The C3–C4 state moves lineages into C4-like environments, bridging the ecological gap between C3 and C4 species and facilitating C4 evolution.
Keywords: Biomes, C3–C4 intermediate, C4 photosynthesis, ecology, evolution, phylogeny
Abstract
C4 photosynthesis is a physiological innovation involving several anatomical and biochemical components that emerged recurrently in flowering plants. This complex trait evolved via a series of physiological intermediates, broadly termed ‘C3–C4’, which have been widely studied to understand C4 origins. While this research program has focused on biochemistry, physiology, and anatomy, the ecology of these intermediates remains largely unexplored. Here, we use global occurrence data and local habitat descriptions to characterize the niches of multiple C3–C4 lineages, as well as their close C3 and C4 relatives. While C3–C4 taxa tend to occur in warm climates, their abiotic niches are spread along other dimensions, making it impossible to define a universal C3–C4 niche. Phylogeny-based comparisons suggest that, despite shifts associated with photosynthetic types, the precipitation component of the C3–C4 niche is particularly lineage specific, being highly correlated with that of closely related C3 and C4 taxa. Our large-scale analyses suggest that C3–C4 lineages converged toward warm habitats, which may have facilitated the transition to C4 photosynthesis, effectively bridging the ecological gap between C3 and C4 plants. The intermediates retained some precipitation aspects of their C3 ancestors’ habitat, and likely transmitted them to their C4 descendants, contributing to the diversity among C4 lineages seen today.
Introduction
The C4 photosynthetic pathway relies on a coordinated system of anatomical and biochemical traits that function to concentrate CO2 around Rubisco, which in most C4 plants is localized to the bundle sheath cells (Hatch, 1987). The enhanced CO2 concentration substantially suppresses O2 fixation and subsequent photorespiration, compared with the ancestral C3 photosynthetic pathway, making C4 photosynthesis advantageous in conditions that increase photorespiration (Chollet and Ogren, 1975; Hatch and Osmond, 1976). C4 photosynthesis is consequently prevalent in the open biomes of warm regions where it boosts growth (Sage et al., 1999; Osborne and Freckleton, 2009; Atkinson et al., 2016), to ultimately shape entire ecosystems, such as the emblematic savannas (Sage and Stata, 2015).
It has been widely reported that some plants possess only a subset of the anatomical and/or biochemical components of the C4 pump. These plants tend to be physiologically somewhere in between typical C3 and C4 plants and, as such, are termed C3–C4 intermediates (Kennedy and Laetsch, 1974; Monson and Moore, 1989; Sage, 2004; Schlüter and Weber, 2016). These physiologically intermediate plants use a photorespiratory CO2 pump, or glycine shuttle, to rescue CO2 released from mesophyll photorespiratory activity and transport it into the bundle sheath for re-use in the Calvin cycle located there (Hylton et al., 1988). Thus, the C3–C4 system establishes a CO2 recycling mechanism based on the spatial segregation of metabolic reactions, the migration of the Calvin cycle to the bundle sheath, and the dual-compartment coordination that are characteristic of the C4 pathway. These modifications improve the physiological performance of C3–C4 plants over the C3 system in conditions that promote photorespiration, as they lessen the total carbon lost via photorespiration to improve net carbon assimilation (Vogan and Sage, 2011; Way et al., 2014). In addition to the glycine shuttle, a number of C3–C4 plants engage a weak C4 cycle (Ku et al., 1983), which further reduces photorespiration and is predicted to increase biomass accumulation (Mallmann et al., 2014). Thus, this variation in C4-associated traits forms a continuum between the C3 condition and a diversity of C4 phenotypes (Bauwe, 1984; McKown and Dengler, 2007; Lundgren et al., 2014; Bräutigam and Gowik, 2016).
Because C3–C4 plants share many anatomical, biochemical, and physiological traits with C4 plants, they are often assumed to represent an evolutionary step facilitating C4 evolution (Hylton et al., 1988; Sage, 2004; Sage et al., 2012; Bräutigam and Gowik, 2016), a hypothesis confirmed by the close relationships between C3–C4 and C4 taxa in some groups (McKown et al., 2005; Christin et al., 2011b; Khoshravesh et al., 2012; Sage et al., 2012; Fisher et al., 2015). They are consequently widely studied and incorporated into models of C4 evolution, which show that C3–C4 phenotypes can bridge the gap between C3 and C4 states by providing a series of stages that are advantageous over the preceding ones (Heckmann et al., 2013; Williams et al., 2013; Mallmann et al., 2014; Bräutigam and Gowik, 2016). This research program has been extremely successful in tracking the changes in leaf anatomy, organelles, metabolism, genes, and enzymes that likely took place during C4 evolution, particularly in the eudicot genus Flaveria (e.g. Bauwe and Chollet, 1986; Svensson et al., 2003; McKown and Dengler, 2007, 2009; Sage et al., 2013). However, previous research failed to address the ecological consequences of these intermediate stages. Indeed, while models that predict the carbon gains of the intermediate stages exist (Heckmann et al., 2013; Mallmann et al., 2014), studies of natural distributions of extant C3–C4 taxa are nearly non-existent (but see Sudderth et al., 2009).
The differing geographical and environmental distributions of C3 and C4 species have been widely studied (Teeri and Stowe, 1976; Rundel, 1980; Williams et al., 1995; Ehleringer et al., 1997, Epstein et al., 1997; Edwards and Still, 2008), with later incorporation of phylogenetic data providing estimates of the ecological shifts that happened before, during, or after photosynthetic transitions (Osborne and Freckleton, 2009; Edwards and Smith, 2010; Edwards and Ogburn, 2012; Kadereit et al., 2012; Lundgren et al., 2015). However, these efforts focused on comparisons between C3 and C4 plants, which are much more frequent and abundant than C3–C4 taxa. Previous discussions of C3–C4 ecology characterized their distributions in hot, sandy, and disturbed habitats with little competition (Powell 1978; Hedge and Patil, 1980; Prendergast and Hattersley, 1985; Vogan et al., 2007; Feodorova et al., 2010; Christin et al., 2011b; Sage et al., 2011, 2012). However, other groups with C3–C4 intermediates thrive in apparently very different habitats, with C3–C4Flaveria inhabiting a broad range of environments from open fields and scrublands (F. angustifolia) to pine forests (F. anomala), wetlands (F. floridana), and warm mineral springs (F. sonorensis; Powell 1978), yet field data failed to identify differences in the distributions of different photosynthetic types in Flaveria (Sudderth et al., 2009). The monocot C3–C4 intermediates of Eleocharis and Steinchisma thrive in wetland habitats (USDA/NRCS, 2016), C3–C4Alloteropsis grow in shady, deciduous forests of tropical Africa (Lundgren et al., 2015), and the recently identified intermediates in Homolepis (Khoshravesh et al., 2016) grow at the margins of South American rainforests. These disparate characterizations urge a careful, data based evaluation of the C3–C4 niche, its variation among evolutionary lineages, and its relation to that of C3 and C4 relatives.
In this study, we use available global occurrence data and local habitat descriptions to characterize the niche of C3–C4 lineages, along with their close C3 and C4 relatives. The ecological data are used to (i) quantitatively and objectively describe the abiotic habits of C3–C4 taxa and determine whether they inhabit uniform conditions, (ii) test whether phylogenetic effects partially explain the ecological sorting of C3–C4 lineages and whether their sorting explains the diversity in the ecology of C4 relatives, and (iii) test whether, when controlling for phylogenetic effects, the C3–C4 physiology affects the niche, potentially bringing the plants closer to the C4 niche. Our large-scale analyses, which consider all described C3–C4 lineages and their relatives, show that C3–C4 plants inhabit a large array of habitats, and that physiology closely interacts with evolutionary history to shape the niches of C3–C4, but also C4, taxa.
Methods
Ecological distribution of individual C3–C4 species
A list of 56 C3–C4 intermediate taxa was assembled from the literature, and included 11 eudicot and two monocot families (Table 1). Occurrence data for each taxon were downloaded from the Global Biodiversity Information Facility (GBIF, http://www.gbif.org) using the RGBIF package in R (Chamberlain et al., 2016; data accessed 1 and 2 July 2016). Occurrence data for the Zambezian C3–C4 within Alloteropsis semialata were taken from Lundgren et al. (2015, 2016). All occurrence data were cleaned by removing any anomalous latitude or longitude points, points falling outside of a landmass, and any points close to GBIF headquarters in Copenhagen, Denmark, which may result from erroneous geolocation. To avoid repeated occurrences, latitude and longitude decimal degree values were rounded to two decimal places, and any duplicates at this resolution were removed. These filters are commonly applied to data extracted from GBIF (Zanne et al., 2014).
Table 1.
