In baboons with the levonorgestrel-releasing intrauterine system and Chlamydia trachomatis infection, progression to pelvic inflammatory disease may not be affected, but infection lasts longer and yields higher titers relative to infected controls. Defects in early activation of the interleukin 1 pathway may account for this difference.
Keywords: Chlamydia trachomatis, intrauterine contraception, levonorgestrel, baboon, immune responses
Abstract
Background
Understanding the relationship between the levonorgestrel (LNG)–releasing intrauterine system (IUS) and sexually transmitted infections (STIs) is increasingly important as use of the LNG-IUS grows to include women at higher risk for STIs. This study assessed the impact of the LNG-IUS on development of Chlamydia trachomatis pelvic inflammatory disease, using a baboon model.
Methods
Baboons with and those without the LNG-IUS were cervically inoculated with C. trachomatis and monitored daily, and cervical and fallopian tube swab specimens were collected weekly for C. trachomatis quantitation by nucleic acid amplification testing and culture. Vaginal swab specimens were collected for cytokine analysis, and serum samples were obtained for detection of C. trachomatis antibodies.
Results
The LNG-IUS resulted in an increased C. trachomatis burden in the cervix, with the bacterial burden in the LNG-IUS group diverging from that in the non–LNG-IUS group by 6 weeks after infection. One of 7 baboons in the non–LNG-IUS group and 2 of 6 in the LNG-IUS group developed pelvic inflammatory disease, while 3 animals in each group met criteria suggestive of pelvic inflammatory disease. LNG-IUS increased baseline interleukin 8 levels but failed to further upregulate interleukin 8 during infection. In LNG-IUS recipients, early perturbations in the interleukin 1β axis corresponded to decreased C. trachomatis clearance and increased T-helper type 2 immune responses.
Conclusion
LNG-IUS use results in delayed clearance of C. trachomatis and might alter the reproductive tract immune environment.
Sexually transmitted infections (STIs) and unplanned pregnancies are interconnected global health crises affecting millions of women and men. Moreover, countries with the largest STI burden have the highest rates of maternal and infant mortality and the greatest need for effective contraception. The levonorgestrel (LNG)–releasing intrauterine system (IUS), a form of long-acting reversible contraception, provides women with user-independent contraception that can increase the interval between births and decrease the number of unintended pregnancies. However, use is limited worldwide because of fear of side effects, including development of pelvic inflammatory disease (PID), as observed with previous generations of intrauterine devices, such as the Dalkon Shield [1, 2]. PID is an infectious disease of the female upper reproductive tract that affects nearly 800000 women annually in the United States [3]. Potential consequences of PID include infertility, ectopic pregnancy, chronic pelvic pain, and tubo-ovarian abscess [3]. Chlamydia trachomatis is one of the most common pathogens associated with PID and is the most common sexually transmitted bacterial pathogen worldwide [4].
A primate model that effectively recapitulates PID allows prospective interrogation of the association between chlamydial infection and intrauterine device use, as well as investigation of basic immune mechanisms, pathogenesis, and interventional strategies. In macaques (Macaca species), chlamydial infection can be reliably produced, but use of the LNG-IUS in this model is limited by the size of the animal and the presence of a tortuous cervical canal, making intracervical insertion challenging. Baboons (Papio anubis) have a rectilinear cervical canal that allows for ease of transcervical procedures [5, 6]. Previously our group demonstrated that baboons are susceptible to infection with C. trachomatis and develop a range of clinical manifestations that include DNA detection with no clinical signs, cervicitis, and PID with tubal enlargement and inflammation [7]. In this study, we evaluated C. trachomatis infection in baboons with and those without the LNG-IUS to determine the influence of this device on C. trachomatis infection, persistence, and progression to PID.
METHODS
Regulatory Approval
The Institutional Review Committee at the Institute of Primate Research in Nairobi, Kenya, approved all aspects of live animal work in this study, in compliance with pertinent Kenyan law (Cap 360: Prevention of Cruelty to Animals Act) and the International Guiding Principles for Biomedical Research Involving Animals [8]. This study received an off-site exemption from the University Committee for the Care and Use of Animals at the University of Michigan. A Kenyan export permit (in accordance with the Convention on International Trade in Endangered Species of Wild Fauna and Flora) was obtained from the Kenya Wildlife Service, and permits for import were obtained from the Centers for Disease Control and Prevention (as described elsewhere [9]).
Study Site, Population, and Design
This study was conducted at the Institute of Primate Research and has been previously described [9]. Briefly, 18 wild-caught sexually mature female olive baboons (P. anubis) were used in this study, quarantined, examined, and housed as described elsewhere [9]. Animals were then randomly assigned to an experimental group (6 received an LNG-IUS [the LNG group], and 8 did not [the non-LNG group]) or an uninfected control group (2 received an LNG-IUS, and 8 did not). All animals underwent baseline collection of serum and cervical swab specimens for detection of C. trachomatis by culture and nucleic acid amplification testing (NAAT). For the LNG group, the device (Mirena) was inserted transcervically, using our previously described protocol [10] and the manufacturer-provided insertion device, as well as a small Pederson vaginal speculum for visualization [9, 11], in anesthetized baboons. This group was monitored for 24 weeks for problems associated with the LNG-IUS (eg, expulsion and perforation). Following this observation period, animals underwent C. trachomatis infection. The non-LNG group did not undergo the postquarantine observation period and went immediately into the infection phase. A total of 1 × 107 inclusion-forming units of a clinical C. trachomatis serovar E isolate or an equal volume of SPG buffer was administered by pipette directly onto the ectocervix at weekly intervals for a total of 5 inoculations as previously described [7, 9].
