A nonhemolytic and nonpigmented group B streptococcus clinical isolate exhibits hypervirulence and has a CovS mutation that confers constitutive kinase signaling.
Keywords: hemolysis, hyaluronidase, nonhemolytic, pigment, Streptococcus agalactiae
Abstract
Group B streptococci (GBS) are Gram-positive bacteria that are a leading cause of neonatal infections. Most invasive isolates are β-hemolytic, and hemolytic activity is critical for GBS virulence. Although nonhemolytic GBS strains are occasionally isolated, they are often thought to be virulence attenuated. In this study, we show that a nonhemolytic GBS strain (GB37) isolated from a septic neonate exhibits hypervirulence. Substitution of tryptophan to leucine (W297L) in the sensor histidine kinase CovS results in constitutive kinase signaling, leading to decreased hemolysis and increased activity of the GBS hyaluronidase, HylB. These results describe how nonpigmented and nonhemolytic GBS strains can exhibit hypervirulence.
Group B streptococci (GBS) are β-hemolytic, Gram-positive bacteria that are a leading cause of neonatal infections. Group B streptococci also cause infections in adults including the elderly, diabetic, and immunocompromised. Invasive GBS isolates are characteristically β-hemolytic. The ornithine rhamnolipid pigment encoded by the cyl operon is responsible for both the orange pigmentation and GBS hemolysis [1]. Expression of the hemolysin/hemolytic pigment is critical for GBS virulence as isogenic nonhemolytic strains exhibit attenuated virulence [2] and hyperhemolytic strains exhibit enhanced virulence [3]. The two-component system comprising the sensor histidine kinase CovS and the response regulator CovR repress the transcription of cyl genes encoding the hemolytic pigment [1, 4]. In addition, the multi-transmembrane protein Abx1 interacts with the membrane-spanning sensory region of CovS and facilitates phosphatase activity of CovS through protein-protein interactions [5]. Deletion of Abx1 results in enhanced repression of hemolysin genes [5].
Despite the importance of the hemolysin/hemolytic pigment to virulence of GBS, 3% of clinically invasive GBS strains are nonhemolytic/nonpigmented [6, 7]. Virulence factors that mediate the pathogenesis of nonpigmented GBS strains remain unknown. A nonpigmented strain (GB0037, hereafter called GB37) was recovered from the blood of a neonate with early-onset GBS disease [8, 9]. We show that GB37 is highly virulent in animal models of systemic infection, and increased hyaluronidase activity contributes to its virulence. An amino acid substitution in CovS (W297L) renders GB37 with constitutive CovR/S kinase signaling leading to enhanced repression of hemolysin and expression of hyaluronidase.
METHODS
Group B streptococci were grown in Tryptic Soy Broth (Difco) in 5% CO2 at 37°C. Escherichia coli were grown in Luria-Bertani broth (Difco) at 37°C. GB37, A909, NEM316, and COH1 are GBS clinical isolates obtained from infected newborns, and GB590 is a rectovaginal isolate [8, 10]. A909ΔcovR and COH1ΔcovR were described previously [1]. GB37ΔhylB and GB37CovS297W were derived using methods described previously [11].
Hyaluronidase assays were performed as described previously [11]. In brief, 50 µL of supernatants from overnight cultures were added to 200 µL hyaluronic acid solution (1.25 mg/mL Rooster Comb Hyaluronic Acid [Sigma]) and incubated at 37°C for 45 minutes. Subsequent derivation and quantification were performed as described previously [11]
All animal experiments were approved by the Institutional Animal Care and Use Committee, Seattle Children’s Research Institute (Protocol no. 13311) and performed using accepted veterinary standards. Wild-type (WT) C57BL/6J mice were purchased from Jackson Laboratories.
For survival studies, 6- to 8-week-old WT mice were given intravenous (IV) injection with ~1 × 108 colony-forming units (CFU) of either GB37, A909, A909ΔcovR, COH1, COH1ΔcovR, or GB590, and survival was monitored up to 14 days. To compare bacterial dissemination and leukocyte infiltration, GBS strains were infected intraperitoneally (IP), and experimental endpoint was 2 days postinfection, when the mice typically do not succumb to the infection. Bacterial counts in the brain and spleen homogenates were determined by serial dilution and plating. Inflammatory cytokine release was measured using Luminex (eBiosciences). For flow cytometry, reagents and antibodies were obtained from BD Biosciences; peritoneal cells were Fc blocked (clone 2.4G2) for 15 minutes, stained for 30 minutes with anti-CD45 (APC-Cy7 conjugated, clone 30-F11) and anti-Ly6G (fluorescein isothiocyanate conjugated, clone 1A8) antibodies, collected on an LSRII flow cytometer, and analyzed using FlowJo version 10.
