Abstract
Staphylococcus aureus is a severe pathogen found in the community and in hospitals. Most notably, methicillin-resistant S. aureus (MRSA) is resistant to almost all antibiotics, which is a growing public health concern. The emergence of drug-resistant strains has prompted the search for alternative treatments such as immunotherapeutic approaches. Previous research showed that S. aureus exploit the immunomodulatory attributes of adenosine to escape host immunity. In this study, we investigated adenosine synthase A (AdsA), an S. aureus cell wall–anchored enzyme as possible targets for immunotherapy. Mice vaccinated with aluminum hydroxide–formulated recombinant AdsA (rAdsA) induced high-titer anti-AdsA antibodies, thereby providing consistent protection in 3 mouse infection models when challenged with 2 S. aureus strains. The importance of anti-AdsA antibody in protection was demonstrated by passive transfer experiments. Moreover, AdsA-specific antisera promote killing S. aureus by immune cells. Altogether, our data demonstrate that the AdsA is a promising target for vaccines and therapeutics development to alleviate severe S. aureus diseases.
Keywords: S. aureus, adenosine synthase A, AdsA, immunotherapy
Staphylococcus aureus is a common pathogen in the community and frequently found in hospitals [1–4]. Particularly, methicillin-resistant S. aureus (MRSA) is one of the more dangerous antibiotic-resistant S. aureus strains. The strains are prevalent in hospitals and are fast becoming a common community-acquired infection [5]. For this reason, research into the development of immunotherapeutic approaches, either active or passive, has seen a resurgence in recent years [6]. At least 13 secreted proteins and 24 surface adhesion proteins from S. aureus have been implicated in the bacterial immune evasion, many of which have been evaluated as potential antigens [7–16]. Current and past S. aureus vaccines or therapeutic antibody strategies have mainly focused on virulence factors, capsular polysaccharide (CPS) and iron-regulated proteins, including ess extracellular A (EsxA) and ess extracellular B (EsxB) [17], alpha toxin (nontoxic derivative of H35L) [8, 18], clumping factor A (ClfA) [19], fibronectin binding protein (FnBPA or FnBPB) [13], Panton-Valentine leukocidin (PVL) [20], and protein A [12]. Iron-regulated proteins have also been investigated as another possible target for vaccines against S. aureus, such as Merck V710, which is based on the iron-regulated surface determinant B (IsdB) [7, 21]. To date, most of the clinical trials for vaccines or passive immunization against S. aureus have ended in failure. Therefore, investigations of other potential antigens are very important to develop S. aureus vaccine.
S. aureus has a unique ability to escape a variety of innate immune responses, such as phagocytic killing and complement and antimicrobial peptides, thereby leading to survival in blood or other host tissues, causing persistent infections [22]. S. aureus deploys a range of mechanisms, such as secretion of virulent factors and toxins, to escape or subvert innate immune responses [23]. Previous research has indicated that adenosine synthase A (AdsA), an S. aureus cell wall–anchored enzyme, acts as an immune evasion factor [22]. When both wild-type and adsA-deficient S. aureus are mixed with fresh mouse or human blood, they are phagocytized by polymorphonuclear leukocytes (PMNs), particularly phagocytic neutrophils; however, wild-type S. aureus survives within PMNs but adsA mutants do not. Furthermore, adsA mutant S. aureus were cleared more easily from the BALB/c mice bloodstream than wild-type strain, correlating with the reduced ability to grow during infection and/or seed abscesses [22]. S. aureus generates adenosine by converting from adenosine monophosphates (AMP) or adenosine di-phosphates (ADP) after infecting humans or mammals. In mammals, it is a 2-step process to catalyze adenosine triphosphate to adenosine. First, ectonucleoside triphosphate diphosphohydrolases (ecto-NTDPases) hydrolyze ATP or ADP to produce AMP. AdsA contains two 5ʹ-nucleotidase signature regions, which then catalyses the conversion of AMP to adenosine [24].
Bacterial invasion of human or animal tissues activates granulocytes to release DNA, thereby fixing pathogens for subsequent clearance by macrophage phagocytosis. These networks are called neutrophil extracellular traps (NETs), which also act a first line of defense against foreign microbes. Recently, Thammavongsa et al showed that S. aureus could degrade NET function and induce immune cell death [25]. When S. aureus infects host tissues, the bacteria express 2 enzymes, nuclease and AdsA. Nuclease can degrade NET DNA into deoxyadenylate (dAMP), which is subsequently converted to produce 2’-deoxyadenosine (dAdo) by AdsA. dAdo can sufficient activate caspase-3 pathway to promote apoptosis of macrophages. Therefore, S. aureus can confine macrophages from crossing the immune cell cuff into the abscess communities. This is the mechanism preventing phagocytosis, thereby establishes persistent S. aureus infections [25].
In this study, we tested whether active or passive immunization directed at AdsA could alleviate disease severity in 3 infection BALB/c models.
MATERIALS AND METHODS
Bacterial Strains and Culture Conditions
Escherichia coli strain BL21 (DE3) was used for protein expression. The S. aureus Newman [26] and USA300 strains [27] are a gift from Dr Richard Yi-Tsun Kao (Department of Microbiology, University of Hong Kong). S. aureus strains were grown at 37°C in BHI broth or agar. For preparation of bacterial challenge inocula for infection studies in animals, S. aureus USA300 strains were grown 12 hours at 37°C in brain-heart infusion (BHI). The next day, inoculation of 50 μL preculture USA300 strains into 5 mL BHI to subculture at 37°C for 2 hours until mid-exponential phase is reached (optical density at 600 nm). The bacteria were harvested using endotoxin-free phosphate-buffered saline (PBS), washed twice, and resuspended with the desired amount of colony-forming units (CFU) depending on the model.