Sampling
Cervical, tubal, and blood samples were obtained from, physical examination was performed on, and laparoscopic tubal evaluation was conducted on anesthetized animals, as described elsewhere [9] and summarized in Figure 1A. Animals were euthanized and final samples collected 16 weeks after initial inoculation. Tubal and cervical NAATs, C. trachomatis cultures, serological analyses, and histological analyses were performed as previously described [7, 9]. The presence of antichlamydial antibodies in serum was determined using the Medac pELISA kit [12] (Medac, Wedel, Germany). Quantitative polymerase chain reaction (PCR) analysis for C. trachomatis was performed using the Abbott RealTime Ct/NG assay (Promega, Madison, WI) at the University of Michigan Clinical Microbiology Laboratory. Testing was performed with the Abbott m2000rt system, using the mSample Preparation DNA kit for sample extraction on the m2000sp system. Internal controls from the Abbott RealTime CT/NG kit were added to lysis buffer before extraction. When extraction was complete, sample eluate and master mix were added to the 96-well PCR plate, which was then transferred to the m2000 system for real-time PCR analysis. Histological evaluation was performed by a board-certified veterinary pathologist blinded to the experimental groups at the time of evaluation, using a previously described 4-point histological scoring system [7]. PID was diagnosed on the basis of criteria reported elsewhere [7], with modifications, as shown in Table 2.
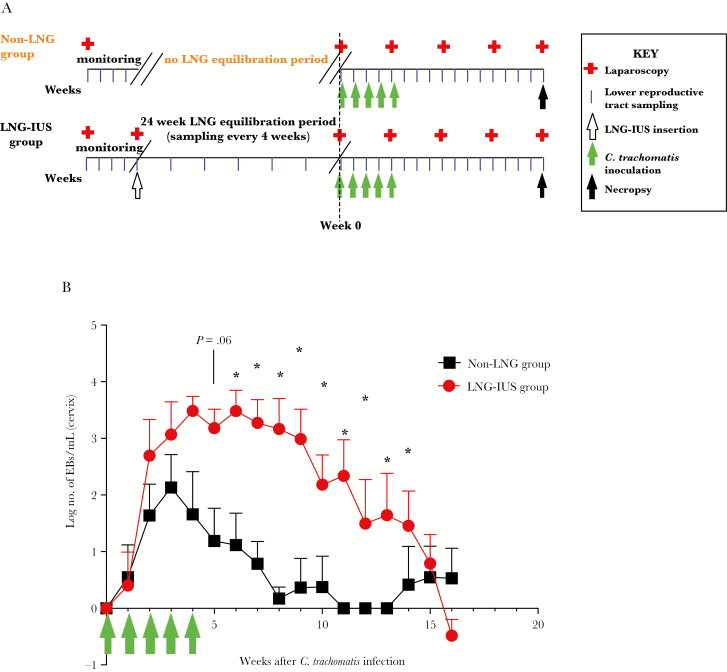
Figure 1.
Chlamydia trachomatis bacterial burden between baboons with and those without the levonorgestrel (LNG)–releasing intrauterine system (LNG-IUS). A, Baseline monitoring, equilibration, infection, and sampling schematic. All animals had 4 weeks of baseline monitoring with laparoscopic examination, followed by weekly sampling of the lower female reproductive tract. Baboons randomly assigned to the LNG-IUS group had a 24-week equilibration period, with sampling every 4 weeks before infection at week 0. Baboons in the non-LNG group did not have the 24-week IUD equilibration period and were inoculated with C. trachomatis immediately after the baseline monitoring period. All infected animals received 5 cervical inoculations of 1 × 107 inclusion-forming units of serovar E C. trachomatis. During the infection period, animals had samples collected noninvasively each week and were monitored laparoscopically every 4 weeks until necropsy at 16 weeks after infection. B, Quantitative nucleic acid amplification testing was performed weekly during infection on cervical samples obtained from animals. Green arrows indicate the multiple inoculation time course. Data are for 6–7 animals per time point. EB, elementary body. *P < .05.
Table 2.
Satisfaction of Criteria for or Suggestive of Pelvic Inflammatory Disease (PID) or Lower Reproductive Tract Infection Only, by Study Group
| Criteria | Non-LNG Group | LNG-IUS Group | ||||
|---|---|---|---|---|---|---|
| PID (n = 1) | Suggestive of PID (n = 3) | Lower Tract Infection Only (n = 3) | PID (n = 2) | Suggestive of PID (n = 3) | Lower Tract Infection Only (n = 1) | |
| Major | ||||||
| Tubal NAAT positivity | + | − | − | + | − | − |
| Tubal enlargement or adhesion | + | + | − | + | + | − |
| Histologically evident salpingitis | + | − | − | + | − | − |
| Minor | ||||||
| Cervical NAAT positivity | + | + | + | + | + | + |
| Culture | + | + | + | + | + | + |
The presence of ≥2 major criteria and ≥1 minor criterion was sufficient for PID. The presence of 1 major criterion and ≥1 minor criterion was suggestive of PID. The presence of ≥1 minor criterion was sufficient for lower reproductive tract infection only. There was 1 animal with PID in the non-LNG group, 3 with criteria suggestive of PID, and 3 with infections in the lower reproductive tract only. There were 2 animals in the LNG-IUS group with PID, 3 with criteria suggestive of PID, and 1 with infection in the lower reproductive tract only.
Abbreviations: LNG, levonorgestrel; LNG-IUS, levonorgestrel-releasing intrauterine system; −, absent; +, present.
Assessment of Vaginal Cytokines
For cytokine analysis, vaginal swab specimens were clipped into Spin-X centrifuge filter tubes (Costar, Corning, Corning, NY), equilibrated with 300 µL of extraction buffer (phosphate-buffered saline [PBS], 0.25 M NaCl, 0.1 mg/mL aprotinin, and 0.001% sodium azide) for 30 minutes on ice, and centrifuged at 16000×g for 20 minutes at 4°C. Equilibration and centrifugation were repeated, and then 50 µL of sample was transferred to a 96-well plate for analysis by a multiplex enzyme-linked immunosorbent assay (ELISA) in accordance with the manufacturer’s protocol (NHP Milliplex cytokine panel [PRCYTOMAG-40K-13], EMD Millipore/Merck, Darmstadt, Germany). A total of 100 µL of sample was further used for analysis of interleukin 1β (IL-1β), using an individual IL-1β DuoSet ELISA in accordance with the manufacturer’s protocol (R&D Systems, Minneapolis, MN), and a 10-µL specimen was used for protein quantification by the Bradford assay in accordance with the manufacturer’s protocol (Thermo-Pierce, Rockford, IL).
