High and multiple resistance to insecticides are recorded in the 2 main malaria vectors in the Democratic Republic of the Congo, leading to a significant loss of efficacy of conventional bed nets in the presence of alarmingly high Plasmodium infection rate, suggesting high malaria transmission.
Keywords: malaria, Plasmodium falciparum, insecticide resistance, Anopheles, Democratic Republic of Congo
Abstract
Accounting for approximately 11% of all malaria cases, the Democratic Republic of the Congo (DRC) is central to malaria elimination efforts. To support vector control interventions in DRC, we characterized the dynamics and impact of insecticide resistance in major malaria vectors in 2015. High Plasmodium infection rates were recorded in Anopheles gambiae and Anopheles funestus, with Plasmodium falciparum predominant over Plasmodium malariae. Both mosquito species exhibited high and multiple resistance to major public health insecticide classes. The extremely high resistance to permethrin and DDT (dichlorodiphenyltrichloroethane) in An. gambiae (low mortalities after 6 hours exposure) is worrisome, and is supported by a reduced insecticidal effect of bed nets against both mosquito species in laboratory tests. Metabolic and target site insensitivity mechanisms are driving this resistance in An. gambiae, but only the former was observed in An. funestus. These findings highlight the urgent need for actions to prolong the effectiveness of insecticide-based interventions in DRC.
With 11% of all worldwide malaria cases occurring in the Democratic Republic of the Congo (DRC) [1], this country is key for malaria elimination. Control efforts in DRC extensively rely on the use of long-lasting insecticidal nets (LLINs). However, the development of insecticide resistance is threatening the effectiveness of this control tool, calling for urgent action to implement suitable resistance management strategies [2]. Unfortunately, the scarcity of information on the extent and impact of resistance prevents the design of such strategies in DRC.
Resistance to pyrethroids and organochlorines has previously been reported in DRC, although exclusively in the major malaria vector Anopheles gambiae [1, 3, 4]. Nevertheless, the extent and intensity of this resistance remain unclear and completely unknown for other major vectors such as Anopheles funestus. Additionally, the resistance profile to other insecticide classes has not been clearly established, limiting the ability of national malaria control programs to make informed decisions for resistance management. Indeed, so far, only limited evidence of resistance to carbamates and organophosphates has been reported in An. gambiae from DRC [1], with the exception of possible resistance to malathion observed in a single locality [3]. Furthermore, it also remains unclear how pyrethroid resistance in malaria vectors currently impacts the efficacy of both conventional and piperonyl butoxide (PBO)–based LLINs, although a low efficacy has been reported previously for the OlysetNet [5].
Limited investigation of resistance mechanisms in DRC has detected the presence of the kdr mutation in An. gambiae in Kinshasa [5]. The role of metabolic resistance has not been explored further. To support and facilitate the success of the ongoing and future vector control programs in DRC, the present study extensively evaluated the current insecticide resistance profile of An. gambiae and An. funestus, the 2 main malaria vectors in Kinshasa, the capital city. Furthermore, the efficacy of several LLINs was assessed, in addition to the molecular mechanisms driving resistance. Additionally, the Plasmodium infection rate in malaria vectors was also assessed.
METHODS
Study Area and Mosquito Sampling
Indoor-resting mosquitoes were collected using electrical aspirators from 10–15 households each day for a week at Ndjili-Brasserie, a suburb of Kinshasa (4°19′39″S, 15°18′48″E), in May 2015. This collection site was chosen due to the presence of suitable breeding sites for the 2 main malaria vectors, An. gambiae and An. funestus, through the Ndjili River and its flooded shores. Blood-fed and half-gravid female Anopheles, morphologically identified as belonging to the An. funestus or An. gambiae complex [6], were kept in holding cages for 4–6 days and forced to lay eggs individually during 2–3 extra days [7]. One hundred thirty-three An. gambiae sensu lato (s.l.) and 79 An. funestus s.l. laid eggs. F0 dead females and F1 eggs were transported to the Liverpool School of Tropical Medicine for the subsequent analysis under a license from the Department for Environment, Food and Rural Affairs (DEFRA) (PATH/125/2012, UK).
Species Identification
Genomic DNA from 111 An. gambiae s.l. and 81 F0An. funestus s.l. whole female mosquitoes was extracted, using the Livak protocol [8], and identified to species level using a cocktail polymerase chain reaction (PCR) assay [9, 10]. Larvae were transferred to plastic trays according to species group for rearing, as previously described [7, 11].
Plasmodium Infection Rate
The Plasmodium infection rate was estimated using the mosquito’s whole body extracts by detecting the presence of Plasmodium falciparum (F+) and/or Plasmodium ovale, Plasmodium vivax, and Plasmodium malariae (OVM+) in 109 An. gambiae s.l. and 81 An. funestus sensu stricto (s.s.) field-collected F0 females individually using the TaqMan assay, as previously described [12, 13]. Results of TaqMan assay were confirmed by performing a nested PCR assay as previously described [14].
Insecticide Susceptibility Assays
World Health Organization (WHO) tube bioassays were performed using the F1 generation to assess the insecticide resistance profile of An. gambiae s.l. and An. funestus s.s. following WHO guidelines [15]. Insecticides tested include permethrin (0.75%), deltamethrin (0.05%), bendiocarb (0.1%), dichlorodiphenyltrichloroethane (DDT) (4%), malathion (5%), and fenitrothion (1%). Due to a low number of An. funestus s.s. F1 obtained, only males were tested for malathion and fenitrothion. Additionally, An. gambiae s.l. mosquitoes were exposed to λ-cyhalothrin (0.05%), etofenprox (0.05%), propoxur (0.1%), dieldrin (4%), and pirimiphos-methyl (1%). Control mosquitoes were exposed to papers impregnated only with insecticide carrier oil. The mortality rates were determined 24 hours later. Efficacy of all insecticide-impregnated papers was confirmed using the An. gambiae Kisumu susceptible laboratory strain.