Details of C3–C4 taxa used in this study and their local habitats
| Comparison | Species | n | Habitat | Referencea |
|---|---|---|---|---|
| Acanthaceae | ||||
| Blepharis | B. diversispina | 42 | Deciduous woodland, grasslands, soil sandy and gravelly, disturbed |
Fisher et al., 2015; Hyde et al., 2016a,b; USDA/NRCS, 2016 |
| B. gigantea | 6 | Sandy to stony soils | ||
| B. natalensis | 6 | Rocky slopes | ||
| B. noli-me-tangere | 2 | Sandy soil, dry watercourses | ||
| B. pruinosa | 19 | Sandy to stony soils | ||
| B. sinuata | 4 | Bushland | ||
| B. espinosa | 5 | Deciduous woodland, disturbed, various habitats | ||
| Amaranthaceae | ||||
| Alternanthera | A. ficoidea | 268 | Uplands | Rajendrudu et al., 1986 |
| A. tenella | 446 | |||
| Salsola | S. divaricata | 32 | Semi-arid rocky zones near coastal areas; salt tolerant | Voznesenskaya et al., 2013 |
| Sedobassia | S. sedoides | 3 | Ruderal, sandy, saline habitats |
Eliáš and Dítě, 2014; Freitag and Kadereit, 2014 |
| Asteraceae | ||||
| Flaveria | F. pubescens | 8 | Wetlands, alkaline and saline soils, fine textured soils |
Powell, 1978; Edwards and Ku, 1987; USDA/NRCS, 2016 |
| F. oppositifolia | 36 | |||
| F. angustifolia | 16 | Pastures, fields, roadsides, disturbed | ||
| F. anomala | 44 | |||
| F. chloraefolia | 16 | Wetlands, saline and gypseous soils, disturbed | ||
| F. floridana | 3 | Wetlands, woodlands, sandy, saline, disturbed | ||
| F. linearis | 77 | Wetlands, woodlands, sandy, disturbed | ||
| F. ramosissima | 6 | |||
| F. sonorensis | 3 | Disturbed, semiarid soils | ||
| Parthenium | P. hysterophorus | 11 | Disturbed, mainly dry or saline soils |
Hedge and Patil, 1980; Moore et al., 1987 |
| Boraginaceae | ||||
| Heliotropium | H. convolvulaceum | 164 | Sand dune specialist |
Frohlich, 1978; Vogan et al., 2007 |
| H. lagoense | 5 | |||
| H. greggii | 49 | Open site, lay, gravel soils | ||
| Brassicaceae | ||||
| Diplotaxis | D. erucoides | 2328 | Disturbed |
Apel et al., 1997; USDA/NRCS, 2016 |
| D. muralis | 4828 | Grazed grasslands, disturbed | ||
| D. tenuifolia | 7206 | Wetlands, wet woods, mountain slopes, sandy, disturbed | ||
| Moricandia | M. nitens | 285 |
Holaday and Chollet, 1984; USDA/NRCS, 2016 |
|
| M. sinaica | 14 | |||
| M. spinosa | 1 | |||
| M. suffruticosa | 32 | |||
| M. arvensis | 821 | Grainfields, orchards, disturbed | ||
| Cleomaceae | ||||
| Cleome | C. paradoxa | 7 | Arid, rocky soils |
Voznesenskaya et al., 2007; Feodorova et al., 2010 |
| Euphorbiaceae | ||||
| Euphorbia | E. acuta | 7 | Dry limestone uplands, semi-arid scrublands, disturbed | Sage et al., 2011 |
| E. johnstonii | 1 | Dry limestone uplands, semi-arid scrublands; calcareous soils, caliche outcrops | ||
| E. lata | 52 | Dry limestone uplands, semi-arid scrublands; calcareous soils, sandy plains | ||
| Molluginaceae | ||||
| Hypertelis | Hypertelis spergulacea | 16 |
Edwards and Ku, 1987; Christin et al., 2011b ; USDA/NRCS, 2016 |
|
| Paramollugo nudicaulis | 203 | Ruderal habitats lacking competition | ||
| Mollugo | M. verticillata | 1686 | Fields, gardens, disturbed, moist to dry soils; lacking competition | |
| Portulacaceae | ||||
| Portulaca | P. cryptopetala | 35 | Moist, warm habitats | Voznesenskaya et al., 2010 |
| Scrophulariaceae | ||||
| Anticharis | A. ebracteata | 5 | Quartz gravel | Khoshravesh et al., 2012 |
| A. juncea | 7 | Farm, granite rocks | ||
| Cyperaceae | ||||
| Eleocharis | E. atropurpurea | 355 | Wetlands, disturbed |
Roalson et al., 2010; USDA/NRCS, 2016 |
| E. brainii | 6 | |||
| E. flavescens | 182 | Wetlands | ||
| E. nigrescens | 53 | Wetlands, woodlands, sandy and peaty soils | ||
| E. subfoliata | 6 | |||
| Poaceae | ||||
| Alloteropsis | Zambezian A. semialata | 13 | Shady, miombo woodlands | Lundgren et al., 2015; 2016 |
| Homolepis | H. aturensis | 411 | Rainforest | Khoshravesh et al., 2016 |
| Neurachne | N. minor | 69 | Arid soils, often shallow |
Prendergast and Hattersley, 1985; Hattersley et al., 1986 |
| Steinchisma | S. cuprea | 8 |
Edwards and Ku, 1987; USDA/NRCS, 2016 |
|
| S. decipiens | 130 | |||
| S. hians | 285 | Wetlands | ||
| S. spathellosum | 57 | |||
| S. stenophyllum | 6 | Wetlands | ||
a References describe local habit. Those characterising C3–C4 intermediate status are italicized.
Environmental parameters that have been predicted to potentially explain the sorting of C3, C3–C4, and C4 photosynthetic types were selected (Christin and Osborne, 2014; Supplementary Table S1). Geographic distributions are characterized with latitudinal and altitudinal ranges, and broad climatic distributions are characterized via mean annual precipitation (MAP) and mean annual temperature (MAT) variables. The growing season temperature (i.e. temperature of the wettest quarter), minimum temperature (i.e. minimum temperature of the coldest month), number of annual frost days, minimum precipitation (i.e. precipitation of the driest month), number of annual wet days, percentage of maximum possible sunshine, rainfall seasonality, and fire return interval (FRI) were also used to characterize the environment. The rainfall seasonality data, which come from Lehmann et al. (2011), are based on an index that indicates how evenly dispersed rainfall is throughout a year, with zero indicating equal rain in all months and a value of 100 indicating that all annual rain fell within a single month (see Supplementary Table S1). The FRI data, which come from Archibald et al. (2013), are based on an index that indicates the growth time available to plants between fires, with greater FRI values indicating less frequent fire regimes and longer regrowth periods (Supplementary Table S1). Climate and soil fertility data were obtained by overlaying the occurrence coordinates onto high-resolution raster layers obtained from WorldClim (http://www.worldclim.org; Hijmans et al., 2005), Climatic Research Unit (New et al., 2002; http://www.ipcc-data.org), and the Harmonized World Soils Database (HWSD; FAO/IIASA/ISRIC/ISSCAS/JRC, 2012; http://webarchive.iiasa.ac.at; Supplementary Table S1).
Data from the dominant soil type of the topsoil layer were extracted from the HWSD raster layers. Specifically, four soil parameters were used to characterize soil fertility and are described below as per the HWSD classifications (FAO/IIASA/ISRIC/ISSCAS/JRC, 2012). First, the percentage of organic carbon (OC) in the topsoil is a particularly good indicator of soil health, with moderate to high OC present in fertile, well-structured soils. Soils with less than 0.2% or 0.6% OC are considered very poor and poor, respectively, and soils with greater than 2% OC are considered fertile. Total exchangeable bases (TEB) is the sum of exchangeable cations of sodium, calcium, magnesium, and potassium in the topsoil and, as such, soils with more TEB have better fertility. The cation exchange capacity (CEC) of the topsoil indicates the total nutrient fixing capacity of the soil, with low CEC soils, such as sandy soils with CEC less than 4 cmol kg–1, having little resilience and low nutrient stores, while soils with greater than 10 cmol kg–1 have high nutrient fixing capacity and are suitable for crops. The pH of the topsoil indicates the acidity and alkalinity of the soil, with pH values less than 4.5, as found in mangrove soils or acid sulfate soils, being extremely acid and poorly draining, pH values of 5.5–7.2 are considered neutral, and those above 8.5 are alkaline and consequently may inhibit the bio-availability of nutrients in the soils.
The variation among environmental variables at individual plant occurrence points was summarized using a principal component analysis (PCA), as implemented in the FactoMineR package in R (Lê et al., 2008). A first PCA was conducted on climate variables, as described in Lundgren et al. (2015), and a second PCA was completed on the four soil fertility variables.