Statistical Analysis
Statistical analysis was performed using Prism, version 6.0 (GraphPad Software, La Jolla, CA). The Student t test or analysis of variance was used where applicable, and nonparametric data were log transformed. The threshold for statistical significance was defined a priori as a P value of < .05.
RESULTS
Eighteen animals began the study; 2 were euthanized before the end of the 16 week period after initial inoculation, administered at week 0. Four additional inoculations were administered weekly at 1, 2, 3, and 4 weeks. One animal in the non-LNG group (animal 3920) was euthanized after the fourth inoculation owing to development of a tubo-ovarian abscess, a PID end point. Another animal (3910) was euthanized on week 6 after initial inoculation because of a postlaparoscopy wound infection. This animal did not have gross or clinical evidence of PID but was later found to have positive PCR results and histologically evident tubal inflammation consistent with PID (see below). Individual animals and their study outcomes are listed in Table 1.
Table 1.
Results of Nucleic Acid Amplification Testing (NAAT) for Chlamydia trachomatis Nucleic Acid and Enzyme-Linked Immunosorbent Assay (ELISA) for C. trachomatis–Specific Immunoglobulin G Seroconversion
| Animal, Parameter | Wk 0 | Wk 1 | Wk 2 | Wk 3 | Wk 4 | Wk 5 | Wk 6 | Wk 7 | Wk 8 | Wk 9 | Wk 10 | Wk 11 | Wk 12 | Wk 13 | Wk 14 | Wk 15 | Wk 16 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-LNG group | |||||||||||||||||
| PAN 3804 | |||||||||||||||||
| NAAT results | |||||||||||||||||
| Cervix | − | − | + | + | + | + | + | + | + | + | + | + | − | − | − | − | − |
| Tubes | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| Seroconversion | − | − | − | − | − | − | + | + | + | + | − | − | − | − | − | − | − |
| PAN 3835 | |||||||||||||||||
| NAAT results | |||||||||||||||||
| Cervix | − | + | − | − | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Tubes | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| Seroconversion | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| PAN 3847 | |||||||||||||||||
| NAAT results | |||||||||||||||||
| Cervix | − | + | + | + | + | + | + | + | + | − | − | − | − | − | − | − | − |
| Tubes | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| Seroconversion | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| PAN 3859 | |||||||||||||||||
| NAAT results | |||||||||||||||||
| Cervix | − | − | + | + | + | + | + | − | − | − | − | − | − | − | − | − | − |
| Tubes | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| Seroconversion | − | − | − | − | − | − | + | + | + | + | + | + | − | − | − | − | − |
| PAN 3882 | |||||||||||||||||
| NAAT results | |||||||||||||||||
| Cervix | − | + | + | + | + | + | − | − | − | − | − | − | − | − | − | − | − |
| Tubes | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| Seroconversion | − | − | − | − | +a | − | − | − | − | − | − | − | − | − | − | − | − |
| PAN 3920a | |||||||||||||||||
| NAAT results | |||||||||||||||||
| Cervix | − | + | + | + | + | … | … | … | … | … | … | … | … | … | … | … | … |
| Tubes | − | − | + | + | + | … | … | … | … | … | … | … | … | … | … | … | … |
| Seroconversion | − | − | − | + | + | … | … | … | … | … | … | … | … | … | … | … | … |
| PAN 3921 | |||||||||||||||||
| NAAT results | |||||||||||||||||
| Cervix | − | + | + | + | + | − | − | − | − | − | − | − | − | − | − | − | − |
| Tubes | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| Seroconversion | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| LNG-IUS group | |||||||||||||||||
| PAN 3893 | |||||||||||||||||
| NAAT results | |||||||||||||||||
| Cervix | − | + | + | + | + | + | + | + | + | + | + | + | + | + | + | − | − |
| Tubes | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| Seroconversion | − | − | − | − | − | − | + | + | +a | +a | + | + | + | +a | + | +a | + |
| PAN 3905 | |||||||||||||||||
| NAAT results | |||||||||||||||||
| Cervix | − | − | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Tubes | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| Seroconversion | − | − | − | − | − | + | + | + | + | + | + | + | + | + | + | + | + |
| PAN 3910c | |||||||||||||||||
| NAAT results | |||||||||||||||||
| Cervix | − | − | + | + | + | + | … | … | … | … | … | … | … | … | … | … | … |
| Tubes | − | − | − | − | − | + | … | … | … | … | … | … | … | … | … | … | … |
| Seroconversion | − | − | − | − | − | − | … | … | … | … | … | … | … | … | … | … | … |
| PAN 3926 | |||||||||||||||||
| NAAT results | |||||||||||||||||
| Cervix | − | − | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Tubes | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| Seroconversion | − | − | − | − | − | − | − | − | − | − | − | − | +a | − | − | − | − |
| PAN 3991 | |||||||||||||||||
| NAAT results | |||||||||||||||||
| Cervix | − | − | + | + | + | + | + | + | + | + | + | + | − | − | − | − | − |
| Tubes | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| Seroconversion | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| PAN 4007 | |||||||||||||||||
| NAAT results | |||||||||||||||||
| Cervix | − | − | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Tubes | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| Seroconversion | − | − | − | − | − | + | + | + | + | + | + | + | +a | +a | + | + | + |
Abbreviations: −, negative; +, positive.
Absorbance units were at or minimally above the ELISA’s lower limit of detection (ie, results were equivocal).
The animal was euthanized during week 5.
The animal was euthanized during week 6.
