Carbonic anhydrase and mesophyll conductance are both limiting factors affecting CO2 assimilation rates at low pCO2 as examined in stably transformed lines of the C4 species, Setaria viridis.
Keywords: Carbonic anhydrase, C18O16O isotope discrimination, C4 photosynthesis, mesophyll conductance, Setaria viridis, transformation
Abstract
In C4 species, the major β-carbonic anhydrase (β-CA) localized in the mesophyll cytosol catalyses the hydration of CO2 to HCO3−, which phosphoenolpyruvate carboxylase uses in the first step of C4 photosynthesis. To address the role of CA in C4 photosynthesis, we generated transgenic Setaria viridis depleted in β-CA. Independent lines were identified with as little as 13% of wild-type CA. No photosynthetic defect was observed in the transformed lines at ambient CO2 partial pressure (pCO2). At low pCO2, a strong correlation between CO2 assimilation rates and CA hydration rates was observed. C18O16O isotope discrimination was used to estimate the mesophyll conductance to CO2 diffusion from the intercellular air space to the mesophyll cytosol (gm) in control plants, which allowed us to calculate CA activities in the mesophyll cytosol (Cm). This revealed a strong relationship between the initial slope of the response of the CO2 assimilation rate to cytosolic pCO2 (ACm) and cytosolic CA activity. However, the relationship between the initial slope of the response of CO2 assimilation to intercellular pCO2 (ACi) and cytosolic CA activity was curvilinear. This indicated that in S. viridis, mesophyll conductance may be a contributing limiting factor alongside CA activity to CO2 assimilation rates at low pCO2.
Introduction
C4 plants have evolved a CO2-concentrating mechanism (CCM) that enables the elevation of CO2 around the active sites of Rubisco by a combination of anatomical and biochemical specialization (Hatch, 1987). C4 photosynthesis has independently evolved >60 times, providing one of the most widespread and effective solutions for remedying the catalytic inefficiency of Rubisco (Sage et al., 2012; Christin and Osborne, 2013). The key carboxylases in C4 plants are localized to different cellular compartments. Phosphoenolpyruvate carboxylase (PEPC) is localized to the cytosol of mesophyll cells and Rubisco to the chloroplasts of bundle sheath cells. For the CCM to operate effectively, PEPC activity must exceed Rubisco activity to balance leakage of CO2 out of the bundle sheath compartment. This maintains a high bundle sheath CO2 level but prevents wasteful overcycling of the mesophyll CO2 ‘pump’ (von Caemmerer and Furbank, 2003). As PEPC utilizes HCO3− and not CO2, the first committed enzyme of the C4 pathway is carbonic anhydrase (CA) which catalyses the reversible conversion of CO2 and HCO3− in the cytosol of mesophyll cells. C4 acids produced by PEPC then diffuse into the bundle sheath cells where they are decarboxylated, supplying CO2 for Rubisco.
Within higher plants, there are multiple forms of the α-CA, β-CA, and γ-CA families which share little sequence homology (Moroney et al., 2001). β-CAs are the most prevalent CA family in land plants. CA is an abundant enzyme in C3 plants, representing up to 2% of the soluble leaf protein (Okabe et al., 1984). In C3 plants, the role of CA is unclear (Badger and Price, 1994) as it does not appear to limit photosynthesis but does influence stomatal conductance, guard cell movement, and amino acid biosynthesis (Hu et al., 2010; DiMario et al., 2016; Engineer et al., 2016).
It has long been contended that the uncatalysed rate of CO2 conversion to HCO3− is insufficient to support C4 photosynthetic flux (Hatch and Burnell, 1990; Badger and Price, 1994). This hypothesis was supported by experiments in the C4 dicot Flaveria bidentis, where antisense plants with <10% of wild-type CA activity required high CO2 for growth and showed reduced CO2 assimilation rates (von Caemmerer et al., 2004; Cousins et al., 2006). However, in the C4 monocot Zea mays mutant plants with reduced CA activity (3% of wild type), no limitation to CO2 assimilation rates at ambient CO2 was observed (Studer et al., 2014). CA activity has been shown to vary widely between species (Cousins et al., 2008), and it is unclear whether CA activities are limiting at high CO2 assimilation rates, as has previously been suggested (Hatch and Burnell, 1990; Gillon and Yakir, 2000).
We examined the role of CA in the model C4 monocot species Setaria viridis (green foxtail millet). Setaria viridis is closely related to agronomically important C4 crops including Z. mays (maize), Sorghum bicolor (sorghum), and Saccharum officinarum (sugarcane) (Brutnell et al., 2010). It is an ideal model species due to its rapid generation time, small stature, high seed production, diploid status, and small genome that is sequenced and publicly available (Doust et al., 2009; Brutnell et al., 2010; Li and Brutnell, 2011). Here we used a stable transformation approach to examine the role of CA in S. viridis and could show that S. viridis is a useful model species that lends itself to molecular manipulation of the C4 photosynthetic pathway. Two constructs both targeting the major leaf β-CA (Si003882m.g) were used to generate three independent transformed lines with reduced CA activity. A strong correlation between the CO2 assimilation rate at low pCO2 and CA activity was observed. Our combined measurements of mesophyll conductance and CA activity suggest that increasing mesophyll conductance may be an important way to increase the CO2 assimilation rate at low intercellular pCO2, as may occur under drought.
Materials and methods
Plant growth conditions
T1 seeds were incubated in 5% liquid smoke (Wrights) for 24h to promote germination, and germinated in garden soil mix fertilized with Osmocote (Scotts, Australia) in small containers before being transferred to individual 2 litre pots. Plants were grown in controlled environmental chambers, irradiance 500 µmol photons m−2 s−1, 16h photoperiod, 28 °C day, 24 °C night, 2% CO2. Pots were watered daily.
Construct generation
Two different constructs were used to generate three lines of reduced CA activity. First, an RNAi was targeted to the primary leaf β-CA Si003882m which generated lines 2.1 and 5.3. A region of Si003882m.g was amplified by PCR using gene-specific primers (Supplementary Table S1 at JXB online) and reverse-transcribed RNA from S. viridis leaves ligated into pENTR/D-TOPO (ThermoFisher), and verified by sequencing. The fragment was inserted via a double Gateway system LR reaction (Invitrogen) into the hairpin RNAi binary vector pSTARGATE (Greenup et al., 2010) to form a stem–loop region under the control of the ubiquitin promoter/intron (UBI) and octopine synthase (OCS) terminator to form the RNAi vector pSG/CAa.
Secondly, an overexpression approach, which resulted in gene silencing, generated the third transformed line, 1.1. The coding sequence of the maize β-CA gene (GRMZM2G348512), ZmCA2 (Studer et al., 2014), was amplified by reverse transcription–PCR (RT–PCR) from total RNA extracted from B73 maize. Total RNA was isolated using hot acid phenol and chloroform, and then treated with RQ1 RNase-free DNase (Promega). The reverse transcription and PCRs were performed as per the manufacturer’s protocols with Superscript II (ThermoFisher) and Phusion High-Fidelity DNA polymerase (NEB), respectively (for primers, see Supplementary Table S1). The sequence encoding an AcV5 epitope tag (Lawrence et al., 2003) was added to the C-terminal end of ZmCA2. The resulting ZmCA2 amplicon was cloned into pENTR/D-TOPO and verified by sequencing. LR Gateway cloning (ThermoFisher) was used to insert the ZmCA2 coding sequence into the overexpression vector, pSC110. pSC110 was created by Gibson Assembly (Gibson et al., 2009) from two modified pMDC164 vectors (Curtis and Grossniklaus, 2003), kindly provided to us by Udo Gowik (Heinrich-Heine University, Dusseldorf, Germany). ZmCA2 expression from pSC110 was driven by the B73 ZmPEPC promoter. pSC110 and pSC110/ZmCA2 were verified by sequencing.
Both constructs were transformed into Agrobacterium tumefaciens strain AGL1 for stable plant transformation.
Callus induction and plant transformation
Stable transformation of S. viridis (accession A10.1) was carried out as described by Brutnell et al. (2010). Seed coats were mechanically removed from mature S. viridis seeds to improve germination. Seeds were sterilized before plating on callus induction medium [CIM; 4.3g l−1 Murashige and Skoog (MS) salts, pH 5.8, 10ml l−1 100× MS vitamins stock, 40g l−1 maltose, 35mg l−1 ZnSO4·7H2O, 0.6mg l−1 CuSO4·5H2O, 4g l−1 Gelzan, 0.5mg l−1 kinetin, 2mg l−1 2,4-D]. After 4 weeks in the dark at 24 °C, any seedling structures or gelatinous calli were removed and remaining calli transferred to fresh CIM. After a further 2 weeks, calli were divided and replated onto fresh CIM. One week later, transformations were performed.
AGL1 containing the construct of interest were grown in the presence of 50 µg l−1 kanamycin and 50 µg l−1 rifampicin at 28 °C to OD600=0.5 and then resuspended in CIM without Gelzan and hormones. Acetosyringone (200mM) and synperonic [0.01% (w/v)] were added to the Agrobacterium solution before incubating the calli in the medium for 5min at room temperature. The calli were blotted dry on sterile filter paper and incubated at 22 °C for 3 d in the dark. The calli were then transferred to selective CIM (CIM containing 40mg l−1 hygromycin, 150mg l−1 timentin) and incubated in the dark at 24 °C for 16 d. Calli were then transferred to selective plant regeneration medium (PRM) containing 4.3g l−1 MS salts, pH 5.8, 10ml l−1 100× MS vitamins, 20g l−1 sucrose, 7g l−1 Phytoblend, 2mg l−1 kinetin, 150mg l−1 timentin, 15mg l−1 hygromycin. Calli were maintained at 24 °C under a 16h light:8h dark photoperiod and a light intensity of 60 µmol photons m−2 s−1. Developing shoots were transferred to selective rooting medium (RM) containing 2.15g l−1 MS salts, pH 5.7, 10ml l−1 100× MS vitamins, 30g l−1 sucrose, 7g l−1 Phytoblend, 150mg l−1 timentin, 20mg l−1 hygromycin. Shoots that survived and developed roots were genotyped using primers against the hygromycin phosphotransferase gene (Supplementary Table S1) by PCR, and positive transformants were transplanted to soil.