Active Immunization
Six-week-old female BALB/c mice (n = 10 per group) were immunized with 25 μg of rAdsA (endotoxin [lipopolysaccharide] was <10 EU/mg, host cell protein was <2 ng/mg, and DNA was <0.2 ng/mg) proteins by intramuscular injection. The recombinant proteins were formulated at a ratio of 9:1 with aluminium hydroxide gel (AHG; Alhydrogel) (100 μL of 2% AHG per 900 μL of antigen). These treatments were administered to mice on days 0, 14, and 28. Blood samples were drawn from the tail vein on days 0, 21, and 35.
Furthermore, to produce polyclonal antibodies, New Zealand rabbits (n = 3) were immunized by intradermal injection with rAdsA (250 μg of purified protein) adsorbed to AHG (2 mg/mL) on days 0, 21, and 42. The rAdsA antibody titer was detected by enzyme-linked immunosorbent assay (ELISA) as described elsewhere.
Passive Immunization
Rabbit immune sera (200 μL) were injected into the tail vein of 8-week-old BALB/C mice 24 hours prior to challenge with S. aureus infection according to the skin infection or peritonitis models. Control mice were injected with the same volume of sera from rabbits that had been immunized with PBS and aluminum hydroxide.
Murine Skin Infection Model
Experiments using the mouse skin infection model were performed as previously described [28]. Female BALB/c mice were anesthetized with isoflurane ketamine (100 mg/kg) and xylazine (5 mg/kg) and inoculated by subcutaneous injection in the right shaved flank with 1 × 107 CFU S. aureus USA300 or Newman strain in 50 μL PBS. Mass and abscess formation (size and dermonecrosis) were monitored at 24 hours intervals over a course of 2 weeks. The size of an abscess and associated overlying dermonecrotic lesion was determined using a standard formula for area [A = (π/2) × l × w].
Peritonitis Infection Model
We used a previously described mouse peritonitis model [28]. Active (10 days after second immunization) or passive immunized BALB/C mice were challenged by intraperitoneal injection of S. aureus. Mice were infected with 1 × 108 CFU of S. aureus (specific inoculum varied depending on the challenge strain) and monitored daily for 14 days.
Blood Infection Model
Active (10 days after second immunization) or passive immunized BALB/C mice were challenged by intravenous injection of S. aureus [17, 28]. Mice were infected with 5 × 107 CFU of S. aureus and monitored daily for 14 days.
S. aureus Infection In Vitro
RAW264.7 cells were seeded on 24-well culture plates at 1 × 106 cell/well in RPMI Medium 1640 supplemented with 10% (v/v) fetal bovine serum. Cells were treated with 20 μL AdsA-specific rabbit antisera and infected simultaneously with S. aureus at multiplicity of infection 10. After incubation for 60 and 120 minutes, the extracellular and intracellular bacteria were enumerated by serial dilution and spread on BHI agar plates.
Bacterial Survival in Blood
Whole blood was collected by cardiac puncture of BALB/c mice, and 5 μg/mL of lepirudin anticoagulant was immediately added. One hundred microliters of 106 CFU/mL of S. aureus strain USA300 was mixed with 900 μL of BALB/C mouse blood. The tubes were incubated at 37°C with slow rotation for the time points indicated in the figures, at which time aliquots were incubated on ice for 30 minutes in a final concentration of 0.5% saponin/PBS to lyse eukaryotic cells. Dilutions of staphylococci were plated on BHI agar plates for enumeration of surviving CFUs.
Flow Cytometry
Flow cytometry was used to detect the apoptotic and necrotic rate. RAW264.7 (1 × 106) cells were treated with AdsA-specific rabbit antisera or normal rabbit serum and simultaneously infected with S. aureus strain USA300 (10 MOI). After the RAW264.7 cells had been exposed to S. aureus strain USA300 for 3 hours in vitro, Then the cells were harvested and incubated with Annexin V conjugated to green-fluorescent FITC dye and propidium iodide (PI), and then analyzed at 525 nm for FITC and at 630 nm for PI using BD Accuri C6 flow cytometer (BD).
Statistical Analysis
Log-rank (Mantel-Cox) analysis was used to analyze the statistical significance of the data from the lethal challenge experiment. Analyses were performed using GraphPad Prism 5 (GraphPad Software, United States) and a P value <.05 was determined to be statistically significant.
Research Ethics
SPF BALB/c mice and New Zealand rabbits were supplied by the Laboratory Animal Unit of the University of Hong Kong. All animal experiments were approved by the Committee on the Use of Live Animal in Teaching and Research of the University of Hong Kong (CULATR 2596-11).