Testing for phylogenetic effects on the ecological sorting of C3–C4 lineages
To determine whether the ecological sorting of C3–C4 taxa is partially determined by the phylogenetic lineage to which they belong, we tested for an effect of the abiotic environment of the closest C3 relatives on the sorting of C3–C4 lineages, and for an effect of the C3–C4 habitat on the sorting of the C4 relatives. For this purpose, we identified sets of C3–C4 species and their C3 and C4 sister groups. An angiosperm-wide phylogeny including all of the C3–C4 groups and their relatives was unavailable, and thus groups were defined based on phylogenetic trees published for the different clades (see Supplementary Table S2). This endeavor was complicated by taxa with unknown photosynthetic types. In addition, while some small groups have well resolved phylogenetic trees with clearly identified photosynthetic types (e.g. Flaveria; McKown et al., 2005), many other systems have only been partially sampled or phenotyped. Nodes separating clearly identified C3 and C3–C4, or C3–C4 and C4 groups were selected, ignoring any groups with unknown photosynthetic types. For some C3–C4 lineages, either the C3 or the C4 sister group could not be identified. For example, Portulaca cryptopetala is nested in a group of C4 species and the related species are potentially CAM (Ocampo and Columbus, 2010; Arakaki et al., 2011), and several C3–C4 intermediates lack close C4 relatives (Supplementary Table S2). In cases where C3–C4 taxa were mixed with species of unknown type, the C3–C4 taxa were grouped and compared with a more distant clade with clearly established C3 taxa (e.g. Eleocharis; Roalson et al., 2010; Paramollugo; Christin et al., 2011b), and C3–C4 groups forming paraphyletic clades with respect to C4 species were merged (e.g. Flaveria; McKown et al., 2005; Lyu et al., 2015). However, C3–C4 belonging to the same family, but with distinct C3 and C4 relatives were considered separately (Supplementary Table S2). In other cases, where the phylogeny or photosynthetic categorization for a genus was incomplete, only taxa with clearly assigned photosynthetic types were considered and grouped based on the photosynthetic type independently of the phylogenetic relationships (e.g. Heliotropium; Supplementary Table S2). This approach decreases the number of contrasts, as closely related, yet independent C3–C4 lineages might have been merged. However, it ensures that no erroneous comparisons are included, for example when available plastid phylogenies do not perfectly match genome-wide relationships (e.g. Lyu et al., 2015). Indeed, our analyses only compare photosynthetic types within groups that are monophyletic, even if these are incompletely sampled. In conclusion, while the incomplete phylogenetic knowledge probably decreases our analytical power, our approach is statistically conservative.
The abiotic environment of C3 and C4 relatives of C3–C4 lineages was assessed as described for C3–C4 taxa. For each species and each variable, the median was used to avoid extreme values, which could be misidentifications or erroneously reported occurrence points. To obtain one value per group, the average of the medians was calculated for each C3–C4 lineage, its C3 sister group, and its C4 sister group. A phylogenetic effect on the sorting of C3–C4 taxa was evaluated with correlation tests between the climatic environment of the C3 group and the environment inhabited by its closely related C3–C4 group. In the absence of phylogenetic effects, the values for C3–C4 taxa should be independent from those observed in the closely related C3 group. These analyses were repeated by testing for a correlation between the environment of the C3–C4 lineage and that of the closely related C4 group. Because many variables failed the Shapiro–Wilk test for normality, correlations were tested using the non-parametric Kendall rank correlation, which does not assume normality and is unbiased by small sample sizes. These tests were performed on the primary axis of the climate and soil PCAs, as well as on four climate variables (i.e. growing season temperature, minimum temperature, minimum precipitation, and rainfall seasonality) and two soil fertility variables (i.e. topsoil organic matter and TEB). These variables were selected to capture both temperature and precipitation patterns, which have classically been linked to photosynthetic types (reviewed in Christin and Osborne, 2014), and the two soil variables were selected to characterize the overall soil fertility. P-values of all tests were compared with a threshold corrected for eight comparisons (two PCA primary axes and six independent environmental variables; 0.00625).
Testing for differences among photosynthetic types, while controlling for phylogeny
Phylogenetic effects and photosynthetic types can both potentially contribute to the ecological sorting of plants. We consequently tested for differences among photosynthetic types, while controlling for phylogenetic effects. A sister group approach was adopted to compare C3, C3–C4, and C4 photosynthetic types within each lineage (see Supplementary Table S2), an approach that removes phylogenetic effects in a similar manner to phylogenetic independent contrasts (Garland et al., 1992). Indeed, a directional shift consistently associated with a given photosynthetic type within each group is strongly indicative of non-random processes (Vamosi and Vamosi, 2005; Edwards and Still, 2008; Edwards and Smith, 2010; Spriggs et al., 2014). The age of the different groups varies (Christin et al., 2011a, 2014), which means that the amount of divergence between the photosynthetic types is not necessarily constant among groups. However, our analyses are based on rank or sign tests and are therefore unaffected by variation in the magnitude of differences between photosynthetic types within each group. Consistent shifts between photosynthetic types were evaluated as the number of clades where the mean of the medians of the type of interest (either C3–C4 or C4) was larger than the mean of the medians of the comparison (C3 and C3–C4, respectively). The probability of observing such a shift with a random process (i.e. a probability of success of 0.5) was calculated based on a binomial distribution, in a two-tailed sign test. These tests were performed on the same eight variables used to assess the phylogenetic effects on C3–C4 sorting, and using the same corrections for multiple testing.
Results
Geographic distribution of C3–C4 intermediates
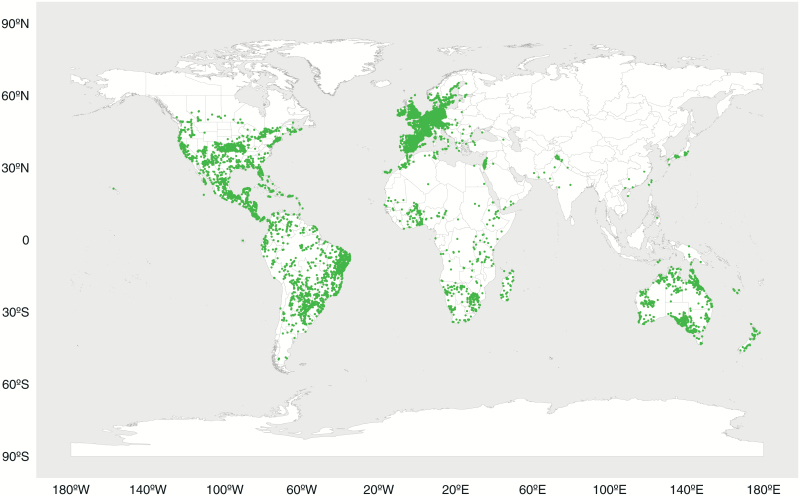
As a whole, C3–C4 intermediates are broadly distributed across Australia, Asia, Europe, Africa, and the Americas (Fig. 1). While the sampling is clearly biased toward western Europe, Central America, and specific countries (e.g. Israel), the data clearly indicate that intermediates can occur in most tropical and temperate regions. The C3–C4 occurrences span a latitudinal belt between 50°S and 65°N (Fig. 1, Table 2, and Supplementary Dataset S1), with Diplotaxis intermediates reaching from northern Europe to the south of Australia, Africa, and South America (see Supplementary Fig. S1). Eleocharis and Mollugo C3–C4 plants are similarly widespread, spreading across the Americas, Europe, Africa, Asia, and Australia (Table 2, Supplementary Figs S1 and S2, and Supplementary Dataset S1). Other intermediate lineages, such as Alloteropsis, Neurachne, Blepharis, and Sebodassia, have smaller geographic ranges, according to the available occurrence data (Table 2, Supplementary Figs S1 and S2, and Supplementary Dataset S1). Many intermediates occur well below sea level, along the Dead Sea (e.g. Diplotaxis erucoides, Moricandia sinaica, and Parthenium hysterophorus), in The Netherlands (e.g. Diplotaxis tenuifolia and Diplotaxis muralis), or along the Gulf of Mexico (e.g. Flaveria linearis, Eleocharis atropurpurea; Table 2, Supplementary Figs S1 and S2, and Supplementary Dataset S1). C3–C4 intermediates also occur at high elevations, along the Andes mountains (e.g. Steinchisma decipiens, Steinchisma hians, Mollugo verticillata, Diplotaxis muralis), in Lesotho (e.g. Diplotaxis muralis, Blepharis espinosa), and in the highlands of Mexico (e.g. Mollugo verticillata, Berkheya spinosissma; Table 2; Supplementary Figs S1 and S2; Supplementary Dataset S1).
Fig. 1.
Global distribution of C3–C4 taxa. Each dot represents an occurrence point for a single C3–C4 intermediate plant.
Table 2.