Selection of plants for analysis
The progeny of three independent T0 transformation events were analysed for CA hydration rates (Supplementary Fig. S1). One T1 plant with low CA hydration rates was selected from each transformation event (labelled 5.3, 2.1, and 1.1) and its progeny (T2) used for all future analysis. Two sets of experiments were performed on the T2 plants. First, gas exchange and biochemical analysis on lines 5.3, 2.1, and 1.1 (Table 1) and, secondly, gas exchange and oxygen discrimination on lines 5.3 and 1.1 (Table 2). Each T2 plant was genotyped prior to experiments using primers against the hygromycin phosphotransferase gene (Supplementary Table S1). The progeny of a plant which went through the S. viridis transformation process and tested negative for the hygromycin phosphotransferase gene were used as null controls.
Table 1.
Physiological and biochemical characteristics of CA transformants under ambient CO2 conditions
Net CO2 assimilation rate (A), stomatal conductance (gs), mesophyll pCO2 (Cm), the rate constant of CA hydration (kCA), and enzyme activities were measured from the uppermost, fully expanded leaf of 5-week-old plants grown at 2% CO2. Gas exchange measurements were made at 25 °C leaf temperature, flow rate at 500 µmol m−2 s−1, and irradiance of 1500 µmol photons m−2 s−1. Three T2 plants from three different transformation events were measured.
| A | g s | C m | k CA | Rubisco | PEPC | NADP-ME | |
|---|---|---|---|---|---|---|---|
| µmol m−2 s−1 | mol m−2 s−1 | µbar | mol m−2 s−1 bar−1 | µmol m−2 s−1 | µmol m−2 s−1 | µmol m−2 s−1 | |
| Null | 22.5±0.6 a | 0.19±0.01 a | 132.4±3.3 a | 6.1±0.8 a | 18.7±1.5 a | 229.6±19.3 a | 59.8±4.3 a |
| 5.3 | 21.7±2.6 a | 0.2±0.02 a | 118.9±13.1 a | 3.3±0.2 b | 18.8±1.8 a | 249.3±24.6 a | 54.5±5.8 a |
| 2.1 | 18.5±1.9 a | 0.16±0.01 a | 152.9±15.2 a | 2.0±0.2 b,c | 20.9±2.9 a | 181.5±25.4 a | 47.3±2.6 a |
| 1.1 | 19.1±1.2 a | 0.19±0.02 a | 153.9±4.4 a | 0.8±0.1 c | 19.7±1.8 a | 180.3±18.4 a | 43.6±3.9 a |
Significant differences are based on one-way ANOVA and Tukey post-hoc analysis (SPSS statistics version 22; P=0.05).
Table 2.
Physiological characteristics of CA transformants at ambient CO2 measured using LI-6400XT coupled to a tunable diode laser
Net CO2 assimilation rate (A), stomatal conductance (gs), mesophyll pCO2 (Cm), the ratio of intercellular to ambient pCO2 (Ci/Ca), the rate constant of CA hydration (kCA), online Δ18O discrimination, and the length of mesophyll cells exposed to intercellular airspace (Sm) were measured on the uppermost, fully expanded leaf of 5-week-old plants grown at 2% CO2. Gas exchange measurements were made at 2% O2, 25 °C leaf temperature, flow rate at 500 µmol m−2 s−1, and irradiance of 1500 µmol photons m−2 s−1. Three T2 plants from two different transformation events were measured.
| A | g s | C m | C i/Ca | k CA | Δ18O | S m | |
|---|---|---|---|---|---|---|---|
| µmol m−2 s−1 | mol m−2 s−1 | µbar | µbar | mol m−2 s−1 bar−1 | ‰ | m2 m−2 | |
| Null | 30.0±1.4 a | 0.30±0.03 a | 144.6±5.9 a | 0.39±0.03 a | 8.4±0.7 a | 18.0±1.4 a | 10.2±0.4 a |
| 5.3 | 29.2±0.9 a | 0.29±0.02 a | 157.9±10.5 a | 0.34±0.01 a | 2.5±0.3 b | 13.6±0.7 a,b | – |
| 1.1 | 24.5±1.6 a | 0.26±0.03 a | 178.1±13.5 a | 0.43±0.02 a | 0.8±0.2 b | 10.9±0.6 b | 10.2±0.9 a |
Significant differences are based on one-way ANOVA and Tukey post-hoc analysis (SPSS statistics version 22; P=0.05).
Insertion number estimation
DNA was isolated from a fully expanded leaf using a CTAB (cetyltrimethylammonium bromide) extraction buffer [2% CTAB (v/v), 20mM Tris–HCl pH 8, 1.4M NaCl, 20mM EDTA, 1% polyvinylpyrrolidone (PVP)-40 (w/v), 0.2% (v/v) β-mercaptoethanol] followed by extraction with phenol/chloroform/isoamylalcohol (25:24:1) and ethanol clean-up. DNA quality and quantity was determined using a NanoDrop spectrophotometer (Thermo Scientific).
IDNA genetics (UK) performed quantitative real-time PCR (qPCR) analysis to estimate the numbers of transgene copies in the CA transformed lines following the procedure described in Bartlett et al. (2008) with some modifications. The hygromycin phosphotransferase gene (with a FAM reporter) and the internal positive control (IPC, with a VIC reporter) were amplified together in a multiplex reaction (15min denaturation, then 40 cycles of 15s at 95 °C and 60s at 60 °C) in an ABI1900 real-time PCR machine. Fluorescence from the FAM and VIC fluorochromes was measured during each 60 °C step and the Ct values obtained. The difference between the Ct values for the hygromycin phosphotransferase gene and the IPC (the Delta Ct) was used to allocate the assayed samples into groups with the same gene copy number.
RNA extraction and reverse transcription–quantitative PCR (RT–qPCR)
Leaf discs (0.78cm2) frozen and stored at −80 °C were lysed using the Qiagen TissueLyser II. RNA was extracted using the Trizol extraction method and in the presence of RNase inhibitor (Ambion). DNA was removed using the TURBO DNA free kit (Ambion), and RNA quantity and quality were determined using a NanoDrop (Thermo Scientific).
RNA (200ng) was reverse transcribed into cDNA using Qiagen’s RT2 HT First Strand cDNA synthesis kit. RT–qPCR and melt curve analysis were performed on a Viia7 Real-time PCR system using the Power SYBR green PCR Master Mix (Thermo Fisher) according to the manufacturer’s instructions. Primers (Supplementary Table S1) were designed using Primer3 in Geneious R7.1.6, ensuring products spanned an intron. Primer amplification efficiencies were determined by the Ct slope method; efficiencies for all primer pairs were comparable (~95%) and no amplification was detected in the no template control. Relative fold change was calculated by the ΔΔCt method, using the average of three nulls as reference, as described by Livak and Schmittgen (2001). The geometric mean of the Ct values for three reference genes was used for normalization (Vandesompele et al., 2002). Statistics were performed with SigmaPlot (version 11.0).
Determination of enzyme activities
For CA activity, leaf discs (0.78cm2) were collected from the uppermost fully expanded leaf of 5-week-old S. viridis plants and frozen in liquid nitrogen. Soluble protein was extracted by grinding one frozen leaf disc in ice-cold glass homogenizers (Tenbroek) in 500 µl of extraction buffer [50mM HEPES, pH 7.8, 1% (w/v) PVP, 1mM EDTA, 10mM dithiothreitol, 0.1% (v/v) Triton X-100, 2% (v/v) protease inhibitor cocktail (Sigma)]. Crude extracts were centrifuged at 4 °C for 1min at 13 000 g and the supernatant collected for the soluble CA assay. Activity was measured on a membrane inlet mass spectrometer to measure the rates of 18O exchange from labelled 13C18O2 to H216O at 25 °C (Badger and Price, 1989; von Caemmerer et al., 2004). The hydration rates were calculated as described by Jenkins et al. (1989).
For Rubisco, PEPC, and NADP-malic enzyme (ME) activities, soluble protein was extracted from fresh leaf discs collected from leaves used for gas exchange analysis. Spectrophotometric assays were then performed as described previously (Pengelly et al., 2010, 2012; Sharwood et al., 2016).
Gas exchange measurements
Net photosynthesis (A) was measured over a range of intercellular pCO2 (Ci) on the uppermost, fully expanded leaf of 5-week-old S. viridis plants using a portable gas exchange system LI-COR 6400XT (LI-COR Biosciences). Measurements were made after leaves had equilibrated at 380 µbar, flow rate 500 µmol s−1, leaf temperature 25 °C, and irradiance 1500 µmol photons m−2 s−1. CO2 response curves were measured in a stepwise increase (3min intervals) in CO2 partial pressure 380, 0, 23.75, 47.5, 71.25, 95, 142.5, 190, 285, 380, 570, 760, and 950 µbar whilst maintaining leaf temperature and irradiance conditions.