The genomes for GB37 [9] and control strains, A909 (PRJNA326), COH1 (PRJNA15609), NEM316 (PRJNA334), 2603V/R (PRJNA330), 18RS21 (PRJNA15605), 515 (PRJNA15606), ATCC 13813 (PRJNA53057), CJB111 (PRJNA15607), FSL S3-026 (PRJNA86137), and ZQ0910 (PRJNA158929), were downloaded from the National Center for Biotechnology Information. Sequences were extracted using BLAST and aligned by MUSCLE in MEGA6. The assembled genomes were also mapped using Bowtie2 to their reads to estimate sequence coverage and check for ambiguity; tablet was used to view the assemblies.
For homology alignment, CovS sequences were extracted from the Protein Data Bank (PDB) file generated by Phyre2 using UCSF chimera. For homology modeling, the HHpred program was used for profile-profile searches. Structure similarity searches were performed using DaliLite. Secondary structures were predicted using JPred. Structural visualization and manipulations were performed using PyMol. Homology modeling was performed using the ProMod II program via the SWISS-MODEL server, which uses a GROMOS energy minimization protocol.
All experimental replicates represent biological replicates. Statistical tests were performed using GraphPad Prism (5.0) and are indicated. P value <.05 was considered significant.
RESULTS
Hypervirulence of a Nonhemolytic and Nonpigmented Group B Streptococcus Strains
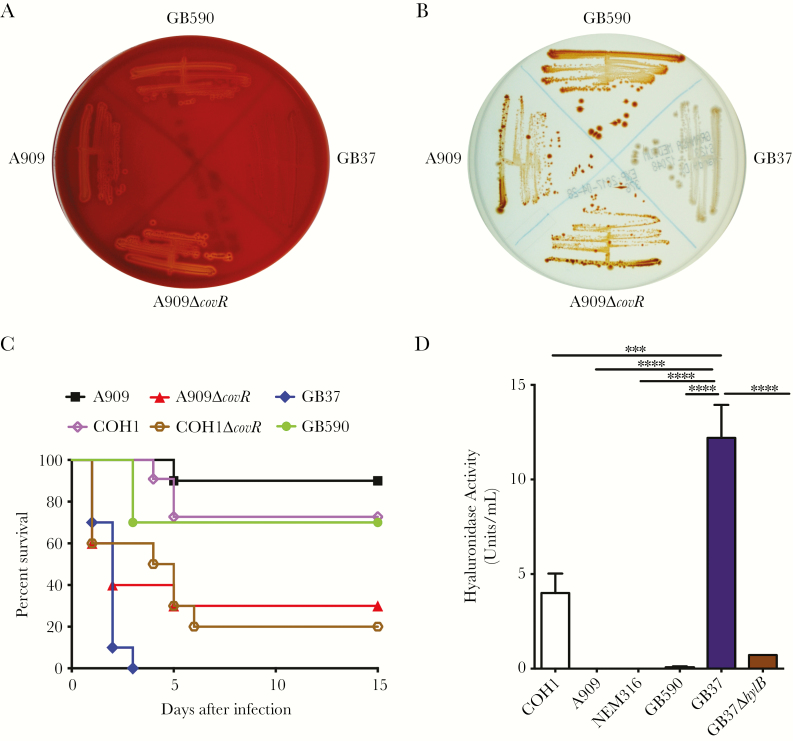
A comparison of hemolysis and pigmentation of GB37 to other GBS strains, such as the moderately hemolytic strain A909, isogenic A909∆covR (lacking the hemolysin repressor CovR/S [1]) or a rectovaginal isolate GB590 [10] indicated that hemolysis and pigmentation are not observed for GB37, unlike GB590, A909, and A909∆covR (Figure 1A and B). Virulence of GB37 was compared with these strains by infecting adult, WT C57BL/6J mice (n = 10/group) IV with ~1 × 108 CFU as described previously [3]. The mildly hemolytic WT GBS strain COH1 and its isogenic hyperhemolytic/hypervirulent COH1∆covR were also included in these analyses [1]. We were surprised to find that GB37 was significantly more virulent than A909, COH1, or GB590 and was similar to hyperhemolytic/hypervirulent ∆covR strains [3] (Figure 1C). Group B streptococci CFU recovered from GB37-infected mice remained nonhemolytic/nonpigmented, indicating that there was no reversion to hemolysis, which could have promoted virulence. Although sialylation of the GBS capsular polysaccharide is critical for virulence [12], sialic acid levels on capsular polysaccharide was similar between GB37 and the WT GBS strains above (data not shown).
