Contrary to the current paradigm, competition experiments showed that green algae defeated cyanobacteria at low CO2 levels, whereas cyanobacteria with high-flux carbon uptake systems became stronger competitors at elevated CO2.
Keywords: Carbon dioxide, climate change, competition model, CO2-concentrating mechanism, cyanobacteria, green algae, harmful algal blooms, lakes, Microcystis aeruginosa
Abstract
Traditionally, it has often been hypothesized that cyanobacteria are superior competitors at low CO2 and high pH in comparison with eukaryotic algae, owing to their effective CO2-concentrating mechanism (CCM). However, recent work indicates that green algae can also have a sophisticated CCM tuned to low CO2 levels. Conversely, cyanobacteria with the high-flux bicarbonate uptake system BicA appear well adapted to high inorganic carbon concentrations. To investigate these ideas we studied competition between three species of green algae and a bicA strain of the harmful cyanobacterium Microcystis aeruginosa at low (100 ppm) and high (2000 ppm) CO2. Two of the green algae were competitively superior to the cyanobacterium at low CO2, whereas the cyanobacterium increased its competitive ability with respect to the green algae at high CO2. The experiments were supported by a resource competition model linking the population dynamics of the phytoplankton species with dynamic changes in carbon speciation, pH and light. Our results show (i) that competition between phytoplankton species at different CO2 levels can be predicted from species traits in monoculture, (ii) that green algae can be strong competitors under CO2-depleted conditions, and (iii) that bloom-forming cyanobacteria with high-flux bicarbonate uptake systems will benefit from elevated CO2 concentrations.
Introduction
Cyanobacterial blooms have become a major water quality problem in many eutrophic lakes worldwide (Chorus and Bartram, 1999; Verspagen et al., 2006; Guo, 2007; Michalak et al., 2013). They produce taste and odor compounds that may interfere with the recreational function of lakes and the preparation of drinking water (Chorus and Bartram, 1999; Watson et al., 2008). Moreover, cyanobacteria can produce a variety of toxins, causing liver, digestive and neurological diseases when ingested by waterfowl, pets, cattle, and humans (Carmichael, 2001; Codd et al., 2005; Huisman et al., 2005; Merel et al., 2013). Hence, an improved understanding of the environmental conditions that favor the dominance of cyanobacteria over eukaryotic phytoplankton species is desirable.
Dense cyanobacterial blooms often deplete the dissolved CO2 concentration in surface waters, sometimes down to less than 0.1 μmol l–1 corresponding to pCO2 less than 3 parts per million (ppm) (Lazzarino et al., 2009; Balmer and Downing, 2011). CO2 depletion by dense blooms induces high pH values, above 9 or even 10 (Talling, 1976; Ibelings and Maberly, 1998; Verspagen et al., 2014b). At these pH values, most dissolved inorganic carbon (DIC) is in the form of bicarbonate, and with increasing pH an increasing fraction of DIC is converted to carbonate. Under these conditions, CO2 availability can become an important limiting factor for photosynthesis. Cyanobacteria have developed a highly efficient CO2-concentrating mechanism (CCM) to take up CO2 and bicarbonate as inorganic carbon (Ci) source, and to augment the intracellular CO2 level around the ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO) enzyme responsible for carbon fixation (Price et al., 2008; Raven et al., 2012; Burnap et al., 2015). It has therefore been hypothesized that cyanobacteria are superior competitors at low CO2 levels, and will dominate waters in which the dissolved CO2 concentration has been depleted (Shapiro, 1990, 1997). Conversely, eukaryotic phytoplankton might be better competitors at high CO2 levels. This classic paradigm has received support from several competition experiments between cyanobacteria and green algae (Caraco and Miller, 1998; Low-Décarie et al., 2011, 2015; but see Verschoor et al., 2013). If this paradigm is true, the logical corollary is that rising CO2 levels will particularly benefit eukaryotic phytoplankton species at the expense of cyanobacteria.
Yet, recent insights indicate that this classic paradigm might be too simple. Although the details of the eukaryotic CCM are not yet fully understood, green algae can also deploy a sophisticated CCM well adapted to low CO2 levels (Moroney and Ynalvez, 2007; Wang et al., 2011; Meyer and Griffiths, 2013). Furthermore, recent studies have revealed a striking genetic and phenotypic diversity in the CCM of harmful cyanobacteria (Sandrini et al., 2014, 2015b; Visser et al., 2016). All harmful freshwater cyanobacteria investigated so far contain two CO2 uptake systems and the ATP-dependent bicarbonate uptake system BCT1. In addition, however, some cyanobacteria deploy the high-affinity but low-flux bicarbonate uptake system SbtA, whereas other cyanobacteria deploy the low-affinity but high-flux bicarbonate uptake system BicA (Sandrini et al., 2014). Therefore, three Ci uptake genotypes can be distinguished: (i) sbtA strains (high-affinity specialists), (ii) bicA strains (high-flux specialists), and (iii) bicA+sbtA strains (CCM generalists). These three genotypes were first described for the genus Microcystis (Sandrini et al., 2014, 2016), but similar genetic diversity also exists within other harmful cyanobacterial genera such as Dolichospermum (formerly known as Anabaena) and Planktothrix (Visser et al., 2016).
Laboratory selection experiments with mixtures of several Microcystis strains found that the strain composition shifted from bicA+sbtA strains at low pCO2 to bicA strains at high pCO2 (Sandrini et al., 2016). Similarly, in a eutrophic lake, bicA+sbtA strains were dominant when Ci concentrations in the lake were depleted during a dense cyanobacterial bloom, but were replaced by bicA strains when Ci concentrations increased later in the season (Sandrini et al., 2016). These results show that the genetic composition of cyanobacterial communities adapts to changes in Ci availability by means of natural selection, favoring CCM generalists at low CO2 levels while favoring high-flux specialists at high CO2. In natural waters, however, cyanobacteria compete not only amongst each other, but also against eukaryotic phytoplankton. How the competitive abilities of different cyanobacterial Ci uptake genotypes perform against eukaryotic species such as green algae has not yet been investigated.
Resource competition theory provides a theoretical framework to understand and predict how changes in resource availability may affect the species composition (Tilman, 1982; Huisman and Weissing, 1994; Grover, 1997). This body of theory uses the kinetic traits of species measured in monoculture to predict the dynamics and outcome of competition for limiting resources in species mixtures. Resource competition models have been successfully applied to predict competition for nutrients and light, both in qualitative and quantitative terms (Tilman, 1977; Sommer, 1985; Huisman et al., 1999; Litchman et al., 2004; Stomp et al., 2004; Passarge et al., 2006). Competition for inorganic carbon is conceptually more complicated, however, because the species compete for two resources (CO2 and bicarbonate) whose concentrations depend not only on resource uptake but also on pH and alkalinity. Moreover, pH and alkalinity depend in turn on a variety of biogeochemical processes and also change in response to the carbon and nutrient uptake activity by the phytoplankton community (Talling, 1976; Wolf-Gladrow et al., 2007; Verspagen et al., 2014b).
This study investigated competition between a harmful cyanobacterium (Microcystis PCC 7806) and three species of green algae (Monoraphidium griffithii, Scenedesmus obliquus, and Chlorella vulgaris) at low and at high CO2 concentrations. Microcystis PCC 7806 is a bicA strain (Sandrini et al., 2014), and is therefore expected to be a relatively weak competitor at low CO2 levels but a stronger competitor at high CO2. To investigate this hypothesis, we ran monoculture experiments of each species at both low and high CO2 levels (100 and 2000 ppm). The results of these monoculture experiments were used to parameterize a resource competition model, which predicted the competitive interactions between the species based on dynamic changes in inorganic carbon chemistry, alkalinity and pH induced by the growing phytoplankton populations. Next, competition experiments were carried out to test the model predictions. Together, the theory and experiments may help in understanding shifts in phytoplankton community composition in response to rising CO2 levels.
Competition model: theory and development
We first developed a model to investigate competition for inorganic carbon and light among phytoplankton species. The model combined previous theoretical work on growth and competition under light-limited (Huisman and Weissing, 1994; Huisman et al., 1999; Passarge et al., 2006) and carbon-limited conditions (Van de Waal et al., 2011; Verspagen et al., 2014a, b).
The model considers a well-mixed vertical water column, where depth z runs from 0 at the water surface to zmax at the bottom of the water column. The population dynamics of the phytoplankton species are governed by their light-dependent assimilation of carbon dioxide and bicarbonate. The model assumes eutrophic conditions, in which all nutrients are in ample supply and hence do not limit phytoplankton growth. Uptake of inorganic carbon and nutrients induces dynamic changes in pH and alkalinity. These changes in pH and alkalinity affect the availability of the different inorganic carbon species, which feeds back on phytoplankton growth. The growing phytoplankton populations also increase the turbidity of the water column, thereby diminishing the light available for further photosynthesis and growth.
Species dynamics
The model assumes that the specific growth rates of the species depend on their carbon assimilation. Let Xi denote the population density of phytoplankton species i, and let Qi denote its cellular carbon content (also known as carbon quota; sensu Droop, 1973). The population dynamics of a number of n competing species can then be written as:
| (1) |
where µi(Qi) is the specific growth rate of species i as an increasing function of its carbon content, and mi is its loss rate. We assume that each species requires a minimum cellular carbon content in order to function, and reaches its maximum specific growth rate when cells are satiated with carbon (see Supplementary Model S1 at JXB online). In our application, the loss rates of the species will be governed by the dilution rate of the chemostat (i.e. mi=D).