RESULTS
Antibody Quantification
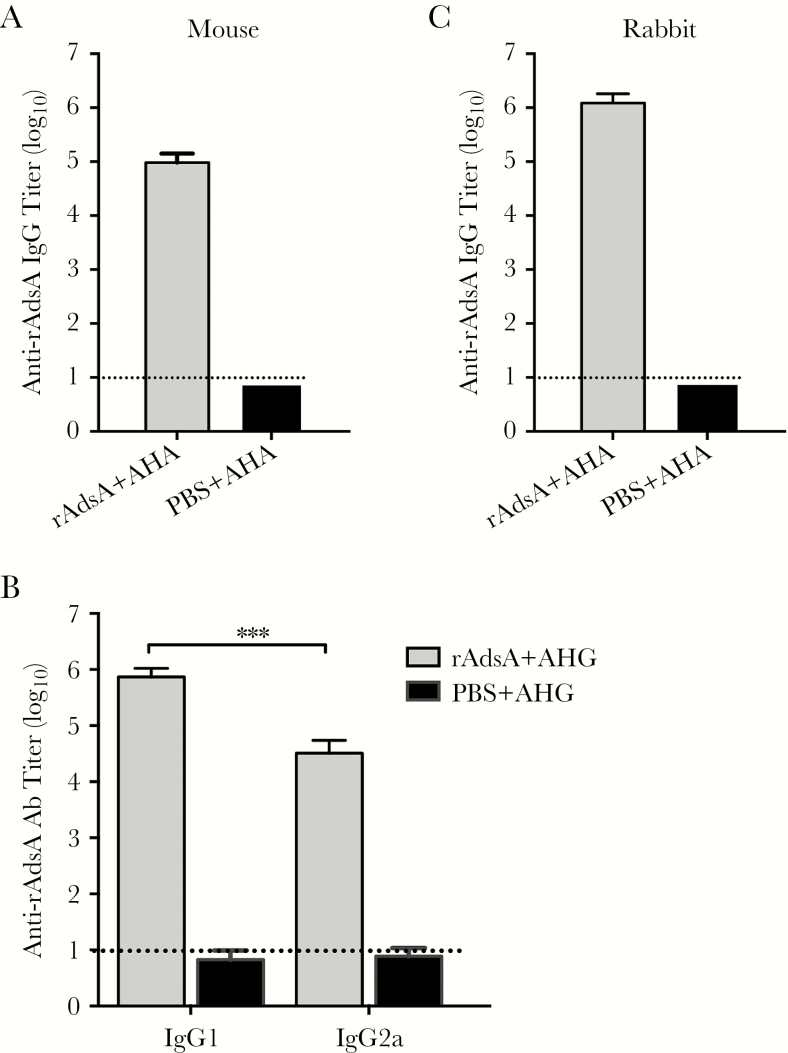
To investigate the antibody titers of AdsA-specific antibodies in the sera of immunized mice, the levels of immunoglobulin G (IgG), IgG1, and IgG2a antibodies were detected by ELISA 7 days after the third immunization. The data showed that immunization with the AdsA recombinant protein induced high levels of anti-AdsA IgG (>1:105; day 42) (Figure 1A). Furthermore, immunization with AdsA in the presence of AHG elicited both T helper 1– and T helper 2–associated AdsA-specific IgG2a and IgG1 antibody responses (Figure 1B). IgG, IgG1, and IgG2a specific for AdsA were not detected in the serum of mice mock immunized with PBS plus AHG. In addition, ELISA of rabbit serum samples was obtained after the second boost (day 42). As shown in Figure 1C, immunization with AdsA induced high levels of anti-AdsA IgG (>1:107) in a rabbit model.
Figure 1.
Adenosine synthase A (AdsA)–specific antibody responses in immunized mice or rabbit. The lower limit of detection (1:10) is shown as dotted lines. The experiment was repeated at least twice. A, AdsA-specific immunoglobulin G (IgG) antibody responses in mouse sera collected at 7 days after the third vaccination. B, AdsA-specific IgG1 and IgG2a antibody responses. C, AdsA -specific antibody responses in rabbit sera obtained 7 days after the third immunization. ***P < .001. Abbreviations: Ab, antibody; AdsA, adenosine synthase A; AHA, xxx; AHG, aluminum hydroxide gel; IgG, immunoglobulin G; PBS, phosphate-buffered saline.