Measurements of C18O16O discrimination (Δ18O)
Simultaneous measurements of exchange of CO2, H2O, C18O16O, and H218O were made by coupling two LI-6400XT gas exchange systems to a tunable diode laser (TDL: TGA200A, Campbell Scientific Inc., Logan, UT, USA) to measure C18O16O and a Cavity Ring-Down Spectrometer (L2130-i, Picarro Inc., Sunnyvale, CA, USA) to measure the oxygen isotope composition of water vapour. The system is essentially that described by Tazoe et al. (2011) except that the TGA100 was replaced by a TGA200A and the additional laser for water vapour measurements has been added together with a 16 port distribution manifold. To generate gas flows to the gas exchange systems, N2 and O2 were mixed by mass flow controllers (Omega Engineering Inc., Stamford, CT, USA) to generate CO2-free air with 2% O2. The humidity of incoming air was adjusted by varying the temperature of water circulating around a Nafion tube (Permapure, MH-110-12P-4) but was kept constant in this set of experiments to supply water vapour of a constant 18O composition. To supply flow to the TDL and the L2130-i from the sample and reference gas streams, two T junctions were inserted into the match valve tubing and in the reference line of the LI-6400XT, respectively. This allowed leaves of two plants to be measured in sequence, with each LI-6400XT sampled by the TDL at 4min intervals for 20s at the sample and reference line. The Picarro Cavity Ring Down spectrometer sampled for 3min, so that leaves were sampled at 6min intervals.
Supplementary Fig. 5 shows the CO2 dependence of the standard error of δ18O of CO2 in the reference gas of repeated measurements on the TGA200A. The 18O isotopic composition of the CO2 calibration gas was 22.17±0.04‰ for Vienna mean oceanic water (VSMOW) and was checked against standards on an Isoprime mass spectrometer. We monitored the 18O composition of water vapour of the reference air streams daily, and the values were −6.07±0.08‰ and −6.34±0.08‰ (VSMOW) for LI-6400XT L1 and L2 references, respectively. We attribute the small difference between the reference lines to differences in the Nafion tubing. At the end of the experiment, the calibration of the Picarro L2130-i was confirmed by collecting water vapour samples from the gas stream of the LI-6400XT reference lines going to the Picarro as described by Cousins et al. (2006) and assaying these water samples against standards on a Picarro 1102i, which was set up to measure the 18O isotopic composition of water samples.
Gas exchange was measured on the uppermost fully expanded leaf of 5-week-old S. viridis plants at 25 °C, and leaves were equilibrated at ambient CO2 (380 µbar), irradiance 1500 µmol photons m−2 s−1, and 2% O2. The flow rate was 200 µmol s−1. CO2 concentration was adjusted from 380 to 760, 570, 380, and 190 µbar at 1h intervals. Immediately following gas exchange measurements, leaf discs were collected and stored at −80 °C until measurements of CA activity were made.
Calculations of C18O16O (Δ18O) discrimination and mesophyll conductance (gm)
Discrimination against 18O in CO2 during photosynthesis, Δ18O, was calculated from the isotopic composition of the CO2 entering δin and exiting δout the leaf chamber and the CO2 concentration entering Cin and exiting Cout (all measured with the TDL) (Evans et al., 1986; Barbour et al., 2016;):
| (1) |
where ξ=Cin/Cin–Cout. Sample streams were passed through a Nafion drying tube before entering the TDL, and CO2 values presented are all at zero water vapour concentration.
Following the derivation by Barbour et al. (2016) and Farquhar and Cernusak (2012) photosynthetic Δ18O discrimination was used to calculate pCO2 in the mesophyll cytosol, Cm, with the assumption that Cm is equal to the pCO2 at the site of CO2–H2O exchange and assuming that cytosolic CO2 is in full isotopic equilibrium with local cytosolic water. This allowed gm to be calculated from
| (2) |
| (3) |
Equation 3 is the same as equation 21 of Barbour et al. (2016), and is a rearrangement of equation 18 of Farquhar and Cernusak (2012) using their notation. The oxygen isotope ratios are expressed relative to the standard, (VSMOW) . Intercellular pCO2 is denoted by Ci, and aw is the discrimination against C16O18O during liquid phase diffusion and dissolution (0.8‰).
The isotopic composition of CO2 being assimilated, δA, is given by
| (4) |
where δa is the isotopic composition of ambient air (in our case δa=δout).
The oxygen isotope composition of CO2 in the intercellular airspaces, δi, including ternary corrections proposed by Farquhar and Cernusak (2012), is given by
| (5) |
where Ca is the pCO2 in the ambient air. The ternary correction factor, t, is given by
| (6) |
where gac is the total conductance to CO2, E the transpiration rate, and a18bs is the weighted discrimination of C16O18O diffusion across the boundary layer and stomata in series given by:
| (7) |
where Cs is the pCO2 at the leaf surface and a18s and a18b are the discriminations against C16O18O through stomata and the boundary layer (8‰ and 5.8‰, respectively).
The isotopic composition of intercellular CO2 ignoring ternary corrections is given by
| (8) |
To calculate Cm, we assume that the isotopic composition of CO2 in the cytosol, δc, is the isotopic composition of CO2 equilibrated with cytosolic water, δcw, and
| (9) |
where δw is the stable oxygen isotope composition of water in the cytosol at the site of evaporation and εw is the isotopic equilibrium between CO2 and water (dependent on temperature TK in K (Barbour et al., 2016, and references therein).
| (10) |
Calculation of the isotopic composition of water at the site of evaporation from the isotopic composition of transpired water
The isotopic composition of water at the site of evaporation, δw, can be estimated from the Craig and Gordon model of evaporative enrichment (Craig and Gordon, 1965; Farquhar and Lloyd, 1993)
| (11) |
where ε* is the equilibrium fractionation during evaporation, εk is the kinetic fractionation during vapour diffusion in air, δt is the oxygen isotopic composition of transpired water, ea/ei is the ratio of ambient to intercellular vapour pressure, and δa is the isotopic composition of ambient air. ε* is dependent on temperature:
| (12) |
εk is dependent on stomatal and boundary layer conductances and associated fractionation factors (Barbour et al., 2016, and references therein):
| (13) |
The isotopic composition of transpired water δt can be calculated from mass balance knowing the isotopic composition of the water entering δwin and exiting δwout the leaf chamber (measured with the Picarro) and the water vapour concentration entering win and exiting wout (measured with the LI-6400XT):
| (14) |
Calculation of the proportion of mesophyll cytosolic CO2 in equilibration with leaf water, θ
If Cm is known, it is possible to calculate the isotopic composition of cytosolic CO2 from measurements of Δ18O using equation 18 from Farquhar and Cernusak (2012):
| (15) |
This can then be compared with δcw (Equation 9), the isotopic composition of CO2 in equilibrium with water at the site of evaporation. We calculated mesophyll conductance, gm, in the S. viridis null plants assuming that δc=δcw and then used this gm to estimate Cm in the S. viridis transgenics to calculate the proportion of cytosolic CO2 in equilibration with leaf water, θ using equations developed by Cernusak et al. (2004)
| (16) |
where a18 is the weighted discrimination of C16O18O diffusion across the boundary layer, stomata, and the liquid phase in series given by:
| (17) |
Leaf anatomical measurements and estimation of gm from anatomical measurements
Fully expanded leaves from 5-week-old T2 plants, null and line 1.1, were collected and cut into ~0.5×2mm pieces. Leaf slices were fixed in 2.5% (v/v) glutaraldehyde, 2% (v/v) paraformaldehyde, 0.1M phosphate buffer, and 0.01% (v/v) Tween-20 under vacuum for 20min, then replaced with buffer containing no Tween-20 and fixed overnight at 4 °C. Leaf pieces were washed in phosphate buffer and post-fixed in 1% (w/v) osmium tetroxide for 2h. Fixed leaf pieces were then dehydrated in an ethanol series (10, 30, 50, 70, 80, 95, 100%) followed by infiltration with LR white. Leaf sections were finally placed in moulds filled with resin and baked at 60 °C for 24h. Sections of 0.5 µm thickness were cut using glass knives on a Reichert ultramicrotome, stained with toluidine blue, and heat fixed to glass slides. Slides were viewed using a Zeiss Axioskop light microscope at ×400 magnification. Three images were taken from each slide for analysis, each containing a leaf cross-section in the same orientation and showing at least two vascular bundles. Fiji quantification software (Schindelin et al., 2012) was used to select regions of interest. Mesophyll surface area exposed to intercellular airspace to leaf area ratio (Sm) was calculated using Equation 18 where CCF is the curvature correction factor of 1.43 (Evans et al., 1994).
| (18) |
The values of Sm together with measurements of cell wall thickness and cytosol thickness were used to derive an estimate of gm from anatomical parameters. The cell wall thickness (0.113±0.005 μm) was kindly estimated from transmission electron micrographs of S. viridis grown under similar conditions by Florence Danila (Danila et al., 2016). Calculations followed equations 1–5 of von Caemmerer and Evans (2015) using the membrane permeability of Gutknecht for a lipid bilayer of 3.5×10–3 m s−1 since only the plasma membrane needs to be transversed for diffusion of CO2 from he intercellular airspace to mesophyll cytosol (Gutknecht et al., 1977) and a cytosol thickness of 0.3 μm (von Caemmerer and Evans, 2015). These calculations give a gm of 0.68mol m−2 s−1 bar−1.
Statistical analysis
One-way ANOVAs with post-hoc Tukey test analyses were performed for all measurements of gas exchange and enzyme activities with P=0.05 using the IBM SPSS Statistics 22 package.
Results
Generation of transgenic S. viridis with reduced β-CA
In S. viridis we identified four β-CA genes: Si002140m.g (with one other isoform Si002148m), Si002669m.g, Si030616m.g (with two other isoforms Si030928m and Si030803m), and Si003882m.g. There is very low sequence identity between these β-CA genes, ~37% (Supplementary Fig. S2). Si003882m.g has been shown to be the major leaf β-CA (Christin et al., 2013; John et al., 2014).