The carbon contents of the species increase through uptake of carbon dioxide (uCO2,i) and bicarbonate (uHCO3,i), and decrease through respiration (ri) and dilution of the carbon content by growth:
| (2) |
We assume that uptake rates of CO2 (uCO2,i) and bicarbonate (uHCO3,i) are increasing functions of the ambient CO2 and bicarbonate concentration according to Michaelis–Menten kinetics. Carbon uptake and assimilation require energy from the light reactions of photosynthesis, and therefore the carbon uptake rates also depend on the photosynthetic activity of the cells and hence on light availability. Furthermore, we incorporated a simple negative feedback loop in which carbon uptake rates decrease with increasing cellular carbon content, such that carbon uptake systems are active under carbon-limiting conditions (Eisenhut et al., 2007; Burnap et al., 2015; Wang et al., 2015) and down-regulated when cells become satiated with carbon (Beardall and Giordano, 2002; Sandrini et al., 2015a). Respiration rates (ri) of the species increase with their cellular carbon content, approaching maximum values when cells become satiated with carbon. The mathematical equations describing these relationships are presented in Supplementary Model S1.
Light conditions
The underwater light gradient is described by the Lambert–Beer law (Huisman et al., 1999):
| (3) |
where I(z) is the light intensity at depth z, Iin is the incident light intensity, Kbg is the background turbidity of the water itself, and ki is the specific light attenuation coefficient of phytoplankton species i. We note that the light gradient changes dynamically, because the light intensity at a given depth decreases with increasing phytoplankton densities. We define Iout as the light intensity reaching the bottom of the water column (i.e. Iout=I(zmax)).
Dissolved inorganic carbon
Changes in the concentration of total dissolved inorganic carbon, [DIC], are described by (Verspagen et al., 2014b):
| (4) |
The first term on the right-hand side of this equation describes changes through the influx ([DIC]in) and efflux of water containing DIC. The second term describes CO2 exchange with the atmosphere, where gCO2 is the CO2 flux across the air–water interface and division by zmax converts the flux per unit surface area into the corresponding change in DIC concentration. The third term describes uptake of dissolved CO2 and bicarbonate by the photosynthetic activity of the phytoplankton community. Finally, the fourth term describes CO2 release by respiration of the phytoplankton species.
The CO2 flux across the air–water interface, gCO2, depends on the difference in partial pressure. More specifically, gCO2 depends on the difference between the expected concentration of dissolved CO2 in water if in equilibrium with the partial pressure in the atmosphere and the actual dissolved CO2 concentration (Siegenthaler and Sarmiento, 1993; Cole et al., 2010):
| (5) |
where v is the gas transfer velocity (also known as piston velocity), K0 is the solubility of CO2 gas in water (also known as Henry’s constant), pCO2 is the partial pressure of CO2 in the atmosphere, and [CO2] is the dissolved CO2 concentration. In chemostats, gas transfer will depend on the gas flow rate (a). We therefore assume that v=ba, where b is a constant of proportionality.
Dissolved CO2, bicarbonate and carbonate concentrations, and pH were calculated from [DIC] and alkalinity (Stumm and Morgan, 1996). Assimilation of nitrate, phosphate and sulfate by phytoplankton involves proton consumption, thus increasing alkalinity (Wolf-Gladrow et al., 2007; Verspagen et al., 2014b). Therefore, the model treats alkalinity as a dynamic variable (see Supplementary Model S1 for details).
Materials and methods
Species
We performed monoculture and competition experiments with four freshwater phytoplankton species: the green algae Monoraphidium griffithii (Berk.) Kom.-Legn. (strain CCAP 202/15A), Scenedesmus obliquus (strain CCAP 276/3A) and Chlorella vulgaris Beyerinck (strain UTEX 259), and the cyanobacterium Microcystis aeruginosa (strain PCC 7806). Microcystis PCC 7806 produces the hepatotoxins microcystin-LR and [Asp3]-microcystin-LR and the potential neurotoxins cyanopeptolin A, C and 970 (Tonk et al., 2009; Faltermann et al., 2014). All four species were unialgal but not axenic. Regular microscopic inspection confirmed that concentrations of heterotrophic bacteria remained low throughout the experiments (<1% of the total biovolume measured with a CASY TTC cell counter; OLS OMNI Life Science, Bremen, Germany).
Chemostat experiments
All experiments were conducted in laboratory-built chemostats, specifically designed to study the population dynamics of phytoplankton species (Huisman et al., 2002; Passarge et al., 2006; Verspagen et al., 2014b). The chemostats allowed full control of light conditions, temperature, pCO2 in the gas flow, and nutrient concentrations in the mineral medium. Each chemostat consisted of a flat culture vessel with an optimal path length (‘mixing depth’) of zmax=5 cm and a working volume of ~1.7 l. The vessel was illuminated from one side to create a unidirectional light gradient, using a constant incident light intensity (Iin) of 50 ± 1 μmol photons m–2 s–1 provided by white fluorescent tubes (Philips PL-L 24W/840/4P, Philips Lighting, Eindhoven, The Netherlands). The temperature was maintained at 20 ± 1 °C with a stainless steel cooling finger inside each chemostat and connected to a Colora thermocryostat. To avoid nutrient limitation, the chemostats were provided with a very nutrient-rich mineral medium (Van de Waal et al., 2009; Verspagen et al., 2014a). The dilution rate was maintained at D=0.125 d–1.
CO2 supply
We applied two CO2 treatments. The chemostats were bubbled with gas containing a CO2 concentration of either 100 ppm (‘low pCO2’) or 2000 ppm (‘high pCO2’) (Table 1). The gas was prepared as a mixture of pressurized air from which the variable CO2 concentration was completely removed using a CO2 scrubber (Ecodry K-MT6; Parker Zander, Lancaster, NY, USA) and subsequently a defined amount of pure CO2 gas was added to obtain the desired concentration using mass flow controllers (GT 1355R-2-15-A316 SS and 5850S, Brooks Instrument, Hatfield, PA, USA). Before entering the chemostats, the mixed gas was filter sterilized (0.2 µm Midisart 2000 Filter, Sartorius Stedim Biotech GmbH, Göttingen, Germany) and moisturized with Milli-Q water to suppress evaporation from the chemostats. The gas was dispersed as fine bubbles supplied from the bottom of the chemostat vessels at a constant flow rate of a=25 l h–1, which also ensured homogeneous mixing of the phytoplankton populations. We checked the CO2 concentration in the gas flow regularly using an environmental gas monitor (EGM-4; PP Systems, Amesbury, MA, USA).
Table 1.
System parameters applied in the experiments
| Parameter | Description | Value | Units |
|---|---|---|---|
| D | Dilution rate | 0.125 | d–1 |
| z max | Mixing depth | 0.05 | m |
| T | Temperature | 20 | °C |
| I in | Incident light intensity | 50 | µmol photons m–2 s–1 |
| K bg | Background turbiditya | 7−11 | m–1 |
| DICin | DIC concentration at influxb | 0.5 and 2.0 | mmol l–1 |
| ALKin | Alkalinity at influxb | 0.79 and 2.29 | mEq l–1 |
| sal | Salinityb | 1.23 and 1.36 | g l–1 |
| pCO2 | CO2 concentration in gas flowb | 100 and 2000 | ppm |
| a | Gas flow rate | 25 | l h–1 |
| v | Gas transfer velocityb | 0.24 and 0.68 | m h–1 |
Background turbidity varied among the chemostat vessels.
The first value refers to the low pCO2 experiments and the second value to the high pCO2 experiments.
Experimental measurements
The experiments were sampled at least every other day. The incident light intensity (Iin) was measured with a LI-COR LI-250 quantum photometer (LI-COR Biosciences, Lincoln, NE, USA) at ten randomly chosen positions at the front surface of the chemostat vessel. Likewise, the light intensity transmitted through the chemostat (Iout) was measured at the back surface of the chemostat vessel (Huisman et al., 2002).
Population densities and biovolumes in samples of the monoculture experiments were measured in triplicate with a CASY TTC automated cell counter (OLS OMNI Life Science, Bremen, Germany) using a 60 µm capillary. The cell counter could not distinguish between the different species. Therefore, population densities in the competition experiments were counted on an Accuri C6 flow cytometer (Accuri Cytometers Inc., Ann Arbor, MI, USA), which distinguished the different species in these experiments on the basis of differences in pigmentation and cell size (side scatter). We did not perform competition experiments between Scenedesmus and Chlorella, because the flow cytometer could not adequately distinguish between these two chlorophytes.
Temperature and pH were measured immediately after sampling, using a SCHOTT pH meter (SCHOTT AG, Mainz, Germany). For DIC analysis, 35 ml samples were transferred to 50 ml falcon tubes, centrifuged for 15 min at 600 g, immediately filtered over 0.45 µm polyethersulfone membrane filters (Sartorius Stedim Biotech GmbH, Göttingen, Germany), transferred to gas-tight urine tubes (Terumo Europe NV, Leuven, Belgium) and stored at 4 ºC until analysis. DIC was analysed by phosphoric acid addition using a Model 700 TOC Analyzer (OI Corp., College Station, TX, USA). Concentrations of CO2(aq), bicarbonate and carbonate were calculated from DIC and pH, based on the dissociation constants of inorganic carbon corrected for temperature and salinity (Stumm and Morgan, 1996; Verspagen et al., 2014b).
To determine the cellular carbon, nitrogen and sulfur content, pellets from the 50 ml falcon tube were transferred into 2 ml Eppendorf tubes, washed three times with a nutrient-free NaCl solution with a salinity equal to our medium, and stored at –20 °C. Subsequently, the pellets were freeze-dried and weighted, and the carbon, nitrogen and sulfur content of homogenized freeze-dried cell powder were analysed with a Vario EL Elemental Analyzer (Elementar Analysensysteme GmbH, Hanau, Germany).
