In this phase 1 human immunodeficiency virus vaccine trial of PENNVAX-G DNA, administered by Biojector 2000 or CELLECTRA electroporation device, boosted by modified vaccinia Ankara–Chiang Mai double recombinant, the vaccine was safe with similar immunogenicity observed between the DNA delivery arms.
Keywords: HIV vaccine, modified vaccinia Ankara, electroporation, needle-free injection
Abstract
Background
We report the first-in-human safety and immunogenicity evaluation of PENNVAX-G DNA/modified vaccinia Ankara–Chiang Mai double recombinant (MVA-CMDR) prime-boost human immuonodeficiency virus (HIV) vaccine, with intramuscular DNA delivery by either Biojector 2000 needle-free injection system (Biojector) or CELLECTRA electroporation device.
Methods
Healthy, HIV-uninfected adults were randomized to receive 4 mg of PENNVAX-G DNA delivered intramuscularly by Biojector or electroporation at baseline and week 4 followed by intramuscular injection of 108 plaque forming units of MVA-CMDR at weeks 12 and 24. The open-label part A was conducted in the United States, followed by a double-blind, placebo-controlled part B in East Africa. Solicited and unsolicited adverse events were recorded, and immune responses were measured.
Results
Eighty-eight of 100 enrolled participants completed all study injections, which were generally safe and well tolerated, with more immediate, but transient, pain in the electroporation group. Cellular responses were observed in 57% of vaccine recipients tested and were CD4 predominant. High rates of binding antibody responses to CRF01_AE antigens, including gp70 V1V2 scaffold, were observed. Neutralizing antibodies were detected in a peripheral blood mononuclear cell assay, and moderate antibody-dependent, cell-mediated cytotoxicity activity was demonstrated.
Discussion
The PVG/MVA-CMDR HIV-1 vaccine regimen is safe and immunogenic. Substantial differences in safety or immunogenicity between modes of DNA delivery were not observed.
Clinical Trials Registration
An effective preventative vaccine is urgently needed to combat the global human immunodeficiency virus (HIV) epidemic. Although several advanced-stage clinical trials have been performed, only the RV144 Thai trial has shown protection against HIV type 1 (HIV-1) infection, achieving modest efficacy with an ALVAC-HIV/AIDSVAX B/E regimen [1–6]. Poxvirus vectors, such as the canarypox ALVAC-HIV, represent a promising strategy for vaccination against HIV-1 [7]. Modified vaccinia Ankara (MVA), a replication-deficient, attenuated vaccinia virus, is a poxvirus vector developed during the smallpox eradication campaign [8]. Human immunodeficiency virus vaccine regimens including MVA-vectored vaccines with and without DNA priming have been demonstrated to be safe and immunogenic in early phase clinical trials [9–17]. Cognate DNA/MVA prime-boost simian immunodeficiency virus vaccine regimens have also shown promise in the macaque model [18–21]. Novel DNA delivery methods may improve the immunogenicity of DNA priming [22–26]: in human clinical trials, both electroporation and needle-free injection devices have demonstrated improved cellular immunogenicity in DNA-containing regimens compared with standard intramuscular injection [23, 27, 28].
In RV262, we evaluated the safety and immunogenicity of PENNVAX-G (PVG) DNA, administered by Biojector 2000 (Biojector) or CELLECTRA electroporation device, boosted by modified vaccinia Ankara–Chiang Mai double recombinant (MVA-CMDR) in healthy HIV-uninfected adults in the United States and East Africa. This study represents the first-in-human experience of PVG DNA delivered by either method and of the PVG DNA/MVA-CMDR prime-boost combination. It is also the first direct comparison of HIV DNA administration by electroporation or needle-free injection device.
METHODS
Participants and Study Design
The study was a multicenter, randomized trial to evaluate safety and immunogenicity of a prime-boost regimen of 4 mg of PVG DNA delivered intramuscularly by needle-free injection or electroporation at baseline (week 0) and 4 weeks, followed by intramuscular injection of 108 plaque-forming units of MVA-CMDR at 12 and 24 weeks (Supplementary Table 1). The open-label part A was conducted in Rockville, Maryland. Safety evaluation of part A was performed before initiation of part B, which was placebo controlled and conducted in Kampala, Uganda; Kericho, Kenya; and Mbeya, Tanzania. Study participants were healthy, at low risk of HIV acquisition, and had normal baseline electrocardiograms. The protocol was approved by institutional and ethical review boards at the Walter Reed Army Institute of Research, Kenya Medical Research Institute, Tanzanian National Institute of Medical Research, and Ugandan National HIV/AIDS Research Committee. Written informed consent was obtained from each participant. The study was registered at ClinicalTrials.gov (NCT01260727). All vaccines were administered in the deltoid muscle. Except for the assessment of immediate pain, reactogenicity was assessed at 45 minutes, 6 hours, and then daily for 6 days after injection. Adverse events were recorded at all study visits from baseline to study completion. After screening, laboratory monitoring included routine hematology, chemistry, and creatine phosphokinase measured at weeks 0, 2, 6, 12, 14, 24, 26, 37, and 50, with troponin I measured 2 weeks after each MVA/placebo injection. Immunogenicity assessments were performed on cryopreserved specimens collected at baseline and at weeks 6, 14, 26, and 50. All participants provided phlebotomy specimens for ELISpot and binding antibody evaluations; a subset were selected for additional evaluation by intracellular cytokine staining (ICS), neutralizing antibody (NAb), and antibody-dependent cell-mediated cytotoxicity (ADCC) assays. Participants were given the option of participating in a mucosal substudy that collected semen and cervico-vaginal mucus for quantification of binding antibodies. Cervico-vaginal mucus was collected using the Instead Softcup (The Flex Company, Venice, CA).
Vaccine Product and Delivery
PENNVAX-G is a mixture of 4 DNA plasmids that encode consensus HIV immunogens selected to develop an internationally relevant DNA vaccine. Plasmids pEY1E1 (pGX1001), pEY3E1 (pGX1002), and pEY4E1 (pGX1004) encode consensus Env (gp140) immunogens for HIV subtypes A, C, and D, respectively [29, 30]. Plasmid pMC-Gag (pGX1005) encodes a multiclade (subtypes A, B, C, and D) consensus Gag immuonogen. All 4 plasmids use the modified pVAX1 expression vector [24](pGX0001) with changes only to the HIV sequences inserted into the gene expression cassette. Participants were randomized to intramuscular PVG administration by either the Biojector 2000 Needle Free Injection System (formerly Biojector, Inc, Bedminster, NJ; presently Inovio Pharmaceuticals, Plymouth Meeting, PA) or the CELLECTRA electroporation device (Inovio Pharmaceuticals). The Biojector 2000 uses sterile, single-use syringes that deliver the study material using a compressed carbon dioxide cartridge. The CELLECTRA electroporation device delivers 3 pulses at 0.5 A constant current, with a 52-millisecond pulse length and a 1-second rest between pulses following injection of the DNA by needle and syringe via a hand-held applicator [23, 31].