Immunization With AdsA Induces Consistent Protective Immunity Against 2 Staphylococcal Strains in 3 Mouse Models
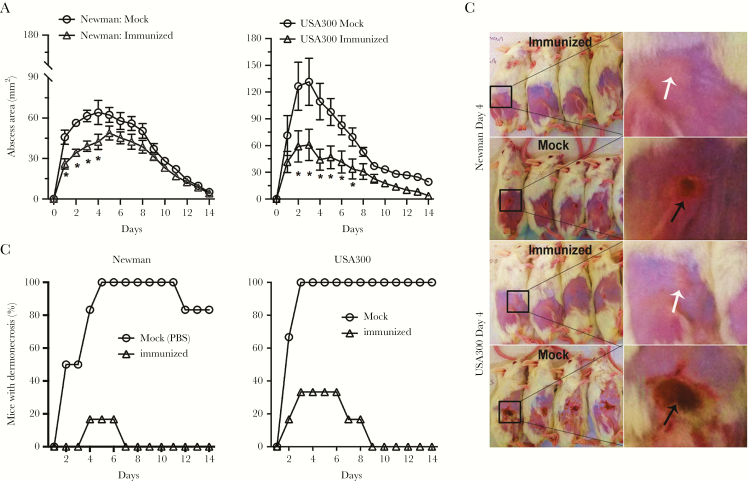
To assess the protective efficacy of AdsA against different S. aureus disease outcomes, we used a skin infection model. In the skin infection model, mice were inoculated by subcutaneous injection in the shaved right flank with S. aureus Newman or USA300 strain. In both the vaccinated and control sets of animals, abscess mass and dermonecrotic area were monitored at 24-hour intervals for 14 days. S. aureus abscess size was reduced significantly in mice vaccinated with AdsA (Figure 2A). In addition, there was little or no dermonecrosis in infected mice that had been vaccinated (Figure 2B and 2C), which demonstrates that active immunization with AdsA alleviates the severity of S. aureus skin infections. The protective efficacy of AdsA was further investigated with 2 additional models. First, after immunization, mice were challenged intravenously with a lethal dose of either USA300 or Newman strain, and we followed mouse survival over a period of 14 days. As shown in Figure 3A, the 14 days protection elicited by AdsA ranged from 37.5% (USA300) to 50% (Newman) and was always significantly superior to that observed in mock. Second, we tested the protective efficacy of AdsA in the peritonitis model, challenging mice with a lethal dose of either USA300 or Newman strain, and we also monitored daily for 14 days. In the model, compared with the control mice treated with PBS + AHG, mice vaccinated with combined AdsA + AHG had significant protective immunity to Newman (62.5%; P = .011, log-rank Mantel-Cox test) and USA300 (50%; P = .017, log-rank Mantel-Cox test) S. aureus strains (Figure 3B).
Figure 2.
Active immunization with adenosine synthase A (AdsA) decreases the size of abscesses caused by USA300 or Newman strains of S. aureus. Mice were injected intramuscularly with aluminum hydroxide gel (AHG) plus phosphate-buffered saline (PBS). A, Abscess formation was monitored once per day after subcutaneous infection with 1 × 107 of the indicated bacteria 49 days after primary immunization. Results are the mean value ± standard error of the mean; n = 12 mice per group. *P < .05 vs wild-type USA300 or Newman strains using a 2-way analysis of variance and Bonferroni posttest. B, Percentage of mice per group that had dermonecrosis on each day. P < .001 for mock immunized mice after infection with either Newman or USA300 strains over the 14-day time course. C, Representative mouse skin lesions on day 4. Black arrows indicate dermonecrosis; white arrows indicate abscess formation without dermonecrosis.
Figure 3.
Survival curve of vaccinated BALB/c mice challenged with S. aureus. Mice were challenged intravenously (A) or intraperitoneally (B) with S. aureus Newman and USA300 strains. The results of 2 independent experiments (mice, n = 10) are shown. The P value represents the likelihood of a significant difference between all groups by pairwise log-rank analysis. *P < .05; **P < .01. Abbreviations: AdsA, adenosine synthase A; AHG, aluminum hydroxide gel; i.p., intraperitoneal; i.v., intravenous.
Passive Immunization With AdsA-Specific Antisera Reduces Staphylococcal Infection in Mouse Models
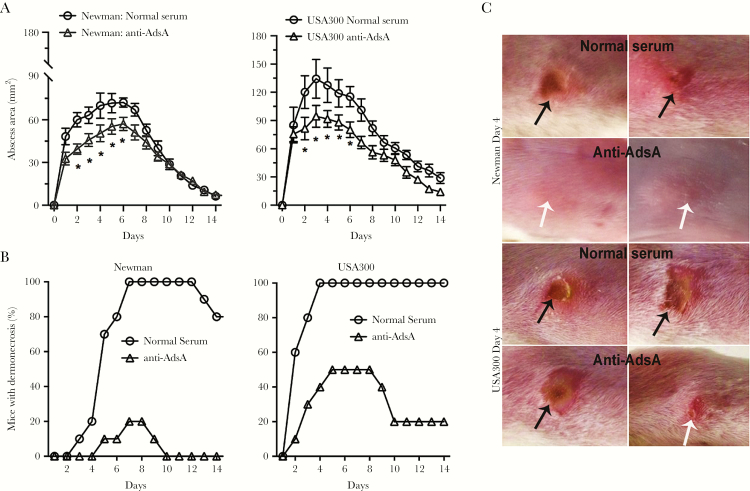
Inasmuch as AdsA contributed to disease severity in the mouse infection model, we further investigated whether passive immunization with AdsA-specific antibody would reduce the severity of S. aureus disease in infected mice. To this aim, 2 rabbits were immunized with AdsA, and subsequently the rabbit serum was used in passive protection experiments. Specifically, 200 μL of serum was administered intraperitoneally to mice, and 2 hours later animals were challenged with S. aureus of either USA300 or Newman strain accordingly in the skin, blood, and peritoneal models. Skin lesions of mice infected with Newman or USA300 strains were significantly smaller after passive immunization with AdsA-specific rabbit antisera, compared with lesions of mice that received preimmune serum samples (Figure 4A). The percentage of dermonecrosis in mice that received the AdsA-specific rabbit antisera was significantly lower than in control animals (Figure 4B and 4C). Likewise, survival of passively immunized mice to the intravenous (Newman: 60% vs 20%; USA300: 30% vs 0) or intraperitoneal (Newman: 50% vs 10%; USA300: 30% vs 0) S. aureus challenge was significantly greater than that of the control group (Figure 5).
Figure 4.