Ranges of geography, climate, and soil characteristics of C3–C4 taxa within each lineage group
| C3–C4 group | n | Latitude | Altitude (m) | MAT (°C) | MAP (mm) | OC (% weight) | TEB (cmol/kg) | CEC (cmol/kg) | pH (–log (H+)) |
|---|---|---|---|---|---|---|---|---|---|
| Eudicots | |||||||||
| Alternanthera | 714 | 35°S–51°N | 0–2873 | 8–29 | 363–4523 | 0.1–16 | 0.2–76 | 1–76 | 3.3–8.4 |
| Anticharis | 12 | 29°S–22°S | 289–1831 | 18–23 | 27–442 | 0.3–0.7 | 1.5–16 | 2–16 | 5.5–8.6 |
| Blepharis | 84 | 33°S–12°S | 182–2555 | 10–23 | 100–1228 | 0.1–1.6 | 0–41 | 0–41 | 4.9–9.8 |
| Cleome | 7 | 11°N–16°N | 23–777 | 25–29 | 38–503 | 0.3–0.7 | 6.8–17 | 6–17 | 6.5–8.1 |
| Diplotaxis a | 14362 | 50°S–65°N | –409 to 3959 | –2 to 26 | 33–2990 | 0.1–39.4 | 0.8–68.2 | 1–87 | 4.1–8.8 |
| Euphorbia | 60 | 25°N–38°N | 59–1913 | 11–23 | 245–736 | 0.4–1.8 | 4.4–31.1 | 5–23 | 6.0–8.4 |
| Flaveria | 209 | 17°N–35°N | –1 to 3116 | 10–27 | 214–1581 | 0.3–14 | 1.7–83 | 4–83 | 4.5–8.4 |
| Heliotropium | 218 | 15°S–40°N | 0–2543 | 9–26 | 63–2183 | 0.1–14 | 1.1–44 | 2–44 | 4.7–8.4 |
| Hypertelis | 16 | 29°S–28°S | 68–1086 | 16–23 | 41–98 | 0.4–0.7 | 4.0–16 | 4–16 | 6.5–8.5 |
| Mollugo a | 1889 | 38°S–53°N | –5 to 4209 | 0–30 | 1–4048 | 0.1–35.3 | 0.2–83 | 2–85 | 3.3–10.2 |
| Moricandia a | 1153 | 35°S–60°N | –251 to 2701 | 6–25 | 10–1328 | 0.2–2.7 | 2.0–46.6 | 3–43 | 4.4–8.7 |
| Parthenium a | 11 | 22°S–33°N | –228 to 904 | 18–23 | 325–1685 | 0.4–1.6 | 1.7–45.2 | 6–44 | 4.9–8.1 |
| Portulaca | 35 | 34°S–17°S | 2–1948 | 15–26 | 308–1749 | 0.4–2.5 | 0.6–43.4 | 2–43 | 4.9–9.0 |
| Salsola | 32 | 28°N–40°N | 5–1066 | 14–21 | 97–545 | 0.5–1.4 | 4.5–24.3 | 5–16 | 6.4–8.0 |
| Sedobassia | 3 | 43°N–48°N | 64–97 | 10–12 | 527–540 | 1.1–1.7 | 38.0–40.9 | 23–43 | 6.9–7.8 |
| Monocots | |||||||||
| Eleocharis | 604 | 35°S–51°N | –1 to 3805 | –1 to 29 | 163–4614 | 0.1–35.3 | 0.2–76 | 2–84 | 3.3–8.9 |
| Alloteropsis | 13 | 13°S–6°S | 958–2264 | 18–24 | 812–1439 | 0.7–2.5 | 0.8–12 | 5–20 | 4.6–6.5 |
| Homolepis | 411 | 18°S–20°N | 0–3548 | 8–28 | 671–7731 | 0.1–28 | 0.2–83 | 1–85 | 3.3–8.3 |
| Neurachne | 69 | 34°S–23°S | 205–637 | 14–24 | 166–1128 | 0.3–2.1 | 2.1–18.1 | 2–15 | 4.5–8.3 |
| Steinchisma a | 486 | 35°S–37°N | 2–4524 | 3–27 | 229–3104 | 0.2–5.3 | 0.2–45.2 | 2–46 | 3.5–9 |
CEC, topsoil cation exchange capacity; MAP, mean annual precipitation; MAT, mean annual temperature; OC, topsoil organic matter content; TEB, topsoil total exchangeable bases.
a C3–C4 lineages lacking close C4 relatives.
Environmental distribution of C3–C4 intermediates
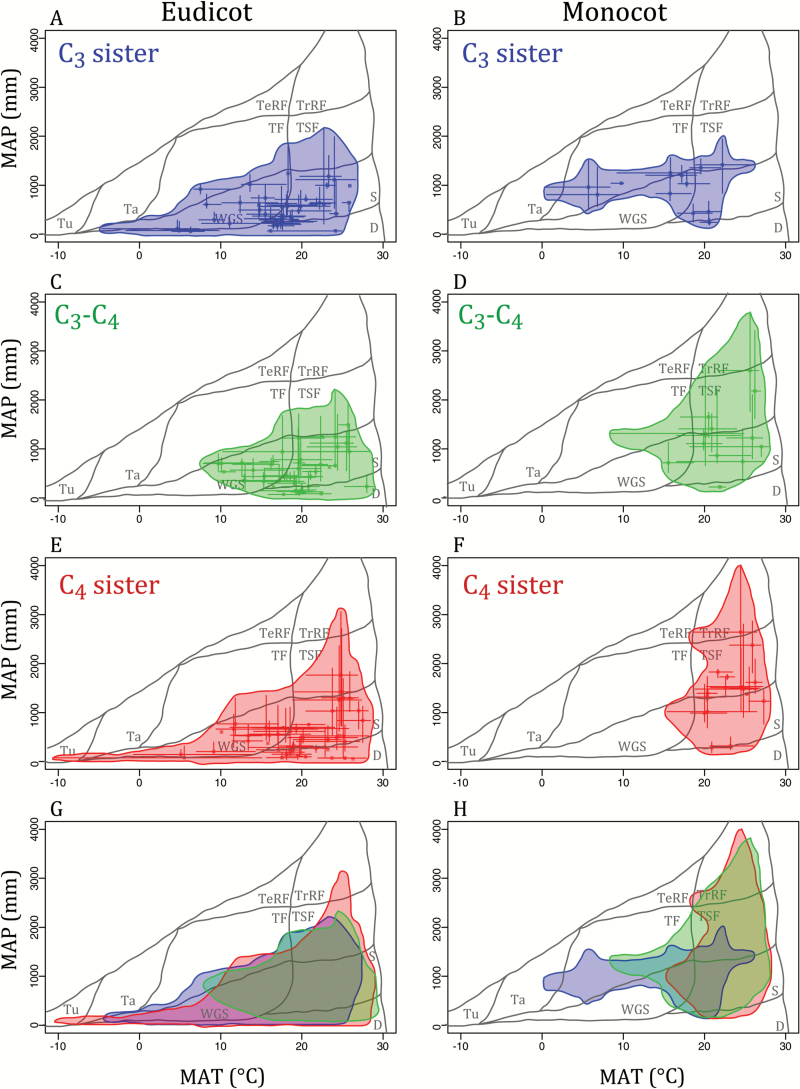
As a whole, C3–C4 taxa are broadly distributed across environments, inhabiting a variety of warm biomes, from tropical rainforests to deserts (Fig. 2C, D and Tables 1 and 2). In particular, C3–C4 eudicots are distributed within tropical seasonal forests, savannas, the woodland/grassland/shrubland habitats, temperate forests, and deserts (Fig. 2C, G). C3–C4 monocots are primarily distributed within tropical seasonal forests and savannas (Fig. 2D, H). Unlike C3–C4 eudicots, they are largely excluded from deserts and are present in tropical rainforests. They also have a smaller presence in the woodland/grassland/shrubland habitats than eudicot intermediates (Fig. 2D, H).
Fig. 2.
Comparative C3–C4 distributions across biomes. The median ± 10th and 90th quantiles for mean annual temperature (MAT) and precipitation (MAP) are plotted for eudicot (left) and monocot (right) C3 sister (blue; A, B), C3–C4 (green, C, D), and C4 sister (red, E, F) taxa. The bottom row overlaps the three distributions for eudicots (left, G) and monocots (right, H). All panels contain biome classifications (see Ricklefs, 2008) for tropical rainforest (TrRF), temperate rainforest (TeRF), temperate forest (TF), tropical seasonal forest (TSF), woodland/grassland/shrubland (WGS), savanna (S), desert (D), taiga (Ta), and tundra (Tu).
While the exact conditions in which the plants grow are not captured by average climatic variables, especially for annuals, annual precipitation may be virtually absent (e.g. Mollugo verticillata in the warm coastal deserts of Peru) or over 7700 mm (e.g. Homolepis aturensis in the tropical rainforests of Colombia) in habitats supporting C3–C4 intermediates (Table 2 and Supplementary Dataset S1). C3–C4 plants can inhabit regions with mean annual temperatures just below zero (e.g. Diplotaxis muralis, Diplotaxis tenuifolia, Eleocharis flavescens), but also as high as 30°C (e.g. Paramollugo nudicaulis; Table 2 and Supplementary Dataset S1). They exist in areas with winter temperatures down to –25°C (e.g. Diplotaxis muralis and Mollugo verticillata in Ontario and Saskatchewan, Canada) and 285 days of frost per year (e.g. Mollugo verticillata in the Rocky Mountains of Colorado and Eleocharis flavescens in the Andes of Chile) and growing season temperatures as low as –10°C (e.g. Eleocharis flavescens in Wyoming) but also above 32°C (e.g. Paramollugo nudicaulis in Pakistan, Heliotropium convolvulacea in California, Eleocharis atropurpurea in Western Australia, and Cleome paradoxa in Ethiopia; Supplementary Dataset S1). These broad climatic variables do not encapsulate the micro-environment of each species. Of the plants that inhabit the coldest climates, Mollugo verticillata and Diplotaxis muralis are annuals, and the perennial Eleocharis flavescens occurs in aquatic environments connected to warm thermal water (Simpson and Simpson, 2015). However, these regional climatic variables do highlight the broad-scale variation among C3–C4 taxa. The broad ecological distribution of C3–C4 taxa found in the global raster datasets is supported by species-specific habitat descriptions from the literature (Table 1). These descriptions report C3–C4 plants from deciduous woodlands, grasslands, wetlands, scrublands, and mountainous slopes, as well as from a variety of soil types (e.g. from fine-textured, to sandy, gravelly, and rocky soils; Table 1).