Three independent transformation events resistant to hygromycin and with reduced CA activity were generated using two different approaches. First, one line (1.1) was generated through gene suppression upon transformation with the overexpression construct pSC110/ZmCA2. The coding sequences of ZmCA2 and Si003882m.g show 87% identity (Supplementary Fig. S3). Most probably, expression of ZmCA2 therefore caused suppression of the primary S. viridis β-CA gene, resulting in reduced CA activity in line 1.1. The second approach was to target Si003882m.g using the RNAi construct pSG/CAa which generated stably transformed lines from two different events (2.1 and 5.3). Plants were grown at high pCO2 for all experiments.
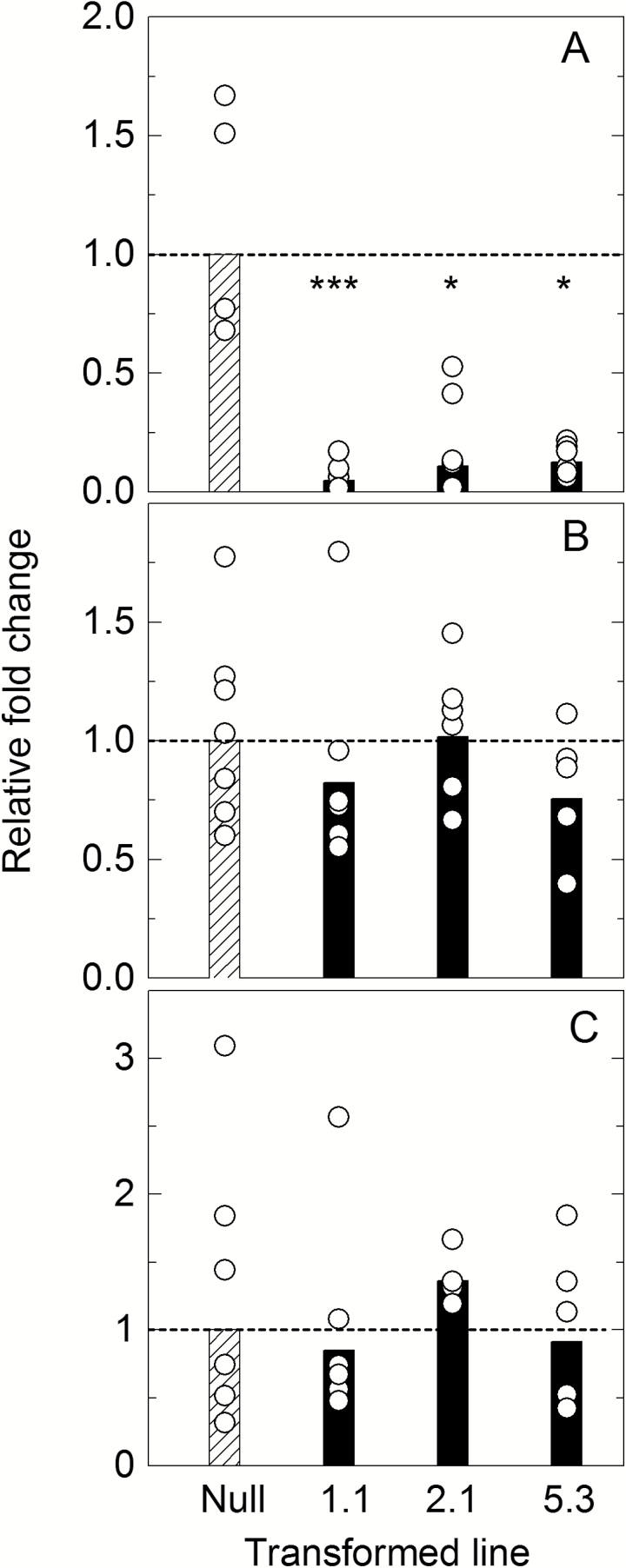
To determine the specificity of the RNAi construct and check which β-CA was suppressed in line 1.1, RT–qPCR was performed against the β-CAs in S. viridis. Expression of the primary leaf β-CA Si003882m.g was significantly down-regulated, between 83% and 96%, in lines from all three transformation events (Fig. 1A). Transcript levels of Si030616m.g and Si002140m.g were unchanged relative to expression in the null plants (Fig. 1B, C) while Si002669m.g transcript was undetectable in all samples (data not shown). Therefore, expression of only the target β-CA gene was affected in the three transformed lines.
Fig. 1.
Expression level of β-CA transcripts. (A) Si003882m.g, (B) Si002140m.g, and (C) Si030616m.g in null control and CA transformed lines 1.1, 2.1, and 5.3 as measured by RT–qPCR and analysed by ΔΔCt. Fold change relative to the null transformant is shown; bars represent mean fold change, and circles show the data range of T2 plants (n=5–7 plants) from each transformation event measured in triplicate. The dotted line indicates average null fold change. Expression level of the major leaf β-CA transcript Si003882m.g (A) is significantly lower compared with the null control in all three transformed lines, calculated using one-way ANOVA.
qPCR was used to estimate the number of insertions in the transgenic plants, based on the number of copies of the hygromycin phosphotransferase gene. Three T2 plants of the three lines were analysed and there were two, four, and more than four transgene insertions detected for plants of line 5.3, 2.1, and 1.1, respectively. The high copy number in the overexpressing line of 1.1 is the likely cause of the suppression of transcript accumulation.
CA and photosynthetic enzyme activity and leaf anatomy
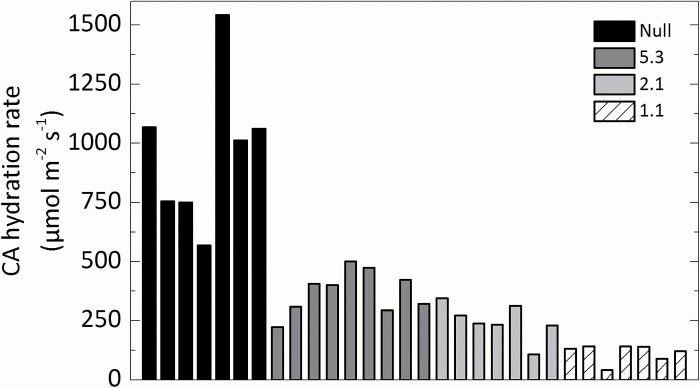
T1 progeny of the three independent transformation events showed a range of CA hydration rates as measured on the soluble leaf fraction on a membrane inlet mass spectrometer. Compared with the null control, lines 1.1, 2.1, and 5.3 had on average (n=7 T2 plants) an 87, 70, and 50% reduction of CA activity, respectively (Fig. 2). The CA hydration rate in the null plants was 934±92 µmol m−2 s−1 as calculated at a mesophyll pCO2 (Cm) of 140 µbar (Equation 2).
Fig. 2.
Range of CA hydration rates at mesophyll pCO2 (Cm) measured using a membrane inlet mass spectrometer in the null control and T2 plants from lines 5.3, 2.1, and 1.1.
The activities of the photosynthetic enzymes Rubisco, PEPC, and NADP-ME were unchanged in lines 5.3, 2.1, and 1.1 compared with the nulls (Table 1) and showed no correlation with CA hydration rates (one-way ANOVA and Tukey post-hoc analysis (SPSS statistics version 22; P=0.05).
No significant differences were observed for the surface area of mesophyll cells exposed to intercellular airspace per unit leaf area (Sm) in embedded leaf sections of nulls (10.22±0.35 m2 m−2) and plants from line 1.1 (10.18±0.95 m2 m−2). These anatomical measurements were used to estimate an anatomical gm of 0.68mol m−2 s−1 bar−1 (see the Materials and methods).