Model parameterization
The model parameters consisted of system parameters and species parameters. System parameters are under experimental control and were regularly measured during the experiments. We had already specified several of the system parameters, such as the incident light intensity (Iin), pCO2 level in the gas flow, and dilution rate (D) of the chemostats. A complete list of all system parameters is provided in Table 1.
Species parameters describe the traits of the species. Some species parameters were measured experimentally. The maximum specific growth rate (µmax,i) and minimum cellular carbon content (Qmin,i) were determined in batch cultures at an Iin of 50 ± 1 μmol photons m–2 s–1 and a temperature of 20 ± 1 ° C. Maximum specific growth rate was measured in batch cultures aerated with gas containing a saturating CO2 concentration of 10 000 ppm. The minimum cellular carbon content was measured as the cellular carbon content in unaerated dense batch cultures that were first grown for a day in medium to which no DIC was added, and were subsequently incubated overnight in the dark. The cellular N:C and S:C ratios (cN,i and cS,i) were calculated from the cellular carbon, nitrogen and sulfur contents measured in the steady-state monocultures of the species. The specific light attenuation coefficients (ki) of the species were estimated from the monoculture experiments using the Lambert–Beer law. For monocultures, Eqn (3) can be written as ln(Iin/Iout)/zmax=Kbg+kiXi. Hence, the specific light attenuation coefficient (ki) was estimated as the slope of a linear regression of ln(Iin/Iout)/zmaxversus the population density Xi, and the background turbidity (Kbg) was estimated as the intercept.
All other species parameters were estimated by fitting the model predictions to the observed dynamics in the monoculture experiments. More specifically, we fitted the time courses of population density, light transmission (Iout), pH and inorganic carbon concentrations predicted by the model to the time courses measured in the monoculture experiments, following the same methodology as in earlier studies (Huisman et al., 1999; Passarge et al., 2006; Verspagen et al., 2014b). To avoid overfitting, the model was fitted simultaneously to both the low pCO2 and high pCO2 monocultures, resulting in eight parameter estimates per species.
The species parameters obtained from the monoculture experiments were combined with the system parameters to predict the population dynamics and inorganic carbon chemistry in the competition experiments.
In the competition experiments, we calculated the rate of competitive displacement (RCD) from the slope of the linear regression of ln(X1/X2) versus time, where X1 and X2 are the population densities of the two competing species (Grover, 1991; Passarge et al., 2006).
Results
Monoculture experiments at low pCO2
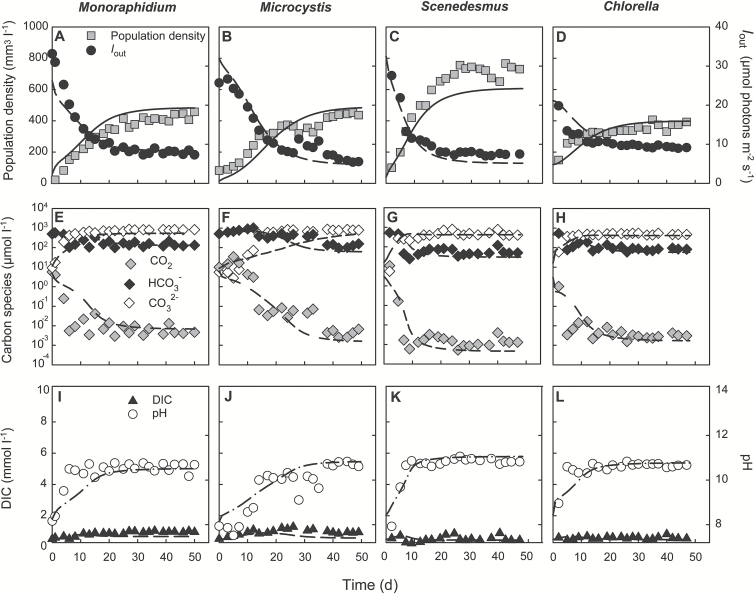
At low pCO2, the phytoplankton species increased until a steady state was reached with population densities (expressed as biovolumes) ranging from 370 mm3 l–1 for Chlorella to 720 mm3 l–1 for Scenedesmus (Fig. 1A–D; Supplementary Table S1). The growing phytoplankton populations reduced the light intensity penetrating through the chemostats (Iout) to 6.2–9.3 µmol photons m–2 s–1 and depleted the dissolved CO2 concentration by several orders of magnitude to <0.01 μmol l–1 (Fig. 1E–H). Bicarbonate concentrations decreased about one order of magnitude, and were offset by a similar increase of the carbonate concentrations, such that the total DIC concentration in the chemostats remained more or less constant. Alkalinity increased to 1.3–2.0 mEq l–1 depending on the species (see Supplementary Table S1 at JXB online). The pH increased by more than two units, from initial values of ~8 to steady state values of ~10.7 (Fig. 1I–L). The relatively high values of Iout in combination with severe CO2 depletion and a high pH indicate that the phytoplankton growth rates in these experiments were carbon limited.
Fig. 1.
Monoculture experiments at low pCO2 (100 ppm). (A–D) Population density (expressed as biovolume) and light intensity Iout penetrating through the chemostat. (E–H) CO2(aq), bicarbonate and carbonate concentrations. (I–L) Dissolved inorganic carbon (DIC) and pH. Different panels represent different species: (A, E, I) Monoraphidium; (B, F, J) Microcystis; (C, G, K) Scenedesmus; (D, H, L) Chlorella. Symbols indicate experimental data, and lines indicate model fits. Parameter values of the model are provided in Table 1 and Table 2.
Monoculture experiments at high pCO2
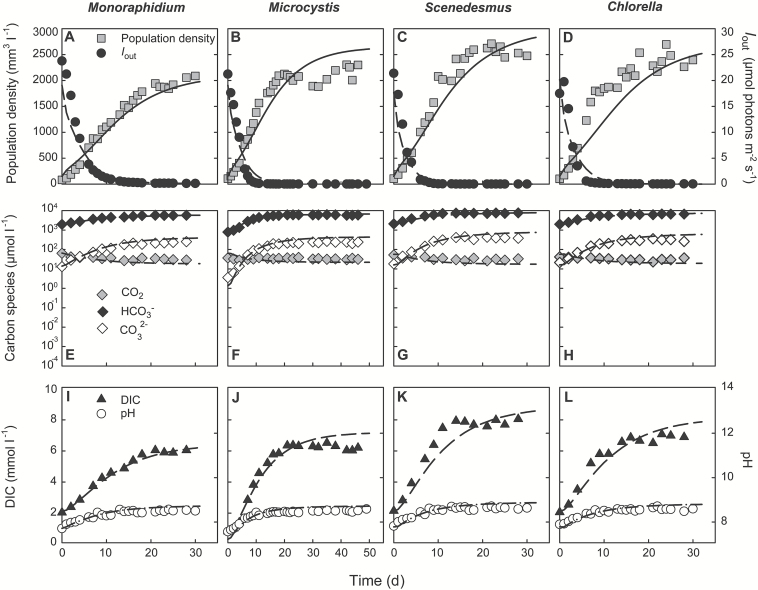
At high pCO2, the phytoplankton species reached population densities ranging from ~2000 mm3 l–1 for Monoraphidium to ~2500 mm3 l–1 for Scenedesmus (Fig. 2A–D; Supplementary Table S1). These values are 3.5–6 times higher than in the low pCO2 experiments (Fig. 1A–D), thus confirming that phytoplankton growth in the low pCO2 experiments was indeed carbon limited. The dense phytoplankton populations absorbed almost all incident light, reducing the light intensity penetrating through the chemostats (Iout) to <0.2 µmol photons m–2 s–1 (Fig. 2A–D). The dissolved CO2 concentration was only slightly reduced to ~30 μmol l–1 (Fig. 2E–H). By contrast, the bicarbonate and carbonate concentration and hence also the total DIC concentration increased during the experiments (Fig. 2I–L). The DIC increase was enabled by a rise in alkalinity, from 2.3 mEq l–1 at the start of the experiments to 6.2–8.4 mEq l–1 at steady state (see Supplementary Table S1). Alkalinity increased during the experiments, because the high uptake rates of nitrate, phosphate, and sulfate by the growing phytoplankton populations are accompanied by proton consumption to maintain charge balance (Goldman and Brewer, 1980; Wolf-Gladrow et al., 2007). A similar increase in DIC and alkalinity induced by dense phytoplankton populations was also observed in earlier chemostat studies (Verspagen et al., 2014b). The pH increased only slightly from ~8 to ~8.6 (Fig. 2I–L). The very low Iout values in combination with high dissolved CO2 and bicarbonate concentrations indicate that the phytoplankton growth rates in these experiments were light limited.
Fig. 2.
Monoculture experiments at high pCO2 (2000 ppm). (A–D) Population density (expressed as biovolume) and light intensity Iout penetrating through the chemostat. (E–H) CO2(aq), bicarbonate and carbonate concentrations. (I–L) Dissolved inorganic carbon (DIC) and pH. Different panels represent different species: (A, E, I) Monoraphidium; (B, F, J) Microcystis; (C, G, K) Scenedesmus; (D, H, L) Chlorella. Symbols indicate experimental data, and lines indicate model fits. Parameter values of the model are provided in Table 1 and Table 2.
Predictions derived from the monoculture experiments
The monoculture experiments showed dynamic changes in population abundances, light conditions, carbon speciation, alkalinity, and pH, which caused concomitant changes in the growth rates of the species. We tried to capture these dynamics by the development of a mathematical model. The results show that the model generally fitted well to the monoculture data, in the experiments at both low pCO2 (Fig. 1) and high pCO2 (Fig. 2). The species parameters estimated from the monoculture experiments are summarized in Table 2, and will be used to predict the dynamics of the competition experiments.
Table 2.