Modified vaccinia Ankara–Chiang Mai double recombinant is a recombinant, live-attenuated modified vaccinia virus-vectored vaccine genetically engineered to express HIV-1 gp150 (CRF01_AE, isolate CM235) and Gag and Pol (integrase-deleted and nonfunctional reverse transcriptase, subtype A, isolate CM240) [32, 33]. The MVA-CMDR was delivered intramuscularly by needle and syringe.
Immunogenicity
ELISpot
Interferon γ (IFN-γ) ELISpot responses were measured to vaccine-matched Env, Gag, and Pol expression products (Supplementary Table 2) at baseline, week 6, and week 26 [5, 34]. Ninety-six–well hydrophobic membrane-bottom plates (Millipore, Billerica, MA) were coated overnight at 4°C with antihuman IFN-γ monoclonal antibody (MAb; Mabtech, Nacka Strand, Sweden). A panel of vaccine insert–matched Env, Gag, and Pol peptide pools was used to stimulate 2 × 105 peripheral blood mononuclear cells (PBMCs) per well overnight at 37°C in 5% carbon dioxide. Captured IFN-γ was incubated with a biotinylated antihuman IFN-γ MAb (Mabtech), and peroxidase staining was performed using 3-amino-9-ethylcarbazole substrate (Vectastain AEC Kit, Vector Laboratories, Burlingame, CA). Results are expressed as spot-forming cells (SFCs) per 106 PBMCs. A positive IFN-γ response was defined as ≥55 SFCs per 106 PBMCs (uncorrected) and at least 4 times the average of the dimethyl sulfoxide–treated wells.
Intracellular Cytokine Staining
Among vaccine recipients with available specimen, 29 ELISpot responders and 9 nonresponders were selected for additional characterization by qualified intracellular cytokine staining for IFN-γ at weeks 0, 14, and 26 [16]. Cells were stimulated with vaccine-matched peptide pools for CMDR Env and CMDR Gag (1 μg/mL for each peptide), with positive control phorbol myristate acetate (PMA, 1μg/mL) and ionomycin (1μg/mL), or with dimethyl sulfoxide containing media alone. Stimulations were performed in the presence of CD107a PE-Cy7 (clone H4A3), CD154 PE-Cy5 (clone TRAP-1), and the costimulatory molecules CD28/CD49d MAbs (Becton Dickenson, San Jose, CA) for 4 hours. Following stimulation, cells were stained with Aqua Live/Dead, followed by surface staining with MAbs for CD14 (clone M5E2), CD19 (clone HIB19), and CD56 clone HCD56—all BV510—and CD4-BV605 (clone RPA-T4). Peripheral blood mononuclear cells were permeabilized and stained intracellularly with MAbs against IFN-γ–eFluor450 (clone 4S.B3) and CD8-PerCp efluor 710 (clone SK1), TNF-α–FITC (clone MAb11), CD3-APC-H7 (clone SK7), IL-4–APC (clone MP4-25D2), and IL-2–PE (clone MQ1-17H12). Cells were acquired on an FACS LSRII SORP cytometer (Becton Dickenson) and analyzed using Flow Jo (TreeStar, Inc, Ashland, OR). A median 110000 (range, 16668–209000) CD3+ lymphocytes were acquired. A positive response was defined by a 2-fold increase over the unstimulated condition and a frequency >0.025% of CD8 or CD4 T cells. The criteria for positivity was not validated but used to increase sensitivity for this assay.
Binding Antibody
Recombinant gp120 CRF01_AE A244 and scaffold gp70 V1V2 Env protein (subtype B case A2 and CRF01_AE 92TH023) antigens were expressed and purified [35–37]. Plasma enzyme-linked immunosorbent assay binding antibody (bAb) endpoint titers were performed [35]. Antibody titers were determined at weeks 0, 6, 14, 26, and 50. Antibody titers were calculated as a reciprocal plasma dilution using serial 2-fold dilutions and expressed as end-point titers; geometric mean titers (GMTs) were calculated from end-point titers.
Neutralizing Antibody
Neutralizing antibody responses were measured in a subset of participants comprised of 10 of 13 participants from part A and roughly half of the participants from part B (n = 20/42 in Uganda, n = 12/22 in Kenya, and n = 12/23 in Tanzania). Participants were selected equally from different administration routes with preferential inclusion of those with high binding antibody titers. Serum was evaluated at weeks 0, 26, and 50. The TZMbl pseudovirus neutralizing assay was performed at a screening serum dilution of 1:20 [38], using a panel of 6 tier 1 pseudoviruses encoding HIV-1 Env genes, including BaL (subtype B), GS015 (subtype C), A03349M1 (subtype D), 271 (CRF02_AG), TH023 (CRF01_AE) and CM235 (CRF01_AE), as well as a pseudovirus expressing the murine leukemia virus Env as a negative control. Neutralization using PBMCs as target cells was also conducted using replication-competent Renilla reniformis luciferase (LucR)–expressing HIV-1 infectious molecular clones encoding HIV-1 Env genes, including CM235, TH023, SF162 (subtype B), and GS015 [39]. All sera were titered to achieve a 50% inhibitory dose in the PBMC/LucR infectious molecular clone assay.
Antibody-Dependent Cell-Mediated Cytotoxicity
Antibody-dependent cell-mediated cytotoxicity responses were measured to the subtype B MN protein and to the CRF01_AE CM235 protein at weeks 0, 6, 26, and 50 in participants with available specimen. The ADCC activity was measured by flow cytometry using a rapid fluorometric ADCC assay [40]. The CEM-NKr T lymphoblast cell line expressing CCR5 was labeled with the intracellular dye CFSE and the membrane dye PKH26 and then pulsed with gp120 proteins (5 µg/mL). Healthy donor PBMCs and plasma from the vaccinated participants were added to the labeled CEM-NKr cells for 6 hours. The cell mix was fixed, and the proportion of cells that maintained membrane expression of PKH26 but lost intracellular CFSE (PKH26+CFSE−) was analyzed by flow cytometry. Data are displayed as percentage of lysed target cells (%PKH26+CFSE−) at a plasma dilution of 1:1000. The cutoff for positivity was defined by the average of the antigen-pulsed PKH26+CFSE− CEM-NKr target cells incubated with normal human serum.