WHO tube bioassays were used to assess the mortality of An. gambiae s.l. after extended periods (3 and 6 hours) of exposure to DDT and deltamethrin. Furthermore, in addition to the 60-minute exposure described above, mortality after 30- and 90-minute exposures to bendiocarb was also assessed. The mortality rates were determined 24 hours later.
Synergist Assays
Synergist assays were performed with PBO using An. gambiae s.l. Four replicates of 20–25 adult mosquitoes (2–5 days old) were preexposed using WHO tube bioassays with PBO-impregnated papers (4 %) for 1 hour. Thereafter, the mosquitoes were immediately exposed to permethrin (0.75%), deltamethrin (0.05%), or DDT (4%) for 60 minutes. Control assays using only 4% PBO papers for 60 minutes were also performed. Mortality was scored after 24 hours, and the results obtained were compared with the mortality without PBO exposure using unpaired Student t test.
Insecticide-Treated Bed Net Efficacy
The efficacy of conventional bed nets against both mosquito populations was estimated by 3-minute exposure cone bioassays following the WHO guidelines with minor modifications, with respect to the number of pieces of net and the number of mosquito per replicate tested [16]. In brief, 5 replicates of 10 F1 females (2–5 days old) were placed in plastic cones attached to different commercially produced nets newly purchased: OlysetNet, OlysetPlus, Permanet 2.0, Permanet 3.0-side and -roof, and an untreated net (as a control). Due to the low number of F1 mosquitoes available, only 1 piece per type of net was tested. After exposure, the mosquitoes were placed in holding paper cups with cotton soaked in 10% sugar solution. Mortality was recorded 24 hours after exposure.
Polymorphism Analysis of the Voltage-Gated Sodium Channel Gene in An. gambiae
To assess the role of target-site knockdown resistance in An. gambiae s.l., 111 F0 female field-collected mosquitoes were genotyped for the L1014F and L1014S kdr mutations by TaqMan assay as previously described [17]. Furthermore, the genetic diversity of the voltage-gated sodium channel (VGSC) gene was investigated for An. gambiae. A fragment of intron 19 of the VGSC gene spanning a portion of exon 20 (including the 1014 codon associated with kdr) was amplified in 11 field-collected An. gambiae s.s. females, cleaned, and sequenced as previously described [7, 18]. Sequences were aligned using ClustalW [19], whereas haplotype reconstruction and polymorphism analysis were done using DnaSPv5.10 [20]. DRC haplotypes were compared to the 4 kdr haplotypes previously detected across Africa as containing either the 1014F (H1/H4) or the 1014S (H2/H3) mutations [21], as well as to a susceptible haplotype from Cameroon [22]. All DNA sequences have been submitted to GenBank (accession numbers KY700707–KY700728).
Genotyping of Resistance Markers in An. funestus
The role of the An. funestus s.s. L119F-GSTe2 and A296S-RDL resistant markers, involved in DDT/permethrin and dieldrin resistance, respectively, was also evaluated by TaqMan assay, genotyping 66 F0 female mosquitoes collected from the field. L119F-GSTe2 and A296S-RDL TaqMan reactions were performed as previously described [23, 24].
Transcription Profile of Resistance Genes in An. funestus
Total RNA was extracted from 3 batches of 10 adults from F1 female An. funestus s.s. mosquitoes nonexposed to insecticides and the FANG susceptible strain, as previously described [24, 25]. The expression patterns of key resistance genes including CYP6P9a, CYP6P9b, CYP6M7, and GSTe2 were assessed by quantitative reverse-transcription PCR (qRT-PCR) [24]. After normalization with housekeeping genes Actin (AFUN006819) and RSP7 (AFUN007153-RA), the relative expression for each gene was calculated according to the 2-ΔΔCT method [26]. The statistical significance between gene expression estimates was performed using unpaired Student t test.
RESULTS
All An. funestus group F0 mosquitoes (81) were confirmed to belong to An. funestus s.s., whereas the majority of a subsample of 100 F0An. gambiae s.l. tested were An. gambiae s.s. (95 of 100), with a low percentage of Anopheles coluzzii (5 of 100 [5%]).
Plasmodium Infection Rate
Using TaqMan assays, 38 An. gambiae s.l. mosquitoes were found to be infected only with P. falciparum (F+; 34.9% [38/109]), whereas 3 mosquitoes were infected with P. ovale, P. vivax, and/or P. malariae (OVM+; 2.8% [3/109]); 4 mosquitoes were coinfected (F+/OVM+; 3.7% [4/109]). The nested PCR confirmed 31 F+ positive mosquitoes and that 2 F+/OVM+ mosquitoes were coinfected with P. falciparum and P. malariae. However, the nested PCR failed to confirm 8 F+ positive mosquitoes, the 3 OVM+ and 2 F+/OVM+ TaqMan-positive mosquitoes, probably because of the lower sensitivity of this method [12].
In the case of An. funestus s.s., 18 mosquitoes were infected only with P. falciparum (22.2% [18/81]), 3 mosquitoes were infected with OVM+ (3.7% [3/81]), and 4 mosquitoes were coinfected: F+/OVM+ (4.9% [4/81]). The nested PCR confirmed all P. falciparum–positive infections of An. funestus s.s. and established that the 3 OVM+ and 3 F+/OVM+ mosquitoes were infected/coinfected with P. malariae, while only 1 F+/OVM+ mosquito was coinfected with P. ovale.