CA activity and CO2 assimilation rates
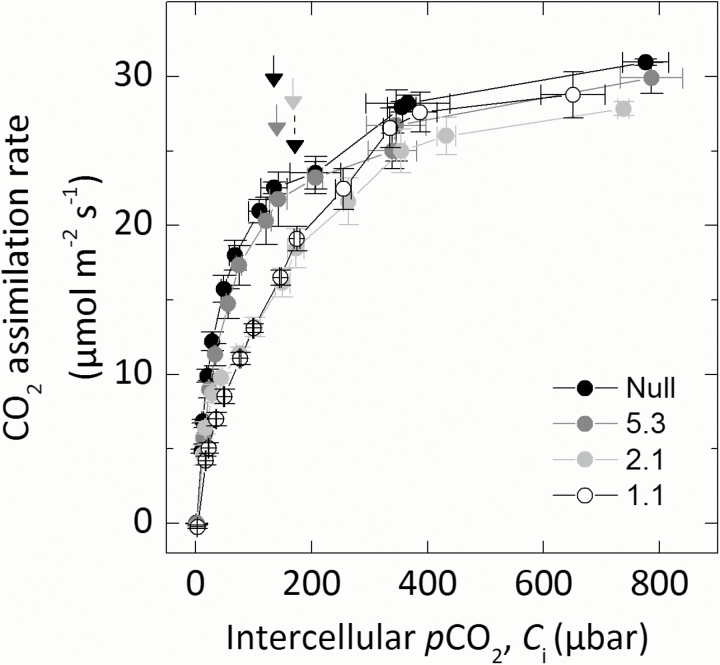
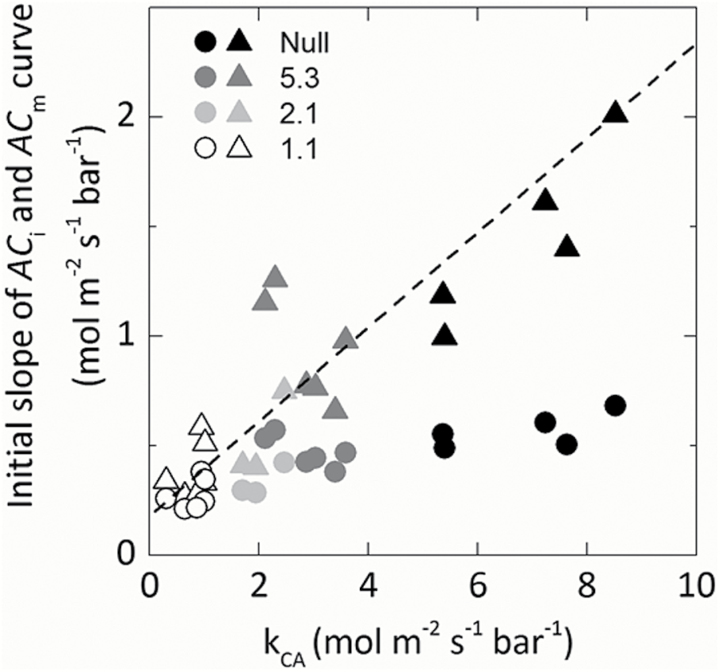
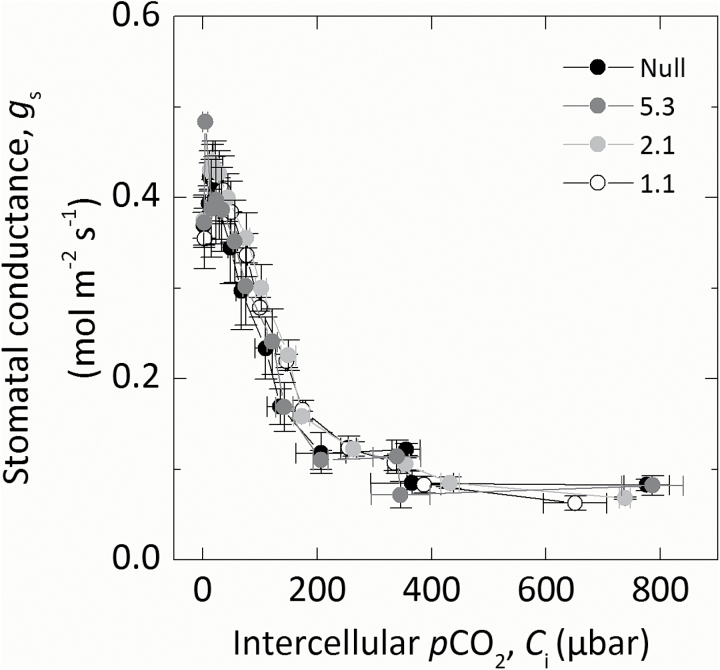
The response of CO2 assimilation rate (A) to increasing intercellular pCO2 (Ci) was investigated to examine the effect of reduced CA activity on CO2 assimilation rates (Fig. 3). There were no statistical differences in the maximum rate of CO2 assimilation under ambient or high CO2 conditions between null control and progeny of transformant lines. At low pCO2, CO2 assimilation rates were reduced to varying degrees in the progeny of the transformed lines compared with the null control. Individuals of line 1.1 with the lowest CA hydration rate had the lowest initial slopes of the ACi curves. The initial slopes of the ACi and ACm curve were plotted against the CA hydration rate constant (kCA; Fig. 4). Mesophyll cytosolic pCO2, Cm, was calculated from Equation 2, using the average null gm (0.9mol m−2 s−1 bar−1) since there was no difference in Sm. A strong correlation between the initial slope from the ACm curve and kCA was observed, with the initial slope increasing as CA hydration rates increase (R2=0.845; Fig. 4). There was a curvilinear response between the initial slope of the ACi curves indicating other limitations. No difference in stomatal conductance (gs) was observed across a range of intercellular pCO2 between null controls and any of the transformed lines during the rapid measurements of CO2 responses (Fig. 5).
Fig. 3.
CO2 assimilation rate of transformed lines over a range of intercellular pCO2 (Ci). Average of three T2 plants from each line. Plants were grown at 2% CO2, and the uppermost, fully expanded leaves of 5-week-old plants were measured using a LI-6400XT at 25 °C leaf temperature at an irradiance of 1500 µmol photons m−2 s−1. Arrows mark ambient pCO2 for each line; note that the dotted arrow is line 1.1.
Fig. 4.
Relationship between the initial slope of the ACm (triangles) or ACi (circles) curves and the rate constant of CA hydration rates (kCA), ACmR2=0.846. Each point represents a measurement made on an individual leaf of a T2 plant.
Fig. 5.
Stomatal conductance (gs) over a range of intercellular pCO2 (Ci). Measurements were made concurrently with those in Fig. 4.
Oxygen isotope discrimination measurements
Oxygen (∆18O) isotope discrimination and CO2 assimilation rates were measured in response to changes in pCO2 using a LI-6400XT coupled to a TDL trace gas analyser to measure C18O16O and a Cavity Ring-Down Spectrometer to measure the oxygen isotope composition of water vapour. Transformed plants with reduced CA hydration rates had lower ∆18O compared with the nulls, but only line 1.1 was significantly lower (Table 2).
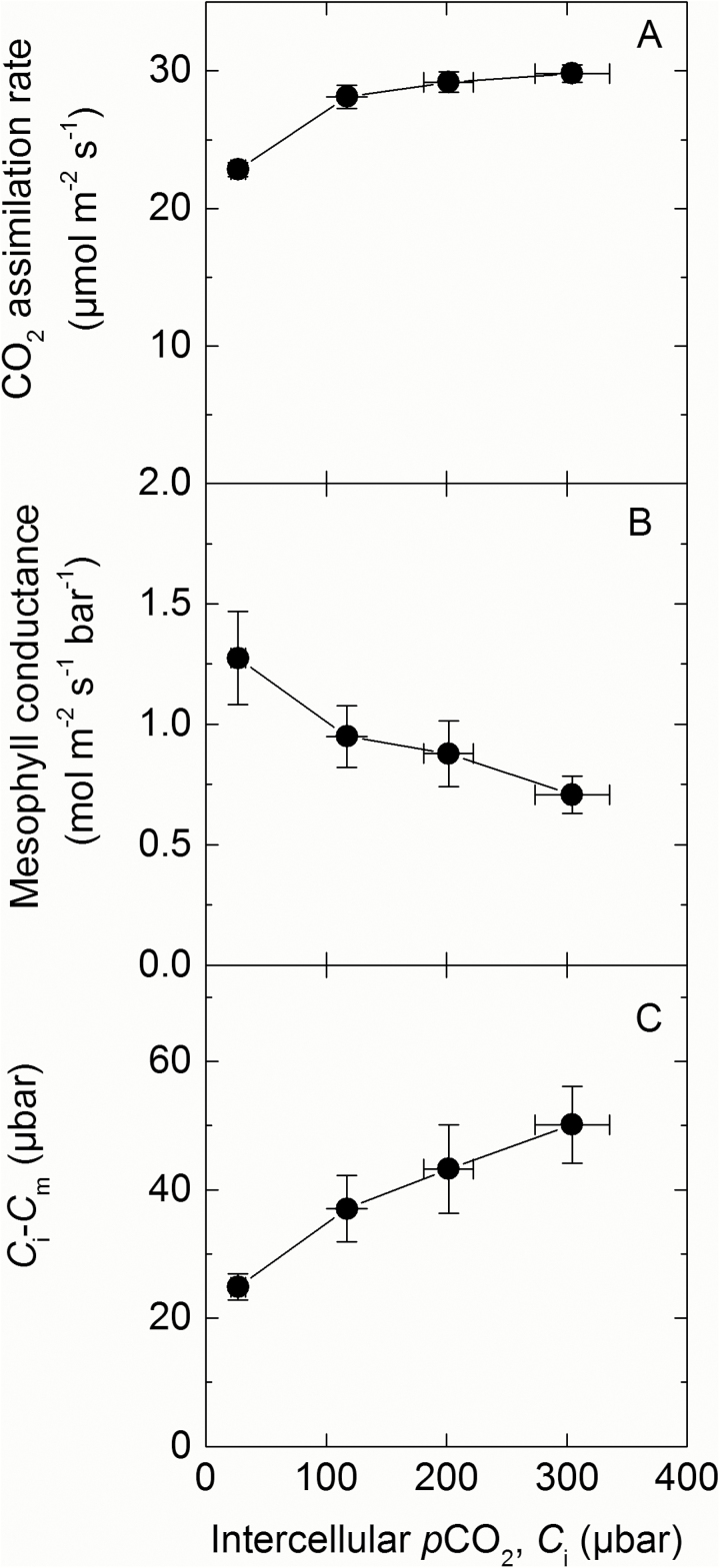
In the null controls, measurements of ∆18O were used to estimate conductance of CO2 from the intercellular airspace to the sites of CO2 and H2O exchange in the cytosol (gm) with the assumption that CO2 was in full isotopic equilibrium with leaf water in the cytosol (Equation 2; Fig. 6). Although gm appeared to increase with decreasing Ci, there were no significant differences between gm estimated at the different Ci, and the average value was 0.94±0.06mol m−2 s−1 bar−1 (Fig. 6B). Ci–Cm indicates the drawdown of CO2 from the intercellular airspace to the site of fixation, and for the null controls there is an increasing gradient of pCO2 as Ci increases (Fig. 6C).
Fig. 6.
(A) CO2 assimilation rate, (B) mesophyll conductance (gm; Equation 2), and (C) Ci–Cm over a range of intercellular pCO2 in null controls measured using a LI-6400XT coupled to a tunable diode laser. Plants were grown at 2% CO2 and the uppermost, fully expanded leaves of 5-week-old plants were measured at 25 °C leaf temperature, flow rate 200 µmol m−2 s−1, 2% O2 at an irradiance of 1500 µmol photons m−2 s−1.