Statistical Analysis
All analyses combined part A and part B study participants. Safety analyses are based on the intent-to-treat principle and include all participants in the group to which they were randomized. For the presentation of reactogenicity data, number and percentage of participants reporting each type of sign or symptom were tabulated by severity and study group. Each participant’s reactogenicity is reported once under the maximum severity for all injection visits, and differences between groups were assessed using Fisher’s exact test. ELISpot responses are presented as responder frequency to individual and cumulative peptide pools across tested visits. Intracellular cytokine staining, binding antibody, neutralizing antibody, and ADCC responses are plotted by DNA delivery device and week number, with week 0 representing baseline values. Flow cytometry analysis and presentation of distributions were performed using SPICE version 5-1.2, downloaded from http://exon.niaid.nih.gov/spice [41]. Comparison of distributions was performed using a Student’s t test and a partial permutation test [41]. All other group comparisons were made using nonparametric tests and SAS (Cary, NC) or GraphPad PRISM (La Jolla, CA) software.
RESULTS
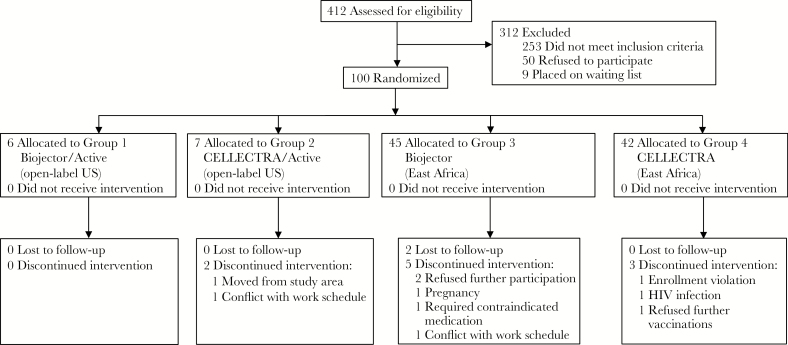
Among the 100 participants enrolled, the median age was 27 years (range, 18–48 years) and 24 (24%) were female. Of 13 part A participants in the United States, 10 (77%) were non-white; all part B participants were African (Table 1). Thirteen participants were enrolled in part A, and 11 completed all injections. Eighty-seven participants were enrolled in part B (n = 42 in Uganda, n = 22 in Kenya, and n = 23 in Tanzania), and 77 completed all injections. Forty-four male participants contributed semen, and 11 female participants provided cervico-vaginal mucus specimens. Reasons for study discontinuation are detailed in Figure 1 and included 1 pregnancy and 1 HIV infection following the second DNA vaccination.
Table 1.
Participant Characteristics
| Characteristic | Biojector active | Electroporation Active | Biojector placebo | Electroporation placebo | P value | Total |
|---|---|---|---|---|---|---|
| Planned enrollment | 38 | 38 | 8 | 8 | 92 | |
| Actual enrollmenta | 43 | 41 | 8 | 8 | 100 | |
| Sex | ||||||
| Male | 31 (72.1) | 33 (80.5) | 5 (62.5) | 7 (87.5) | .56 | 76 |
| Female | 12 (27.9) | 8 (19.5) | 3 (37.5) | 1 (12.5) | 24 | |
| Ethnicity/race | ||||||
| White, non-Hispanic | 2 (4.7) | 1 (2.4) | 0 (0) | 0 (0) | NA | 3 |
| African or African-American, non-Hispanic | 41 (95.4) | 39 (95.1) | 8 (100) | 8 (100) | 96 | |
| Hispanic | 0 (0) | 0 (0) | 0 (0) | 0 | 0 | |
| Other | 0 (0) | 1 (2.4) | 0 (0) | 0 (0) | 1 | |
| Age, y | 26 (18–47) | 29 (18–48) | 22 (18–43) | 27 (20–39) | .40 | 27 (18–48) |
| Body mass index, kg/m2 | 22.1 (18.3–30.1) | 21.9 (18.0–32.7) | 23.0 (19.8–26.6) | 20.7 (18.3–24.7) | .50 | 21.9 (18.0–32.7) |
| Vaccinations received | ||||||
| 1st (PV-G/placebo; month 0) | 43 (100) | 41 (100) | 8 (100) | 8 (100) | 100 | |
| 2nd (PV-G/placebo; month 1) | 40 (93.0) | 41 (100) | 8 (100) | 8 (100) | 97 | |
| 3rd (MVA-CMDR/placebo; month 3) | 38 (88.4) | 36 (87.8) | 7 (87.5) | 8 (100) | 89 | |
| 4th (MVA-CMDR/placebo; month 6) | 37 (86.0) | 36 (87.8) | 7 (87.5) | 8 (100) | 88 | |
| All | 37 (86.0) | 36 (87.8) | 7 (87.5) | 8 (100) | 88 |
Data are n. (%) of participants or median (range). Fisher’s exact test used for comparisons by sex; Wilcoxon rank sum test used for age and body mass comparisons.
Abbreviation: NA, not applicable.
aReplacements were allowed until enrollment was closed at each site.
Figure 1.
Screening, enrollment, vaccinations, and follow-up. Part A and part B volunteer activities are combined. Abbreviation: HIV, human immunodeficiency virus.