C. trachomatis Infection and Quantitation
No animals exhibited gross, microbiological, NAAT-based, or serological evidence of chlamydial infection before inoculation. Seven of 8 inoculated non-LNG recipients and 6 of 6 inoculated LNG recipients became infected with C. trachomatis, as determined by results of cervical NAAT and culture (culture data are consistent with NAAT findings and are not shown) for a minimum of 2 consecutive time points during the study. One of 8 non-LNG recipients (animal 4000) had transient positive NAAT results 3 weeks after initial inoculation but negative results at all other time points; immune response and clearance data exclude this animal because it failed to meet infection criteria. No control animals had tubal or cervical specimens positive for C. trachomatis by NAAT or culture. As we previously reported, C. trachomatis clearance was delayed in LNG recipients [9]. Furthermore, by quantitative PCR from cervical samples, LNG recipients had a higher bacterial burden than non-LNG recipients. Calculated log numbers of elementary bodies per milliliter began to diverge between the groups 3 weeks after initial inoculation (while animals were still being inoculated with C. trachomatis), reaching significant differences 6 weeks after initial inoculation, the week after inoculations ended (Figure 1B).
Upper reproductive tract infection was confirmed by positive NAAT results among tubal samples obtained by laparoscopy after inoculation during week 0, by laparoscopy 4 and 8 weeks after initial inoculation, and by necropsy 16 weeks after initial inoculation (Table 1). Positive tubal NAAT results were observed for the animal with a tubo-ovarian abscess (animal 3920) in the non-LNG group and for 1 LNG recipient (animal 3910). Tubal NAAT results for all other animals were negative at all time points.
Seroconversion
Among animals in the non-LNG group, 3 of 7 were seropositive for anti–C. trachomatis immunoglobulin G for ≥2 consecutive weeks (Table 1). One (animal 3920) had the tubo-ovarian abscess and became seropositive during week 3 after initial inoculation, while the other 2 (animals 3804 and 3859) were seropositive by week 6 after initial inoculation and seronegative by the end of the study. In the LNG group, 3 of 6 inoculated animals (3893, 3905, and 4007) were seropositive by week 6 after initial inoculation (Table 1); these animals remained seropositive for the remainder of the study period. No control animals seroconverted.
Clinical Findings
Yellow or malodorous vaginal discharge was seen at ≥1 time point in 7 of 7 animals in the non-LNG group and in 3 of 6 infected LNG recipients (data not shown). This was not seen in any control animals. Appetite, behavior, and overall clinical appearance remained normal throughout the study in all groups. Of note, the animal (3920) euthanized for a tubo-ovarian abscess did not show clinical signs (eg, altered appetite or depression).
Laparoscopic Observations
Laparoscopic findings were observed in 4 of 7 infected animals in the non-LNG group and in 5 of 6 infected LNG recipients (Table 2). Laparoscopic findings in the non-LNG group were present as early as week 3 after initial inoculation (range, weeks 3–16) and consisted of tubal dilation and adhesions (in animals 3804, 3859, and 3882) and a tubo-ovarian abscess (in animal 3920). The tubo-ovarian abscess was first detected at week 4 after initial inoculation and had a diameter of >20 mm (baseline tubal diameter, 2 mm), involving the right ovary and tubal infundibulum. In the LNG group, 3 animals (3905, 3991, and 4007) had tubal enlargement, and 2 (3910 and 3893) had tubal adhesions.
Histological Findings
Vaginal, cervical, endometrial, and fallopian tube tissue specimens were evaluated for evidence of chronic (ie, lymphoplasmacytic) cervicitis, endometritis, or salpingitis or other manifestations of C. trachomatis infection. At the end point (week 16 after initial inoculation), all but 2 animals in the entire study had mild-to-moderate lymphoplasmacytic cervicovaginal inflammation, including 1 of 2 uninfected non-LNG recipients and 2 of 2 uninfected LNG recipients. This low-level cervicitis/vaginitis has been previously reported as a common background finding in nonhuman primates [13] and was also seen in our previous studies [10, 14].
In the non-LNG group, C. trachomatis–related histological findings were present in only 1 animal (3920) and consisted of a tubo-ovarian abscess within the right ovary and infundibulum (Figure 2). The contralateral (left) tube also had neutrophilic luminal exudate and infiltration within the tubal propria. The predominant leukocyte infiltration in this animal was neutrophilic; this is consistent with an acute manifestation of PID that may be related to C. trachomatis but may also be associated with secondary infection by other bacteria [15, 16]. None of the remaining animals in the non-LNG group had histologically evident inflammation in the endometrium, tubes, or ovary.
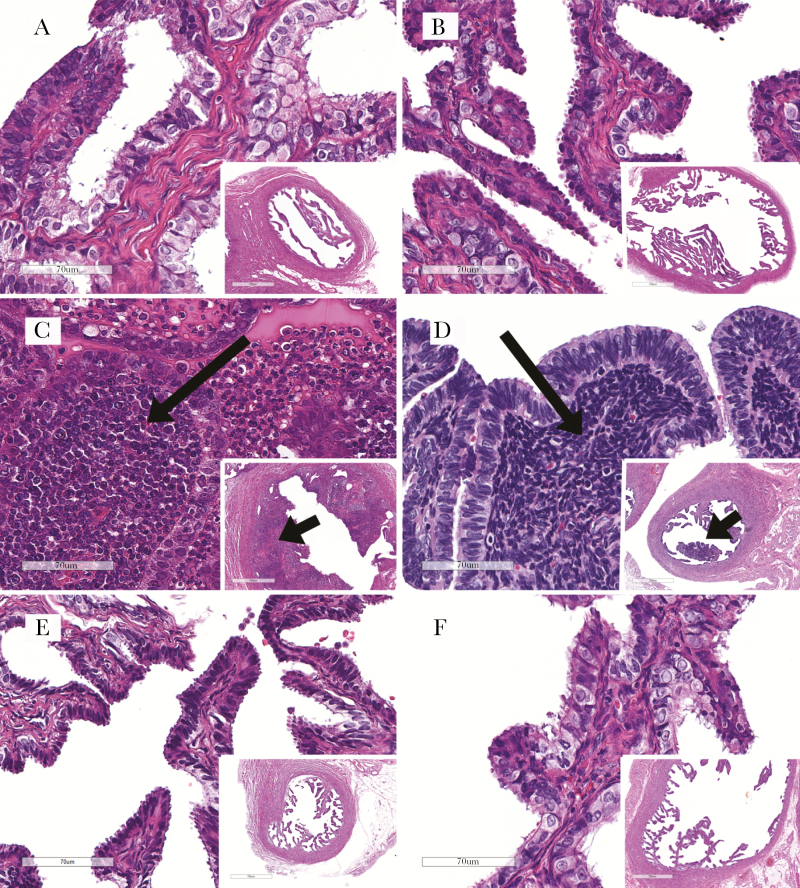
Figure 2.