Insecticide Susceptibility Assays
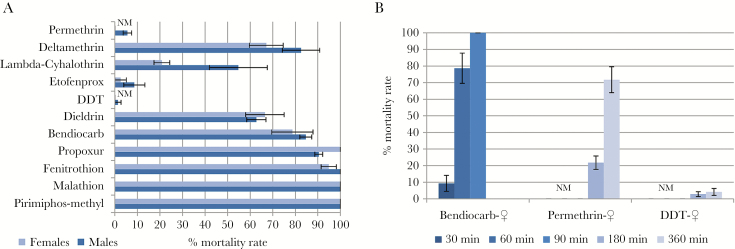
The An. gambiae s.l. population exhibited high resistance to permethrin (type I pyrethroid; 0% mortality), DDT (organochlorine; 0%), the nonester pyrethroid etofenprox (2.6% ± 2.6%), and the type II pyrethroid λ-cyhalothrin (20.9% ± 3.5%), but moderate resistance to the type II pyrethroid deltamethrin (67.2% ± 7.5%) (Figure 1A). Resistance was also observed against the organochlorine dieldrin (66.6% ± 8.5% mortality). Full susceptibility was obtained with the organophosphates malathion and pirimiphos-methyl. An. gambiae s.l. females were fully susceptible to the carbamate propoxur, whereas males were slightly resistant (90.4% ± 1.7% mortality). Resistance of An. gambiae s.l. to bendiocarb, an insecticide commonly used in indoor residual spraying, was also assessed showing a low mortality level after a 30-minute (9.3% ± 4.8%) and 60-minute (78.7% ± 9.2%) exposure, but full susceptibility at a 90-minute exposure. Due to the high resistance observed with permethrin and DDT, bioassays with exposure times of 3 and 6 hours were performed (Figure 1B). Surprisingly, the mortality barely increased for DDT after 6 hours of exposure (3 hours: 2.8% ± 1.4%; 6 hours: 4.2% ± 2.1%), whereas it increased moderately for permethrin (3 hours: 21.8% ± 4.0%; 6 hours: 71.8% ± 7.8%).
Figure 1.
Susceptibility profile of Anopheles gambiae sensu lato population in Kinshasa using World Health Organization insecticide susceptibility tube assays with 60 minutes of exposure (A) and for females at different time points (B). Error bars represent standard error of the mean. Abbreviations: DDT, dichlorodiphenyltrichloroethane; NM, no mortality.
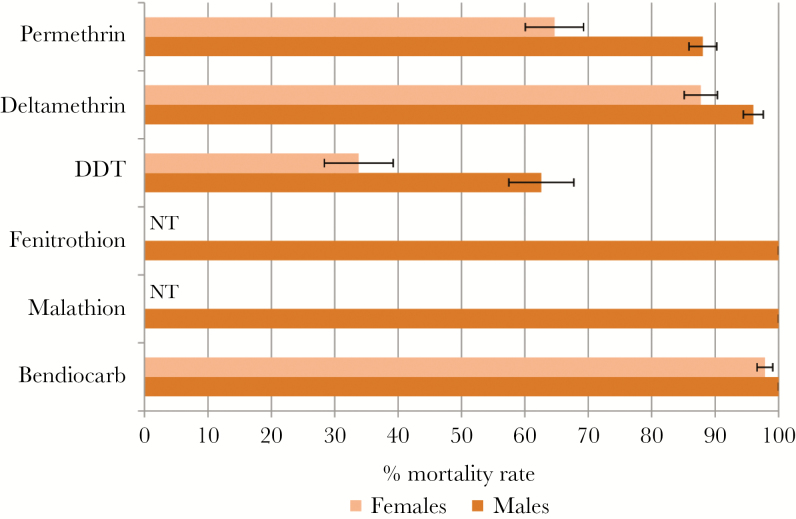
An. funestus s.s. was resistant to DDT (33.8% ± 5.4% mortality) and to permethrin (64.7% ± 4.6%), moderately resistant to deltamethrin (87.7% ± 2.6%), and nearly susceptible to bendiocarb (97.9% ± 1.2%). Only males were tested for fenitrothion and malathion, with a full susceptibility observed (Figure 2).
Figure 2.
Susceptibility profile of Anopheles funestus sensu stricto population using World Health Organization insecticide susceptibility tube assays in Kinshasa. Error bars represent standard error of the mean. Abbreviations: DDT, dichlorodiphenyltrichloroethane; NT, not tested.
Synergist Assays
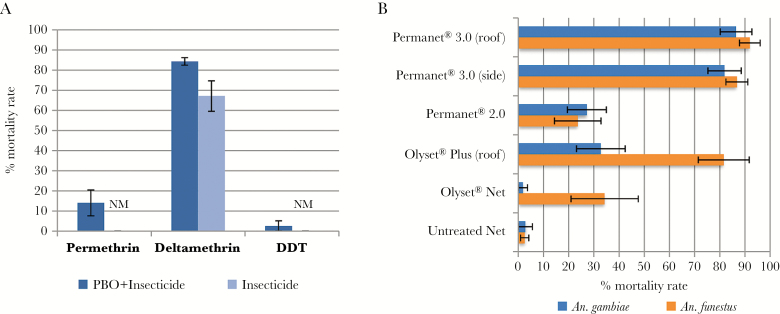
The synergist assay results showed a slight recovery of susceptibility after PBO preexposure for the 2 pyrethroids tested (permethrin: no PBO preexposure [0 ± 0%] mortality vs PBO preexposure [14.0% ± 6.5%], P = .048; deltamethrin: no PBO preexposure [67.1% ± 7.5%] vs PBO preexposure [84.4% ± 1.9%], P = .12), suggesting that cytochrome P450 enzymes may play a minor role in pyrethroid resistance in this population of An. gambiae s.l. (Figure 3A). Tests with DDT also revealed a lack of impact of PBO preexposure with only 2.7% ± 2.7% mortality (P = .29) observed with PBO exposure vs no mortality without exposure, suggesting that DDT resistance is conferred by other mechanisms or gene families. No mortality was observed in control mosquitoes exposed to the synergist PBO only.