Safety and Tolerability
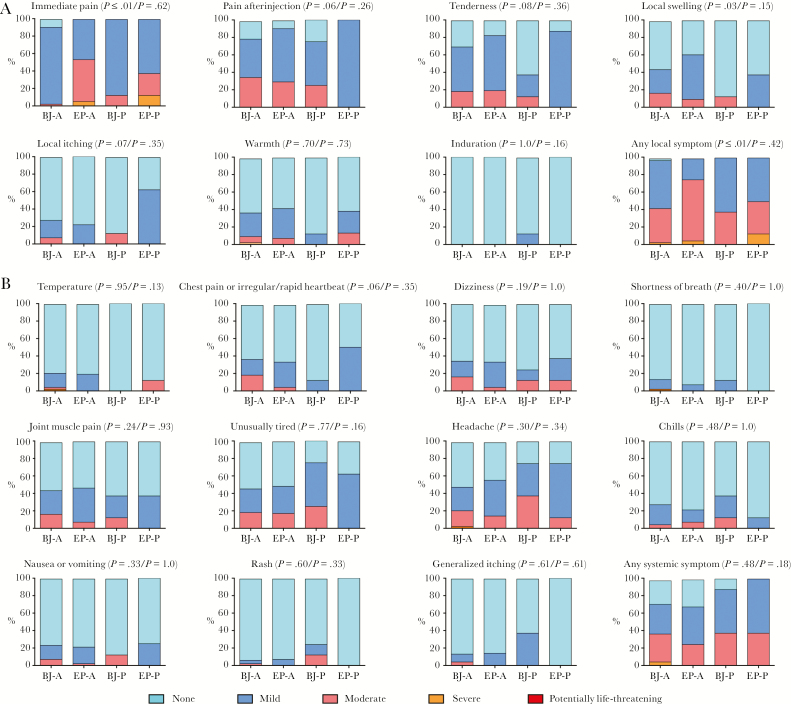
Immunizations were safe and well tolerated. There were no study pauses or study-related serious adverse events. There were no discontinuations due to study-related adverse events. Reactogenicity was predominantly mild to moderate (Figure 2). Grade 3 episodes included an isolated fever of 40.1°C 6 hours after the 4th vaccination, headache on day 3 after the 3rd vaccination, and warmth at the injection site 6 hours after the 2nd vaccination. Immediate pain at the injection site was reported as moderate or severe by 4% (n = 2/51) of Biojector recipients compared with 51% (n = 25/49) of electroporation recipients (P < .0001), and local swelling was also more common in electroporation recipients (P = .03). There were no differences among groups in experiencing local pain or systemic reactogenicity at subsequent timepoints. Study-related adverse events in vaccine recipients were mild or moderate, the latter comprised of epistaxis (n = 1), neutropenia (n = 1), and tonsillitis (n = 1) that were classified as possibly related.
Figure 2.
Maximum local and systemic reactogenicity. Safety assessment showing maximum local reactogenicity (A) and systemic reactogenicity (B) by treatment group. All reactions except immediate pain were assessed at 45 minutes, 6 hours, and then daily for 6 days after injection. P values shown are for Fisher’s exact test for differences between electroporation and Biojector followed by differences between active vaccine and placebo recipients. Abbreviations: A, active vaccine; BJ, Biojector; EP, electroporation; P, placebo.
Immunogenicity
ELISpot
Peripheral blood mononuclear cells from Uganda, Kenya, and the United States were included in the IFN-γ ELISpot analysis, whereas PBMCs from Tanzania were excluded from cellular immunogenicity analyses due to high background activation. The majority of responses were directed against MVA-CMDR Env followed by MVA-CMDR Gag. Fewer responses were observed against PVG DNA inserts, with no responses against MVA-CMDR Pol. One vaccine recipient showed a positive baseline IFN-γ ELISpot response, and 1 placebo recipient showed a positive response at weeks 6 and 26. Interferon γ ELISpot responses were infrequent at week 6 following PVG DNA administration, with 2 of 31 (7%) Biojector recipients and 3 of 29 (10%) electroporation recipients having a response against any Env and/or any Gag. At week 26 after the 2nd MVA injection, 19 of 30 (63%) and 12 of 24 (50%) participants showed a positive IFN-γ ELISpot response against any Env or any Gag with previous PVG DNA delivery by Biojector and electroporation, respectively (P = not significant) (Supplementary Table 3). There were no significant differences between DNA delivery methods in number of responders or in response magnitude.
Intracellular Cytokine Staining
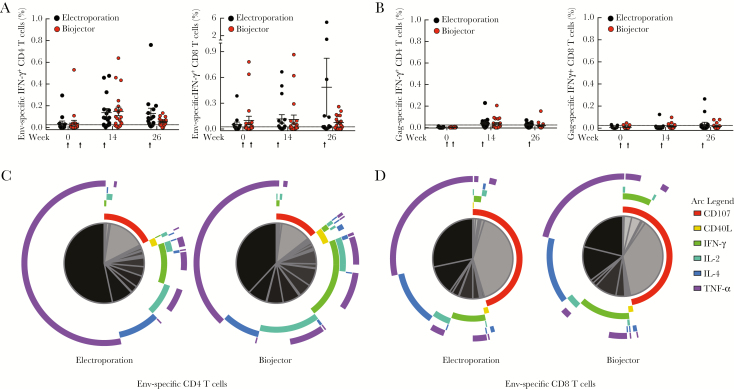
Intracellular cytokine staining was used to further characterize cellular immune responses generated in 38 vaccine recipients: 29 IFN-γ ELISpot responders and 9 ELISpot nonresponders (Figure 3 and Supplementary Table 4). CD4 IFN-γ responses were predominant and peaked at week 26 against CMDR Env, with 13 of 18 (72%) recipients in the electroporation group and 15 of 19 (79%) recipients in the Biojectory group developing responses (Figure 3A). Cumulative Env-specific CD4 T-cell responses developed in 32 of 38 (84%) recipients (Supplementary Table 4). CD8 T-cell IFN-γ responses against HIV-1 CMDR Env were observed, albeit at a lower frequency, in 23 of 38 (61%) participants tested (Figure 3A). CMDR Gag-specific CD4 and CD8 T-cell IFN-γ cumulative responses were demonstrated in 23 of 38 (61%) and 10 of 38 (26%) vaccine recipients, respectively (Figure 3B and Supplementary Table 4). Functional responses (Figure 3C and D) for HIV-1 Env-specific CD4 T cells included TNF-α (>50%) expression by the majority of cells. Other CD4 T-cell responses were distributed among those that expressed IFN-γ, IL-4, IL-2, and CD107a. The majority of Env-specific T cells were monofunctional, expressing only TNF-α, CD107a, or IL-4. The HIV-1 Env-specific CD8 T-cell responses were dominated by those expressing either TNF-α or CD107a; combinations of functions were rarely observed. There were no differences in the number of responders, magnitude of the response, or polyfunctionality between the electroporation and Biojector groups.
Figure 3.