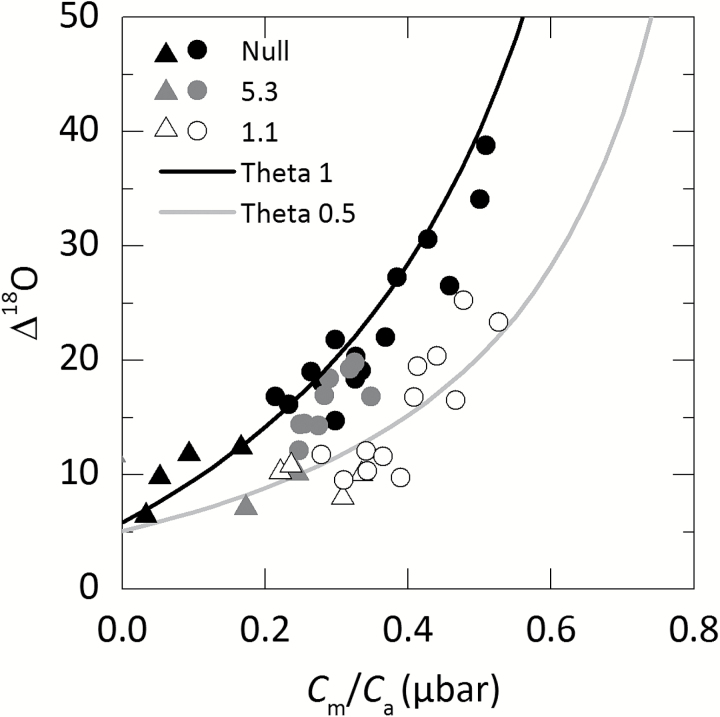
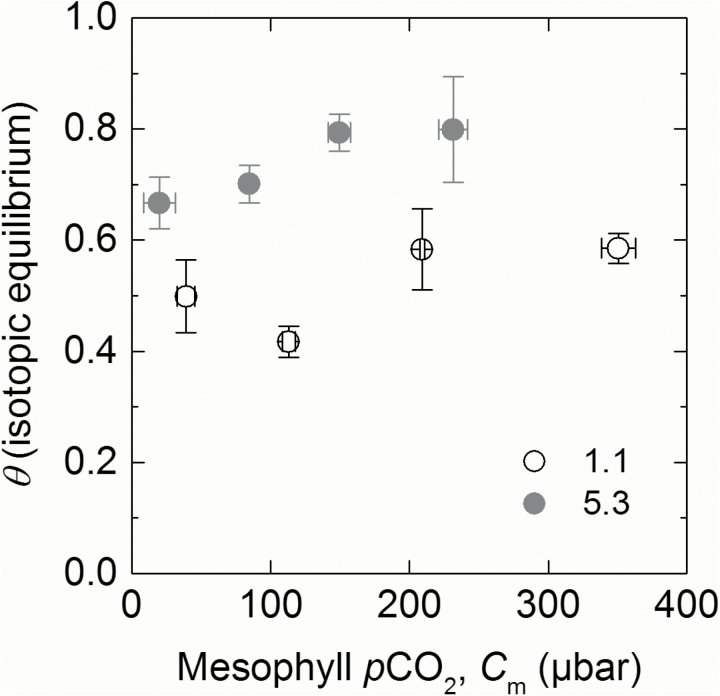
∆18O at ambient pCO2 showed statistically significant differences between line 1.1 (with the lowest CA activity) and null plants (Table 2). When plotted against Cm/Ca, ∆18O measurements closely correspond to theoretical curves representing θ (Equation 16) under different scenarios either where cytosolic CO2 is at full isotopic equilibrium with the cytosolic water (null lines) or where there is only partial equilibrium (such as line 1.1; Fig. 7). Calculated values for line 5.3 which showed a 50% reduction in CA activity relative to the null controls fell in between these two theoretical lines. This is illustrated again with theta (θ) of lines 1.1 and 5.3 over a range of Cm (Fig. 8). When CO2 is at full isotopic equilibrium with the cytosolic water, θ would be 1, whereas in lines 1.1 and 5.3 (with reduced CA hydration rates relative to the null control) θ is <1. There was no CO2 dependence of θ over the range of pCO2 measured.
Fig. 7.
Oxygen isotope discrimination (Δ18O) as a function of the ratio of mesophyll pCO2 to ambient pCO2 (Cm/Ca) in null and lines 5.3 and 1.1. Each point represents a measurement made on an individual leaf of a T2 plant. Triangular symbols represent measurements made at low pCO2. Theoretical curves represent the scenario where cytosolic CO2 is at full isotopic equilibrium with cytosolic water (θ=1, black) or under partial equilibrium (θ=0.5, grey) of 18O in the leaf. The equations for the curves are given by and a18=5.85‰ and δc–δa=33‰ at full equilibration or a18=5.1‰ and δc–δa=15‰ (Farquhar and Lloyd, 1993).
Fig. 8.
Average isotopic equilibrium (theta, θ) over a range of mesophyll pCO2 in two reduced CA lines 5.3 (grey) and 1.1 (white). Measured values of θ were determined from Δ18O using Equation 16. Each point represents the average measurement of three T2 plants.
Discussion
Setaria viridis as a model species to study photosynthetic physiology in a C4 monocot
Flaveria bidentis, a readily transformable model C4 dicot, has been successfully used to study the regulation of C4 photosynthesis using antisense and RNAi technology (Furbank et al., 1997; Matsuoka et al., 2001; von Caemmerer et al., 2004; Pengelly et al., 2012). This work has been crucial in quantifying the rate-limiting steps in the C4 pathway by ‘titrating’ out levels of target enzymes by gene suppression and observing the effects on physiological characteristics of the resultant transgenics (Furbank et al., 1997). There are, however, important differences between C4 dicots and the C4 monocots which make up the majority of agriculturally important C4 species. Setaria viridis has emerged as a new model grass to study C4 photosynthesis in crops and related bioenergy species. Setaria viridis is an appropriate biochemical model species for Z. mays and S. bicolor as all three use NADP-ME as the primary decarboxylation enzyme. We generated transgenic S. viridis plants with reduced CA activity to compare the effect with previous results obtained with F. bidentis and Z. mays (von Caemmerer et al., 2004; Studer et al., 2014) and to explore the effect that a reduction in CA activity has on the initial slope of the ACi and ACm curves. In these lines, only the major leaf isoform of β-CA was reduced (Fig. 1). The transgenic plants had a range of different CA activities (Fig. 2), but showed no changes in PEPC and Rubisco activity (Table 1) or anatomical parameters (Table 2), making these plants ideal for exploring the role of CA activity in S. viridis.
Initial slope of ACi curves in C4 plants
Models of C4 photosynthesis suggest that the initial slope of the ACi curve is determined by three possible limitations: (i) the mesophyll conductance to CO2 diffusion from the intercellular airspace to the mesophyll cytosol (gm); (ii) the rate of CO2 hydration by CA; and (iii) the rate of PEP carboxylation (von Caemmerer, 2000). However, it is not readily known which is the major limitation in C4 species. Studies with PEPC mutants from the C4 dicot Amaranthus edulis indicate that PEPC activity may not be the major limitation as a 60% reduction in PEPC leads to only a 20% reduction in the CO2 assimilation rate at ambient pCO2 accompanied by a small reduction in initial slope for the ACi curves (Dever et al., 1992; Dever, 1997; Cousins et al., 2007). This study with S. viridis confirms that substantial reductions in CA activity are possible before a reduction in steady-state CO2 assimilation rate and initial slope of the ACi curve are observed. This is in accordance with previous observations in F. bidentis and Z. mays (von Caemmerer et al., 2004; Studer et al., 2014).
The Michaelis–Menten constant for CO2 for CA is >2mM (~5% CO2) which makes it appropriate to quantify CA activity by its first-order rate constant (Jenkins et al., 1989; Hatch and Burnell, 1990) and simplifies species comparisons. In S. viridis, the lowest rate constant recorded was 0.8mol m−2 s−1 bar−1 compared with values of 0.1 for the ca1ca2 double mutant in Z. mays and 0.47 for transgenic F. bidentis (von Caemmerer et al., 2004; Studer et al., 2014). With this low rate constant, F. bidentis had very low CO2 assimilation rates and the CO2 response curves did not saturate at high CO2. In contrast, for both S. viridis transgenics and Z. mays mutants, CO2 assimilation rates were only slightly less than in the controls, suggesting that S. viridis is more similar to Z. mays in its CA requirements. This suggests that these two monocot species can make better use of leaf CA activity or that in vivo CA activity is greater than that estimated in vitro.
Mesophyll conductance and the initial slope of ACm curves
Next, we used recently established techniques that utilize 18O discrimination measurements to quantify gm in our null controls (Fig. 6B; Barbour et al., 2016). This estimates the diffusion of CO2 from the intercellular airspace through the cell wall, plasma membrane, and cytosol to the sites of CA activity. At ambient pCO2, the gm observed for the null plants were similar to those reported by Barbour et al. (2016). A key assumption for the calculation of gm is that CA activity is not limiting and that CO2 is in isotopic equilibrium with HCO3−; consequently gm was not measured in the transgenic lines with reduced CA activity. In C3 species, gm (in this instance from the intercellular airspace to the chloroplast stroma) has been shown to be proportional to the chloroplast surface area appressing the intercellular airspace per unit leaf area (Evans et al., 1994). Evans and von Caemmerer (1996) hypothesized that in C4 species gm may correlate with the mesophyll surface area exposed to intercellular airspace per unit leaf area (Sm). Since Sm was similar between the nulls and line 1.1 plants, we assumed that gm may also be similar between the plants. In C3 species, gm has been shown to, in some instances, increase with decreasing pCO2 (Flexas et al., 2007; Tazoe et al., 2011; Alonso-Cantabrana and von Caemmerer, 2016). These changes to gm which may be important in regulating and maintaining photosynthesis were also observed here in the S. viridis null plants, with gm increasing slightly at low pCO2. However, because the differences in gm at different pCO2 were not significant, we used the average gm estimated for the null plants to calculate mesophyll cytosolic pCO2 (Cm) in the transgenics.