Figure 3.
Synergist and bed net efficacy tests. A, Susceptibility profile of Anopheles gambiae sensu lato after synergist assay with piperonyl butoxide. B, Bioefficacy of different commercial long-lasting insecticidal nets against An. gambiae sensu lato and Anopheles funestus sensu stricto. Error bars represent standard error of the mean. Abbreviations: DDT, dichlorodiphenyltrichloroethane; NM, no mortality; PBO, piperonyl butoxide.
Insecticide-Treated Bed Net Efficacy
Cone assays were conducted to evaluate the efficacy of conventional bed nets (Figure 3B). A low efficacy of both OlysetNet and PermaNet 2.0 was observed for both mosquito species (An. gambiae s.l.: OlysetNet: 1.8% ± 1.8% mortality, PermaNet 2.0: 27.2% ± 7.2%; An. funestus s.s.: OlysetNet: 34.3% ± 13.4%, PermaNet 2.0: 23.6% ± 9.3%). The efficacy of the bed nets treated with PBO, OlysetPlus, and PermaNet 3.0 increased markedly for An. funestus s.s. (OlysetPlus: 81.6% ± 10.1% mortality, PermaNet 3.0-side: 86.8% ± 4.3%, PermaNet 3.0-roof: 91.9% ± 4.1%). For An. gambiae s.l., a higher efficacy was observed for both panels of the PermaNet 3.0 (PermaNet 3.0-side: 81.9% ± 6.6% mortality, PermaNet 3.0-roof: 86.5% ± 6.3%) than with the OlysetPlus (32.8% ± 9.6%). This difference is in line with the resistance profile showing a higher resistance level for permethrin, used for OlysetPlus impregnation, than for deltamethrin, used by PermaNet 3.0. Furthermore, for both species, OlysetPlus and PermaNet 3.0 are considerably more effective than OlysetNet and PermaNet 2.0 in cone bioassays, when unwashed.
Polymorphism Analysis of the VGSC Gene in An. gambiae
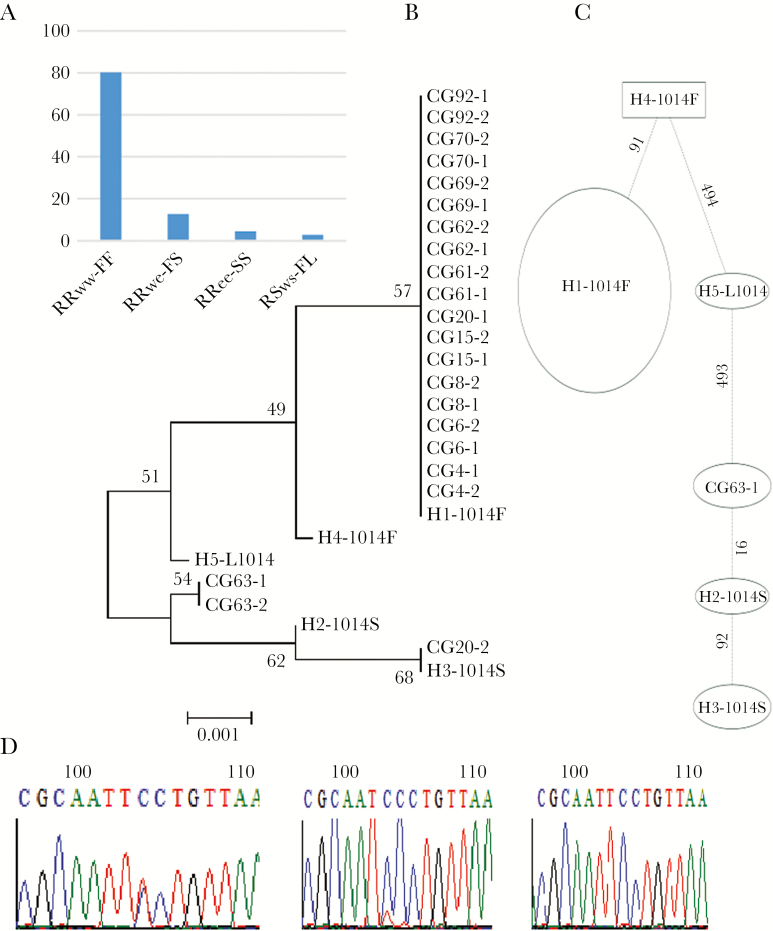
The TaqMan genotyping of kdr target-site resistance mutations in VGSC, which confers resistance to pyrethroids and DDT in An. gambiae s.l. [27, 28], revealed that the 1014F kdr allele was at a high frequency (87.8% [195/222]), the 1014S allele frequency was lower (10.8% [24/222]), and the susceptible allele was virtually absent (1.4% [3/222]) (Figure 4A).
Figure 4.
Analysis of the polymorphism of a portion of the voltage-gated sodium channel (VGSC) gene spanning the L1014F/S mutation. A, Distribution of the genotypes at the 1014 codon position. B, Maximum likelihood phylogenetic tree of VGSC fragment with previously recorded 1014F/S haplotypes across Africa [21]. C, Templeton-Crandall-Singh network for the VGSC haplotypes between susceptible and resistant permethrin samples in Kinshasa. Haplotypes are represented as an oval or a rectangle, scaled to reflect their frequencies. Lines connecting haplotypes and each node represent a single mutation event (respective polymorphic positions are given above branches). D, Sequencing traces showing the polymorphic positions 91 and 92 generating the third 1014S haplotype newly detected in Congo and suggesting an independent occurrence of 1014S.