Figure 1.
GB37 exhibits decreased hemolysis and pigmentation but increased virulence and hyaluronidase activity. (A) The zone of clearing around the colonies on sheep blood agar indicates hemolytic activity. GB37 exhibits little-to-no hemolysis compared with GB590, A909, or A909ΔcovR. (B) Hemolytic group B streptococci (GBS) strains are pigmented, and nonhemolytic strains are nonpigmented on Granada Media. GB37 exhibits little-to-no pigmentation compared with GB590, A909, or A909ΔcovR. (C) Ten, 6- to 8-week-old wild-type (WT) C57BL/6J mice were given intravenous injections with 1 × 108 colony-forming units of GB37, GB590, A909, COH1, or hyperhemolytic strains (A909ΔcovR, COH1ΔcovR). Kalpan-Meier survival curve shows percentage survival of mice after the infection. Note that mice infected with GB37 succumbed to the infection within 4 days postinoculation in contrast those infected with A909, COH1, and GB590 (P < .0001, log-rank test), and GB37 virulence was similar to the ΔcovR strains. (D) Hyaluronidase activity of GB37 was compared with other GBS clinical isolates such as A909, COH1 NEM316, and GB590 and to GB37ΔhylB. Data shown represent the average of 3 independent experiments (error bars ± standard error of the mean). ***, P = .0004 and *****, P < .0001, Sidak’s multiple comparison test after analysis of variance.
GB37 Exhibits Increased Hyaluronidase (HylB) Activity That Contributes to Its Virulence
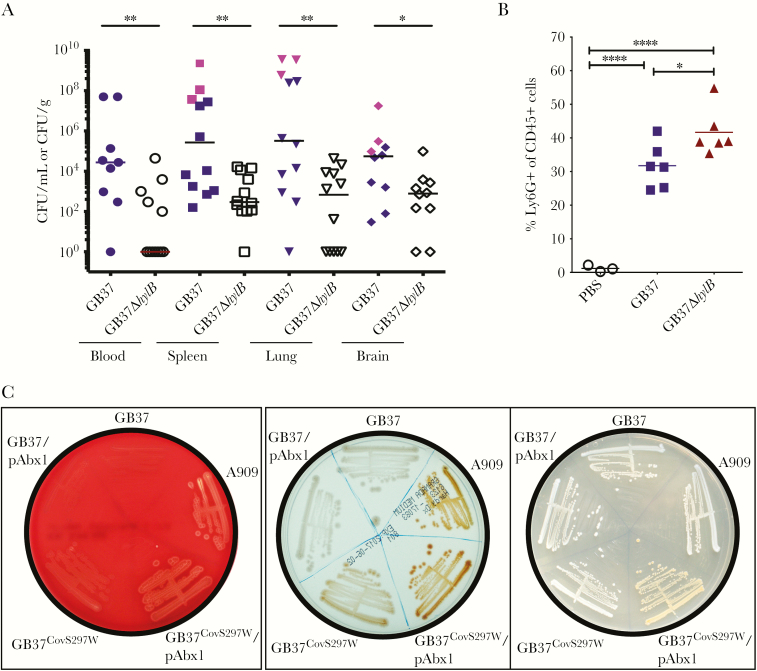
Group B streptococci secrete an enzyme known as hyaluronidase, or HylB, that promotes virulence by degrading host hyaluronan into immunosuppressive disaccharides [13, 14]. A comparison of hyaluronidase activity showed that GB37 had significantly greater hyaluronidase activity compared with other GBS strains (Figure 1D). To test whether HylB contributed to virulence of GB37, we derived a hylB mutant using methods described previously [11]. The GB37∆hylB exhibited significantly diminished hyaluronidase activity (Figure 1D) and was not hemolytic or pigmented, similar to GB37 (data not shown). To determine whether HylB contributes to virulence of GB37, WT mice were infected IP with ~107 CFU of GB37 or GB37∆hylB (n = 12/group). At 48 hours postinfection, blood and systemic organs were harvested to determine bacterial burden. Figure 2A shows that increased dissemination of GB37 was observed in multiple organs of infected mice when compared with GB37∆hylB, and a few GB37-infected mice even succumbed to the infection at this early time point (pink symbols in Figure 2A). However, no significant difference in tumor necrosis factor-α or interleukin-6 levels was observed in the organs of infected mice (Supplementary Figure S1). To determine whether HylB alters neutrophil recruitment early during infection, mice were infected IP with ~108 CFU of GB37, GB37∆hylB, or phosphate-buffered saline (n = 6/group). At 2 hours postinfection, peritoneal fluids were collected and peritoneal cells were stained with antibodies for neutrophils markers (CD45+Ly6G+) and analyzed using flow cytometry. Figure 2B shows that neutrophil recruitment was significantly lower in the peritoneal cavity of GB37-infected mice compared with GB37∆hylB. These results indicate that HylB suppresses neutrophil migration that may contribute to increased systemic infection.