As shown in Fig. 4, a strong almost linear relationship was found between ACm and kCA, whereas a saturating relationship was observed with ACi. This indicates that the CO2 assimilation rate is limited by cytosolic CA activity, with the relationship becoming clearer after accounting for gm. It is tempting to speculate that the differences between the two monocot species and F. bidentis relate to differences in limitations imposed by gm which affects cytosolic pCO2 and hence in vivo CA activity, but this is not borne out by comparative measurements of gm made by Barbour et al. (2016). CA activity increases with increasing pH, so variation in cytosolic pH can also contribute to variations in in vivo CA activity; however, these effects are not large (Jenkins et al., 1989). The interaction of β-CA and a CO2-permeable aquaporin in Arabidopsis thaliana has indicated that CA can be localized near the plasma membrane rather than dispersed throughout the mesophyll cytosol (Wang et al., 2016). This may also impact on CA activity and result in another difference between the C4 species. Other possibilities pertain to differences in anatomical characteristics of leaves. Both CA and PEPC are cytosolic enzymes, and differences in Sm may affect the efficiency with which CA is used. Our results suggest that increasing gm may be an important way to increase the CO2 assimilation rate at low intercellular pCO2, a scenario that may, for example, occur under drought.
Oxygen isotope discrimination and the CO2 dependence of isotopic equilibrium
As had previously been observed, Δ18O decreased with reductions in CA activity as CA facilitates the exchange of O2 between cytosolic water and CO2 (Fig. 7; Williams et al., 1996; Cousins et al., 2006). Previous reports, which have estimated the proportion of cytosolic CO2 in equilibrium with leaf water (θ) in C4 species, have generally assumed a relatively large gm value and this then led to lower estimates of θ (Cousins et al., 2006, 2008). Here we assumed that in the S. viridis null plants there is sufficient CA for isotopic equilibrium to be reached, as discussed by Barbour et al. (2016). For comparison, we also estimated gm from anatomical estimates of Sm, and cell wall and cytosolic thickness following calculations outlined by von Caemmerer and Evans (2015). This gives a gm value of 0.68mol m−2 s−1 bar−1 which is less than the value of 0.9mol m−2 s−1 bar−1 calculated from Δ18O measurements and highlights the anatomical constraints for CO2 diffusion dictated by the photosynthetic pathway in leaves of C4 plants (von Caemmerer et al., 2007).
Reduction in CA activity led to significant reductions in θ but it is interesting to note that θ did not vary significantly with pCO2. This is explained by the fact that CA activity increases linearly with pCO2 so that although there is more CO2 that needs to equilibrate with leaf water, there is also proportionally more CA activity. The fact that neither transgenic line showed a CO2 dependence suggests that the decrease in the ratio of CA hydrations to PEP carboxylations is not affecting the isotopic equilibration of CO2 with leaf water. These results have important implications for the interpretation of the 18O signature of atmosopheric CO2 (Yakir and Sternberg, 2000; Gillon and Yakir, 2001; Wingate et al., 2009).
Reduction of CA in S. viridis does not alter the stomatal reponse to CO2
The CO2 regulation of stomatal conductance remains an open question (Engineer et al., 2016). It has been previously shown that in the ca1/ca4 double mutant of A. thaliana, the degree of stomatal closure in response to increasing pCO2 was reduced (Hu et al., 2010; Wang et al., 2016). It is clear that CA is part of a complex signal transduction network. However, nothing is currently known about the role of CA in stomatal CO2 responses in C4 species. In our study, where only one β-CA isoform was reduced, we found no change in the response of stomatal conductance to CO2. The S. viridis β-CA reduced here (Si003882m.g) has low sequence identity (<50%) to all of the Arabidopsis β-CAs, but we would predict that multiple reductions in β-CA isoforms would be required to observe a similar stomatal phenotype in S. viridis.
Conclusion
Under current atmospheric conditions, CA activity was not rate limiting for C4 photosynthesis in S. viridis. At lower Ci, which may, for example, occur under conditions of drought, our results suggest that gm may pose a greater limitation than CA activity. However, it is important to investigate the role of CA on C4 photosynthesis under a range of environmental conditions such as high temperatures which have recently been suggested to deactivate CA activity in S. viridis (Boyd et al., 2015). Here we have shown that S. viridis is a useful model monocot C4 species that lends itself to molecular manipulation of the C4 photosynthetic pathway.
Supplementary Data
Supplementary data are available at JXB online.
Table S1. Primers used in this study
Figure S1. CA hydration rates at mesophyll pCO2 in the T1 plants.
Figure S2. Very low sequence identity (~37%) between the four main S. viridis β-CAs.
Figure S3. High sequence identity (87%) of Si003882m.g to the ZmCA2 (GRMZM2G348512).
Figure S4. CO2 assimilation rate of the TDL experiment.
Figure S5. Standard error of δ18O in the reference gas of repeated measurements with the TGA200A.
Supplementary Material
Acknowledgements
We thank Jasper Pengelly for assisting with construct generation, Xueqin Wang for assisting with S. viridis transformations, Soumi Bala for help with biochemical assays, gas exchange, and TDL measurements, and Murray Badger for making the MIMS available for measurements of CA activity. We thank Hilary Stuart-Williams for calibrating standard gases and water samples, and Joyce van Eck and Tom Brutnell for helpful discussions regarding S. viridis transformations. We thank Joanne Lee and the Centre for Advanced Microscopy at ANU for technical assistance with microscopy. This research was supported by the Bill and Melinda Gates Foundation’s funding for the C4 Rice consortium and by the Australian Research Council Centre of Excellence for Translational Photosynthesis (CE140100015). RES is funded by ARC DECRA (DE130101760).
References
- Alonso-Cantabrana H, von Caemmerer S. 2016. Carbon isotope discrimination as a diagnostic tool for C4 photosynthesis in C3–C4 intermediate species. Journal of Experimental Botany 67, 3109–3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger MR, Price GD. 1989. Carbonic anhydrase activity associated with the cyanobacterium Synechococcus PCC7942. Plant Physiology 89, 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger MR, Price GD. 1994. The role of carbonic anhydrase in photosynthesis. Annual Review of Plant Biology 45, 369–392. [Google Scholar]
- Barbour MM, Evans JR, Simonin KA, von Caemmerer S. 2016. Online CO2 and H2O oxygen isotope fractionation allows estimation of mesophyll conductance in C4 plants, and reveals that mesophyll conductance decreases as leaves age in both C4 and C3 plants. New Phytologist 210, 875–889 [DOI] [PubMed] [Google Scholar]
- Bartlett JG, Alves SC, Smedley M, Snape JW, Harwood WA. 2008. High-throughput Agrobacterium-mediated barley transformation. Plant Methods 4, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd RA, Gandin A, Cousins AB. 2015. Temperature response of C4 photosynthesis: biochemical analysis of Rubisco, phosphoenolpyruvate carboxylase and carbonic anhydrase in Setaria viridis. Plant Physiology 160, 1850–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brutnell TP, Wang L, Swartwood K, Goldschmidt A, Jackson D, Zhu X-G, Kellogg E, Van Eck J. 2010. Setaria viridis: a model for C4 photosynthesis. The Plant Cell 22, 2537–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernusak LA, Farquhar GD, Wong SC, Stuart-Williams H. 2004. Measurement and interpretation of the oxygen isotope composition of carbon dioxide respired by leaves in the dark. Plant Physiology 136, 3350–3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christin P-A, Boxall SF, Gregory R, Edwards EJ, Hartwell J, Osborne CP. 2013. Parallel recruitment of multiple genes into C4 photosynthesis. Genome Biology and Evolution 5, 2174–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christin PA, Osborne CP. 2013. The recurrent assembly of C4 photosynthesis, an evolutionary tale. Photosynthesis Research 117, 163–175. [DOI] [PubMed] [Google Scholar]
- Cousins AB, Badger MR, von Caemmerer S. 2006. A transgenic approach to understanding the influence of carbonic anhydrase on C18OO discrimination during C4 photosynthesis. Plant Physiology 142, 662–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins AB, Badger MR, von Caemmerer S. 2008. C4 photosynthetic isotope exchange in NAD-ME-and NADP-ME-type grasses. Journal of Experimental Botany 59, 1695–1703. [DOI] [PubMed] [Google Scholar]
- Cousins AB, Baroli I, Badger MR, Ivakov A, Lea P, Leegood RC, von Caemmerer S. 2007. The role of phosphenolpyruvate carboxylase during C4 photosynthetic isotope exchange and stomatal conductance. Plant Physiology 145, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig H, Gordon LI. 1965. Deuterium and oxygen 18 variations in the ocean and the marine atmosphere. In: Tongiorgi E, ed. Proceedings of a Conference on stable isotopes in oceanographic studies and paleotemperatures. Pisa, Italy: Consiglio Nazionale delle Ricerche, Laboratorio di Geologia Nucleare, 9–130. [Google Scholar]
- Curtis MD, Grossniklaus U. 2003. A Gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiology 133, 462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danila F, Quick WP, White RG, Furbank RT, von Caemmerer S. 2016. The metabolite pathway between bundle sheath and mesophyll: quantification of plasmodesmata in leaves of C3 and C4 monocots. The Plant Cell 28, 1461–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dever LV. 1997. Control of photosynthesis in Amaranthus edulis mutants with reduced amounts of PEP carboxylase. Australian Journal of Plant Physiology 24, 469–476. [Google Scholar]