In addition, sequencing of a 510-bp fragment of VGSC gene spanning the 1014 codon revealed a reduced genetic diversity with only 4 polymorphic sites and 3 haplotypes, including a predominant 1014F haplotype (19/22), in line with the near fixation of the 1014F allele in this population. Comparison of the DRC-VGSC haplotypes with 4 kdr bearing haplotypes previously detected across Africa revealed that all 1014F haplotypes from Kinshasa belong to the H1-1014F haplotype, predominant in West/Central Africa [21] (Figure 4B). The 1014S haplotypes belong to the H3-1014S haplotype previously described in East Africa [21], whereas the other haplotype (CG63) exhibited a single mutational step difference (position 91) from the previously described H2-1014S. This is further supported by the Templeton-Crandall-Singh haplotype network tree (Figure 4C), showing that the 1014S haplotypes are separated by 1 or 2 confirmed mutational steps (Figure 4D), suggesting an independent occurrence of the CG63-1014S haplotype in DRC, potentially from local selection.
Genotyping An. funestus L119F-GSTe2 and A296S-RDL Resistant Markers
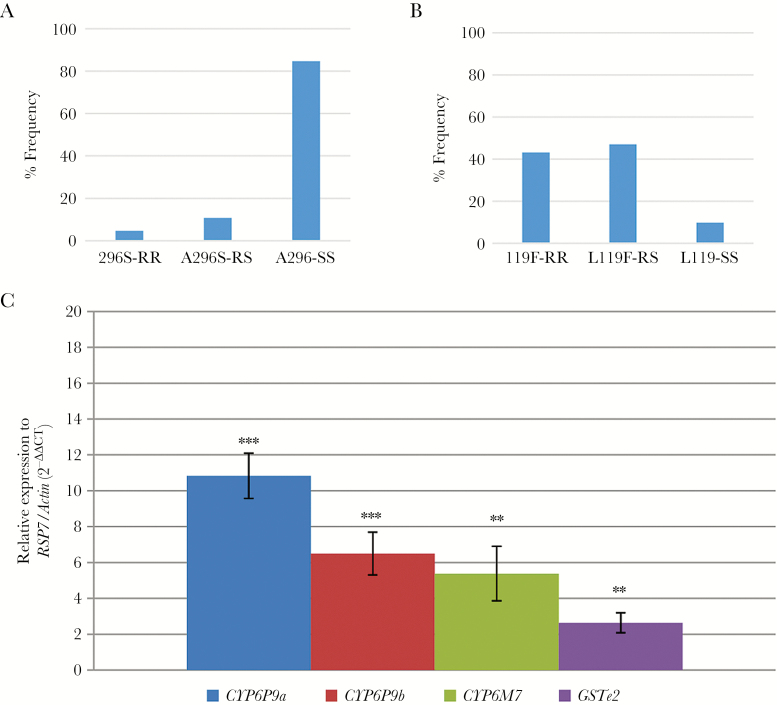
In An. funestus s.s., the A296S-RDL GABA receptor mutation which is known to confer resistance to dieldrin [29], was detected. However, the 296S resistant (R) allele frequency was low (10%) (Figure 5A). In contrast, for the L119F-GSTe2 marker conferring metabolic resistance to DDT/permethrin [23], a high frequency of the resistant 119F-GSTe2 (R) allele was observed (66.7%); 43.1% of the individuals genotyped were RR and 47.1% were RS (Figure 5B).
Figure 5.
Investigation of molecular basis of resistance in Anopheles funestus. A, Distribution of the genotypes at the A296S RDL resistance marker. B, Distribution of the genotypes at the L119F resistance marker of the GSTE2 gene. C, Differential expression by quantitative reverse-transcription polymerase chain reaction of the major insecticide resistance genes in An. funestus sensu stricto in Kinshasa compared with the susceptible An. funestus sensu stricto strain FANG. Error bars represent standard error of the mean. **P < 0.01; ***P < 0. 001.
Transcription Profile of Resistance Genes in An. funestus
The transcription analyses of 3 cytochrome P450 genes, CYP6P9a, CYP6P9b, and CYP6M7, known to be involved in pyrethroid resistance in An. funestus [30–32], and 1 glutathione-s-transferase, GSTe2, previously shown to confer DDT and permethrin resistance [23], were assessed by qRT-PCR in nonexposed mosquitoes (Figure 5C). The results reveal that these genes are significantly up-regulated in the field-collected An. funestus s.s., in comparison with the susceptible laboratory strain FANG.
DISCUSSION
Contribution of Vectors to Malaria Transmission
The high number of An. gambiae and An. funestus infected with Plasmodium (37.6% and 30.9%, respectively) suggests a high burden of malaria in this location. These infection rates are far higher than those obtained by Coene in 1993 in urban and rural areas of Kinshasa using the enzyme-linked immunosorbent assay (ELISA) detection method [33]. However, it should be noted that we were detecting all stages of the development of Plasmodium, whereas ELISA detects only the infective sporozoite stage. Furthermore, the infection rates observed in Kinshasa are considerably higher than those recently reported in other locations using the same method (Benin [18.2%], Ghana [12.5%], Malawi [5.3%], Nigeria [8.0%], and Uganda [5.3%]), as well as those obtained using other approaches (Guinea-Bissau [12.4%], Kenya [10%–18.5%], and Cameroon [6.5%–8.1%]) [13, 24, 34–39]. Our findings suggest that P. falciparum is the predominant malaria parasite in Kinshasa, although control and elimination efforts should not ignore P. malariae and P. ovale, present at low frequency, as previously reported [1, 40].