Figure 2.
Hyaluronidase activity promotes GB37 virulence, and a CovS W297L substitution in GB37 contributes to the decreased hemolysis and increased hyaluronidase activity. (A) Twelve, 6- to 8-week-old wild-type (WT) C57BL/6J mice were given intraperitoneal (IP) injections with ~1 × 107 colony-forming units (CFU) of either GB37 or GB37ΔhylB. At approximately 48 hours postinfection, blood, spleens, lungs, and brains were harvested from the infected mice and CFU were enumerated. Pink symbols for GB37 indicate mice that succumbed to the infection within 48 hours. Note that mice infected with GB37 have increased CFU compared with GB37ΔhylB. *, P < .05 and **, P < .01, Mann-Whitney test. (B) Wild-type C57BL/6J mice were infected IP with ~108 CFU of GB37, GB37∆hylB (n = 6/group), or phosphate-buffered saline ([PBS] n = 3/group). At 2 hours postinfection, peritoneal fluids were collected and peritoneal cells were stained with antibodies for neutrophils markers (CD45+Ly6G+) and analyzed using flow cytometry. ****, P < .0001 and *, P < .05, Sidak’s multiple comparison test after analysis of variance. (C) Group B streptococci strains on red blood agar, Granada Media, and tryptic soy agar. Introduction of Abx1 into GB37 (GB37/pAbx1) did not restore hemolysis or pigment expression. Correction of the CovS substitution back to WT, ie, GB37CovS297W restored hemolysis and pigmentation, which was enhanced when Abx1 was present (compare GB37CovS297W with GB37CovS297W/pAbx1).
A W297L Substitution in CovS Contributes to Constitutive Kinase Signaling Resulting in Decreased Hemolysis and Increased Hyaluronidase
The CovR/S two component system typically controls the expression of ~200 genes including those encoding the hemolytic pigment, and in some GBS strains CovR/S activates hylB (hyaluronidase) expression [3]. We therefore hypothesized that opposing regulation of hyaluronidase and the hemolytic pigment in GB37 may be due to mutations in CovR/S and/or in the Abx1 protein that promotes phosphatase activity of CovS. Analysis of the GB37 genome [9] revealed a nucleotide deletion in abx1 at position 97 bp that results in premature truncation of Abx1 at amino acid 46. However, complementation of GB37 with a plasmid-expressing Abx1 (pAbx1) did not restore hemolysis or pigmentation (see GB37/pAbx1; Figure 2C). Analysis of the CovR/S sequence in GB37 indicated the presence of a unique, nonsynonymous G to T transversion in covS at nucleotide position 890, resulting in a predicted tryptophan to leucine substitution at position 297. When the covS mutation was repaired, the resulting GB37CovS297W strain exhibited increased hemolysis and pigmentation (Figure 2C), compared with GB37. Introduction of Abx1 into GB37CovS297W further enhanced hemolysis and pigmentation (see GB37CovS297W/pAbx1 in Figure 2C). Taken together, these data indicate that the tryptophan residue at position 297 is necessary for Abx1 modulation of CovS and that the W297L substitution overrides the ability of Abx1 to impact CovS.