Species parameters estimated from the monoculture experiments
| Parameter | Description | Monoraphidium | Microcystis | Scenedesmus | Chlorella | Units |
|---|---|---|---|---|---|---|
| µmax | Maximum specific growth ratea | 1.27 ± 0.05 | 1.04 ± 0.02 | 1.39 ± 0.05 | 1.28 ± 0.03 | d–1 |
| k | Specific light attenuation coefficienta | 6 × 10–5±0.7 × 10–6 | 8 × 10–5±1.0 × 10–6 | 6 × 10–5±1.0 × 10–6 | 6 × 10–5±1.0 × 10–6 | m2 mm–3 |
| H | Half-saturation constant for lightb | 50 | 11 | 30 | 48 | µmol photons m–2 s–1 |
| H CO2 | Half-saturation constant for CO2b | 12.5 | 1.0 | 2.0 | 2.5 | µmol l–1 |
| H HCO3 | Half-saturation constant for HCO3–b | 80 | 20 | 30 | 40 | µmol l–1 |
| r max | Maximum respiration rateb | 2.2 | 0.9 | 2.2 | 2.2 | µmol mm–3 d–1 |
| u max,CO2 | Maximum uptake rate of CO2b | 17.1 | 20.1 | 14.7 | 22.5 | µmol mm–3 d–1 |
| u max,HCO3 | Maximum uptake rate of HCO3–b | 14.0 | 10.8 | 14.7 | 15.7 | µmol mm–3 d–1 |
| Q min | Minimum cellular carbon contenta | 14.0 ± 1.1 | 9.0 ± 2.7 | 12.5 ± 1.0 | 11.5 ± 0.8 | µmol mm –3 |
| Q max | Maximum cellular carbon contentb | 24.5 | 29.9 | 21.1 | 29.9 | µmol mm –3 |
| c N | Cellular N:C ratioa | 0.114 ± 0.002 | 0.130 ± 0.003 | 0.125 ± 0.003 | 0.100 ± 0.002 | Molar ratio |
| c P | Cellular P:C ratiob | 7.1 × 10–3 | 8.1 × 10–3 | 7.8 × 10–3 | 6.3 × 10–3 | Molar ratio |
| c S | Cellular S:C ratioa | 3.8 × 10–3±0.2 × 10–3 | 7.5 × 10–3±0.3 × 10–3 | 6.0 × 10–3±0.3 × 10–3 | 8.2 × 10–3±0.3 × 10–3 | Molar ratio |
Parameter values measured experimentally, given as mean±standard error.
Parameter values estimated by fitting the model predictions to time courses of the experiments.
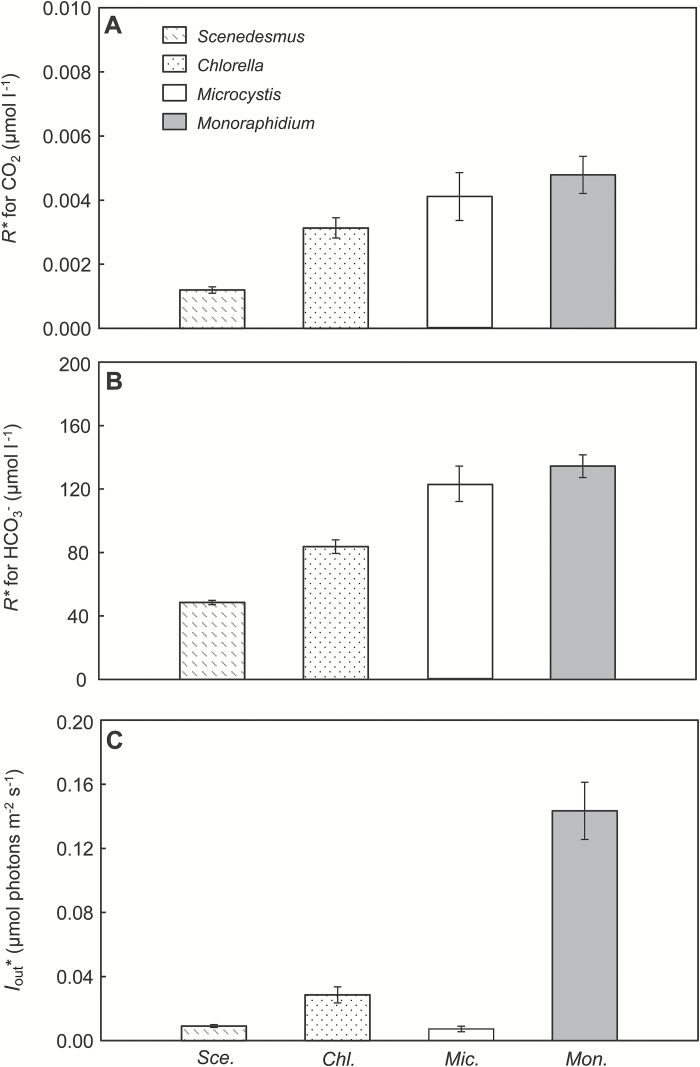
Resource competition theory can provide some further insights. Consider several species competing for a single limiting resource. Each species has its own critical R*, defined as the resource availability at which the specific growth rate of a species equals its loss rate. During competition, resource availability diminishes as the resource is consumed by the species. One by one, species start to decline when resource availability is depleted below their R* values. This process continues, until eventually the species with lowest R* has competitively displaced all other species. Hence, resource competition theory predicts that the species with lowest R* will be the superior competitor (Tilman, 1982; Grover, 1997).
In many applications, the R* value of each species is measured as the steady-state concentration of the limiting resource in monoculture (e.g. Tilman, 1981; Passarge et al., 2006; Wilson et al., 2007). In our application, however, CO2 and bicarbonate provide two alternative inorganic carbon sources that are rapidly interconverted by the chemical reaction of CO2 with water, which makes it difficult to measure the R* for CO2 and R* for bicarbonate independently. Nevertheless, the steady-state concentrations of CO2 and bicarbonate in carbon-limited monoculture may provide useful information on the competitive abilities for inorganic carbon of the species. That is, if we assume that a low steady-state CO2 concentration in monoculture implies a high competitive ability for CO2, then the species can be ranked according to their competitive ability for CO2 as (Fig. 3A): Scenedesmus > Chlorella > Microcystis > Monoraphidium. Similarly, based on the steady-state bicarbonate concentrations, the species can be ranked according to their competitive ability for bicarbonate as (Fig. 3B): Scenedesmus > Chlorella > Microcystis > Monoraphidium. Accordingly, the ranking of the species is the same for both CO2 and bicarbonate, indicating that Scenedesmus will be the best competitor for inorganic carbon, followed by Chlorella and then Microcystis, while Monoraphidium is the worst competitor for inorganic carbon.
Fig. 3.
R* values of the species. (A, B) R* values for CO2(aq) (A) and bicarbonate (B) of the species, indicative of their competitive abilities for inorganic carbon. R* values were estimated from the CO2 and bicarbonate concentrations measured in the steady-state monocultures at low pCO2. (C) Critical light intensities (I*out) of the species, indicative of their competitive abilities for light. Critical light intensities were estimated from the light intensities penetrating through the steady-state monocultures at high pCO2. All estimates are based on the mean±SD of the last five data points of each monoculture experiment. Sce, Scenedesmus; Chl, Chlorella; Mic, Microcystis; Mon, Monoraphidium.
At high CO2 levels, CO2 and bicarbonate were in ample supply, but light becomes a limiting resource (Fig. 2A–D). Analogous to R*, competition theory predicts that the species with lowest critical light intensity (I*out) is the superior competitor for light (Huisman and Weissing, 1994; Huisman et al., 1999). The critical light intensities of the species were measured as the steady-state values of Iout in the monoculture experiments at high pCO2. Based on their critical light intensities, the species can be ranked according to their competitive ability for light as (Fig. 3C): Microcystis ≈ Scenedesmus > Chlorella > Monoraphidium. Hence, from the monoculture data, Microcystis and Scenedesmus are predicted to be the best competitors for light with an approximately similar competitive ability, followed by Chlorella, while Monoraphidium is the worst competitor for light.
Comparison of the above species rankings indicates that Microcystis will become a stronger competitor when the species interactions shift from competition for inorganic carbon at low pCO2 to competition for light at high pCO2. The relative ranking among the three green algae remains unaltered with rising pCO2.