Multiple Insecticide Resistance in Malaria Vector in Kinshasa
This study revealed a high frequency of resistance to multiple insecticide classes in An. gambiae and An. funestus which, together with their high level of Plasmodium infection rate, calls for urgent actions to be taken to control malaria in this region. Both malaria vectors exhibit resistance toward pyrethroids, the only insecticide class recommended for use on LLINs [41]. The insecticide resistance pattern observed in An. gambiae differs from that found in the locality of Kingasani (province of Kinshasa) in 2009, which showed higher mortalities [1, 3]. This difference in resistance may be due to the effect of environmental and physiological factors such as the temporal selection caused by insecticide pressures, as well as to the genetic heterogeneity of the mosquito populations in DRC. The Kinshasa An. funestus population also exhibits resistance to pyrethroids and DDT, but at a lower level than An. gambiae. In contrast, An. funestus is susceptible to the carbamate bendiocarb. The resistance profile of this An. funestus population is similar to the situation observed in East Africa, where full susceptibility to bendiocarb is reported [7, 18]. As observed in other African populations of malaria vectors, organophosphates are the only insecticide class to which there is full susceptibility, and these could be recommended for indoor residual spraying around Kinshasa.
The commonly used OlysetNet and Permanet 2.0 LLINs presented a very low bioefficacy under laboratory conditions. The low efficacy of OlysetNet, treated with permethrin only, is particularly evident against An. gambiae. This observation correlates well with the very high permethrin resistance observed for An. gambiae in Kinshasa. A previous assessment of efficacy of the OlysetNet in other parts of Kinshasa had reported a higher efficacy of the OlysetNet, suggesting either a heterogeneity of the response to this net or that resistance has worsened with time [5]. Even when the synergist PBO is combined with permethrin (OlysetPlus), the mortality of An. gambiae was low, indicating that P450 genes might not be the major drivers of the observed permethrin resistance, but rather the kdr mutation, which is nearly fixed in this population.
Molecular Mechanisms Involved in Insecticide Resistance
The extremely high resistance to permethrin and DDT in An. gambiae correlates with the high frequency of the L1014F kdr mutation (87.8%). This mutation has been previously detected in DRC [3, 5]. Furthermore, the fact that 17% of mosquitoes possess the 1014S resistance allele further contributes to maintain the high resistance level. The likely independent selection of the 1014S mutation suggests that both migration and local selection forces are driving the development of pyrethroid/DDT resistance in DRC. A de novo occurrence of kdr mutations has previously been described across Africa with two 1014F and two 1014S haplotypes reported [21]. Similar to the low efficacy of Olyset Plus, the lack of notable recovery after PBO exposure in the synergist assays further supports the assumption that cytochrome P450s only play a minor role in this resistance, with kdr mutations likely driving resistance to pyrethroids/DDT. Although both type I and II pyrethroids have the same molecular target, the VGSC, resistance to permethrin (type I) was higher than to deltamethrin (type II). Several studies based on modeling, electrophysiology, and in vivo bioassay analyses have shown that the different L1014 polymorphisms may present different responses to the pyrethroids type I and type II [42–44]. For An. gambiae, similar results have been reported in a population with a high 1014F frequency in Burkina Faso (West Africa) [45], but also in Tanzania and Uganda (East Africa), where the presence of L1014S is predominant [46, 47]. Nevertheless, it is necessary to bear in mind that the differences in response to the type of pyrethroids may be also influenced by other minor mutations in the VGSC that may enhance the level of resistance associated with L1014 polymorphism [43, 48].
In the absence of kdr mutations in An. funestus s.s. [49], this study explored the contribution of metabolic resistance in the multiple resistance observed. Previous reports have confirmed the important role of the duplicated CYP6P9a/CYP6P9b and CYP6M7 genes in pyrethroid resistance [30, 31]. The significant up-regulation of these genes in this population supports the predominant role of metabolic enzymes in the pyrethroid resistance. Nevertheless, the FC values obtained here are considerably lower than in southern African populations [24], suggesting that mechanisms are different as recently shown between African regions for this species [50]. On the other hand, up-regulation for GSTe2 gene and the action of L119F-GSTe2 mutation have been associated mainly with DDT resistance [23]. The 119F-GSTe2 mutation, which has been shown to play a key role in DDT resistance in West and Central Africa (23), was found in high frequency, which is consistent with the DDT resistance observed in Kinshasa. Due to this, and along with the high frequency of kdr in An. gambiae, the use of DDT as an alternative to pyrethroids would not be recommended for vector control. Additionally, the presence of the 296S-RDL resistant to dieldrin allele is reported for the first time in DRC and could be a consequence of past use of this insecticide or ongoing use in the agricultural sector.
CONCLUSIONS
Besides the high Plasmodium infection rate in both An. funestus and An. gambiae, the results from this study reveal high and multiple insecticide resistance patterns, together with an alarmingly low efficacy of conventional LLINs without the synergist PBO under laboratory conditions. This study highlights the urgent need for actions to better manage the issue of insecticide resistance in order to prolong the effectiveness of the ongoing and future malaria control programs in DRC.
Notes
Author contributions. C. S. W. designed the study. J. M. R., H. I., and C. S. W. performed the experiments and analyzed the data. C. S. W., S. I., and F. T. W. supervised the fieldwork. J. M. R., S. I., and C. S. W. wrote the manuscript.
Acknowledgments. John Gimnig is thanked for his critical reading of the manuscript.
Disclaimer. The opinions expressed by authors contributing to this journal do not necessarily reflect the opinions of the Centers for Disease Control and Prevention or the institutions with which the authors are affiliated.