Human immunodeficiency virus type 1 (HIV-1)−specific T-cell responses measured by intracellular cytokine staining. HIV-1 Env Chiang Mai double recombinant (CMDR)–specific (A) and Gag CMDR–specific (B) interferon γ (IFN-y)+CD4 T cells (left) and IFN-y+CD8 T cells (right) were measured in vaccine recipients at weeks 0, 14, and 26. Vaccination timepoints are indicated with an arrow. The responses were measured in both electroporation (black) and Biojector (red) groups, and mean (+ standard error) is indicated. Positive responses (for both CD4 and CD8) were defined as cytokine responses that had a 2-fold increase over the unstimulated condition and >0.025%. Multifunctional flow cytometry analysis of HIV-1 Env-specific responses at week 14 (2 weeks after 1st modified vaccinia Ankara) with the different pie chart arcs representing each cytokine function (purple = tumor necrosis factor α, blue = interleukin 4, turquoise = interleukin 2, green = IFN-γ, yellow = CD40L, red = CD107a). Relative sizes for subsets of cells expressing the various combinations of functions are proportionally represented in the pie chart with individual slices of pie colored from gray to black. Specific functional components of each slice of pie are denoted based on arc. HIV-1 Env-specific CD4 T-cell responses (C) and CD8 T-cell responses (D) are shown for both electroporation (left) and Biojector (right) groups. Abbreviations: IFN-γ, interferon γ; IL-2, interleukin 2; IL-4, interleukin 4; TNF-α, tumor necrosis factor α.
Binding Antibody
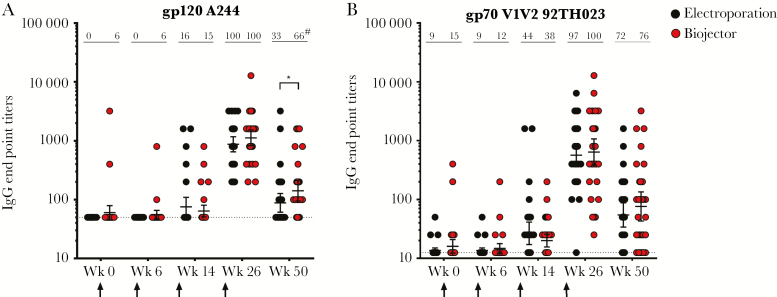
Binding antibody responses were minimal after DNA administration and peaked after the 2nd MVA injection (Figure 4). All vaccinated participants had bAb responses to gp120 A244 at week 26 with GMTs of 1120 and 872 for Biojector and electroporation recipients, respectively, and those responses declined significantly by week 50. Geometric mean titers (141 vs 88; P = .02) and response rates (66% vs 33%; P = .01) to A244 gp120 were higher in the Biojector group compared with the electroporation group at week 50 (Figure 4A). Responses to gp70 V1V2 (92TH023) scaffold were demonstrated in 100% of Biojector and 97% of electroporation recipients at week 26 with GMTs of 639 and 566, respectively (Figure 4B). V1V2 antibody responses also declined in both groups by week 50, with no differences between Biojector and electroporation delivery (Figure 4B). Antibodies to gp70 V1V2 subtype B case A2 scaffold were weak and were only detected in 3 US participants at peak immunogenicity (data not shown). Furthermore, no bAb responses were detected in cervico-vaginal mucus or seminal plasma at the lowest dilution tested (1:25; data not shown).
Figure 4.
Plasma binding antibody responses. Plasma binding antibody (bAb) titers were determined by enzyme-linked immunosorbent assay using gp120 (A244) (A) and gp70 (B) V1V2 scaffold (92TH023) CRF01_AE antigens. Samples were evaluated at weeks 0, 6, 14, 26, and 50 for both electroporation (black) and Biojector (red) groups. Responses are shown for vaccine recipients only; response rates as percentage of responses are depicted across the top of each column, with geometric mean titers and 95% confidence intervals indicated in the figure. Baseline values for each antigen are shown as a dotted line. Vaccination timepoints are indicated with an arrow. *P = .02 by 2-tailed Mann-Whitney test. #P = .01 by Fisher’s exact test. Abbreviation: IgG, immunoglobulin G.
Neutralizing Antibody
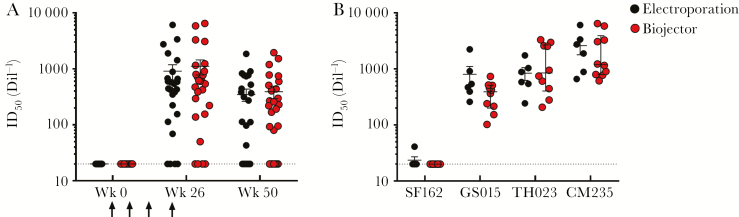
TZMbl screening neutralization assays were negative for all participants tested against 6 tier 1 pseudoviruses, including CM235 (data not shown). The PBMC neutralization assay using the CRF01_AE CM235 LucR infectious molecular clone detected neutralization in 84% (n = 43/51) of participants at week 26 in both the Biojector and electroporation groups (Figure 5A); prevaccination sera were negative in this assay. Neutralization activity observed at week 26 was shown to be antibody mediated and partially NK-cell dependent. Titers were reduced to baseline when immunoglobulin G was depleted from the serum (supplementary figure 1A), and neutralization activity was reduced when NK cells were depleted from the PBMC target population (supplementary figure 1B). Participants with the highest titers to CM235 (n = 10 in the Biojector group and n = 6 in the electroporation group) were further evaluated using additional LucR infectious molecular clones at week 26. The subtype C GS015 and CRF01_AE TH023 infectious molecular clones were neutralized by sera from all 16 participants evaluated, whereas minimal neutralization was observed with the subtype B SF162 infectious molecular clone (Figure 5B). Statistical differences were not observed between immunization routes.
Figure 5.
Antibody-mediated inhibition of human immunodeficiency virus infection in the peripheral blood mononuclear cell (PBMC) neutralizing antibody (NAb) assay. Vaccine recipient sera were titered in the PBMC NAb assay; reported values are the mean of 2 independent experiments with 50% inhibitory dose values within a 5-fold range. Sera from weeks 0, 26, and 50 were tested against the subtype CRF01_AE CM235 rnLuc infectious molecular clone (n = 25 for electroporation; n = 26 for Biojector; panel A). Vaccination timepoints are indicated with an arrow. Sera from 16 vaccine recipients at the peak immune response (week 26) were titered against 3 additional infectious molecular clones from multiple subtypes, including SF162 (subtype B), GS015 (subtype C), and TH023 (CRF01_AE) (n = 6 for electroporation; n = 10 for Biojector; panel B). Data are plotted by DNA immunization route, electroporation (black) or Biojector (red), and mean (+ standard error) is indicated. Abbreviation: Dil, dilution; ID50, 50% inhibitory dose.