Phylogenetic effects on the sorting of C3–C4 taxa and their C4 relatives
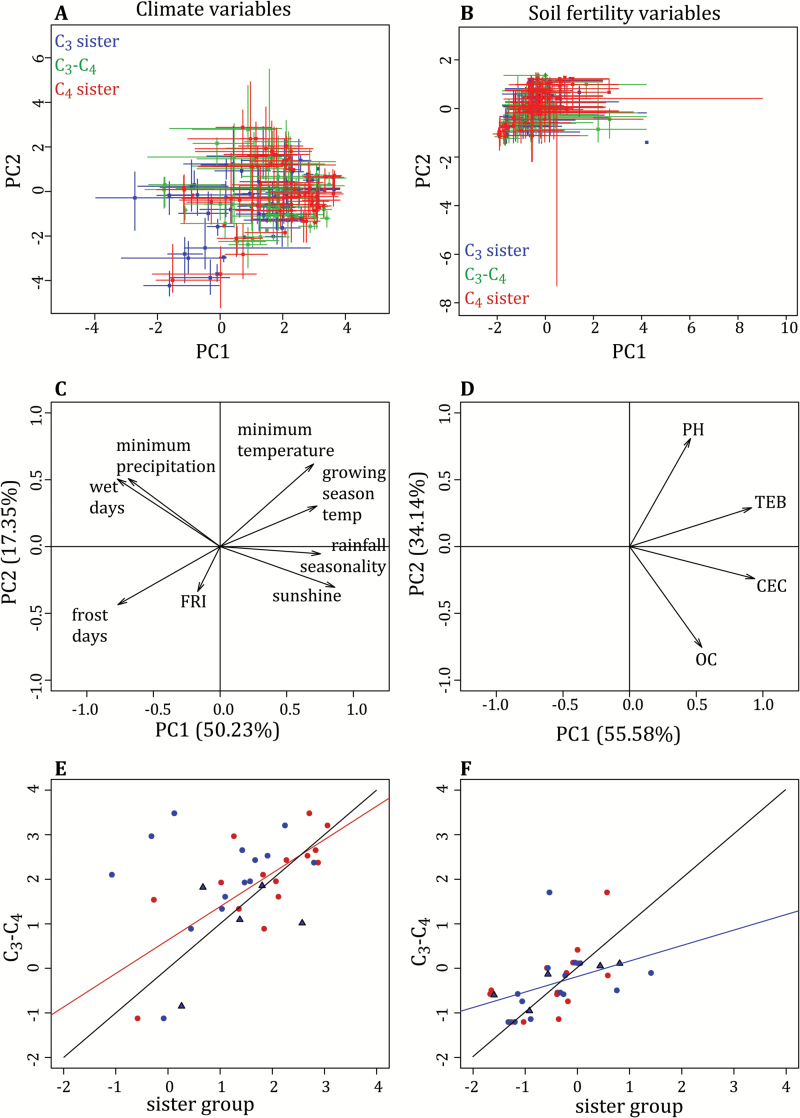
The C3 relatives of C3–C4 lineages occur in a variety of temperature regimes from dry habitats to moderately wet ones, a pattern that is similar in monocot and eudicot systems (Fig. 2A, B). The medians of the C3–C4 lineages are widely distributed along the primary PCA axis for climatic variables, which explains 50.23% of the variation, and these are not correlated to those of their close C3 relatives (Fig. 3A, C, E and Table 3). However, the soil fertility conditions experienced by C3–C4 plants, extracted from the primary PCA axis for soil variables, which explains 55.58% of the variation, are correlated to those of their C3 relatives, which might be driven by topsoil TEB (Fig. 3B, D, F and Tables 3 and 4). Similarly, variation in minimum precipitation experienced by C3–C4 lineages is correlated to that of closely related C3 lineages (Fig. 4C and Table 3), indicating a strong phylogenetic effect.
Fig. 3.
Distribution of photosynthetic types in ecological space. The median ± 10th and 90th quantiles for the first two principal component axes (PC1 and PC2) of the climate (A) and soil fertility (B) PCAs for C3 sister (blue), C3–C4 (green), and C4 sister (red) taxa. The associated variable factor maps for the climate and soil fertility PCAs are shown in (C, D). Shifts in the primary axis of the climatic (E) and soil fertility (F) PCAs, as comparisons between C3–C4 taxa and their closely related C3 (blue) and C4 (red) sister taxa within each phylogenetic group. Comparisons of C3–C4 taxa and their C3 relatives in groups that lack close C4 relatives are presented as blue triangles. Black lines indicate the 1:1 relationship. Linear relationships are shown for correlations significant after correction for multiple testing (P<0.00625), in the relevant color (see Table 3).
Table 3.
Kendall correlation tests for environmental medians among photosynthetic types across angiosperms
| C3–C4vs. C3 | C4vs. C3–C4 | |||
|---|---|---|---|---|
| Variable | P-value | tau | P-value | tau |
| Climate PCA axis 1 | 0.27 | 0.19 | 0.0059* | 0.52 |
| Soils PCA axis 1 | 0.0032* | 0.50 | 0.02 | 0.46 |
| Growth season temperature | 0.14 | 0.25 | 0.03 | 0.42 |
| Minimum temperature | 0.78 | –0.05 | 0.85 | 0.05 |
| Minimum precipitation | 0.0041* | 0.48 | 0.0025* | 0.59 |
| Rainfall seasonality | 0.07 | 0.31 | 0.0011* | 0.63 |
| Topsoil organic content | 0.92 | 0.02 | 0.02 | 0.47 |
| Total exchangeable bases | 0.04 | 0.34 | 0.03 | 0.42 |
* Tests that were considered significant, using a threshold of 0.00625, which corresponds to a 0.05 threshold corrected for eight tests.
Table 4.
Tests for environmental shifts among photosynthetic types across angiosperms
| C3–C4vs. C3 (all lineages) |
C3–C4vs. C3 (only lineages with close C4 relatives) |
C4vs. C3–C4 | ||||
|---|---|---|---|---|---|---|
| Variable | Observeda | P-value | Observeda | P-value | Observeda | P-value |
| Climate PCA axis 1 | 14/19 | 0.019 | 12/14 | 0.0018* | 8/15 | 0.61 |
| Soil fertility PCA axis 1 | 10/19 | 0.65 | 8/14 | 0.42 | 6/15 | 0.61 |
| Growth season temperature | 14/19 | 0.019 | 13/14 | 0.00012* | 5/15 | 0.30 |
| Minimum temperature | 13/19 | 0.064 | 12/14 | 0.0018* | 8/15 | 0.61 |
| Minimum precipitation | 7/19 | 0.36 | 3/14 | 0.057 | 7/15 | 1 |
| Rainfall seasonality | 14/19 | 0.019 | 12/14 | 0.0018* | 6/15 | 0.61 |
| Topsoil organic content | 11/19 | 0.36 | 6/14 | 0.79 | 5/15 | 0.30 |
| Total exchangeable bases | 8/19 | 0.65 | 5/14 | 0.42 | 6/15 | 0.61 |
a The number of points higher in the focal group is indicated.
* Tests that were considered significant, using a threshold of 0.00625, which corresponds to a 0.05 threshold corrected for eight tests.