Financial support. This work was supported by a Wellcome Trust Senior Research Fellowship in Biomedical Sciences to C. S. W. (award number 101893/Z/13/Z). S. I. is funded by the US President’s Malaria Initiative.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. US President’s Malaria Initiative. Democratic Republic of the Congo malaria operational plan, fiscal year 2016. https://www.pmi.gov/docs/default-source/default-document- library/malaria-operational-plans/fy16/fy-2016- democratic-republic-of-the-congo-malaria-operational-pan.pdf?sfvrsn=6. Accessed 14 April 2017. [Google Scholar]
- 2. World Health Organization. Global plan for insecticide resistance management in malaria vectors. Geneva, Switzerland: WHO, 2012. [Google Scholar]
- 3. Basilua Kanza JP, El Fahime E, Alaoui S et al. Pyrethroid, DDT and malathion resistance in the malaria vector Anopheles gambiae from the Democratic Republic of Congo. Trans R Soc Trop Med Hyg 2013; 107:8–14. [DOI] [PubMed] [Google Scholar]
- 4. US President’s Malaria Initiative. Democratic Republic of the Congo malaria operational plan, fiscal year 2014. https://www.pmi.gov/docs/default-source/default-document-library/malaria-operational-plans/fy14/drc_mop_fy14.pdf?sfvrsn=16. Accessed 14 April 2017. [Google Scholar]
- 5. Bobanga T, Ayieko W, Zanga M et al. Field efficacy and acceptability of PermaNet® 3.0 and OlysetNet® in Kinshasa, Democratic Republic of the Congo. J Vector Borne Dis 2013; 50:206–14. [PubMed] [Google Scholar]
- 6. Gillies M, Coetzee M. A supplement to the Anophelinae of Africa South of the Sahara. Publ S Afr Inst Med Res 1987; 55:1–143. [Google Scholar]
- 7. Morgan JC, Irving H, Okedi LM, Steven A, Wondji CS. Pyrethroid resistance in an Anopheles funestus population from Uganda. PLoS One 2010; 5:e11872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Livak KJ. Organization and mapping of a sequence on the Drosophila melanogaster X and Y chromosomes that is transcribed during spermatogenesis. Genetics 1984; 107:611–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Koekemoer LL, Kamau L, Hunt RH, Coetzee M. A cocktail polymerase chain reaction assay to identify members of the Anopheles funestus (Diptera: Culicidae) group. Am J Trop Med Hyg 2002; 66:804–11. [DOI] [PubMed] [Google Scholar]
- 10. Santolamazza F, Mancini E, Simard F, Qi Y, Tu Z, della Torre A. Insertion polymorphisms of SINE200 retrotransposons within speciation islands of Anopheles gambiae molecular forms. Malar J 2008; 7:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cuamba N, Morgan JC, Irving H, Steven A, Wondji CS. High level of pyrethroid resistance in an Anopheles funestus population of the Chokwe District in Mozambique. PLoS One 2010; 5:e11010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bass C, Nikou D, Blagborough AM et al. PCR-based detection of Plasmodium in Anopheles mosquitoes: a comparison of a new high-throughput assay with existing methods. Malar J 2008; 7:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mulamba C, Irving H, Riveron JM, Mukwaya LG, Birungi J, Wondji CS. Contrasting Plasmodium infection rates and insecticide susceptibility profiles between the sympatric sibling species Anopheles parensis and Anopheles funestus s.s: a potential challenge for malaria vector control in Uganda. Parasit Vectors 2014; 7:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Snounou G, Viriyakosol S, Zhu XP et al. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol Biochem Parasitol 1993; 61:315–20. [DOI] [PubMed] [Google Scholar]
- 15. World Health Organization. Test procedures for insecticide resistance monitoring in malaria vector mosquitoes. Geneva, Switzerland: WHO, 2013. [Google Scholar]
- 16. World Health Organization. Guidelines for laboratory and field-testing of long-lasting insecticidal nets. Geneva, Switzerland: WHO, 2013. [Google Scholar]
- 17. Bass C, Nikou D, Donnelly MJ et al. Detection of knockdown resistance (kdr) mutations in Anopheles gambiae: a comparison of two new high-throughput assays with existing methods. Malar J 2007; 6:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mulamba C, Riveron JM, Ibrahim SS et al. Widespread pyrethroid and DDT resistance in the major malaria vector Anopheles funestus in East Africa is driven by metabolic resistance mechanisms. PLoS One 2014; 9:e110058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 1994; 22:4673–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Librado P, Rozas J. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009; 25:1451–2. [DOI] [PubMed] [Google Scholar]
- 21. Pinto J, Lynd A, Vicente JL et al. Multiple origins of knockdown resistance mutations in the afrotropical mosquito vector Anopheles gambiae. PLoS One 2007; 2:e1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Antonio-Nkondjio C, Tene Fossog B, Kopya E et al. Rapid evolution of pyrethroid resistance prevalence in Anopheles gambiae populations from the cities of Douala and Yaoundé (Cameroon). Malar J 2015; 14:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Riveron JM, Yunta C, Ibrahim SS et al. A single mutation in the GSTe2 gene allows tracking of metabolically based insecticide resistance in a major malaria vector. Genome Biol 2014; 15:R27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Riveron JM, Chiumia M, Menze BD et al. Rise of multiple insecticide resistance in Anopheles funestus in Malawi: a major concern for malaria vector control. Malar J 2015; 14:344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jones CM, Machin C, Mohammed K et al. Insecticide resistance in Culex quinquefasciatus from Zanzibar: implications for vector control programmes. Parasit Vectors 2012; 5:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 2008; 3:1101–8. [DOI] [PubMed] [Google Scholar]
- 27. Martinez-Torres D, Chandre F, Williamson MS et al. Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae s.s. Insect Mol Biol 1998; 7:179–84. [DOI] [PubMed] [Google Scholar]
