine sepsis model. Hum Vaccin Immunother 2012; 8:336–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Spellberg B, Ibrahim AS, Yeaman MR, et al. The antifungal vaccine derived from the recombinant N terminus of Als3p protects mice against the bacterium Staphylococcus aureus. Infect Immun 2008; 76:4574–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Missiakas D, Schneewind O. Staphylococcus aureus vaccines: deviating from the carol. J Exp Med 2016; 213:1645–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fowler VG, Allen KB, Moreira ED, et al. Effect of an investigational vaccine for preventing Staphylococcus aureus infections after cardiothoracic surgery: a randomized trial. JAMA 2013; 309:1368–78. [DOI] [PubMed] [Google Scholar]
- 13. Datta SK, Okamoto S, Hayashi T, et al. Vaccination with irradiated Listeria induces protective T cell immunity. Immunity 2006; 25:143–52. [DOI] [PubMed] [Google Scholar]
- 14. Datta SK, Sabet M, Nguyen KP, et al. Mucosal adjuvant activity of cholera toxin requires Th17 cells and protects against inhalation anthrax. Proc Natl Acad Sci U S A 2010; 107:10638–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Myles IA, Fontecilla NM, Valdez PA, et al. Signaling via the IL-20 receptor inhibits cutaneous production of IL-1β and IL-17A to promote infection with methicillin-resistant Staphylococcus aureus. Nat Immunol 2013; 14:804–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yoong P, Pier GB. Antibody-mediated enhancement of community-acquired methicillin-resistant Staphylococcus aureus infection. Proc Natl Acad Sci U S A 2010; 107:2241–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lin L, Ibrahim AS, Xu X, et al. Th1-Th17 cells mediate protective adaptive immunity against Staphylococcus aureus and Candida albicans infection in mice. PLoS Pathog 2009; 5:e1000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Penaloza-MacMaster P, Barber DL, Wherry EJ, et al. Vaccine-elicited CD4 T cells induce immunopathology after chronic LCMV infection. Science 2015; 347:278–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Varga SM, Wang X, Welsh RM, Braciale TJ. Immunopathology in RSV infection is mediated by a discrete oligoclonal subset of antigen-specific CD4+ T cells. Immunity 2001; 15:637–46. [DOI] [PubMed] [Google Scholar]
- 20. Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol 2003; 3:133–46. [DOI] [PubMed] [Google Scholar]
- 21. Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations. Annu Rev Immunol 2010; 28:445–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kolata JB, Kühbandner I, Link C, et al. The fall of a dogma? unexpected high T-cell memory response to Staphylococcus aureus in humans. J Infect Dis 2015; 212:830–8. [DOI] [PubMed] [Google Scholar]
- 23. McNeely TB, Shah NA, Fridman A, et al. Mortality among recipients of the Merck V710 Staphylococcus aureus vaccine after postoperative S. aureus infections: an analysis of possible contributing host factors. Hum Vaccin Immunother 2014; 10:3513–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brown AF, Murphy AG, Lalor SJ, et al. Memory Th1 cells are protective in invasive Staphylococcus aureus infection. PLoS Pathog 2015; 11:e1005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Choi SJ, Kim MH, Jeon J, et al. Active immunization with extracellular vesicles derived from Staphylococcus aureus effectively protects against staphylococcal lung infections, mainly via Th1 cell-mediated immunity. PLoS One 2015; 10:e0136021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Narita K, Hu DL, Mori F, Wakabayashi K, Iwakura Y, Nakane A. Role of interleukin-17A in cell-mediated protection against Staphylococcus aureus infection in mice immunized with the fibrinogen-binding domain of clumping factor A. Infect Immun 2010; 78:4234–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Teng MW, Bowman EP, McElwee JJ, et al. IL-12 and IL-23 cytokines: from discovery to targeted therapies for immune-mediated inflammatory diseases. Nat Med 2015; 21:719–29. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.