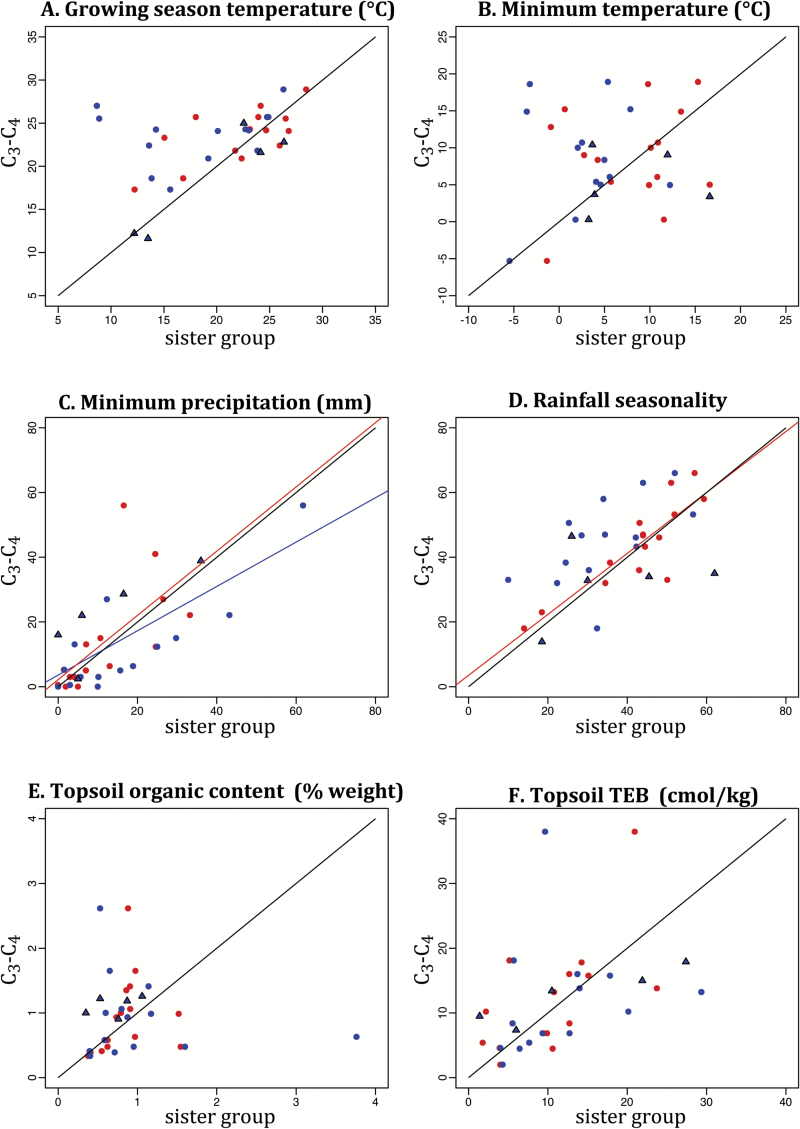
Fig. 4.
Ecological shifts between photosynthetic types. Shifts in growing season temperature, minimum temperature of the coldest month, minimum precipitation of the driest month, rainfall seasonality, topsoil organic matter content, and topsoil total exchangeable bases (as labelled) between C3–C4 taxa and their C3 (blue) and C4 (red) close relatives were evaluated. Each point represents an average for all species within each comparison group (see Methods). Comparisons of C3–C4 taxa and their C3 relatives in groups that lack close C4 relatives are presented as blue triangles. Black lines indicate the 1:1 relationship. Linear relationships are shown for correlations significant after correction for multiple testing (P<0.00625), in the relevant color (see Table 3).
The close C4 relatives of C3–C4 plants exist along a broad range of temperatures in eudicots, but are restricted to warmer areas in monocot species, resulting in less overlap between photosynthetic types in the latter than in the former (Fig. 2E–H). The variation among C4 lineages on the first axis of the climate variable PCA is correlated with that of their C3–C4 relatives (Fig. 3A, E and Table 3), indicating an overall phylogenetic effect on the sorting of C4 lineages. The soil fertility conditions experienced by C4 plants, assessed with the PCA on soil variables, is weakly correlated to that of their C3–C4 relatives (Fig. 3B, F and Table 3). Among the individual variables, the minimum precipitation and rainfall seasonality experienced by C4 lineages are correlated to that of their C3–C4 relatives (Fig. 4C, D and Table 3). Moreover, the growth season temperature and topsoil properties of C4 lineages are also weakly correlated to those of their close C3–C4 relatives; however, these do not remain significant after correcting for multiple tests (Fig. 4A, E, F and Table 3). Thus, the precipitation, and possibly the temperature and soil fertility, preferences of C4 lineages depend, to varying degrees, on phylogenetic effects.
Effects of photosynthetic types after correcting for phylogenetic signals
The five C3–C4 lineages without close C4 relatives do not behave in the same manner as the lineages that did evolve C4 photosynthesis. With the exception of Eleocharis, which contains aquatic plants that grow in warm waters within cold climates, four of these five lineages are those that occupy the coldest environments experienced by intermediate plants (Table 2) and are primarily in habitats with higher minimum precipitation than their C3 relatives (Fig. 4C). All five of these C3–C4 lineages inhabit areas with more organic soils than their close C3 relatives (Fig. 4E). These lineages without C4 relatives are also among the most widely distributed of all intermediates groups (i.e. Diplotaxis, Mollugo verticillata; Supplementary Figs S1 and S2), which likely reflects an ability to tolerate diverse ecological conditions.
Considering the C3–C4 lineages with close C4 relatives, their distributions are significantly shifted toward positive values of the primary axis of the climate variable PCA, which corresponds to drier and warmer environments, compared with their paired C3 relatives (Fig. 3A, C, E and Table 4). This shift is reflected within the individual variables, with C3–C4 lineages occupying regions with warmer growing season temperatures, higher minimum winter temperatures, and more seasonal rainfall patterns than their C3 relatives (Table 4). Therefore C3–C4 intermediates tend to inhabit relatively warm regions, regardless of the habitat in which their C3 relatives occur, while their preference for habitat aridity does depend on the minimum precipitation experienced by their C3 relatives (Fig. 4A–C and Table 4).
None of the studied environmental parameters, including both of the composite PCA variables and the six individual environmental variables, show a significant shift between close C3–C4 and C4 relatives (Table 4). Therefore, with the data available here, the C4 physiology is not linked to consistent ecological shifts when controlling for phylogenetic effects.
Discussion
A uniform C3–C4 niche does not exist
C3–C4 taxa are remarkably widespread across geographical and environmental space, maintaining the ability to exist in both typical C3 and C4 niches (Figs 1–3 and Supplementary Figs S1 and S2). It should be noted that the GBIF occurrence data, if anything, represent a subset of the total geographic range for each species and the realized geographical and environmental ranges of these taxa may be larger than presented here, especially for groups distributed in poorly sampled areas, such as Africa and southeast Asia. However, since related taxa tend to occur in similar regions, a sampling bias would likely affect the different photosynthetic types within a lineage to a similar degree, and the dataset is therefore still representative of the relative distribution of each type. Furthermore, several of the C3–C4 groups likely include more intermediate species than we present here, as we considered only those taxa for which the photosynthetic type has been assessed with confidence. For instance, the photosynthetic type of only one species within the genus Homolepis has been determined (Khoshravesh et al., 2016), while the remaining five congeners have not yet been characterized. The same is true of Eleocharis, where several species have been characterized as only possible intermediates (Roalson et al., 2010) and, as such, were not included in the study. Finally, it is unknown whether the various occurrences for each taxon are using the same photosynthetic type, or whether these vary intraspecifically across space or environments, as has been observed in the grass Alloteropsis semialata (Lundgren et al., 2015, 2016), and suggested for other taxa (e.g. Khoshravesh et al., 2012). When this variation had been reported but not clarified, the taxon was ignored, but in most cases, only a limited number of plants have been screened per species. With these caveats in mind, it is clear that the physiology of C3–C4 plants does not strongly restrict the migration of species geographically or into new environments.
Evolutionary history influences the realized ecology
While differences between sister groups can result from shifts in either group, they do allow for comparisons among character states independent of phylogeny. Interestingly, these analyses clearly show that the precipitation niches of C3–C4 taxa are statistically correlated to those of their close C3 relatives, specifically with respect to minimum precipitation. This suggests that C3–C4 plants can occur in arid habitats if their C3 relatives are already adapted to do so, and not specifically as a result of the C3–C4 physiology. Similarly, statistical evidence indicates that soil preferences of C3–C4 are correlated to those of their close C3 relatives. C3–C4 physiology is only part of the attributes that a plant can use to tolerate environmental conditions, which tend to be similar among relatives (Christin and Osborne, 2014). These attributes, which can include life-history traits, growth strategies, and other non-photosynthetic characters, lead to a certain niche conservatism. Moreover, related taxa tend to occur within the same regions as a function of their biogeography, which increases the likelihood of being found in similar environments. Both precipitation variables are similarly correlated between C3–C4 and C4 relatives, likely explaining previously reported differences among C4 lineages in aridity preferences (Teeri and Stowe, 1976; Stowe and Teeri, 1978; Taub, 2000; Christin and Osborne, 2014). The influence of evolutionary history on the realized C4 niche could go beyond precipitation preference, as our data suggest that temperature and soil fertility between C3–C4 and closely related C4 groups are also associated, although this was not significant with our small species sampling.
C3–C4 species shift closer to the C4 niche
In some cases, C3–C4 lineages emerged from groups that already inhabited warm climates, as reported in C4 grasses (Edwards and Smith, 2010), while in others cases, C3 relatives exist in cold areas (Fig. 3A, C). Independent of C3 ecology, the C3–C4 lineages occupy warm habitats, which might reflect the increased temperature tolerance conferred by the C3–C4 physiology (Schuster and Monson, 1990). Despite some C3–C4 taxa persisting in cold regions, the convergence of physiological intermediates in warmer areas, whether that be in wet forests or dry deserts, may have increased the likelihood of further transitions to a C4 state that occupies a similar temperature niche. Therefore, in terms of temperature, the C3–C4 state brings lineages into warmer habitats that should promote photorespiration and, thus, may encourage selection for C4 physiology, thereby representing a true bridge between the ancestral C3 state and C4 origins. As more detailed phylogenies and updated lists of C3–C4 species become available, further comparative work might be able to distinguish whether this happens via an increase in C3–C4 migrations toward warmer habitats or a decrease in their migrations outside of such habitats, since both scenarios would result in a concentration of C3–C4 lineages in warmer habitats than their C3 relatives.