To understand how the W297L substitution may affect CovS signaling, we used known histidine kinase structures to generate a homology model. In particular, the histidine kinase from Lactobacillus plantarum (PDB no. 5C93) was completely collinear in the region of the mutation and had an excellent alignment (e-value = 5 × 10–30) with the dimerization and histidine-containing phosphotransfer (DHp) and histidine kinase domain of CovS. The mutated CovS residue is located in the juncture between the two helices spanning the DHp motif, which contains the histidine (H278) that is autophosphorylated and the threonine (T282) residue necessary for phosphatase activity [5] (Supplementary Figure S2). Comparison with structures that contain complexes of the receiver domain with the histidine kinase module revealed that W297 falls in the region that mediates an interaction with the receiver domain of the response regulator. Closer examination revealed that the side chain of W297 is likely to adopt an exposed conformation, which makes it available for direct interaction with H24 of CovR. Thus, the W297L mutation in CovS would alter the predicted contact with H24 in CovR, which would involve Pi-Pi stacking and cation-Pi interactions. We interpret that this change in interaction with the receiver domain as altering the threshold for the conformational change in the histidine kinase, allowing for excessive phosphorylation of CovR and accounting for increased repression of the cyl genes in GB37.
Although virulence analysis of mice infected with GB37 or GB37CovS297W did not indicate statistically significant differences between them (Supplementary Figure S3), the virulence assay maybe limiting for evaluating differences between hypervirulent GBS strains. Taken together, our results indicate that spontaneous mutations in CovS (eg, W297L) results in constitutive CovR/S phosphorylation that leads to enhanced repression of hemolytic pigment but also alters expression of other virulence genes such as the hyaluronidase rendering GBS hypervirulent.
Discussion
Group B streptococci remain a significant cause of infections in newborns and adults. Clinical isolates with spontaneous mutations in CovR/S typically exhibit hyperhemolyis and hyperpigmentation that promote hypervirulence [1, 15]. In this study, we describe a GBS strain with a unique, spontaneous CovS W297L mutation that significantly decreased hemolysis and pigmentation. It is interesting to note that Abx1 was able to alleviate CovS repression of hemolysin only when the covS mutation was repaired, suggesting that the W297L substitution results in CovS/R signaling that bypasses Abx1. Although truncations in Abx1 have been reported for clinical GBS strains exhibiting reduced hemolysis such as CCH1350 and others [6], these strains do not harbor the CovS W297L substitution and introduction of Abx1 restored hemolysis to CCH1350 [6]. However, in GB37, we observed that the CovS W297L mutation had to be repaired for restoration of hemolytic pigment expression and repression of hyaluronidase.
These observations taken together with analysis of our homology model for CovS help us reconstruct multiple aspects of the complex signaling via the CovS-CovR-Abx1 system. We posit that the region including the mutated W297 plays a key role in interacting with the receiver domain of CovR, and the strength of this interaction is critical for setting the threshold for the transmission of the conformational change to the bound CovR receiver domain upon signal reception by CovS. Defining mutations that contribute to hypervirulence is critical, particularly in clinical settings wherein nonpigmented GBS strains may have the potential for invasive infections.
Conclusions
Although we demonstrate a role for HylB in virulence of GB37, the CovR/S regulon comprises over 200 genes. Therefore, it is possible that other CovR/S regulated genes and/or other single-nucleotide polymorphisms or factors also contribute to the hypervirulence of GB37. While nonpigmented GBS strains are infrequently isolated from invasive settings [6, 7] and some carry mutations in the cyl operon where only hemolysis is affected [6], our results provide new insight into the diversity of these GBS isolates and their pathogenesis. In summary, we show that increased expression of hyaluronidase promotes virulence of a nonpigmented GBS strain with constitutively active kinase signaling via the CovR/S system.