Representative fallopian tube histological staining with hematoxylin and eosin for uninfected animals, animals with pelvic inflammatory disease (PID), and animals with lower reproductive tract infection, by levonorgestrel (LNG)–releasing intrauterine system (LNG-IUS) receipt. PID, when present, was manifested as leukocyte infiltration to the tubal propria (salpingitis). A and B, Uninfected animals without (A) and with (B) the LNG-IUS, showing high magnification of thin tubal propria lined by epithelium (inset shows the low-magnification overview). C and D, Infected animals positive for PID, showing infiltrating leukocytes (long arrows) in animals without (C) and with (D) an LNG-IUS. Leukocyte infiltration is seen as proprial thickening (short arrows) at low magnification (insets) in both animals. E and F, Infected animals with infection confined to the lower reproductive tract, showing normal thin propria (similar to uninfected controls) in animals without (E) and with (F) an LNG-IUS. See the text for a description of leukocytic infiltrations. Scale bars denote 70 um (panels) and 700 um (insets).
C. trachomatis–related histological findings in the LNG group were present in 2 animals (3893 and 3910) and consisted of focal tubal leukocyte infiltration (Figure 2). One animal (3910) had a unilateral mixed neutrophilic-to-mononuclear (macrophagic and lymphoplasmacytic) infiltrate at week 6 after initial inoculation (the animal was euthanized early, at this time point, because of a surgical wound infection). The other (animal 3893) had a bilateral lymphocytic tubal infiltrate at the study end point (ie, week 16 after initial inoculation), consistent with mild chronic salpingitis. There was no histologically evident endometrial, tubal, or ovarian inflammation in any other animal in the LNG group, including the 2 uninfected controls (Figure 2).
In addition to the changes described above, all animals in the LNG group, including uninfected controls, also had LNG-related histological changes independent of C. trachomatis infection. These consisted of extensive endometrial decidualization and multifocal neutrophilic and mononuclear endometrial infiltration (Supplementary Figure 1). These findings were consistent with previous reports of LNG-related histological findings in women, including endometrial inflammatory infiltrates [17].
Evidence of PID
The presence of PID was determined on the basis of all clinical, laparoscopic, molecular, and histological evidence. In the non-LNG group, 1 animal (3920) met the diagnostic criteria for PID (Table 2), with the presence of a tubo-ovarian abscess and NAAT positivity for C. trachomatis. In the non-LNG group, 3 animals (3804, 3859, and 3882) met criteria suggestive of PID, since they had tubal enlargement/adhesions and positive cervical NAAT results but lacked upper tract disease (ie, positive results of tubal NAAT or positive histological findings). Three animals in this group (3835, 3847, and 3921) had lower reproductive tract infection only (ie, positive results of cervical NAAT). In the LNG group, 2 animals (3910 and 3893) met the diagnostic criteria for PID (ie, focal chronic salpingitis, tubal NAAT positivity, and tubal enlargement or adhesion), and 3 (animals 3905, 3991, and 4007) met criteria suggestive of PID. One LNG recipient (animal 3926) had infection confined to the lower reproductive tract.
Cervical Immune Response to C. trachomatis Infection
To define the local immune response to C. trachomatis during the course of infection, we analyzed the production of the cytokines interleukin 1RA (IL-1RA), interleukin 8 (IL-8), CCL2, and CCL3, by multiplex ELISA, and IL-1β, individually, using swab specimens obtained from the cervixes of infected baboons at baseline and weekly throughout the course of infection. Non-LNG recipients had high IL-1RA levels at baseline (before infection), while LNG recipients had suppressed IL-1RA levels at baseline (Figure 3A). After infection, all groups had low IL-1RA levels. This suggested that LNG modulated the IL-1β axis even in the absence of infection. Both non-LNG and the LNG groups had low IL-1β in the absence of infection. In the non-LNG group, statistically significant induction of IL-1β was observed at weeks 1, 2, and 4 after initial inoculation, while the animals were still undergoing inoculations. LNG recipients had delayed induction, and IL-1β levels were consistently low or below the limit of detection (Figure 3B).
Figure 3.
Cytokine responses to Chlamydia trachomatis infection at baseline and during infection. Vaginal swab specimens were collected weekly for cytokine analysis. A, Baseline interleukin 1RA (IL-1RA) production was high in nonrecipients of the levonorgestrel (LNG)–releasing intrauterine system (the non-LNG group) but was repressed after C. trachomatis infection; the LNG-IUS group had low expression of IL-1RA at baseline and during infection. B, Interleukin 1β (IL-1β) production increased immediately after C. trachomatis infection in the non-LNG group and remained increased until infection began to clear; the LNG-IUS group had delayed production of IL-1β. C, The interleukin 8 (IL-8) level was significantly elevated at baseline in the LNG-IUS group in the absence of infection. D, Induction of IL-8, expressed as the fold increase from the baseline level, increased in the non-LNG group, while the LNG-IUS group, which had high baseline IL-8 levels, had decreased production of IL-8 during infection, relative to their baseline levels. Data are for 6–7 animals sample (some points are superimposed). NS, not significant; ND, none detected. *P < .05; **P < .01.
In non-LNG recipients, the level of the neutrophil chemoattractant IL-8 was low at baseline, while that in LNG recipients trended higher (Figure 3C). Upon normalization of individual animals to each animal’s baseline levels of IL-8, non-LNG recipients had a 3–5-fold increase in IL-8 production throughout infection, both during and after the inoculation period, while LNG recipients experienced unchanged or decreased IL-8 production (Figure 3C).