While precipitation preferences vary tremendously across C3–C4 lineages as a function of evolutionary history, these intermediate lineages shifted toward habitats with more rainfall seasonality than their close C3 relatives, yet no consistent shift was observed between C3–C4 plants and their C4 relatives (Table 4). Phylogenetic models in grasses have previously reported that C4 origins were accompanied by consistent shifts into drier habitats (Edwards and Smith, 2010), a trend that we suggest is initiated in C3–C4 taxa. Direct measurements and modelling efforts have failed to identify increases in water-use efficiency in intermediates of Flaveria, which suggests that the C3–C4 advantage is mainly linked to carbon gain, not water saving (Monson, 1989; Vogan and Sage, 2011; Way et al., 2014). However, the xylem architecture was altered during the transition from C3 to C3–C4 species in Flaveria, providing protection against cavitation and hence increased drought tolerance (Kocacinar et al., 2008). Such alterations of leaf hydraulics, if consistently associated with the C3–C4 type, might explain their observed propensity to migrate to habitats with higher rainfall seasonality, habitats that would promote episodes of water limitations, potentially increasing the pressure for further evolutionary transitions to C4 photosynthesis (Osborne and Sack, 2012), especially in warm habitats where C3–C4 plants tend to occur.
The fate of C3–C4 lineages lacking C4 relatives
Since all of the taxa included in this study still naturally occur in the wild, they have all persisted in a C3–C4 state since their early emergence from C3 ancestors, which is estimated to be as recent as 2 and as old as 20 Ma, depending on the group (Christin et al., 2011a). However, most of the known C3–C4 lineages are related to some C4 groups, which prove that their ancestors had the ability, at least at some point, to produce C4 descendants. Clear exceptions include the closely related groups Diplotaxis and Moricandia, which belong to a large family completely lacking C4 taxa (Brassicaceae). While three other C3–C4 groups (Steinchisma, Mollugo verticillata, Parthenium) belong to families with C4 origins, which are included here for other C3–C4 groups (Poaceae, Molluginaceae, Asteraceae), they are sufficiently distant from any C4 group in their phylogenies that one cannot be sure whether their ancestors were able at any point to produce C4 descendants (Christin et al., 2011b; Grass Phylogeny Working Group II, 2012). It is therefore reasonable to ask whether some attributes of these groups decreased the likelihood of C4 evolution. While genomics, anatomy, and physiology might play a role (Christin et al., 2013; Bräutigam and Gowik, 2016), the ecology might also affect these evolutionary trajectories. For instance, C3–C4Moricandia occurs mainly in colder climates, which might decrease pressure for C4 evolution. Three of the other four C3–C4 groups lacking close C4 relatives are among the most widespread geographically (see Supplementary Figs S1 and S2), and these groups tend to occur in habitats with relatively high minimum precipitation and fertile soil. While none of these factors should prevent C4 evolution in itself, it is possible that the realization of the C3–C4 phenotype in these groups was successful enough to limit selective pressures for further transitions in photosynthesis.
Conclusions
In this study, we present the first systematic description of the geographical and ecological distribution of C3–C4 intermediates. Our investigations reveal that C3–C4 taxa are found in a very large range of conditions and habitats, from dry deserts to tropical rainforests and cold wetlands. This variation is partially explained by evolutionary history, with C3–C4 lineages tending to grow in habitats with similar precipitation to their C3 relatives, a conservatism that is further reported onto C4 lineages. However, C3–C4 taxa inhabit warm climates, independent of the ancestral condition, and shift toward more seasonal rainfall habitats. Our findings indicate that the C3–C4 condition moves lineages into environments that promote photorespiration and, as such, may facilitate the evolution of a full C4 pathway. There is, in our dataset, no clear difference between C3–C4 and C4 in any of the environmental preferences. However, different C4 groups might shift in various directions or extend their niche in ways that are not universal across flowering plants as, for example, it has been suggested that C4 evolution was linked to different pressures in grasses and chenopods (Osborne and Freckleton, 2009; Kadereit et al., 2012). While group-specific detailed analyses might reveal peculiarities of each lineage, our angiosperm-wide joint analysis of C3, C3–C4, and C4 groups helps to disentangle the ecological changes that happened during consecutive phases of C4 evolution. Indeed, shifts toward drier and warmer habitats occurred in C3–C4 lineages, but others, such as geographic expansions, might be specific to the C4 state. When detailed phenotype information becomes available for a larger number of taxa, similar analyses might identify the changes linked to each individual C4 component, bringing together anatomy, biochemistry, physiology, and evolutionary ecology.
Supplementary data
Supplementary data are available at JXB online.
Dataset S1. Occurrence and environmental data for C3–C4 taxa and their close C3 and C4 relatives used in this study.
Fig. S1. Distribution of C3 sister (blue), C3–C4 (green), and C4 sister (red) taxa in eudicot comparison groups.
Fig. S2. Distribution of C3 sister (blue), C3–C4 (green), and C4 sister (red) taxa in monocot comparison groups.
Table S1. Details on the environmental data used in this study.
Table S2. Details of C3–C4 species used in this study and the C3 and C4 sister taxa within each comparison group.
Supplementary Material
Acknowledgements
This work was supported by a European Research Council grant (ERC-2014-STG-638333) and a Royal Society University Research Fellowship (URF120119).
Glossary
Abbreviations:
- CEC
cation exchange capacity
- FRI
fire return interval
- MAP
mean annual precipitation
- MAT
mean annual temperature
- OC
organic carbon
- TEB
total exchangeable bases
- PCA
principal component analysis.
References
- Apel P, Horstmann C, Pfeffer M. 1997. The Moricandia syndrome in species of the Brassicaceae—evolutionary aspects. Photosynthetica 33, 205–215. [Google Scholar]
- Arakaki M, Christin PA, Nyffeler R, Lendel A, Eggli U, Ogburn RM, Spriggs E, Moore MJ, Edwards EJ. 2011. Contemporaneous and recent radiations of the world’s major succulent plant lineages. Proceedings of the National Academy of Sciences of the United States of America 108, 8379–8384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald S, Lehmann CER, Gómez-Dans JL, Bradstock RA. 2013. Defining pyromes and global syndromes of fire regimes. Proceedings of the National Academy of Sciences of the United States of America 110, 6442–6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson RR, Mockford EJ, Bennett C, Christin PA, Spriggs EL, Freckleton RP, Thompson K, Rees M, Osborne CP. 2016. C4 photosynthesis boosts growth by altering physiology, allocation and size. Nature Plants 2, 16038. [DOI] [PubMed] [Google Scholar]
- Bauwe H. 1984. Photosynthetic enzyme activities and immunofluorescence studies on the localization of ribulose-1,5-bisphosphate carboxylase/oxygenase in leaves of C3, C4, and C3-C4 intermediate species of Flaveria (Asteraceae). Biochemie und Physiologie der Pflanzen 179, 253–268. [Google Scholar]
- Bauwe H, Chollet R. 1986. Kinetic properties of phosphoenolpyruvate carboxylase from C3, C4, and C3-C4 intermediate species of Flaveria (Asteraceae). Plant Physiology 82, 695–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bräutigam A, Gowik U. 2016. Photorespiration connects C3 and C4 photosynthesis. Journal of Experimental Botany 67, 2953–2962. [DOI] [PubMed] [Google Scholar]
- Chamberlain S, Ram K, Barve V, Mcglinn D. 2016. rgbif: Interface to the Global ‘Biodiversity’ Information Facility ‘API’. R package version 0.9.3 https://CRAN.R-project.org/package=rgbif.