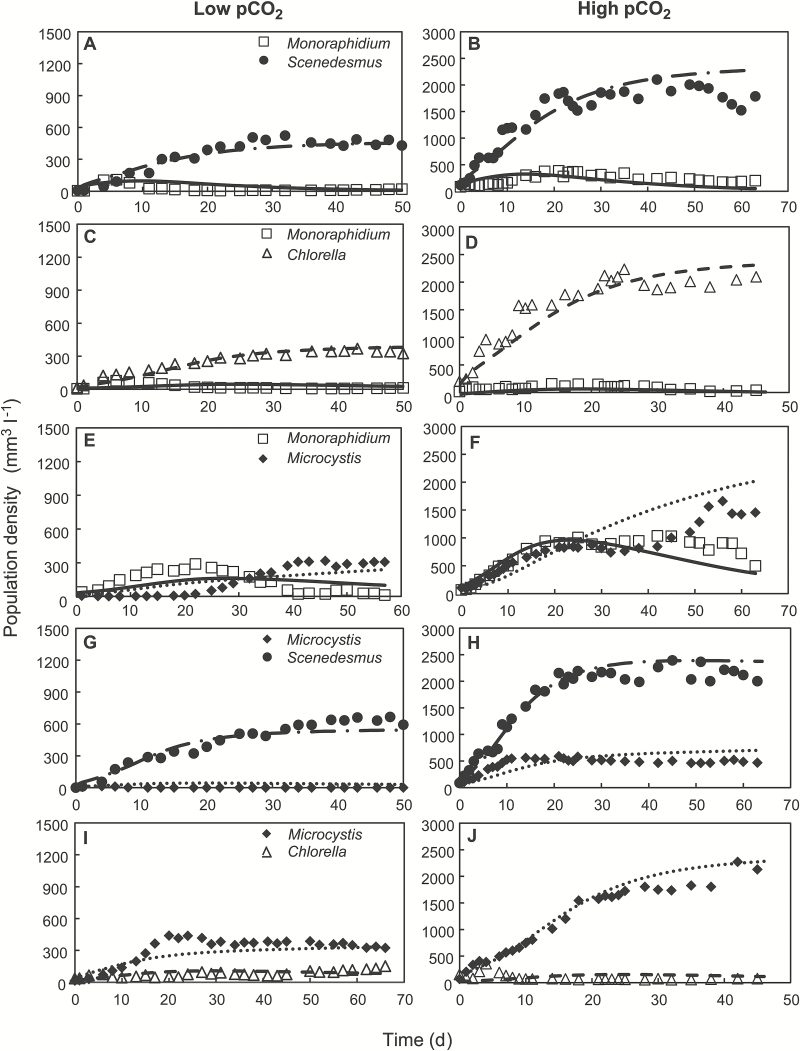
Competition experiments
The competition experiments largely confirmed the predictions derived from the monoculture experiments. For instance, in the competition experiment at low pCO2 between Monoraphidium and Scenedesmus, both species increased during the first 6 d (Fig. 4A). Meanwhile, pH increased to 10.8 (see Supplementary Fig. S1A), the dissolved CO2 concentration was depleted to <0.004 μmol l–1 and the bicarbonate concentration decreased to ~100 μmol l–1 (Supplementary Fig. S2A). These concentrations are below the R* values for CO2 and bicarbonate of Monoraphidium (Fig. 3A, B). Hence, as predicted, Monoraphidium started to decline after the first week, and was competitively displaced by Scenedesmus (Fig. 4A).
Fig. 4.
Competition experiments. The competition experiments were performed at low pCO2 (100 ppm; left panels) and high pCO2 (2000 ppm; right panels). (A, B) Competition between Monoraphidium and Scenedesmus. (C, D) Competition between Monoraphidium and Chlorella. (E, F) Competition between Monoraphidium and Microcystis. (G, H) Competition between Microcystis and Scenedesmus. (I, J) Competition between Microcystis and Chlorella. Dynamic changes in light conditions, carbon speciation and pH during the competition experiments are presented in Supplementary Figs S1 and S2. Symbols indicate experimental data, and lines indicate model predictions. Parameter values of the model are provided in Tables 1 and 2.
At low pCO2, Monoraphidium was also outcompeted by Chlorella (Fig. 4C) and Microcystis (Fig. 4E). Hence, Monoraphidium was the weakest competitor. Furthermore, in line with expectation, Scenedesmus competitively displaced Microcystis (Fig. 4G). Contrary to expectation, Chlorella and Microcystis appeared to coexist at low pCO2 (Fig. 4I). During the last 20 d of this experiment, however, Chlorella tended to increase slowly at the cost of Microcystis, although the experiment didn’t last long enough to witness the final outcome. The latter result is in agreement with the rate of competitive displacement (Table 3), which indicated that Chlorella was indeed slowly displacing Microcystis. According to the competition experiments, the competitive ranking of the species at low pCO2 can thus be summarized as follows: Scenedesmus > Chlorella ≥ Microcystis > Monoraphidium. This is in good agreement with the rankings based on the R* values for CO2 and bicarbonate estimated in the monoculture experiments.
Table 3.
Rates of competitive displacement (RCD±standard error) in the competition experiments
RCD was calculated as the slope of a linear regression of ln(X1/X2) versus time, where X1 and X2 are the population densities of species 1 and species 2. The coefficient of determination (R2), number of data points (n) and significance (P) of the linear regression are indicated.
| Competition experiment | RCD (d–1) | R 2 | n | P |
|---|---|---|---|---|
| Monoraphidium vs Microcystis | ||||
| Low pCO2 | –0.250 ± 0.008 | 0.99 | 14 | <0.001 |
| High pCO2 | –0.043 ± 0.005 | 0.88 | 11 | <0.001 |
| Monoraphidium vs Scenedesmus | ||||
| Low pCO2 | –0.292 ± 0.021 | 0.97 | 8 | <0.001 |
| High pCO2 | –0.021 ± 0.002 | 0.84 | 20 | <0.001 |
| Monoraphidium vs Chlorella | ||||
| Low pCO2 | –0.100 ± 0.008 | 0.93 | 16 | <0.001 |
| High pCO2 | –0.062 ± 0.008 | 0.82 | 15 | <0.001 |
| Scenedesmus vs Microcystis | ||||
| Low pCO2 | +0.654 ± 0.071 | 0.95 | 6 | <0.001 |
| High pCO2 | 0.000 ± 0.003 | 0.00 | 11 | n.s. |
| Microcystis vs Chlorella | ||||
| Low pCO2 | –0.039 ± 0.071 | 0.96 | 11 | <0.001 |
| High pCO2 | +0.197 ± 0.025 | 0.92 | 11 | <0.001 |
In their competition experiment at high pCO2, both Monoraphidium and Scenedesmus increased during the first 20 d (Fig. 4B). Meanwhile, pH stabilized at ~8.5 (see Supplementary Fig. S1B at JXB online), the dissolved CO2 concentration slightly decreased to ~20 μmol l–1 and the bicarbonate concentration increased to >3,000 μmol l–1 (Supplementary Fig. S2B). However, the incident light was almost completely absorbed by the dense species mixture, with <0.1 µmol photons m–2 s–1 penetrating through the chemostat (Supplementary Fig. S1B). This value is below the critical light intensity of Monoraphidium (Fig. 3C). Hence, after the first 20 d, Monoraphidium declined, and was gradually displaced by Scenedesmus (Fig. 4B).
At high pCO2, Monoraphidium was also outcompeted by Chlorella (Fig. 4D) and Microcystis (Fig. 4F), and hence it was again the weakest competitor. Interestingly, Scenedesmus and Microcystis appeared to coexist (Fig. 4H), in agreement with the similar critical light intensities of these two species (Fig. 3C). Their coexistence at high pCO2 was confirmed by the rate of competitive displacement, which did not differ significantly from zero for this species pair (Table 3). In line with expectation, Microcystis competitively displaced Chlorella (Fig. 4J). Hence, according to the competition experiments, the competitive abilities of the species at high pCO2 can be ranked as follows: Microcystis ≈ Scenedesmus > Chlorella > Monoraphidium.
This matches the species ranking based on their critical light intensities in monoculture.
Also quantitatively, the population dynamics predicted by the competition model agreed well with the results of the competition experiments (compare lines versus symbols in Fig. 4, and Supplementary Figs S1 and S2), both at low pCO2 and at high pCO2.
Discussion
Cyanobacteria versus green algae
Both our model predictions and our experimental results contradict the classic view (Shapiro, 1990; Caraco and Miller, 1998; Low-Décarie et al., 2011, 2015) that cyanobacteria are strong competitors at low CO2 levels, whereas eukaryotic phytoplankton such as green algae are better competitors at elevated CO2. We found the opposite. At low CO2 levels, the cyanobacterium Microcystis was a relatively poor competitor. It lost in competition with Scenedesmus, was slowly replaced by Chlorella, and won only against Monoraphidium. At high CO2 levels, Microcystis was a stronger competitor. It won in competition with both Monoraphidium and Chlorella, and coexisted with Scenedesmus. Microcystis was the only species that increased its competitive ranking at elevated CO2; the relative ranking among the three species of green algae did not change.
Competition at low CO2
In the competition experiments at low pCO2, the growing phytoplankton populations depleted the dissolved CO2 concentration within the first 1–3 weeks of the experiments and also the bicarbonate concentration declined. As a consequence, the growth rates of the phytoplankton populations slowed down, and one of the species in the experiments started to displace the other. Interestingly, both the ranking of the R* values estimated from the monocultures and the competitive replacements observed in the competition experiments show that the green algae Scenedesmus and Chlorella were stronger competitors for inorganic carbon than the cyanobacterium Microcystis.
These results can to a large extent be explained by the CCMs of the species. In particular, the cyanobacterium Microcystis PCC 7806 used in this study is a bicA strain (sensuSandrini et al., 2014). It contains the bicarbonate uptake systems BicA and BCT1, but lacks the high-affinity bicarbonate uptake system SbtA. Batch experiments have shown that Microcystis PCC 7806 has a lower growth rate at low Ci levels than strains that do have SbtA (Sandrini et al., 2014). BCT1 is induced when Microcystis PCC 7806 grows at low CO2 levels (Sandrini et al., 2015a, b), but this uptake system appears to have a slightly lower affinity for bicarbonate than SbtA, at least in the cyanobacteria Synechocystis PCC 6803 and Synechococcus PCC 7002 (Price et al., 2004, 2008). Moreover, bicarbonate uptake by BCT1 is ATP dependent and therefore energetically quite expensive. The Na+-dependent bicarbonate uptake system BicA has a high flux rate but low affinity for bicarbonate (Price et al., 2004). Hence, the lack of SbtA offers a plausible explanation of why Microcystis PCC 7806 had a selective disadvantage under Ci-limited conditions. Previous experiments have indeed shown that such bicA strains were selectively displaced by bicA+sbtA strains under Ci-limited conditions (Sandrini et al., 2016). Our results show that cyanobacteria that lack the high-affinity bicarbonate uptake system SbtA are relatively poor competitors under Ci-limited conditions, not only in comparison with other cyanobacteria but also in comparison with green algae such as Scenedesmus and Chlorella.