Antibody-Dependent Cell-Mediated Cytotoxicity
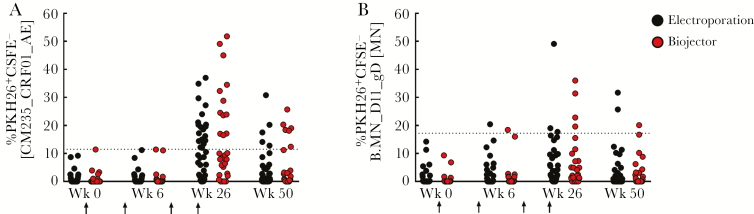
No ADCC activity was detected in the placebo recipients at any time point tested or in evaluated vaccine recipients at baseline. There was no CRF01_AE CM235–specific ADCC activity detected in vaccine recipients at week 6, whereas at week 26, 12 of 29 (41%) Biojector recipients and 19 of 36 (53%) electroporation recipients showed ADCC activity, respectively (Figure 6A). The median magnitude of PKH26+CFSE− CEM-NKr target cells among responders was 26.7% and 19.4% for Biojector and electroporation administration of PENNVAX-G DNA, respectively (P = .02, Mann-Whitney test). Antibody-dependent cell-mediated cytotoxicity activity against CM235 correlated with neutralization activity demonstrated in the PBMC assay (P = .002) (supplementary figure 1C). In contrast, the B.MN_D11_gD−specific ADCC response, which is not matched to the vaccine insert, was less frequent at week 26 with 4 of 29 (14%) in the Biojector and 3 of 36 (8%) in the electroporation group displaying a positive response (Figure 6B).
Figure 6.
Antibody-dependent cell-mediated cytotoxicity (ADCC). Magnitude of ADCC response in vaccine recipients was measured using gp120-coated targets labeled with PKH26 and CFSE at weeks 0, 6, 26, and 50. The frequency of lysed cells was defined by the loss of CFSE and retention of PKH26, resulting in the emergence of a “killed” PKH26+CSFE− population. Antibody-dependent cell-mediated cytotoxicity response using gp120-coated target cells with CRF01_A/E (CM235) (A) and subtype B (MN) (B) were used, with the threshold for positive responses at 11.5% and 17.2%, respectively. Vaccination timepoints are indicated with an arrow. Data are plotted by DNA immunization route, electroporation (black) or biojector (red). Response rates were not significantly different between DNA delivery groups.
DISCUSSION
The PVG/MVA-CMDR prime-boost regimen was generally safe and well tolerated across DNA delivery methods. We observed minimal reactogenicity, with systemic symptoms occurring at a rate similar to placebo. Participants in the electroporation arm reported significantly more local swelling and pain, but difference in local pain was restricted to the short time immediately after product administration and resolved quickly, consistent with previous observations [31]. Local pain measured at the diary time points was not significantly different between DNA delivery methods. We did not observe cardiac symptoms nor evidence of myo/pericarditis, adding to the growing body of literature documenting the cardiac safety of MVA-vectored HIV vaccines [42].
As seen in other DNA/MVA prime-boost HIV vaccines, the PENNVAX-G DNA/MVA-CMDR regimen induced T-cell responses in the majority of vaccine recipients, including CD4 and CD8 responses. However, in this study, they were both primarily directed towards Env. Gag-specific ICS responses were less robust than those observed in early phase trials of other DNA/MVA HIV vaccines [12, 14, 15]. The nature of the HIV Env-specific CD4 T-cell response in this study was more monofunctional and included higher levels of CD107a-producing cells compared with a study of MVA-CMDR administration alone [16]. CD4 responses expressing CD40L, IL-2, and IL-4, which were associated with decreased risk of HIV infection in RV144 [43], were not frequently observed. There were high numbers of baseline responders measured by ICS (up to 39%). The high level of baseline activity could be reflective of less stringent criteria for positivity, compared with a more common 3 times background and a frequency >0.05% of CD4 or CD8 T cells [16, 44], or may represent potential cross-reactivity of peptides in the peptide pools.
In the RV144 trial, V1V2 scaffold binding antibody responses were a correlate of decreased risk for HIV acquisition [5, 45]. Therefore, it is intriguing that recipients of the PVG/MVA-CMDR regimen had frequent responses to V1V2 scaffold as well as to gp120, particularly for CRF01_AE antigens. Further, gp120 responses were higher and more frequent at study conclusion in the Biojector group, suggesting improved durability for that particular response with DNA delivery by needle-free injection. However, bAb responses were generally low in titer and NAb responses were not detectable in the TZMbl assay. Responses were detectable in the PBMC neutralization assay, both to homologous and cross-subtype heterologous infectious molecular clones. Neutralizing activity in the PBMC assay may reflect NK cell effector function (supplementary figure 1B) [39]. Indeed, PBMC NAb results correlated with observed ADCC responses, which were moderate and highest to vaccine-homologous antigen (supplementary figure 1C). For enhanced humoral responses, subsequent prime-boost regimens may benefit from the addition of a protein component.
Because this study lacked an MVA-only arm, we are unable to demonstrate the independent contribution of the DNA prime to post-boost immune responses. However, we can observe that, across cellular and humoral immunogenicity measures, there was no appreciable advantage of electroporation over needle-free device delivery of the DNA prime for this PVG DNA/MVA-CMDR regimen. Although not directly comparable, this is consistent with data from the TaMoVac02 Trial, which evaluated a DNA/MVA-CMDR approach with or without protein boost and demonstrated no augmentation of immune responses with intradermal electroporation (Derma Vax) when it was added to intradermal DNA delivery by needle-free jet injection [46].
In the HVTN 070/080 studies evaluating the role of electroporation delivery and interleukin 12 adjuvant with PENNVAX-B DNA, a third DNA vaccine dose was important for induction of HIV-specific cellular responses [23]. Additional evaluation is warranted to assess the role of the DNA prime, optimize additional delivery parameters and number of DNA vaccinations, and determine if a protein component in the regimen, either as a boost or coadministered with DNA priming, can improve the magnitude and functional characteristics of the vaccine-elicited immune response.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. We thank the RV262 study participants and study teams in the United States, Uganda, Tanzania, and Kenya for their contribution to the conduct of the study; Safety Monitoring Committee members, led by Chair Dr Eric Sandstrom, for their commitment to volunteer safety; US Military HIV Research Program and Armed Forces Research Institute of Medical Sciences immunomonitoring laboratory technicians for their production of immunogenicity data; Dr Michael Pensiero, Ms Tina Tong, Dr Elizabeth Adams, Dr Ana Martinez, Dr Phillip Renzullo of the Division of AIDS/National Institute of Allerfy and Infectious Diseases (NIAID) and COL (Dr) Robert O’Connell, and Dr Mark de Souza of AFRIMS for their expert support and contributions to study design and conduct.