- 28. Ranson H, Jensen B, Vulule JM, Wang X, Hemingway J, Collins FH. Identification of a point mutation in the voltage-gated sodium channel gene of Kenyan Anopheles gambiae associated with resistance to DDT and pyrethroids. Insect Mol Biol 2000; 9:491–7. [DOI] [PubMed] [Google Scholar]
- 29. Wondji CS, Dabire RK, Tukur Z, Irving H, Djouaka R, Morgan JC. Identification and distribution of a GABA receptor mutation conferring dieldrin resistance in the malaria vector Anopheles funestus in Africa. Insect Biochem Mol Biol 2011; 41:484–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Riveron JM, Irving H, Ndula M et al. Directionally selected cytochrome P450 alleles are driving the spread of pyrethroid resistance in the major malaria vector Anopheles funestus. Proc Natl Acad Sci U S A 2013; 110:252–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Riveron JM, Ibrahim SS, Chanda E et al. The highly polymorphic CYP6M7 cytochrome P450 gene partners with the directionally selected CYP6P9a and CYP6P9b genes to expand the pyrethroid resistance front in the malaria vector Anopheles funestus in Africa. BMC Genomics 2014; 15:817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ibrahim SS, Riveron JM, Bibby J et al. Allelic variation of cytochrome P450s drives resistance to bednet insecticides in a major malaria vector. PLoS Genet 2015; 11:e1005618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Coene J. Malaria in urban and rural Kinshasa: the entomological input. Med Vet Entomol 1993; 7:127–37. [DOI] [PubMed] [Google Scholar]
- 34. Cohuet A, Simard F, Wondji CS, Antonio-Nkondjio C, Awono-Ambene P, Fontenille D. High malaria transmission intensity due to Anopheles funestus (Diptera: Culicidae) in a village of savannah-forest transition area in Cameroon. J Med Entomol 2004; 41:901–5. [DOI] [PubMed] [Google Scholar]
- 35. Sanford MR, Cornel AJ, Nieman CC et al. Plasmodium falciparum infection rates for some Anopheles spp. from Guinea-Bissau, West Africa. F1000Res 2014; 3:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Riveron JM, Osae M, Egyir-Yawson A, Irving H, Ibrahim SS, Wondji CS. Multiple insecticide resistance in the major malaria vector Anopheles funestus in southern Ghana: implications for malaria control. Parasit Vectors 2016; 9:504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Djouaka R, Riveron JM, Yessoufou A et al. Multiple insecticide resistance in an infected population of the malaria vector Anopheles funestus in Benin. Parasit Vectors 2016; 9:453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Djouaka RJ, Atoyebi SM, Tchigossou GM et al. Evidence of a multiple insecticide resistance in the malaria vector Anopheles funestus in south west Nigeria. Malar J 2016; 15:565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ogola E, Villinger J, Mabuka D et al. Composition of Anopheles mosquitoes, their blood-meal hosts, and Plasmodium falciparum infection rates in three islands with disparate bed net coverage in Lake Victoria, Kenya. Malar J 2017; 16:360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Doctor SM, Liu Y, Anderson OG et al. Low prevalence of Plasmodium malariae and Plasmodium ovale mono-infections among children in the Democratic Republic of the Congo: a population-based, cross-sectional study. Malar J 2016; 15:350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hougard JM, Duchon S, Darriet F, Zaim M, Rogier C, Guillet P. Comparative performances, under laboratory conditions, of seven pyrethroid insecticides used for impregnation of mosquito nets. Bull World Health Organ 2003; 81:324–33. [PMC free article] [PubMed] [Google Scholar]
- 42. Vais H, Williamson MS, Goodson SJ et al. Activation of Drosophila sodium channels promotes modification by deltamethrin. Reductions in affinity caused by knock-down resistance mutations. J Gen Physiol 2000; 115:305–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Davies TE, O’Reilly AO, Field LM, Wallace B, Williamson MS. Knockdown resistance to DDT and pyrethroids: from target-site mutations to molecular modelling. Pest Manag Sci 2008; 64:1126–30. [DOI] [PubMed] [Google Scholar]
- 44. Burton MJ, Mellor IR, Duce IR, Davies TG, Field LM, Williamson MS. Differential resistance of insect sodium channels with kdr mutations to deltamethrin, permethrin and DDT. Insect Biochem Mol Biol 2011; 41:723–32. [DOI] [PubMed] [Google Scholar]
- 45. Kwiatkowska RM, Platt N, Poupardin R et al. Dissecting the mechanisms responsible for the multiple insecticide resistance phenotype in Anopheles gambiae s.s., M form, from Vallée du Kou, Burkina Faso. Gene 2013; 519:98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ramphul U, Boase T, Bass C, Okedi LM, Donnelly MJ, Müller P. Insecticide resistance and its association with target-site mutations in natural populations of Anopheles gambiae from eastern Uganda. Trans R Soc Trop Med Hyg 2009; 103:1121–6. [DOI] [PubMed] [Google Scholar]
- 47. Protopopoff N, Matowo J, Malima R et al. High level of resistance in the mosquito Anopheles gambiae to pyrethroid insecticides and reduced susceptibility to bendiocarb in north-western Tanzania. Malar J 2013; 12:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jones CM, Liyanapathirana M, Agossa FR et al. Footprints of positive selection associated with a mutation (N1575Y) in the voltage-gated sodium channel of Anopheles gambiae. Proc Natl Acad Sci U S A 2012; 109:6614–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Riveron JM, Chiumia M, Menze BD et al. Rise of multiple insecticide resistance in Anopheles funestus in Malawi: a major concern for malaria vector control. Malar J 2015; 14:344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Riveron JM, Ibrahim SS, Mulamba C et al. Genome-wide transcription and functional analyses reveal heterogeneous molecular mechanisms driving pyrethroids resistance in the major malaria vector Anopheles funestus across Africa. G3 (Bethesda) 2017; 7:1819–32. [DOI] [PMC free article] [PubMed] [Google Scholar]