Our results do not of course imply that cyanobacteria are generally poor competitors under Ci-limited conditions. Microcystis strains containing the high-affinity bicarbonate uptake system SbtA sustain higher growth rates at low Ci concentrations than bicA strains such as Microcystis PCC 7806 (Sandrini et al., 2014). Moreover, recent selection experiments and lake data show that bicA+sbtA strains have a competitive advantage over bicA strains at low CO2 levels (Sandrini et al., 2016). Whether SbtA-containing cyanobacteria or green algae such as Scenedesmus and Chlorella are better competitors at low CO2 levels thus remains an interesting open question.
Compared with cyanobacteria, less is known about the functioning of the CCMs in green algae. Our current understanding of eukaryotic CCMs comes largely from studies with the model organism Chlamydomonas reinhardtii (Moroney and Ynalvez 2007; Spalding, 2008; Wang et al., 2011; Meyer and Griffiths, 2013). Briefly, the key components of the eukaryotic CCM are quite similar to the cyanobacterial CCM and involve active CO2 and bicarbonate uptake, interconversion between these two Ci species by carbonic anhydrases (CAs), and a microcompartment that contains RuBisCO (Wang et al., 2015). In Chlamydomonas, five Ci transporters have been localized, including two confirmed bicarbonate transporters (HLA3 on the plasma membrane and LCIA on the chloroplast envelope) and three undefined Ci transporters (LCIl on the plasma membrane and CCAP1/2 on the chloroplast envelope). Inside the chloroplast, RuBisCO is densely packed in a microcompartment named the pyrenoid (Kuchitsu et al., 1988; Engel et al., 2015). Within the pyrenoid, bicarbonate is dehydrated to CO2 by a CA and released to RuBisCO (Moroney and Ynalvez, 2007).
So far, there are no studies on the CCM genes of the green algal strains we used in this study, but there is some recent work on the CCM genes of a different species of Chlorella, C. pyrenoidosa, indicating that this species has a CCM similar to that of Chlamydomonas (Fan et al., 2016). For instance, when shifting C. pyrenoidosa from high CO2 to low CO2 conditions, CCM-related genes such as LCIA, LCIB, and HLA3 showed increased expression, similar to the response of C. reinhardtii (Fan et al., 2015). Interestingly, earlier work indicates that there is substantial variation in CCM activity within the Chlorella genus and even between different strains of the same species of Chlorella. For example, C. vulgaris strain 11 h and strain UTEX 263 seem to utilize only CO2 as a carbon source (Miyachi et al., 1983, Tu et al., 1986), whereas C. vulgaris strain C-3, strain UTEX 259 and C. pyrenoidosa can use both CO2 and bicarbonate (Miyachi et al., 1983). Our results indicate that the C. vulgaris strain we used can utilize bicarbonate: if this green alga could only use CO2, it would not be able to grow in monoculture in our chemostats at a dilution rate of 0.125 d–1 and dissolved CO2 concentrations lower than 0.01 µmol l–1 (Fig. 1).
Similarly, the fact that S. obliquus and M. griffithii were also able to grow at CO2 concentrations below 0.01 µmol l–1 in our chemostat experiments indicates that these green algae can also utilize bicarbonate as carbon source. Earlier studies confirm the presence of a functional CCM in a different strain of S. obliquus. WT strain D3 has increased intracellular and extracellular CA activity, as well as a higher affinity for the uptake of CO2 and bicarbonate at low compared with high CO2 concentrations (Palmqvist et al., 1994). Furthermore, in our experiments S. obliquus could deplete dissolved CO2 and bicarbonate concentrations to even lower levels than Microcystis PCC 7806 (Fig. 3), and displace Microcystis in the competition experiments at low CO2 (Fig. 4). Similar results were obtained in CO2-limited mesocosm experiments by Verschoor et al. (2013), where the same strain of S. obliquus displaced Synechocystis PCC 6803, a cyanobacterium that contains all five Ci uptake systems (Badger et al., 2006). So far, very little is known about the CCM of Monoraphidium griffithii, but a related species, M. braunii, can photoactivate a blue-light-dependent bicarbonate transport system under CO2-limiting conditions (Mora et al 2002).
In total, these physiological studies indicate that many green algae are well adapted to cope with Ci limitation. Similar to cyanobacteria, many species of green algae are able to induce a CCM under CO2-limiting conditions (Meyer and Griffiths, 2013). Furthermore, in eutrophic lakes, not only cyanobacteria but also green algae have been observed to develop dense blooms at low CO2 concentrations and high pH (Jeppesen et al., 1990; Jensen et al., 1994; Beklioglu and Moss, 1995). The results of our competition experiments are in agreement with these physiological studies and field observations, and demonstrate that green algae can indeed be very effective competitors at low CO2 levels.
Competition at elevated CO2
The notion that Microcystis PCC 7806 lacks the high-affinity uptake system SbtA explains not only why it was a poor competitor at low CO2 levels, but may also help to understand why it was more successful at high CO2 levels. In bicA+sbtA strains of Microcystis, the bicarbonate uptake genes bicA and sbtA are located on the same operon and are co-transcribed (Sandrini et al., 2014). Transcription of sbtA will be inefficient and costly, however, when inorganic concentrations are high and hence the high-affinity but low-flux system SbtA is no longer needed. This reasoning is supported by recent selection experiments, which have shown that bicA strains have a competitive advantage over bicA+sbtA strains at elevated CO2 levels (Sandrini et al., 2016).
Yet, the presence of the high-flux bicarbonate uptake system BicA is probably not sufficient to explain its competitive success at elevated CO2 levels. Elevated CO2 increased the dissolved Ci concentrations, but also yielded 3- to 8-fold higher population densities, which in turn generated very low light availabilities (Figs 1 and 2, and Supplementary Table S1). At these high CO2 but low light levels, carbon availability is no longer a major limiting factor and bicarbonate uptake systems tend to be down-regulated (Beardall, 1991; Sandrini et al., 2015a). Instead, the intense shading induced by the dense phytoplankton populations will favor species adapted to low light environments (Huisman et al., 1999). A range of studies have suggested that cyanobacteria are superior competitors for light (Mur et al., 1977; Reynolds et al., 1987; Huisman et al., 2004; Yang and Jin, 2008; Schwaderer et al., 2011). Comparison of the traits of the species investigated in this study shows that Microcystis PCC 7806 had the lowest half-saturation constant for light-limited growth (Table 2), indicating that it can sustain a relatively high growth rate at low light levels in comparison with the other species. Scenedesmus also had a relatively low half-saturation constant for light-limited growth, albeit higher than Microcystis, and had the highest maximum growth rate of all four species (Table 2). Indeed, the competitive ability of the cyanobacterium Microcystis increased at elevated CO2, and together with Scenedesmus it became the best competitor for light (Fig. 3).
Model predictions
Comparison of the model predictions and experimental results shows that the impact of elevated CO2 on phytoplankton competition can be quite accurately predicted under controlled laboratory conditions. First, the system parameters and several species parameters were measured in monoculture experiments, while the remaining species parameters were estimated from least-squares fits of the model predictions to the monoculture dynamics (Figs 1 and 2). Hence, the model is calibrated with the monoculture data. Subsequently, the parameter estimates from the monocultures were used to predict the time course and outcome of competition in the species mixtures. Accordingly, the model predictions are validated with the competition experiments (Fig. 4, and Supplementary Figs S1 and S2).
Our model and experiments are of course still a major simplification in comparison with the complexities of the real CCMs of cyanobacteria and green algae competing in natural waters. For instance, the model brushes over many of the physiological details involved in the regulation of CCMs in response to changes in carbon and light availability (e.g. Beardall and Giordano, 2002; Burnap et al., 2015). Furthermore, the experiments were limited to only a small number of phytoplankton species under controlled laboratory conditions, in isolation from a multitude of other hydrological, biogeochemical and ecological processes that are important in lakes. Species might be superior competitors for inorganic carbon or light, but if they are also preferentially grazed by zooplankton they are still unlikely to gain dominance in natural waters. Hence, further improvement of the model predictions might be obtained by further refinement of the underlying physiological and ecological processes.
Nevertheless, the results are promising. Our model and experiments seemed to capture the basic ingredients required to predict phytoplankton competition at different CO2 levels. Similar to several previous studies (e.g. Caraco and Miller, 1998; Low-Décarie et al., 2011; Trimborn et al., 2013), we have shown that species traits measured in monoculture can be used to predict changes in phytoplankton species composition at elevated CO2. To our knowledge, our study is the first experimental demonstration that a mathematical model can quantitatively predict dynamic changes in phytoplankton species composition, carbon speciation, pH, alkalinity and light during the competition process.
Conclusions
Our experimental results call for a revision of the classic paradigm that cyanobacteria are superior competitors at low CO2 levels and high pH, whereas eukaryotic phytoplankton such as green algae are superior competitors at elevated CO2. Such simple dichotomies do not capture the diversity of CCMs that have been found among and within different phytoplankton taxa. First, our results demonstrate that green algae can also be very effective competitors at low CO2 levels. Second, our results show that some cyanobacterial strains are relatively poor competitors when CO2 is limiting but become stronger competitors at elevated CO2. We therefore urge the CCM scientific community to further elucidate and compare the performance of different CCMs, and their selective advantages and disadvantages across a wide range of different species at the molecular, physiological and ecological level. Such a comparative approach will be essential if we are to understand and predict how the species composition of natural phytoplankton communities will respond to the anticipated rise in CO2 levels.
Supplementary data
Supplementary data are available at JXB online.
Model S1. Detailed description of the model.
Fig. S1. Dynamic changes in light, DIC and pH during the competition experiments.