- Dever LV, Lea PJ, Blackwell RD, Leegood RC. 1992. The isolation of mutants of C4 photosynthesis. In: Murata N, ed. Research in Photosynthesis, Vol. 111 Kluwer Academic Publishers, 891–894. [Google Scholar]
- DiMario RJ, Quebedeaux JC, Longstreth D, Dassanayake M, Hartman MM, Moroney JV. 2016. The cytoplasmic carbonic anhydrases βCA2 and βCA4 are required for optimal plant growth at low CO2. Plant Physiology 171, 280–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doust AN, Kellogg EA, Devos KM, Bennetzen JL. 2009. Foxtail millet: a sequence-driven grass model system. Plant Physiology 149, 137–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engineer CB, Hashimoto-Sugimoto M, Negi J, Israelsson-Nordstrom M, Azoulay-Shemer T, Rappel WJ, Iba K, Schroeder JI. 2016. CO2 sensing and CO2 regulation of stomatal conductance: advances and open questions. Trends in Plant Science 21, 16–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J, Sharkey T, Berry J, Farquhar G. 1986. Carbon isotope discrimination measured concurrently with gas exchange to investigate CO2 diffusion in leaves of higher plants. Functional Plant Biology 13, 281–292. [Google Scholar]
- Evans JR, Caemmerer S, Setchell BA, Hudson GS. 1994. The relationship between CO2 transfer conductance and leaf anatomy in transgenic tobacco with a reduced content of Rubisco. Functional Plant Biology 21, 475–495. [Google Scholar]
- Evans JR, von Caemmerer S. 1996. Carbon dioxide diffusion inside leaves. Plant Physiology 110, 339–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar GD, Cernusak LA. 2012. Ternary effects on the gas exchange of isotopologues of carbon dioxide. Plant, Cell and Environment 35, 1221–1231. [DOI] [PubMed] [Google Scholar]
- Farquhar G, Lloyd J. 1993. Carbon and oxygen isotope effects in the exchange of carbon dioxide between terrestrial plants and the atmosphere. In: Elheringer JR, Hall AE, Farquhar G, eds. Stable isotopes and plant carbon–water relations. New York: Academic Press, 47–70. [Google Scholar]
- Flexas J, Diaz-Espejo A, GalmES J, Kaldenhoff R, Medrano H, Ribas-Carbo M. 2007. Rapid variations of mesophyll conductance in response to changes in CO2 concentration around leaves. Plant, Cell and Environment 30, 1284–1298. [DOI] [PubMed] [Google Scholar]
- Furbank RT, Chitty JA, Jenkins CLD, Taylor WC, Trevanion SJ, von Caemmerer S, Ashton AR. 1997. Genetic manipulation of key photosynthetic enzymes in the C4 plant Flaveria bidentis. Australian Journal of Plant Physiology 24, 477–485. [Google Scholar]
- Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA, 3rd, Smith HO. 2009. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nature Methods 6, 343–345. [DOI] [PubMed] [Google Scholar]
- Gillon J, Yakir D. 2001. Influence of carbonic anhydrase activity in terrestrial vegetation on the 18O content of atmospheric CO2. Science 291, 2584–2587. [DOI] [PubMed] [Google Scholar]
- Gillon JS, Yakir D. 2000. Naturally low carbonic anhydrase activity in C4 and C3 plants limits discrimination against C18OO during photosynthesis. Plant, Cell and Environment 23, 903–915. [Google Scholar]
- Greenup AG, Sasani S, Oliver SN, Talbot MJ, Dennis ES, Hemming MN, Trevaskis B. 2010. ODDSOC2 is a MADS box floral repressor that is down-regulated by vernalization in temperate cereals. Plant Physiology 153, 1062–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutknecht J, Bisson MA, Tosteson FC. 1977. Diffusion of carbon dioxide through lipid bilayer membranes. Effects of carbonic anhydrase, bicarbonate, and unstirred layers. Journal of General Physiology 69, 779–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch MD. 1987. C4 photosynthesis: a unique blend of modified biochemistry, anatomy and ultrastructure. Biochimica et Biophysica Acta 895, 81–106. [Google Scholar]
- Hatch MD, Burnell JN. 1990. Carbonic anhydrase activity in leaves and its role in the first step of C4 photosynthesis. Plant Physiology 93, 825–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Boisson-Dernier A, Israelsson-Nordstrom M, Bohmer M, Xue S, Ries A, Godoski J, Kuhn JM, Schroeder JI. 2010. Carbonic anhydrases are upstream regulators of CO2-controlled stomatal movements in guard cells. Nature Cell Biology 12, 87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins CLD, Furbank RT, Hatch MD. 1989. Mechanism of C4 photosynthesis: a model describing the inorganic carbon pool in bundle sheath cells. Plant Physiology 91, 1372–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John CR, Smith-Unna RD, Woodfield H, Covshoff S, Hibberd JM. 2014. Evolutionary convergence of cell-specific gene expression in independent lineages of C4 grasses. Plant Physiology 165, 62–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence SD, Novak NG, Slack JM. 2003. Epitope tagging: a monoclonal antibody specific for recombinant fusion proteins in plants. Biotechniques 35, 488–492. [DOI] [PubMed] [Google Scholar]
- Li P, Brutnell TP. 2011. Setaria viridis and Setaria italica, model genetic systems for the Panicoid grasses. Journal of Experimental Botany 62, 3031–3037. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Matsuoka M, Furbank RT, Fukayama H, Miyao M. 2001. Molecular engineering of C4 photosynthesis. Annual Review of Plant Physiology and Plant Molecular Biology 52, 297–314. [DOI] [PubMed] [Google Scholar]
- Moroney JV, Bartlett SG, Samuelsson G. 2001. Carbonic anhydrases in plants and algae. Plant, Cell and Environment 24, 141–153. [Google Scholar]
- Okabe K, Yang S-Y, Tsuzuki M, Miyachi S. 1984. Carbonic anhydrase: its content in spinach leaves and its taxonomic diversity studied with anti-spinach leaf carbonic anhydrase antibody. Plant Science Letters 33, 145–153. [Google Scholar]
- Pengelly JJL, Sirault XRR, Tazoe Y, Evans JR, Furbank RT, von Caemmerer S. 2010. Growth of the C4 dicot Flaveria bidentis: photosynthetic acclimation to low light through shifts in leaf anatomy and biochemistry. Journal of Experimental Botany 61, 4109–4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pengelly JJL, Tan J, Furbank RT, von Caemmerer S. 2012. Antisense reduction of NADP-malic enzyme in Flaveria bidentis reduces flow of CO2 through the C4 cycle. Plant Physiology 160, 1070–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage RF, Sage TL, Kocacinar F. 2012. Photorespiration and the evolution of C4 photosynthesis. Annual Review of Plant Biology 63, 19–47. [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, et al. 2012. Fiji: an open-source platform for biological-image analysis. Nature Methods 9, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharwood RE, Sonawane BV, Ghannoum O, Whitney SM. 2016. Improved analysis of C4 and C3 photosynthesis via refined in vitro assays of their carbon fixation biochemistry. Journal of Experimental Botany 67, 3137–3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studer AJ, Gandin A, Kolbe AR, Wang L, Cousins AB, Brutnell TP. 2014. A limited role for carbonic anhydrase in C4 photosynthesis as revealed by a ca1ca2 double mutant in maize. Plant Physiology 165, 608–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazoe Y, von Caemmerer S, Estavillo GM, Evans JR. 2011. Using tunable diode laser spectroscopy to measure carbon isotope discrimination and mesophyll conductance to CO2 diffusion dynamically at different CO2 concentrations. Plant, Cell and Environment 34, 580–591. [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. 2002. Accurate normalization of real-time quantitative RT–PCR data by geometric averaging of multiple internal control genes. Genome Biology 3, Research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Caemmerer S. 2000. Biochemical models of leaf photosynthesis. Collingwood, Australia: CSIRO Publishing. [Google Scholar]
- von Caemmerer S, Evans JR. 2015. Temperature responses of mesophyll conductance differ greatly between species. Plant, Cell and Environment 38, 629–637. [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Evans JR, Cousins AB, Badger MR, Furbank RT. 2007. C4 photosynthesis and CO2 diffusion. In: Sheehy JE, Mitchell PL, Hardy B, eds. Charting new pathways to C4 rice. Philippines: IRRI, 95–115. [Google Scholar]
- von Caemmerer S, Furbank RT. 2003. The C4 pathway: an efficient CO2 pump. Photosynthesis Research 77, 191–207. [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Quinn V, Hancock N, Price G, Furbank R, Ludwig M. 2004. Carbonic anhydrase and C4 photosynthesis: a transgenic analysis. Plant, Cell and Environment 27, 697–703. [Google Scholar]
- Wang C, Hu H, Qin X, Zeise B, Xu D, Rappel W-J, Boron WF, Schroeder JI. 2016. Reconstitution of CO2 regulation of SLAC1 anion channel and function of CO2-permeable PIP2;1 aquaporin as CARBONIC ANHYDRASE4 interactor. The Plant Cell 28, 568–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TG, Flanagan LB, Coleman JR. 1996. Photosynthetic gas exchange and discrimination against 13CO2 and C18O16O in tobacco plants modified by an antisense construct to have low chloroplastic carbonic anhydrase. Plant Physioogy 112, 319–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingate L, Ogee J, Cuntz M, et al. 2009. The impact of soil microorganisms on the global budget of delta 18O in atmospheric CO2. Proceedings of the National Academy of Sciences, USA 106, 22411–22415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakir D, Sternberg LDL. 2000. The use of stable isotopes to study ecosystem gas exchange. Oecologia 123, 297–311. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.