Evaluation of the therapeutic effects of anti–adenosine synthase A (AdsA) rabbit serum in the BALB/c mouse model. Mice were challenged by intravenous injection of S. aureus. After 2 hours, the experimental groups were treated with anti- AdsA rabbit serum, whereas control mice were injected with normal rabbit serum. The P value represents the likelihood of a significant difference between all groups by pairwise log-rank analysis. A, Abscess formation was monitored once per day after subcutaneous infection with 1 × 107 of the indicated bacteria 2 hours after passive immunization. Results are the mean value ± standard error of the mean; n = 12 mice per group. *P < .05 vs wild-type USA300 or Newman strains using a 2-way analysis of variance and Bonferroni posttest. B, Percentage of mice per group that had dermonecrosis on each day. P < .001 for mock-immunized mice after infection with either Newman or USA300 strains over the 14-day time course. C, Representative mouse skin lesions on day 4. Black arrows indicate dermonecrosis; white arrows indicate abscess formation without dermonecrosis.
Figure 5.
Graph of the evaluation of the therapeutic effects of anti–adenosine synthase A (AdsA) rabbit serum in the BALB/c mouse model. Mice were challenged intravenously (i.v.) or intraperitoneally (i.p.) with S. aureus Newman or USA300. After 2 hours, the experimental groups were treated with anti-AdsA rabbit serum, whereas control mice were injected with normal rabbit serum. *P values represent the likelihood of a significant difference between all groups by pairwise log-rank analysis.
AdsA-Specific Antisera Promotes Killing S. aureus by Immune Cell
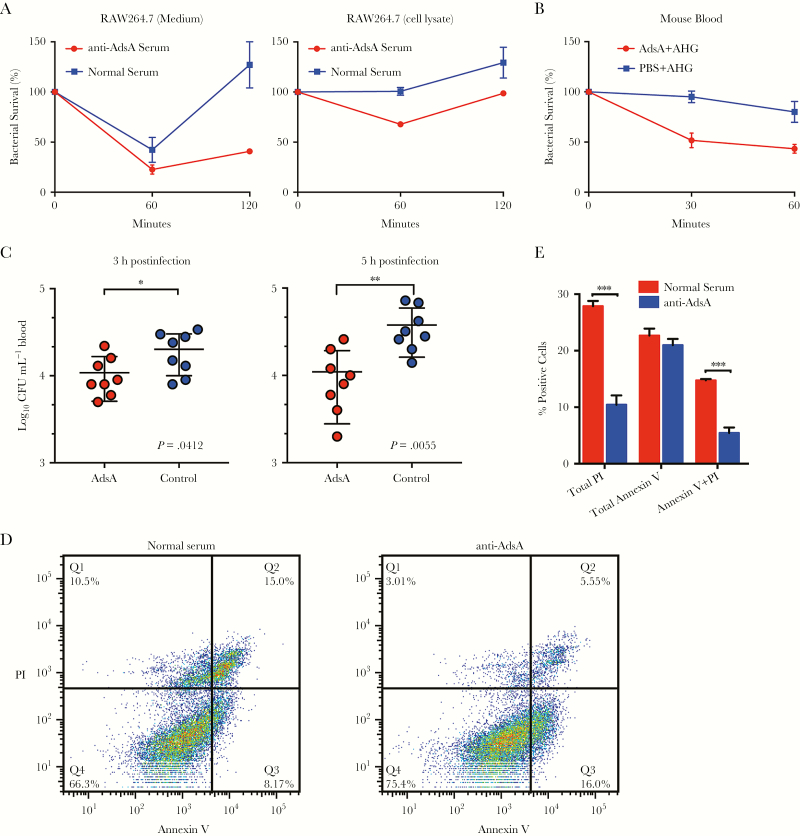
Specially, we examined the protective effect of anti-AdsA antibodies in vitro. A murine macrophage cell line, RAW264.7, was treated with AdsA-specific rabbit antisera and simultaneously infected with S. aureus strain USA300. Extracellular and intracellular bacterial number was enumerated at 2 time points. As shown in Figure 6A and 6B, the anti-AdsA antibody is able to modulate bacterial number in culture medium and invasion into RAW264.7 cells. The results demonstrated that the anti-AdsA antibodies reduce extracellular and intracellular number of S. aureus in murine macrophages. To further identify whether the AdsA-specific antisera functionally affects bacterial–host cell interactions, we detected the ability of S. aureus strain USA300 to survive in whole blood collected from AdsA- or Mock (PBS + AHG)–immunized BALB/c mice by recording bacterial load at timed intervals via the formation of colonies on BHI agar plate. As expected, the blood from Mock-immunized BALB/c mice, which lack AdsA-specific antibodies, was unable to kill the bacteria (Figure 6B). In the contrast to the blood from Mock mice, immunized mice acquiring the AdsA-specific antibodies were blocked in staphylococcal escape from phagocytic killing (P < .05; Figure 6B). To discern whether AdsA-specific antibodies enhance bacterial clearance in the bloodstream, BALB/c mice were infected by intravenous inoculation with 5 × 107 CFU and peripheral blood was enumerated with CFU at 3 and 5 hours postinfection. As shown in Figure 6C, significantly lower CFUs of AdsA-immunized mice were observed at 3 hours (P = .0412) and 5 hours (P = .0055) postinfection as compared with the Mock-immunized group. Altogether, these data suggest that the anti-AdsA antibody can reduce virulence of staphylococci, thereby decreasing the bacteria survival in blood.