Disclaimer. Material has been reviewed by the Walter Reed Army Institute of Research. There is no objection to its publication. The investigators have adhered to the policies for protection of human subjects as prescribed in AR 70–25. The opinions or assertions contained herein are the private views of the author and are not to be construed as official or as reflecting true views of the Department of the Army or the Department of Defense.
Mary Marovich participated in this research while employed by the US Military HIV Research Program, WRAIR. Dr Marovich is currently with Division of AIDS, NIAID. The opinions expressed in this article are the author’s own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the United States government.
Financial support. This work was supported by the Department of Defense through cooperative agreements (W81XWH-07-2-0067 and W81XWH-11–0174) with the Henry M. Jackson Foundation for the Advancement of Military Medicine and by the NIAID through an interagency agreement with the US Army Medical Research and Materiel Command (Y1-AI-2642-17). N. Y. S., A. S. K., J. Y., and D. B. W. gratefully acknowledge funding support for HIV vaccine development from NIH/NIAID/Division of AIDS under an HIV Vaccine Design and Development Team contract awarded to Inovio Pharmaceuticals (HHSN272200800063C).
Potential conflicts of interest. N. Y. S., A. S. K., and J. Y. are employees of Inovio Pharmaceuticals, Inc. D. B. W. has received personal fees and nonfinancial support from Inovio Pharmaceuticals, Inc, outside the submitted work and has intellectual property in connection with PENNVAX-G. J. Y. reports grants from Inovio Pharmaceuticals and related intellectual property. B. M. is an inventor on US government-owned patents on basic poxvirus vector technology. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: HIV Research for Prevention 2014, Cape Town, South Africa, October 29, 2014; Abstract P26.02.
References
- 1. Hammer SM, Sobieszczyk ME, Janes H et al. ; HVTN 505 Study Team Efficacy trial of a DNA/rAd5 HIV-1 preventive vaccine. N Engl J Med 2013; 369:2083–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gray GE, Allen M, Moodie Z et al. ; HVTN 503/Phambili study team Safety and efficacy of the HVTN 503/Phambili study of a clade-B-based HIV-1 vaccine in South Africa: a double-blind, randomised, placebo-controlled test-of-concept phase 2b study. Lancet Infect Dis 2011; 11:507–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Flynn NM, Forthal DN, Harro CD, Judson FN, Mayer KH, Para MF; rgp120 HIV Vaccine Study Group Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J Infect Dis 2005; 191:654–65. [DOI] [PubMed] [Google Scholar]
- 4. Pitisuttithum P, Gilbert P, Gurwith M et al. ; Bangkok Vaccine Evaluation Group Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J Infect Dis 2006; 194:1661–71. [DOI] [PubMed] [Google Scholar]
- 5. Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S et al. ; MOPH-TAVEG Investigators Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med 2009; 361:2209–20. [DOI] [PubMed] [Google Scholar]
- 6. Buchbinder SP, Mehrotra DV, Duerr A et al. ; Step Study Protocol Team Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 2008; 372:1881–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pantaleo G, Esteban M, Jacobs B, Tartaglia J. Poxvirus vector-based HIV vaccines. Curr Opin HIV AIDS 2010; 5:391–6. [DOI] [PubMed] [Google Scholar]
- 8. Mayr A, Stickl H, Müller HK, Danner K, Singer H. The smallpox vaccination strain MVA: marker, genetic structure, experience gained with the parenteral vaccination and behavior in organisms with a debilitated defence mechanism. Zentralbl Bakteriol B 1978; 167:375–90. [PubMed] [Google Scholar]
- 9. Aboud S, Nilsson C, Karlén K et al. Strong HIV-specific CD4+ and CD8+ T-lymphocyte proliferative responses in healthy individuals immunized with an HIV-1 DNA vaccine and boosted with recombinant modified vaccinia virus ankara expressing HIV-1 genes. Clin Vaccine Immunol 2010; 17:1124–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gudmundsdotter L, Nilsson C, Brave A et al. Recombinant modified vaccinia Ankara (MVA) effectively boosts DNA-primed HIV-specific immune responses in humans despite pre-existing vaccinia immunity. Vaccine 2009; 27:4468–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bakari M, Aboud S, Nilsson C et al. Broad and potent immune responses to a low dose intradermal HIV-1 DNA boosted with HIV-1 recombinant MVA among healthy adults in Tanzania. Vaccine 2011; 29:8417–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goepfert PA, Elizaga ML, Sato A et al. ; National Institute of Allergy and Infectious Diseases HIV Vaccine Trials Network Phase 1 safety and immunogenicity testing of DNA and recombinant modified vaccinia Ankara vaccines expressing HIV-1 virus-like particles. J Infect Dis 2011; 203:610–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goepfert PA, Elizaga ML, Seaton K et al. ; HVTN 205 Study Group; National Institutes of Allergy and Infectious Diseases HIV Vaccines Trials Network Specificity and 6-month durability of immune responses induced by DNA and recombinant modified vaccinia Ankara vaccines expressing HIV-1 virus-like particles. J Infect Dis 2014; 210:99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nilsson C, Hejdeman B, Godoy-Ramirez K et al. HIV-DNA given with or without intradermal electroporation is safe and highly immunogenic in healthy Swedish HIV-1 DNA/MVA vaccinees: a phase I randomized trial. PLoS One 2015; 10:e0131748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sandström E, Nilsson C, Hejdeman B et al. ; HIV Immunogenicity Study 01/02 Team Broad immunogenicity of a multigene, multiclade HIV-1 DNA vaccine boosted with heterologous HIV-1 recombinant modified vaccinia virus Ankara. J Infect Dis 2008; 198:1482–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Currier JR, Ngauy V, de Souza MS et al. Phase I safety and immunogenicity evaluation of MVA-CMDR, a multigenic, recombinant modified vaccinia Ankara-HIV-1 vaccine candidate. PLoS One 2010; 5:e13983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Munseri PJ, Kroidl A, Nilsson C et al. Priming with a simplified intradermal HIV-1 DNA vaccine regimen followed by boosting with recombinant HIV-1 MVA vaccine is safe and immunogenic: a phase IIa randomized clinical trial. PLoS One 2015; 10:e0119629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kannanganant S, Gangadhara S, Lai L et al. Local control of repeated-dose rectal challenges in DNA/MVA-vaccinated macaques protected against a first series of simian immunodeficiency virus challenges. J Virol 2014; 88:5864–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lai L, Kwa S, Kozlowski PA et al. Prevention of infection by a granulocyte-macrophage colony-stimulating factor co-expressing DNA/modified vaccinia Ankara simian immunodeficiency virus vaccine. J Infect Dis 2011; 204:164–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Amara RR, Sharma S, Patel M et al. Studies on the cross-clade and cross-species conservation of HIV-1 Gag-specific CD8 and CD4 T cell responses elicited by a clade B DNA/MVA vaccine in macaques. Virology 2005; 334:124–33. [DOI] [PubMed] [Google Scholar]