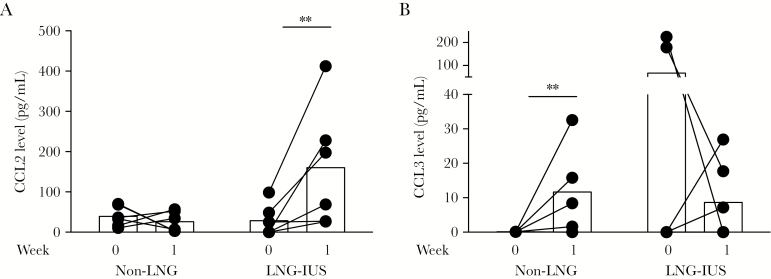
Production of the T-cell chemoattractants CCL2 and CCL3 differed between groups early during infection, while inoculations were still ongoing. Non-LNG recipients did not induce the T-helper type 2 (Th2) chemoattractant CCL2, while LNG recipients induced CCL2 at week 1 after initial inoculation (Figure 4A). In contrast, non-LNG recipients induced the Th1 chemoattractant CCL3, while LNG recipients did not (Figure 4B).
Figure 4.
Effect of levonorgestrel (LNG) on T-helper type 1 (Th1)/Th2 polarization during infection. Vaginal swab specimens were collected weekly for cytokine analysis. Baseline values represent the cytokine environment immediately before infection and were compared to cytokine values 1 week after infection. Animals that received the LNG-releasing intrauterine system (LNG-IUS) had induction of the primarily Th2-recruiting chemokine CCL2 (MCP-1) during the week after initial inoculation (ie, at week 1; A), while the non-LNG group had induction of the primarily Th1-recruiting chemokine CCL3 (MIP-1α) at week 1 (B). Week 0 corresponds to the baseline (uninfected) state. Data are for 6–7 animals per sample (some points are superimposed). *P < .05; **P < .01.
DISCUSSION
In this study, the presence of an LNG was associated with delayed bacterial clearance (in 6 of 6 animals), a higher bacterial burden in early infection (weeks 3–6 after initial inoculation), and an increased incidence of chronic salpingitis (in 2 of 6 animals) in a prospective model of C. trachomatis infection. In contrast, among non-LNG recipients, 6 of 7 infected animals cleared C. trachomatis infection and lacked histological indication of chronic salpingitis. One animal in the non-LNG group was euthanized at an early time point because it developed a tubo-ovarian abscess, a less common presentation that may be related to secondary bacterial infections in addition to C. trachomatis [15, 16]. The findings of decreased clearance, increased bacterial load, and higher incidence of chronic PID in the LNG group suggest that LNG may have an immunomodulatory function that influences C. trachomatis clearance and infection outcome.
In our laboratory’s previously published work [9], we used data from the present study to demonstrate that the vaginal microbial diversity in these baboons is consistent with other studies detailing vaginal microbiomes in wild-caught baboons [18]. Baseline differences in vaginal microbiomes between groups, changes in the microbiome during the LNG-IUS equilibration period, and changes in the C. trachomatis infection period could not account for the decreased clearance of C. trachomatis from the LNG group [9].
Because the early bacterial burden (during weeks 3–6 after initial inoculation) was impacted by the presence of LNG, we investigated differences in immune responses, focusing on baseline and early time points. Before infection, the presence of the LNG-IUS resulted in elevation of IL-8 levels and suppression of IL-1RA levels. Studies have shown IL-8 induction in the first 1–6 months of LNG-IUS use in women, with gradual tapering of IL-8 production [19]. Our baseline time point was 24 weeks after insertion, which is within the window of observed LNG-IUS–dependent IL-8 induction. Similarly, IL-1RA is induced in the follicular phase of the menstrual cycle in vaginal secretions, whereas it is suppressed in the luteal phase [20]. This may explain the low IL-1RA levels in LNG-IUS recipients, as high levels of LNG acting on progesterone receptors may mimic the luteal phase.
The ratio of the IL-1RA to IL-1β levels is an indicator of the inflammatory state [21]: a low IL-1RA to IL-1β ratio indicates a proinflammatory state, while a high IL-1RA to IL-1β ratio indicates an antiinflammatory state. All groups had low IL-1β levels before infection. However, non-LNG recipients had high IL-1RA levels at baseline, whereas LNG recipients had low IL-1RA levels at baseline, suggesting a more inflammatory baseline state and a potentially rapid response to C. trachomatis infection in the LNG group. Here, we observed the opposite: a delayed immune response. LNG-ISU recipients had delayed production of IL-1β (Figure 3B) and several cytokines downstream of IL-1β (ie, IL-8 and CCL3) (Figure 3C and Figure 4B). Suppression of IL-1RA levels at baseline by the LNG-IUS may be independent of suppression of IL-1β levels during C. trachomatis infection, but it suggests broad dysregulation of the IL-1 axis by the LNG-IUS. The mechanism of this dysregulation may be tied to promiscuous receptor binding: when present in high concentrations, progestogens can act via the glucocorticoid receptor to suppress inflammation, particularly IL-1β production [22]. The extent to which LNG can bind the glucocorticoid receptor is controversial [23]. However, the LNG-IUS induces suppression of both estrogen and progesterone receptors in the reproductive tract [24, 25]. Suppression of the progesterone receptor could skew LNG receptor binding by effectively increasing the LNG to progesterone receptor ratio, with an effect similar to that of increasing the concentration of LNG, resulting in increased LNG-mediated immune modulation via the glucocorticoid receptor.