Figure 6.
Adenosine synthase A (AdsA)–specific antisera promotes killing Staphylococcus aureus by immune cell. Comparison of the survival of 107 colony-forming units (CFU) of anti-AdsA rabbit serum or normal rabbit serum in RAW264.7 cells (A). To assess the relative contribution of anti-AdsA antibody to block staphylococci survival in blood, 105 CFU of S. aureus strain USA300 was incubated in blood from AdsA-immunized or mock-immunized mice for 60 minutes (B). Data are the mean, and error bars represent the standard deviation (SD) from 2 independent analyses. C, Staphylococcal burden: S. aureus strain USA300 in blood of BALB/c mice (AdsA immunized or mock immunized mice) (n = 8) was measured as log10 CFU per 1 mL of blood retrieved by cardiac puncture at 3 and 5 hours after intravenous challenge with 3.5 × 107 CFU. Horizontal bars represent mean CFUs in each cohort. Data are representative of 2 independent analyses using cohorts of 8 mice per bacterial strain per time point. RAW264.7 cells were treated with AdsA-specific rabbit antisera or normal rabbit serum and simultaneously infected with S. aureus strain USA300 for 3 hours, and then analyzed by anti–Annexin V, and PI staining. D, Representative contour plots showing exposed cell populations analyzed by Annexin V and propidium iodide (PI) staining. E, Annexin V, PI, and dual-positive cells were quantified. For E, results show mean and SD for 2 independent experiments. *P < .05; **P < .01; ***P < .0001, unpaired Student t test. Abbreviations: AdsA, adenosine synthase A; AHG, aluminum hydroxide gel; CFU, colony-forming units; PBS, phosphate-buffered saline; PI, propidium iodide.
Moreover, to examine whether inhibited AdsA activity reduces cell damage correlated with levels of apoptosis and necrosis, RAW264.7 cells were treated with AdsA-specific rabbit antisera and simultaneously infected with S. aureus strain USA300. After the RAW264.7 cells had been exposed to S. aureus strain USA300 for 3 hours in vitro, we analyzed cells for apoptosis, namely externalization of phospholipids (an early event detected by Annexin V) and necrosis (permeability to PI). As shown in Figure 6D, the percentage of normal rabbit serum–treated cells (healthy cells: ∼66%) was significantly more cell death than anti-AdsA–treated cells (healthy cells: ∼75%); Additionally, the anti-AdsA–treated group had low proportion of Annexin V and PI dual-positive cells (late apoptotic or secondary necrotic,∼5.5%) and PI-positive cells (necrotic, ∼3%) (Figure 6D and 6E). Interestingly, the anti-AdsA–treated group had the highest proportion of Annexin V–positive cells (early apoptotic: ∼16%) compared with normal rabbit serum–treated cells (early apoptotic: ∼8%) (Figure 6D and 6E). Overall, these results indicate that the anti-AdsA antibody may delay apoptosis and protect necrosis during S. aureus infection.
DISCUSSION
S. aureus is a leading cause of skin and soft tissue infections, sepsis, bacteremia, osteomyelitis, meningitis, pneumonia, and endocarditis [23]. A large proportion of cases and deaths have been attributed to the ability of S. aureus to escape innate responses. In mammals, adenosine plays a key role in regulating innate and acquired immune responses [29]. Strong or superabundant host immune responses exacerbate the tissue damage caused by invading pathogens [29] such as S. aureus infection. Successful immune clearance of pathogens involves the balancing of proinflammatory and anti-inflammatory cytokines. The cytokines interleukin (IL) 13, IL-10, IL-4, and transforming growth factor–β function to restrict strong inflammation, whereas adenosine has the ability to totally suppress the immune response [30]. Phagocytes express 4 adenosine transmembrane receptors according to their activation state: A1AR, A2aAR, A2bAR, and A3AR [31]. Adenosine, mediated by A2aAR in monocytes [32] and dendritic cells [33], inhibits proinflammatory cytokine production, particularly IL-12 and tumor necrosis factor–α, while increasing the level of the anti-inflammatory cytokine IL-10. Extracellular adenosine is necessary for the regulation of inflammation, but excess production of adenosine is also harmful as in S. aureus infections [22]. S. aureus AdsA produces excessive adenosine, which disrupts the balance of the proinflammatory and anti-inflammatory response. S. aureus survival in PMNs depends on adenosine receptor–mediated signaling. In addition, adenosine may also suppress adaptive immune responses by interfering with the antigen-presenting cells to present S. aureus antigens [34].