Supplementary Material
Notes
Author contributions. C. G., J. V., B. A., P. S., C. W., S. M., D. K., R. P., L. M. R., P. Q., S. D. M., D. M. A., and L. R. designed the research and/or performed the experiments. L. M. I. and L. A. performed computer modeling. S. D. M., D. M. A., and L. R. wrote the paper with input from other authors. All authors reviewed the final version of the paper.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or other funders. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Finanical support. This work was supported by funding from the following: the National Institutes of Health (NIH), Grant nos. R01AI100989, R01AI112619, and R21AI125907 (to L. R.) and R56AI100903 (to D. M. A. and S. D. M.); the Global Alliance to Prevent Prematurity and Stillbirth (D. M. A., S. D. M.); and the Burroughs Wellcome Fund Investigators in the Pathogenesis of Infectious Disease Award (to D. M. A.). J. V. was supported by NIH Training Grant T32 AI07509 (Principal Invetigator: Lee Ann Campbell). L. M. I. and L. A. are supported by intramural funds from the National Library of Medicine at NIH.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Whidbey C, Harrell MI, Burnside K et al. . A hemolytic pigment of group B streptococcus allows bacterial penetration of human placenta. J Exp Med 2013; 210:1265–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Doran KS, Liu GY, Nizet V. Group B streptococcal beta-hemolysin/cytolysin activates neutrophil signaling pathways in brain endothelium and contributes to development of meningitis. J Clin Invest 2003; 112:736–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lembo A, Gurney MA, Burnside K et al. . Regulation of CovR expression in group B streptococcus impacts blood-brain barrier penetration. Mol Microbiol 2010; 77:431–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lamy MC, Zouine M, Fert J et al. . CovS/CovR of group B streptococcus: a two-component global regulatory system involved in virulence. Mol Microbiol 2004; 54:1250–68. [DOI] [PubMed] [Google Scholar]
- 5. Firon A, Tazi A, Da Cunha V et al. . The Abi-domain protein Abx1 interacts with the CovS histidine kinase to control virulence gene expression in group B streptococcus. PLoS Pathog 2013; 9:e1003179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Six A, Firon A, Plainvert C et al. . Molecular characterization of nonhemolytic and nonpigmented group B streptococci responsible for human invasive infections. J Clin Microbiol 2016; 54:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rodriguez-Granger J, Spellerberg B, Asam D, Rosa-Fraile M. Non-haemolytic and non-pigmented group B streptococcus, an infrequent cause of early onset neonatal sepsis. Pathog Dis 2015; 73:ftv089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Davies HD, Adair C, McGeer A et al. . Antibodies to capsular polysaccharides of group B streptococcus in pregnant Canadian women: relationship to colonization status and infection in the neonate. J Infect Dis 2001; 184:285–91. [DOI] [PubMed] [Google Scholar]
- 9. Singh P, Aronoff DM, Davies HD, Manning SD. Draft genome sequence of an invasive Streptococcus agalactiae isolate lacking pigmentation. Genome Announc 2016; 4: pii: e00015-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Spaetgens R, DeBella K, Ma D, Robertson S, Mucenski M, Davies HD. Perinatal antibiotic usage and changes in colonization and resistance rates of group B streptococcus and other pathogens. Obstet Gynecol 2002; 100:525–33. [DOI] [PubMed] [Google Scholar]
- 11. Vornhagen J, Quach P, Boldenow E et al. . Bacterial hyaluronidase promotes ascending GBS infection and preterm birth. MBio 2016; 7:e00781-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marques MB, Kasper DL, Pangburn MK, Wessels MR. Prevention of C3 deposition by capsular polysaccharide is a virulence mechanism of type III group B streptococci. Infect Immun 1992; 60:3986–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kolar SL, Kyme P, Tseng CW et al. . Group B streptococcus evades host immunity by degrading hyaluronan. Cell Host Microbe 2015; 18:694–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang Z, Guo C, Xu Y, Liu G, Lu C, Liu Y. Two novel functions of hyaluronidase from Streptococcus agalactiae are enhanced intracellular survival and inhibition of proinflammatory cytokine expression. Infect Immun 2014; 82:2615–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sendi P, Johansson L, Dahesh S et al. . Bacterial phenotype variants in group B streptococcal toxic shock syndrome. Emerg Infect Dis 2009; 15:223–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.