Complete profiling of the immune response was complicated in this study by poor availability of validated baboon cross-reactive reagents (eg, interferon γ and interleukin 4). However, our available data suggest that non-LNG recipients have a Th1 response (ie, a high level of CCL3, primarily a Th1 chemoattractant [26]), while LNG promotes Th2 immune responses (ie, high levels of CCL2, primarily a Th2 chemoattractant [27, 28]). The lack of IL-1β induction and the release of pro-Th2 chemokines in early infection suggests that the LNG-IUS skews the immune response toward Th2, but it is unclear how this influences the development of PID. It is possible that an early Th1 response is important in early bacterial clearance and that the lack of this strong Th1 response contributes to chlamydial persistence in the presence of LNG. While modulation of the IL-1β axis by the LNG-IUS during C. trachomatis infection may be responsible for the decreased clearance in LNG-IUS recipients, this could ultimately be protective against PID. IL-1 has been shown to initiate fallopian tube destruction in humans during chlamydial PID [29]. It also showed that disruption of the IL-1 pathway led to complete suppression of IL-8 (as we observed in our study), which could result in decreased inflammatory infiltrate.
One surprising finding was that the level of seroconversion in infected baboons was lower than anticipated. Seroconversion was undetectable in 6 of 13 infection baboons (46%) with C. trachomatis–positive NAAT results. This could reflect the relatively low percentage of animals with symptomatic PID: in C. trachomatis–infected humans, 100% of patients with chlamydial PID have detectable circulating anti–C. trachomatis antibodies [30]; however, among C. trachomatis–infected humans without PID, seroconversion in 20%–35% is undetectable by various current methods [12, 31, 32]. The low rate of seroconversion could also be due to poor species cross-reactivity issues or a high assay threshold for positive titers (2 values not included in the positive count were at the threshold of detection). Nevertheless, animals that met the criteria for PID in each group (animals 3920 and 3893) seroconverted, with the exception of 3910 in the LNG-IUS group, which may have been euthanized before antibody responses developed. The remaining animals that seroconverted had criteria suggestive of PID. There was no seroconversion in any animals with infection confined to the lower reproductive tract. We did not observe a correlation between high antigen load and seroconversion.
Limitations in this study are worth noting. First, although the study design permitted evaluation of C. trachomatis infection and persistence, the ability to make broad statements about the LNG-IUS and PID severity or progression is limited since, as in humans, not every infected animal developed PID. Additionally, the amount of C. trachomatis that was inoculated (1 × 107 inclusion-forming units), along with the number of inoculations undertaken (5 per animal), may represent superphysiologic inoculations, although the amount necessary to cause infection in humans is unknown. This high inoculum and frequent-inoculation regimen was undertaken to maximize the likelihood of infection and increase the likelihood of the relatively rare end point of PID. Studies with PID as an objective have necessitated multiple infections in macaque models [33, 34]. Despite this high dosing level, non-LNG recipients were able to clear infection in this study.
As stated above, we cannot conclude from this study that the LNG-IUS causes an increased incidence of PID during C. trachomatis infection. In animals with C. trachomatis infection, we found that the LNG-IUS resulted in 2 of 6 baboons developing PID, while 1 of 7 non-LNG recipients had PID. Three animals from each group met criteria suggestive of PID. The LNG group had significantly higher bacterial burdens in the cervix throughout a prolonged period of infection, relative to the non-LNG group, and this correlated with decreased induction of IL-1β and downstream cytokines in the LNG-IUS group; the LNG-IUS appeared to promote Th2 immune responses over Th1 response. These findings suggest that the LNG-IUS influences the local immune response to infection, a finding that has implications for persistence and progression of C. trachomatis– related disease. Future work characterizing immune disruption to the female reproductive tract—and elucidating the associated molecular mechanisms—during use of the LNG-IUS is of paramount importance as more women at higher risk for C. trachomatis infection use an LNG-IUS.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank the Animal Care and Resources staff of the Institute of Primate Research, for animal care and necropsy; the University of Washington Chlamydia Laboratory, for supplying the C. trachomatis isolate; Scott McClellan, Michelle Garrasi, and Kathy Lucas of the University of Michigan Clinical Microbiology Laboratory, for serological analysis, culture, and NAAT testing; the University of Michigan Unit for Laboratory Animal Medicine In Vivo Animal Core, for histology support; and Dr Mayu Uchihashi, Nicholas Kiulia, Emily Chen, and Diane Wang, for their hard work in completing this project.
Financial support. This work was supported by the Women’s Reproduction Health Research Program, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (grant K12 HD065257-01) and (UL1TR002240)
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Hubacher D. The checkered history and bright future of intrauterine contraception in the United States. Perspect Sex Reprod Health 2002; 34:98–103. [PubMed] [Google Scholar]
- 2. Hubacher D, Cheng D. Intrauterine devices and reproductive health: American women in feast and famine. Contraception 2004; 69:437–46. [DOI] [PubMed] [Google Scholar]
- 3. Soper DE. Pelvic inflammatory disease. Obstet Gynecol 2010; 116:419–28. [DOI] [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention. Chlamydia—CDC fact sheet. 2011. http://www.cdc.gov/std/chlamydia/stdfact-chlamydia.htm. Accessed 28 July 2017. [Google Scholar]
- 5. D’Hooghe TM, Kyama CM, Chai D et al. Nonhuman primate models for translational research in endometriosis. Reprod Sci 2009; 16:152–61. [DOI] [PubMed] [Google Scholar]
- 6. Chai D, Cuneo S, Falconer H, Mwenda JM, D’Hooghe T. Olive baboon (Papio anubis anubis) as a model for intrauterine research. J Med Primatol 2007; 36:365–9. [DOI] [PubMed] [Google Scholar]
- 7. Bell JD, Bergin IL, Harris LH et al. The effects of a single cervical inoculation of Chlamydia trachomatis on the female reproductive tract of the baboon (Papio anubis). J Infect Dis 2011; 204:1305–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Council for International Organization of Medical Sciences, International Council for Laboratory Animal Science. International guiding principles for biomedical research involving animals. 2012. https://grants.nih.gov/grants/olaw/guiding_principles_2012.pdf. Accessed 28 October 2017.