Fig. S2. Dynamic changes in carbon speciation during the competition experiments.
Table S1. Steady-state characteristics in the monoculture experiments.
Supplementary Material
Acknowledgements
We thank Hanneke van Bree, Sander de Groot, Duke van Velden, Maureen de Wit and Josan Yauw for their help in running the chemostats, Leo Hoitinga for assistance with the TOC-VCPH Analyzer and Elemental Analyzer, Suzanne Naus-Wiezer of the NIOO-KNAW for assistance with flow cytometry, and the reviewers for their helpful comments. This research is supported by the Division of Earth and Life Sciences (ALW) of the Netherlands Organization for Scientific Research (NWO).
Glossary
Abbreviations:
- CCM
CO2-concentrating mechanism
- Ci
inorganic carbon
- CA
carbonic anhydrase
- DIC
dissolved inorganic carbon
- RuBisCO
ribulose-1,5-bisphosphate carboxylase/oxygenase
- ppm
parts per million.
References
- Badger MR, Price GD, Long BM, Woodger FJ. 2006. The environmental plasticity and ecological genomics of the cyanobacterial CO2 concentrating mechanism. Journal of Experimental Botany 57, 249–265. [DOI] [PubMed] [Google Scholar]
- Balmer MB, Downing JA. 2011. Carbon dioxide concentrations in eutrophic lakes: undersaturation implies atmospheric uptake. Inland Waters 1, 125–132. [Google Scholar]
- Beardall J. 1991. Effects of photon flux density on the ‘CO2-concentrating mechanism’ of the cyanobacterium Anabaena variabilis. Journal of Plankton Research 13, 133–141. [Google Scholar]
- Beardall J, Giordano M. 2002. Ecological implications of microalgal and cyanobacterial CO2 concentrating mechanisms, and their regulation. Functional Plant Biology 29, 335–347. [DOI] [PubMed] [Google Scholar]
- Beklioglu M, Moss B. 1995. The impact of pH on interactions among phytoplankton algae, zooplankton and perch (Perca fluviatilis) in a shallow, fertile lake. Freshwater Biology 33, 497–509. [Google Scholar]
- Burnap RL, Hagemann M, Kaplan A. 2015. Regulation of CO2 concentrating mechanism in cyanobacteria. Life 5, 348–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraco NF, Miller R. 1998. Effects of CO2 on competition between a cyanobacterium and eukaryotic phytoplankton. Canadian Journal of Fisheries and Aquatic Sciences 55, 54–62. [Google Scholar]
- Carmichael WW. 2001. Health effects of toxin-producing cyanobacteria: “The CyanoHABs”. Human and Ecological Risk Assessment 7, 1393–1407. [Google Scholar]
- Chorus I, Bartram J. 1999. Toxic cyanobacteria in water: a guide to their public health consequences, monitoring, and management. London: E & FN Spon. [Google Scholar]
- Codd GA, Morrison LF, Metcalf JS. 2005. Cyanobacterial toxins: risk management for health protection. Toxicology and Applied Pharmacology 203, 264–272. [DOI] [PubMed] [Google Scholar]
- Cole JJ, Bade DL, Bastviken D, Pace ML, Van de Bogert M. 2010. Multiple approaches to estimating air-water gas exchange in small lakes. Limnology and Oceanography Methods 8, 285–293. [Google Scholar]
- Droop MR. 1973. Some thoughts on nutrient limitation in algae. Journal of Phycology 9, 264–272. [Google Scholar]
- Eisenhut M, Aguirre von Wobeser EA, Jonas L, Schubert H, Ibelings BW, Bauwe H, Matthijs HCP, Hagemann M. 2007. Long-term response toward inorganic carbon limitation in wild type and glycolate turnover mutants of the cyanobacterium Synechocystis sp. strain PCC 6803. Plant Physiology 144, 1946–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel BD, Schaffer M, Cuellar LK, Villa E, Plitzko JM, Baumeister W. 2015. Native architecture of the Chlamydomonas chloroplast revealed by in situ cryo-electron tomography. eLife 4, e04889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faltermann S, Zucchi S, Kohler E, Blom JF, Pernthaler J, Fent K. 2014. Molecular effects of the cyanobacterial toxin cyanopeptolin (CP1020) occurring in algal blooms: global transcriptome analysis in zebrafish embryos. Aquatic Toxicology 149, 33–39. [DOI] [PubMed] [Google Scholar]
- Fan J, Xu H, Li Y. 2016. Transcriptome-based global analysis of gene expression in response to carbon dioxide deprivation in the green algae Chlorella pyrenoidosa. Algal Research 16, 12–19. [Google Scholar]
- Fan J, Xu H, Luo Y, Wan M, Huang J, Wang W, Li Y. 2015. Impacts of CO2 concentration on growth, lipid accumulation, and carbon-concentrating-mechanism-related gene expression in oleaginous Chlorella. Applied Microbiology and Biotechnology 99, 2451–2462. [DOI] [PubMed] [Google Scholar]
- Goldman JC, Brewer PG. 1980. Effect of nitrogen source and growth rate on phytoplankton-mediated changes in alkalinity. Limnology and Oceanography 25, 352–357. [Google Scholar]
- Grover JP. 1991. Dynamics of competition among microalgae in variable environments: experimental tests of alternative models. Oikos 62, 231–243. [Google Scholar]
- Grover JP. 1997. Resource competition. London: Chapman & Hall. [Google Scholar]
- Guo L. 2007. Doing battle with the green monster of Taihu Lake. Science 317, 1166. [DOI] [PubMed] [Google Scholar]
- Huisman J, Jonker RR, Zonneveld C, Weissing FJ. 1999. Competition for light between phytoplankton species: experimental tests of mechanistic theory. Ecology 80, 211–222. [Google Scholar]
- Huisman J, Matthijs HCP, Visser PM, eds 2005. Harmful cyanobacteria. Dordrecht: Springer Netherlands. [Google Scholar]
- Huisman J, Matthijs HCP, Visser PM, Balke H, Sigon CAM, Passarge J, Weissing FJ, Mur LR. 2002. Principles of the light-limited chemostat: theory and ecological applications. Antonie van Leeuwenhoek 81, 117–133. [DOI] [PubMed] [Google Scholar]
- Huisman J, Sharples J, Stroom JM, Visser PM, Kardinaal WEA, Verspagen JMH, Sommeijer B. 2004. Changes in turbulent mixing shift competition for light between phytoplankton species. Ecology 85, 2960–2970. [Google Scholar]
- Huisman J, Weissing FJ. 1994. Light-limited growth and competition for light in well-mixed aquatic environments: an elementary model. Ecology 75, 507–520. [Google Scholar]
- Ibelings BW, Maberly SC. 1998. Photoinhibition and the availability of inorganic carbon restrict photosynthesis by surface blooms of cyanobacteria. Limnology and Oceanography 43, 408–419. [Google Scholar]
- Jensen JP, Jeppesen E, Olrik K, Kristensen P. 1994. Impact of nutrients and physical factors on the shift from cyanobacterial to chlorophyte dominance in shallow Danish lakes. Canadian Journal of Fisheries and Aquatic Sciences 51, 1692–1699. [Google Scholar]
- Jeppesen E, Sondergaard M, Sortkjaer O, Mortensen E, Kristensen P. 1990. Interactions between phytoplankton, zooplankton and fish in a shallow, hypertrophic lake – a study of phytoplankton collapses in Lake Søbygård, Denmark. Hydrobiologia 191, 149–164. [Google Scholar]
- Kuchitsu K, Tsuzuki M, Miyachi S. 1988. Characterization of the pyrenoid isolated from unicellular green-alga Chlamydomonas reinhardtii: particulate form of RuBisCo protein. Protoplasma 144, 17–24. [Google Scholar]
- Lazzarino JK, Bachmann RW, Hoyer MV, Canfield DE., Jr 2009. Carbon dioxide supersaturation in Florida lakes. Hydrobiologia 627, 169–180. [Google Scholar]
- Litchman E, Klausmeier CA, Bossard P. 2004. Phytoplankton nutrient competition under dynamic light regimes. Limnology and Oceanography 49, 1457–1462. [Google Scholar]
- Low-Décarie E, Bell G, Fussmann GF. 2015. CO2 alters community composition and response to nutrient enrichment of freshwater phytoplankton. Oecologia 177, 875–883. [DOI] [PubMed] [Google Scholar]
- Low-Décarie E, Fussmann GF, Bell G. 2011. The effect of elevated CO2 on growth and competition in experimental phytoplankton communities. Global Change Biology 17, 2525–2535. [Google Scholar]
- Merel S, Walker D, Chicana R, Snyder S, Baurès E, Thomas O. 2013. State of knowledge and concerns on cyanobacterial blooms and cyanotoxins. Environment International 59, 303–327. [DOI] [PubMed] [Google Scholar]
- Meyer M, Griffiths H. 2013. Origins and diversity of eukaryotic CO2-concentrating mechanisms: lessons for the future. Journal of Experimental Botany 64, 769–786. [DOI] [PubMed] [Google Scholar]
- Michalak AM, Anderson EJ, Beletsky D, et al. 2013. Record-setting algal bloom in Lake Erie caused by agricultural and meteorological trends consistent with expected future conditions. Proceedings of the National Academy of Sciences of the United States of America 110, 6448–6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyachi S, Tsuzuki M, Avramova ST. 1983. Utilization modes of inorganic carbon for photosynthesis in various species of Chlorella. Plant and Cell Physiology 24, 441–451. [Google Scholar]
- Mora C, Witt FG, Aparicio PJ, Quiñones MA. 2002. Independent induction of two blue light-dependent monovalent anion transport systems in the plasma membrane of Monoraphidium braunii. Journal of Experimental Botany 53, 1909–1918. [DOI] [PubMed] [Google Scholar]