Bacterial invasion of host tissues triggers neutrophil extracellular traps (NETs), thereby immobilizing pathogens or subsequent clearance. However, AdsA also has an ability to modulate immune responses following the degradation of NETs by promoting macrophage apoptosis [25]. Although a great number of infiltrated neutrophils migrate to the infection sites, host immunity may not be able to eliminate S. aureus from abscess lesions and eventually succumb to persistent infection. The strong impact that AdsA has on the development of S. aureus persistent infection disease identifies the protein as a promising target for drug development. Two routes using AdsA as immunotherapy targets can be envisaged. First, AdsA could be used as an antigen in active vaccination approaches. Second, AdsA could be targeted by antibodies. To test this hypothesis, we decided to use 3 mouse infection models to test AdsA as a candidate antigen for S. aureus vaccine. We showed that mice immunized with AdsA plus AHG adjuvant was protected against S. aureus skin and lethal infection. Although AdsA could decrease the severity of S. aureus diseases, it is not clear whether anti-AdsA antibody-biased immune responses conferred this protection. To dissect the possible mechanisms of protection induced by AdsA, we focused our attention on the analysis of polyclonal antibodies. To this aim, rabbits were immunized with AdsA, and subsequently the rabbit serum was used in passive protection experiments. Our results indicated that anti-AdsA specific antibodies can decrease the severity of S. aureus infection in different mouse models. To reveal how the AdsA-specific antibody functionally affects bacterial–host cell interactions, RAW264.7 cells were treated with AdsA-specific rabbit antisera and simultaneously infected with S. aureus strain USA300. The results demonstrated that the Anti-AdsA antibodies can reduce extracellular and intracellular numbers of S. aureus in murine macrophages. AdsA is required for staphylococcal survival in blood [22]. Therefore, we also examined the ability of anti-AdsA–specific antibodies to block staphylococcal escape from phagocytic killing. As expected, the AdsA-specific antisera not only rescues phagocytic killing of immune cells, but also enhances the bacterial clearance in the bloodstream. S. aureus can cause liquefaction necrosis, AMP levels are expected to increase as a consequence of staphylococcal toxins (eg, leukocidins, α-hemolysin) that form pores in membranes and precipitate cellular lysis [35]. Furthermore, production of dAdo by AdsA is sufficient to induce apoptosis of macrophages via the activation of caspase-3 [25]. Our results suggest that the anti-AdsA antibody may protect necrosis and delay apoptosis during S. aureus infection.
The active and passive immunization data presented here suggest that AdsA is a potential antigen for vaccines or therapeutics designed to alleviate the severity of S. aureus infections. Inasmuch as AdsA is present in all strains and highly conserved, an immunotherapeutic approach directed at AdsA would be relatively broad in scope.
Notes
Author contributions. B. Z. Z., J. D. H., and K.-Y. Y. designed the experiment; B. Z. Z. performed the experiments; J. P. C., B. Y., L. F. X., Q. B. L., C. X., and S. Y. Z. participated in the study; B. Z. Z. and J.-D. H. wrote the manuscript. B. Z. Z., J.-D. H., and K.-Y. Y. analyzed the data; R. K., K. S., and K.-Y. Y. provided essential reagents and critical comments.
Acknowledgments. The authors thank the technicians of J.D.H. and K.-Y.Y. laboratory for their help in offering the resources in running the project.
Financial support. This work was supported by the Research Fund for the Control of Infectious Diseases Commissioned Study of Food and Health Bureau of Hong Kong Government (HK-09-01-21); Health and Medical Research Fund (HKM-15-M09, 14130742); a National Basic Research Program of China (973 Program, 2014CB745200) from the Ministry of Science and Technology of the People’s Republic of China, and by Shenzhen Peacock project (KQTD2015033117210153).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Tacconelli E, Tumbarello M, Cauda R. Staphylococcus aureus infections. N Engl J Med 1998; 339:2026–7. [PubMed] [Google Scholar]
- 2. Conde A. Staphylococcus aureus infections. N Engl J Med 1998; 339:2026; author reply 2027. [PubMed] [Google Scholar]
- 3. Blot S, Vandewoude K, Colardyn F. Staphylococcus aureus infections. N Engl J Med 1998; 339:2025–6; author reply 6–7. [DOI] [PubMed] [Google Scholar]
- 4. Lowy FD. Staphylococcus aureus infections. N Engl J Med 1998; 339:520–32. [DOI] [PubMed] [Google Scholar]
- 5. Gould IM. The clinical significance of methicillin-resistant Staphylococcus aureus. J Hosp Infect 2005; 61:277–82. [DOI] [PubMed] [Google Scholar]
- 6. Schaffer AC, Lee JC. Vaccination and passive immunisation against Staphylococcus aureus. Int J Antimicrob Agents 2008; 32(suppl 1):S71–8. [DOI] [PubMed] [Google Scholar]
- 7. Stranger-Jones YK, Bae T, Schneewind O. Vaccine assembly from surface proteins of Staphylococcus aureus. Proc Natl Acad Sci U S A 2006; 103:16942–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bubeck Wardenburg J, Schneewind O. Vaccine protection against Staphylococcus aureus pneumonia. J Exp Med 2008; 205:287–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yin RL, Li C, Yang ZT et al. Construction and immunogenicity of a DNA vaccine containing clumping factor A of Staphylococcus aureus and bovine IL18. Vet Immunol Immunopathol 2009; 132:270–4. [DOI] [PubMed] [Google Scholar]
- 10. Jonsson IM, Mazmanian SK, Schneewind O, Verdrengh M, Bremell T, Tarkowski A. On the role of Staphylococcus aureus sortase and sortase-catalyzed surface protein anchoring in murine septic arthritis. J Infect Dis 2002; 185:1417–24. [DOI] [PubMed] [Google Scholar]