- 9. Liechty ER, Bergin IL, Bassis CM et al. The levonorgestrel-releasing intrauterine system is associated with delayed endocervical clearance of Chlamydia trachomatis without alterations in vaginal microbiota. Pathog Dis 2015; 73:ftv070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hashway SA, Bergin IL, Bassis CM et al. Impact of a hormone-releasing intrauterine system on the vaginal microbiome: a prospective baboon model. J Med Primatol 2014; 43:89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mirena. Mirena Package Insert. Bayer Healthcare Pharmaceuticals Inc, 2009. [Google Scholar]
- 12. Bax CJ, Mutsaers JA, Jansen CL, Trimbos JB, Dörr PJ, Oostvogel PM. Comparison of serological assays for detection of Chlamydia trachomatis antibodies in different groups of obstetrical and gynecological patients. Clin Diagn Lab Immunol 2003; 10:174–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Spear G, Rothaeulser K, Fritts L, Gillevet PM, Miller CJ. In captive rhesus macaques, cervicovaginal inflammation is common but not associated with the stable polymicrobial microbiome. PLoS One 2012; 7:e52992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bell JD, Bergin IL, Natavio MF et al. Feasibility of LNG-IUS in a baboon model. Contraception 2013; 87:380–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Heinonen PK, Miettinen A. Laparoscopic study on the microbiology and severity of acute pelvic inflammatory disease. Eur J Obstet Gynecol Reprod Biol 1994; 57:85–9. [DOI] [PubMed] [Google Scholar]
- 16. Rice PA, Schachter J. Pathogenesis of pelvic inflammatory disease. What are the questions? JAMA 1991; 266:2587–93. [PubMed] [Google Scholar]
- 17. Dinh A, Sriprasert I, Williams AR, Archer DF. A review of the endometrial histologic effects of progestins and progesterone receptor modulators in reproductive age women. Contraception 2015; 91:360–7. [DOI] [PubMed] [Google Scholar]
- 18. Uchihashi M, Bergin IL, Bassis CM, Hashway SA, Chai D, Bell JD. Influence of age, reproductive cycling status, and menstruation on the vaginal microbiome in baboons (Papio anubis). Am J Primatol 2015; 77:563–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jones RL, Critchley HO. Morphological and functional changes in human endometrium following intrauterine levonorgestrel delivery. Hum Reprod 2000; 15(Suppl 3):162–72. [DOI] [PubMed] [Google Scholar]
- 20. Kyongo JK, Jespers V, Goovaerts O et al. Searching for lower female genital tract soluble and cellular biomarkers: defining levels and predictors in a cohort of healthy Caucasian women. PLoS One 2012; 7:e43951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Arend WP. The balance between IL-1 and IL-1Ra in disease. Cytokine Growth Factor Rev 2002; 13:323–40. [DOI] [PubMed] [Google Scholar]
- 22. Lei K, Chen L, Georgiou EX et al. Progesterone acts via the nuclear glucocorticoid receptor to suppress IL-1β-induced COX-2 expression in human term myometrial cells. PLoS One 2012; 7:e50167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Africander D, Verhoog N, Hapgood JP. Molecular mechanisms of steroid receptor-mediated actions by synthetic progestins used in HRT and contraception. Steroids 2011; 76:636–52. [DOI] [PubMed] [Google Scholar]
- 24. Jänne O, Ylöstalo P. Endometrial estrogen and progestin receptors in women bearing a progesterone-releasing intrauterine device. Contraception 1980; 22:19–23. [DOI] [PubMed] [Google Scholar]
- 25. Zhu P, Liu X, Luo H et al. The effect of a levonorgestrel-releasing intrauterine device on human endometrial oestrogen and progesterone receptors after one year of use. Hum Reprod 1999; 14:970–5. [DOI] [PubMed] [Google Scholar]
- 26. Van Coillie E, Van Damme J, Opdenakker G. The MCP/eotaxin subfamily of CC chemokines. Cytokine Growth Factor Rev 1999; 10:61–86. [DOI] [PubMed] [Google Scholar]
- 27. Chensue SW, Warmington KS, Lukacs NW et al. Monocyte chemotactic protein expression during schistosome egg granuloma formation. Sequence of production, localization, contribution, and regulation. Am J Pathol 1995; 146:130–8. [PMC free article] [PubMed] [Google Scholar]
- 28. Handel TM, Domaille PJ. Heteronuclear (1H, 13C, 15N) NMR assignments and solution structure of the monocyte chemoattractant protein-1 (MCP-1) dimer. Biochemistry 1996; 35:6569–84. [DOI] [PubMed] [Google Scholar]
- 29. Hvid M, Baczynska A, Deleuran B et al. Interleukin-1 is the initiator of Fallopian tube destruction during Chlamydia trachomatis infection. Cell Microbiol 2007; 9:2795–803. [DOI] [PubMed] [Google Scholar]
- 30. Theunissen JJ, Minderhoud-Bassie W, Wagenvoort JH, Stolz E, Michel MF, Huikeshoven FJ. Chlamydia trachomatis-specific antibodies in patients with pelvic inflammatory disease: comparison with isolation in tissue culture or detection with polymerase chain reaction. Genitourin Med 1994; 70:304–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Menon S, Stansfield SH, Walsh M et al. Sero-epidemiological assessment of Chlamydia trachomatis infection and sub-fertility in Samoan women. BMC Infect Dis 2016; 16:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Winstanley CE, Ramsey KH, Marsh P, Clarke IN. Development and evaluation of an enzyme-linked immunosorbent assay for the detection of antibodies to a common urogenital derivative of Chlamydia trachomatis plasmid-encoded PGP3. J Immunol Methods 2017; 445:23–30. [DOI] [PubMed] [Google Scholar]
- 33. Patton Dorothy L, Sweeney Yvonne TC, Stamm Walter E. Significant reduction in inflammatory response in the macaque model of chlamydial pelvic inflammatory disease with azithromycin treatment. J Infect Dis 2005; 192:129–35. [DOI] [PubMed] [Google Scholar]
- 34. Bell JD, Bergin IL, Schmidt K, Zochowski MK, Aronoff DM, Patton DL. Nonhuman primate models used to study pelvic inflammatory disease caused by Chlamydia trachomatis. Infect Dis Obstet Gynecol 2011; 2011:675360. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.