- Moroney JV, Ynalvez RA. 2007. Proposed carbon dioxide concentrating mechanism in Chlamydomonas reinhardtii. Eukaryotic Cell 6, 1251–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mur LR, Gons HJ, Van Liere L. 1977. Some experiments on the competition between green algae and blue-green bacteria in light-limited environments. FEMS Microbiology Letters 1, 335–338. [Google Scholar]
- Palmqvist K, Yu JW, Badger MR. 1994. Carbonic anhydrase activity and inorganic carbon fluxes in low- and high-C1 cells of Chlamydomonas reinhardtii and Scenedesmus obliquus. Physiologia Plantarum 90, 537–547. [Google Scholar]
- Passarge J, Hol S, Escher M, Huisman J. 2006. Competition for nutrients and light: stable coexistence, alternative stable states, or competitive exclusion?Ecological Monographs 76, 57–72. [Google Scholar]
- Price GD, Badger MR, Woodger FJ, Long BM. 2008. Advances in understanding the cyanobacterial CO2-concentrating-mechanism (CCM): functional components, Ci transporters, diversity, genetic regulation and prospects for engineering into plants. Journal of Experimental Botany 59, 1441–1461. [DOI] [PubMed] [Google Scholar]
- Price GD, Woodger FJ, Badger MR, Howitt SM, Tucker L. 2004. Identification of a SulP-type bicarbonate transporter in marine cyanobacteria. Proceedings of the National Academy of Sciences of the United States of America 101, 18228–18233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven JA, Giordano M, Beardall J, Maberly SC. 2012. Algal evolution in relation to atmospheric CO2: carboxylases, carbon-concentrating mechanisms and carbon oxidation cycles. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 367, 493–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CS, Oliver RL, Walsby AE. 1987. Cyanobacterial dominance: the role of buoyancy regulation in dynamic lake environments. New Zealand Journal of Marine and Freshwater Research 21, 379–390. [Google Scholar]
- Sandrini G, Cunsolo S, Schuurmans JM, Matthijs HCP, Huisman J. 2015a. Changes in gene expression, cell physiology and toxicity of the harmful cyanobacterium Microcystis aeruginosa at elevated CO2. Frontiers in Microbiology 6, 401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandrini G, Jakupovic D, Matthijs HCP, Huisman J. 2015b. Strains of the harmful cyanobacterium Microcystis aeruginosa differ in gene expression and activity of inorganic carbon uptake systems at elevated CO2 levels. Applied and Environmental Microbiology 81, 7730–7739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandrini G, Matthijs HCP, Verspagen JMH, Muyzer G, Huisman J. 2014. Genetic diversity of inorganic carbon uptake systems causes variation in CO2 response of the cyanobacterium Microcystis. The ISME Journal 8, 589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandrini G, Ji X, Verspagen JMH, Tann RP, Slot PC, Luimstra VL, Schuurmans JM, Matthijs HCP, Huisman J. 2016. Rapid adaptation of harmful cyanobacteria to rising CO2. Proceedings of the National Academy of Sciences of the United States of America 113, 9315–9320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaderer AS, Yoshiyama K, De Tezanos Pinto P, Swenson NG, Klausmeier CA, Litchman E. 2011. Eco-evolutionary differences in light utilization traits and distributions of freshwater phytoplankton. Limnology and Oceanography 56, 589–598. [Google Scholar]
- Shapiro J. 1990. Current beliefs regarding dominance of bluegreens: the case for the importance of CO2 and pH. Verhandlungen Internationale Vereiniging für Theoretische und Angewandte Limnologie 24, 38–54. [Google Scholar]
- Shapiro J. 1997. The role of carbon dioxide in the initiation and maintenance of blue-green dominance in lakes. Freshwater Biology 37, 307–323. [Google Scholar]
- Siegenthaler U, Sarmiento JL. 1993. Atmospheric carbon dioxide and the ocean. Nature 365, 119–125. [Google Scholar]
- Sommer U. 1985. Comparison between steady state and non-steady state competition: experiments with natural phytoplankton. Limnology and Oceanography 30, 335–346. [Google Scholar]
- Spalding MH. 2008. Microalgal carbon-dioxide-concentrating mechanisms: Chlamydomonas inorganic carbon transporters. Journal of Experimental Botany 59, 1463–1473. [DOI] [PubMed] [Google Scholar]
- Stomp M, Huisman J, De Jongh F, Veraart AJ, Gerla D, Rijkeboer M, Ibelings BW, Wollenzien UIA, Stal LJ. 2004. Adaptive divergence in pigment composition promotes phytoplankton biodiversity. Nature 432, 104–107. [DOI] [PubMed] [Google Scholar]
- Stumm W, Morgan JJ. 1996. Aquatic chemistry: chemical equilibria and rates in natural waters, 3rd edn. New York: John Wiley & Sons. [Google Scholar]
- Talling JF. 1976. The depletion of carbon dioxide from lake water by phytoplankton. Journal of Ecology 64, 79–121. [Google Scholar]
- Tilman D. 1977. Resource competition between plankton algae: an experimental and theoretical approach. Ecology 58, 338–348. [Google Scholar]
- Tilman D. 1981. Tests of resource competition theory using four species of Lake Michigan algae. Ecology 62, 802–815. [Google Scholar]
- Tilman D. 1982. Resource competition and community structure. Princeton: Princeton University Press. [PubMed] [Google Scholar]
- Tonk L, Welker M, Huisman J, Visser PM. 2009. Production of cyanopeptolins, anabaenopeptins, and microcystins by the harmful cyanobacteria Anabaena 90 and Microcystis PCC 7806. Harmful Algae 8, 219–224. [Google Scholar]
- Trimborn S, Brenneis T, Sweet E, Rost B. 2013. Sensitivity of Antarctic phytoplankton species to ocean acidification: growth, carbon acquisition, and species interaction. Limnology and Oceanography 58, 997–1007. [Google Scholar]
- Tu CK, Acevedo-Duncan M, Wynns GC, Silverman DN. 1986. Oxygen-18 exchange as a measure of accessibility of CO2 and HCO3– to carbonic anhydrase in Chlorella vulgaris (UTEX 263). Plant Physiology 80, 997–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Waal DB, Verspagen JMH, Finke JF, et al. 2011. Reversal in competitive dominance of a toxic versus non-toxic cyanobacterium in response to rising CO2. The ISME Journal 5, 1438–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Waal DB, Verspagen JMH, Lürling M, Van Donk E, Visser PM, Huisman J. 2009. The ecological stoichiometry of toxins produced by harmful cyanobacteria: an experimental test of the carbon-nutrient balance hypothesis. Ecology Letters 12, 1326–1335. [DOI] [PubMed] [Google Scholar]
- Verschoor AM, Van Dijk MA, Huisman J, Van Donk E. 2013. Elevated CO2 concentrations affect the elemental stoichiometry and species composition of an experimental phytoplankton community. Freshwater Biology 58, 597–611. [Google Scholar]
- Verspagen JMH, Passarge J, Jöhnk KD, Visser PM, Peperzak L, Boers P, Laanbroek HJ, Huisman J. 2006. Water management strategies against toxic Microcystis blooms in the Dutch delta. Ecological Applications 16, 313–327. [DOI] [PubMed] [Google Scholar]
- Verspagen JMH, Van de Waal DB, Finke JF, Visser PM, Huisman J. 2014a. Contrasting effects of rising CO2 on primary production and ecological stoichiometry at different nutrient levels. Ecology Letters 17, 951–960. [DOI] [PubMed] [Google Scholar]
- Verspagen JMH, Van de Waal DB, Finke JF, Visser PM, Van Donk E, Huisman J. 2014b. Rising CO2 levels will intensify phytoplankton blooms in eutrophic and hypertrophic lakes. PloS ONE 9, e104325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser PM, Verspagen JMH, Sandrini G, Stal LJ, Matthijs HCP, Davis TW, Paerl HW, Huisman J. 2016. How rising CO2 and global warming may stimulate harmful cyanobacterial blooms. Harmful Algae 54, 145–159. [DOI] [PubMed] [Google Scholar]
- Wang Y, Duanmu D, Spalding MH. 2011. Carbon dioxide concentrating mechanism in Chlamydomonas reinhardtii: inorganic carbon transport and CO2 recapture. Photosynthesis Research 109, 115–122. [DOI] [PubMed] [Google Scholar]
- Wang Y, Stessman DJ, Spalding MH. 2015. The CO2 concentrating mechanism and photosynthetic carbon assimilation in limiting CO2: how Chlamydomonas works against the gradient. The Plant Journal 82, 429–448. [DOI] [PubMed] [Google Scholar]
- Watson SB, Ridal J, Boyer GL. 2008. Taste and odour and cyanobacterial toxins: impairment, prediction, and management in the Great Lakes. Canadian Journal of Fisheries and Aquatic Sciences 65, 1779–1796. [Google Scholar]
- Wilson JB, Spijkerman E, Huisman J. 2007. Is there really insufficient support for Tilman’s R* concept? A comment on Miller et al. The American Naturalist 169, 700–706. [DOI] [PubMed] [Google Scholar]
- Wolf-Gladrow DA, Zeebe RE, Klaas C, Körtzinger A, Dickson AG. 2007. Total alkalinity: the explicit conservative expression and its application to biogeochemical processes. Marine Chemistry 106, 287–300. [Google Scholar]
- Yang S, Jin X. 2008. Critical light intensities for Microcystis aeruginosa, Scenedesmus quadricauda and Cyclotella sp. and competitive growth patterns under different light:N:P ratios. Journal of Freshwater Ecology 23, 387–396. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.