- 21. Barouch DH, Liu J, Li H et al. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature 2012; 482:89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sardesai NY, Weiner DB. Electroporation delivery of DNA vaccines: prospects for success. Curr Opin Immunol 2011; 23:421–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kalams SA, Parker SD, Elizaga M et al. ; NIAID HIV Vaccine Trials Network Safety and comparative immunogenicity of an HIV-1 DNA vaccine in combination with plasmid interleukin 12 and impact of intramuscular electroporation for delivery. J Infect Dis 2013; 208:818–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Muthumani K, Wise MC, Broderick KE et al. HIV-1 Env DNA vaccine plus protein boost delivered by EP expands B- and T-cell responses and neutralizing phenotype in vivo. PLoS One 2013; 8:e84234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bråve A, Gudmundsdotter L, Sandström E et al. Biodistribution, persistence and lack of integration of a multigene HIV vaccine delivered by needle-free intradermal injection and electroporation. Vaccine 2010; 28:8203–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bråve A, Boberg A, Gudmundsdotter L et al. A new multi-clade DNA prime/recombinant MVA boost vaccine induces broad and high levels of HIV-1-specific CD8(+) T-cell and humoral responses in mice. Mol Ther 2007; 15:1724–33. [DOI] [PubMed] [Google Scholar]
- 27. Wang R, Epstein J, Baraceros FM et al. Induction of CD4(+) T cell-dependent CD8(+) type 1 responses in humans by a malaria DNA vaccine. Proc Natl Acad Sci U S A 2001; 98:10817–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vasan S, Hurley A, Schlesinger SJ et al. In vivo electroporation enhances the immunogenicity of an HIV-1 DNA vaccine candidate in healthy volunteers. PLoS One 2011; 6:e19252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yan J, Corbitt N, Pankhong P et al. Immunogenicity of a novel engineered HIV-1 clade C synthetic consensus-based envelope DNA vaccine. Vaccine 2011; 29:7173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Morrow MP, Tebas P, Yan J et al. Synthetic consensus HIV-1 DNA induces potent cellular immune responses and synthesis of granzyme B, perforin in HIV infected individuals. Mol Ther 2015; 23:591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Diehl MC, Lee JC, Daniels SE et al. Tolerability of intramuscular and intradermal delivery by CELLECTRA(®) adaptive constant current electroporation device in healthy volunteers. Hum Vaccin Immunother 2013; 9:2246–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Earl PL, Cotter C, Moss B et al. Design and evaluation of multi-gene, multi-clade HIV-1 MVA vaccines. Vaccine 2009; 27:5885–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brown BK, Darden JM, Tovanabutra S et al. Biologic and genetic characterization of a panel of 60 human immunodeficiency virus type 1 isolates, representing clades A, B, C, D, CRF01_AE, and CRF02_AG, for the development and assessment of candidate vaccines. J Virol 2005; 79:6089–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. de Souza MS, Ratto-Kim S, Chuenarom W et al. ; Ministry of Public Health–Thai AIDS Vaccine Evaluation Group Collaborators The Thai phase III trial (RV144) vaccine regimen induces T cell responses that preferentially target epitopes within the V2 region of HIV-1 envelope. J Immunol 2012; 188:5166–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Karasavvas N, Billings E, Rao M et al. ; MOPH TAVEG Collaboration The Thai phase III HIV type 1 vaccine trial (RV144) regimen induces antibodies that target conserved regions within the V2 loop of gp120. AIDS Res Hum Retroviruses 2012; 28:1444–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zolla-Pazner S, deCamp A, Gilbert PB et al. Vaccine-induced IgG antibodies to V1V2 regions of multiple HIV-1 subtypes correlate with decreased risk of HIV-1 infection. PLoS One 2014; 9:e87572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pinter A, Honnen WJ, Kayman SC, Trochev O, Wu Z. Potent neutralization of primary HIV-1 isolates by antibodies directed against epitopes present in the V1/V2 domain of HIV-1 gp120. Vaccine 1998; 16:1803–11. [DOI] [PubMed] [Google Scholar]
- 38. Wieczorek L, Krebs SJ, Kalyanaraman V et al. Comparable antigenicity and immunogenicity of oligomeric forms of a novel, acute HIV-1 subtype C gp145 envelope for use in preclinical and clinical vaccine research. J Virol 2015; 89:7478–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Joachim A, Nilsson C, Aboud S et al. Potent functional antibody responses elicited by HIV-I DNA priming and boosting with heterologous HIV-1 recombinant MVA in healthy Tanzanian adults. PLoS One 2015; 10:e0118486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gómez-Román VR, Florese RH, Patterson LJ et al. A simplified method for the rapid fluorometric assessment of antibody-dependent cell-mediated cytotoxicity. J Immunol Methods 2006; 308:53–67. [DOI] [PubMed] [Google Scholar]
- 41. Roederer M, Nozzi JL, Nason MC. SPICE: exploration and analysis of post-cytometric complex multivariate datasets. Cytometry A 2011; 79:167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Elizaga ML, Vasan S, Marovich MA et al. ; MVA Cardiac Safety Working Group Prospective surveillance for cardiac adverse events in healthy adults receiving modified vaccinia Ankara vaccines: a systematic review. PLoS One 2013; 8:e54407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lin L, Finak G, Ushey K et al. COMPASS identifies T-cell subsets correlated with clinical outcomes. Nat Biotechnol 2015; 33:610–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Horton H, Thomas EP, Stucky JA et al. Optimization and validation of an 8-color intracellular cytokine staining (ICS) assay to quantify antigen-specific T cells induced by vaccination. J Immunol Methods 2007; 323:39–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Haynes BF, Gilbert PB, McElrath MJ et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med 2012; 366:1275–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Viegas EO, Missanga M, Nilsson C et al. Intradermal electroporation of HIV-DNA vaccine followed by HIV-MVA boost wth or without addition of GLA adjuvanted CN54 rgp140: TaMoVac02. Chicago: HIV Research For Prevention; 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.