- 11. Inskeep TK, Stahl C, Odle J et al. Oral vaccine formulations stimulate mucosal and systemic antibody responses against staphylococcal enterotoxin B in a piglet model. Clin Vaccine Immunol 2010; 17:1163–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim HK, Cheng AG, Kim HY, Missiakas DM, Schneewind O. Nontoxigenic protein A vaccine for methicillin-resistant Staphylococcus aureus infections in mice. J Exp Med 2010; 207:1863–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Arrecubieta C, Matsunaga I, Asai T, Naka Y, Deng MC, Lowy FD. Vaccination with clumping factor A and fibronectin binding protein A to prevent Staphylococcus aureus infection of an aortic patch in mice. J Infect Dis 2008; 198:571–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim HK, DeDent A, Cheng AG et al. IsdA and IsdB antibodies protect mice against Staphylococcus aureus abscess formation and lethal challenge. Vaccine 2010; 28:6382–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Roth DM, Senna JP, Machado DC. Evaluation of the humoral immune response in BALB/c mice immunized with a naked DNA vaccine anti-methicillin-resistant Staphylococcus aureus. Genet Mol Res 2006; 5:503–12. [PubMed] [Google Scholar]
- 16. Gaudreau MC, Lacasse P, Talbot BG. Protective immune responses to a multi-gene DNA vaccine against Staphylococcus aureus. Vaccine 2007; 25:814–24. [DOI] [PubMed] [Google Scholar]
- 17. Zhang BZ, Hua YH, Yu B et al. Recombinant ESAT-6-like proteins provoke protective immune responses against invasive Staphylococcus aureus disease in a murine model. Infect Immun 2015; 83:339–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Menzies BE, Kernodle DS. Site-directed mutagenesis of the alpha-toxin gene of Staphylococcus aureus: role of histidines in toxin activity in vitro and in a murine model. Infect Immun 1994; 62:1843–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Spellberg B, Ibrahim AS, Yeaman MR et al. The antifungal vaccine derived from the recombinant N terminus of Als3p protects mice against the bacterium Staphylococcus aureus. Infect Immun 2008; 76:4574–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brown EL, Dumitrescu O, Thomas D et al. The Panton-Valentine leukocidin vaccine protects mice against lung and skin infections caused by Staphylococcus aureus USA300. Clin Microbiol Infect 2009; 15:156–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kuklin NA, Clark DJ, Secore S et al. A novel Staphylococcus aureus vaccine: iron surface determinant B induces rapid antibody responses in rhesus macaques and specific increased survival in a murine S. aureus sepsis model. Infect Immun 2006; 74:2215–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thammavongsa V, Kern JW, Missiakas DM, Schneewind O. Staphylococcus aureus synthesizes adenosine to escape host immune responses. J Exp Med 2009; 206:2417–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thammavongsa V, Kim HK, Missiakas D, Schneewind O. Staphylococcal manipulation of host immune responses. Nat Rev Microbiol 2015; 13:529–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thammavongsa V, Schneewind O, Missiakas DM. Enzymatic properties of Staphylococcus aureus adenosine synthase (AdsA). BMC Biochem 2011; 12:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thammavongsa V, Missiakas DM, Schneewind O. Staphylococcus aureus degrades neutrophil extracellular traps to promote immune cell death. Science 2013; 342:863–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Miller KD, Hetrick DL, Bielefeldt DJ. Production and properties of Staphylococcus aureus (strain Newman D2C) with uniform clumping factor activity. Thromb Res 1977; 10:203–11. [DOI] [PubMed] [Google Scholar]
- 27. Seybold U, Kourbatova EV, Johnson JG et al. Emergence of community-associated methicillin-resistant Staphylococcus aureus USA300 genotype as a major cause of health care-associated blood stream infections. Clin Infect Dis 2006; 42:647–56. [DOI] [PubMed] [Google Scholar]
- 28. Bagnoli F, Fontana MR, Soldaini E et al. Vaccine composition formulated with a novel TLR7-dependent adjuvant induces high and broad protection against Staphylococcus aureus. Proc Natl Acad Sci U S A 2015; 112:3680–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thiel M, Caldwell CC, Sitkovsky MV. The critical role of adenosine A2A receptors in downregulation of inflammation and immunity in the pathogenesis of infectious diseases. Microbes Infect 2003; 5:515–26. [DOI] [PubMed] [Google Scholar]
- 30. Németh ZH, Csóka B, Wilmanski J et al. Adenosine A2A receptor inactivation increases survival in polymicrobial sepsis. J Immunol 2006; 176:5616–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Haskó G, Pacher P. A2A receptors in inflammation and injury: lessons learned from transgenic animals. J Leukoc Biol 2008; 83:447–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Khoa ND, Montesinos MC, Reiss AB, Delano D, Awadallah N, Cronstein BN. Inflammatory cytokines regulate function and expression of adenosine A(2A) receptors in human monocytic THP-1 cells. J Immunol 2001; 167:4026–32. [DOI] [PubMed] [Google Scholar]
- 33. Panther E, Idzko M, Herouy Y et al. Expression and function of adenosine receptors in human dendritic cells. FASEB J 2001; 15:1963–70. [DOI] [PubMed] [Google Scholar]
- 34. Kim HK, Thammavongsa V, Schneewind O, Missiakas D. Recurrent infections and immune evasion strategies of Staphylococcus aureus. Curr Opin Microbiol 2012; 15:92–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Diep BA, Otto M. The role of virulence determinants in community-associated MRSA pathogenesis. Trends Microbiol 2008; 16:361–9. [DOI] [PMC free article] [PubMed] [Google Scholar]