- Christin PA, Osborne CP. 2014. The evolutionary ecology of C4 plants. New Phytologist 204, 765–781. [DOI] [PubMed] [Google Scholar]
- Christin PA, Osborne CP, Chatelet DS, Columbus JT, Besnard G, Hodkinson TR, Garrison LM, Vorontsova MS, Edwards EJ. 2013. Anatomical enablers and the evolution of C4 photosynthesis in grasses. Proceedings of the National Academy of Sciences of the United States of America 110, 1381–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christin PA, Osborne CP, Sage RF, Arakaki M, Edwards EJ. 2011a C4 eudicots are not younger than C4 monocots. Journal of Experimental Botany 62, 3171–3181. [DOI] [PubMed] [Google Scholar]
- Christin PA, Sage TL, Edwards EJ, Ogburn RM, Khoshravesh R, Sage RF. 2011. b. Complex evolutionary transitions and the significance of C3-C4 intermediate forms of photosynthesis in Molluginaceae. Evolution 65, 643–660. [DOI] [PubMed] [Google Scholar]
- Chollet R, Ogren WL. 1975. Regulation of photorespiration in C3 and C4 species. The Botanical Review 41, 137–179. [Google Scholar]
- Edwards EJ, Ogburn RM. 2012. Angiosperm responses to a low-CO2 world: CAM and C4 photosynthesis as parallel evolutionary trajectories. International Journal of Plant Sciences 173, 724–733. [Google Scholar]
- Edwards EJ, Smith SA. 2010. Phylogenetic analyses reveal the shady history of C4 grasses. Proceedings of the National Academy of Sciences of the United States of America 107, 2532–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards EJ, Still CJ. 2008. Climate, phylogeny and the ecological distribution of C4 grasses. Ecology Letters 11, 266–276. [DOI] [PubMed] [Google Scholar]
- Edwards GE, Ku MSB. 1987. Biochemistry of C3–C4 intermediates. In: Hatch MD, Boardman NK, eds. The biochemistry of plants: A comprehensive treatise. Vol 10. Photosynthesis. New York: Academic Press, 275–325. [Google Scholar]
- Ehleringer JR, Cerling TE, Helliker BR. 1997. C4 photosynthesis, atmosphere CO2, and climate. Oecologia 112, 285–299. [DOI] [PubMed] [Google Scholar]
- Eliáš P, Dítě D. 2014. Sedobassia sedoides (Pall.) Freitag & G. Kadereit in Slovakia: native species or alien weed?Acta Fytotechnica et Zootechnica 16, 74–77. [Google Scholar]
- Epstein HE, Lauenroth WK, Burke IC, Coffin DP. 1997. Productivity patterns of C3 and C4 functional types in the U.S. Great Plains. Ecology 78, 722–731. [Google Scholar]
- FAO/IIASA/ISRIC/ISSCAS/JRC 2012. Harmonized World Soil Database (version 1.2). Rome, Italy: FAO; and Laxenburg, Austria: IIASA. [Google Scholar]
- Feodorova TA, Voznesenskaya EV, Edwards GE, Roalson EH. 2010. Biogeographic patterns of diversification and the origins of C4 in Cleome (Cleomaceae). Systematic Botany 35, 811–826. [Google Scholar]
- Fisher AE, McDade LA, Kiel CA, Khoshravesh R, Johnson MA, Stata M, Sage TL, Sage RF. 2015. Evolutionary history of Blepharis (Acanthaceae) and the origin of C4 photosynthesis in section Acanthodium. International Journal of Plant Sciences 176, 770–790. [Google Scholar]
- Freitag H, Kadereit G. 2014. C3 and C4 leaf anatomy types in Camphorosmeae (Camphorosmoideae, Chenopodiaceae). Plant Systematics and Evolution 300, 665–687. [Google Scholar]
- Frohlich MW. 1978. Systematics of Heliotropium section Orthostachys in Mexico. PhD thesis, Harvard University. [Google Scholar]
- Garland T, Harvey PH, Ives AR. 1992. Procedures for the analysis of comparative data using phylogenetically independent contrasts. Systematic Biology 41, 18–32. [Google Scholar]
- Grass Phylogeny Working Group II 2012. New grass phylogeny resolves deep evolutionary relationships and discovers C4 origins. New Phytologist 193, 304–312. [DOI] [PubMed] [Google Scholar]
- Hatch MD. 1987. C4 photosynthesis: a unique blend of modified biochemistry, anatomy and ultrastructure. Biochimica et Biophysica Acta 895, 81–106. [Google Scholar]
- Hatch MD, Osmond CB. 1976. Compartmentation and transport in C4 photosynthesis. Encyclopedia of Plant Physiology 3, 144–184. [Google Scholar]
- Hattersley PW, Wong SC, Perry S, Roksandic Z. 1986. Comparative ultrastructure and gas-exchange characteristics of the C3–C4 intermediate Neurachne minor S. T. Blake (Poaceae). Plant, Cell and Environment 9, 217–233. [Google Scholar]
- Heckmann D, Schulze S, Denton A, Gowik U, Westhoff P, Weber AP, Lercher MJ. 2013. Predicting C4 photosynthesis evolution: modular, individually adaptive steps on a Mount Fuji fitness landscape. Cell 153, 1579–1588. [DOI] [PubMed] [Google Scholar]
- Hedge BA, Patil TM. 1980. Physiological studies on Parthenium hysterophorus under different ecological conditions. Biovigyanam 6, 15–20 [Google Scholar]
- Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. 2005. Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology 25, 1965–1978. [Google Scholar]
- Holaday AS, Chollet R. 1984. Photosynthetic/photorespiratory characteristics of C3–C4 intermediate species. Photosynthesis Research 5, 307–323. [DOI] [PubMed] [Google Scholar]
- Hyde MA, Wursten BT, Ballings P, Coates Palgrave M. 2016a Flora of Mozambique http://www.mozambiqueflora.com/index.php (accessed 22 July 2016).
- Hyde MA, Wursten BT, Ballings P, Coates, Palgrave M. 2016b Flora of Zimbabwe http://www.zimbabweflora.co.zw/index.php (accessed 22 July 2016).
- Hylton CM, Rawsthorne S, Smith AM, Jones DA, Woolhouse HW. 1988. Glycine decarboxylase is confined to the bundle-sheath cells of leaves of C3–C4 intermediate species. Planta 175, 452–459. [DOI] [PubMed] [Google Scholar]
- Kadereit G, Ackerly D, Pirie MD. 2012. A broader model for C4 photosynthesis evolution in plants inferred from the goosefoot family (Chenopodiaceae s.s.). Proceedings of the Royal Society of London. Series B, Biological Sciences 279, 3304–3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy RA, Laetsch WM. 1974. Plant species intermediate for C3, C4 photosynthesis. Science 184, 1087–1089. [DOI] [PubMed] [Google Scholar]
- Khoshravesh R, Hossein A, Sage TL, Nordenstam B, Sage RF. 2012. Phylogeny and photosynthetic pathway distribution in Anticharis Endl. (Scrophulariaceae). Journal of Experimental Botany 63, 5645–5658. [DOI] [PubMed] [Google Scholar]
- Khoshravesh R, Stinson CR, Stata M, Busch FA, Sage RF, Ludwig M, Sage TL. 2016. C3–C4 intermediacy in grasses: organelle enrichment and distribution, glycine decarboxylase expression, and the rise of C2 photosynthesis. Journal of Experimental Botany 67, 3065–3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocacinar F, McKown AD, Sage TL, Sage RF. 2008. Photosynthetic pathway influences xylem structure and function in Flaveria (Asteraceae). Plant, Cell and Environment 31, 1363–1376. [DOI] [PubMed] [Google Scholar]
- Ku MS, Monson RK, Littlejohn RO, Nakamoto H, Fisher DB, Edwards GE. 1983. Photosynthetic characteristics of C3–C4 intermediate Flaveria species: I. Leaf anatomy, photosynthetic responses to O2 and CO2, and activities of key enzymes in the C3 and C4 pathways. Plant Physiology 71, 944–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê S, Josse J, Husson F. 2008. FactoMineR: an R package for multivariate analysis. Journal of Statistical Software 25, 1–18. [Google Scholar]
- Lehmann CE, Archibald SA, Hoffmann WA, Bond WJ. 2011. Deciphering the distribution of the savanna biome. New Phytologist 191, 197–209. [DOI] [PubMed] [Google Scholar]
- Lundgren MR, Besnard G, Ripley BS, et al. 2015. Photosynthetic innovation broadens the niche within a single species. Ecology Letters 18, 1021–1029. [DOI] [PubMed] [Google Scholar]
- Lundgren MR, Christin PA, et al. 2016. Evolutionary implications of C3–C4 intermediates in the grass Alloteropsis semialata. Plant, Cell and Environment 39, 1874–1885. [DOI] [PubMed] [Google Scholar]
- Lundgren MR, Osborne CP, Christin PA. 2014. Deconstructing Kranz anatomy to understand C4 evolution. Journal of Experimental Botany 65, 3357–3369. [DOI] [PubMed] [Google Scholar]
- Lyu MJ, Gowik U, Kelly S, et al. 2015. RNA-Seq based phylogeny recapitulates previous phylogeny of the genus Flaveria (Asteraceae) with some modifications. BMC Evolutionary Biology 15, 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallmann J, Heckmann D, Bräutigam A, Lercher MJ, Weber AP, Westhoff P, Gowik U. 2014. The role of photorespiration during the evolution of C4 photosynthesis in the genus Flaveria. eLife 3, e02478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKown AD, Dengler NG. 2007. Key innovations in the evolution of Kranz anatomy and C4 vein pattern in Flaveria (Asteraceae). American Journal of Botany 94, 382–399. [DOI] [PubMed] [Google Scholar]
- McKown AD, Dengler NG. 2009. Shifts in leaf vein density through accelerated vein formation in C4Flaveria (Asteraceae). Annals of Botany 104, 1085–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKown AD, Moncalvo JM, Dengler NG. 2005. Phylogeny of Flaveria (Asteraceae) and inference of C4 photosynthesis evolution. American Journal of Botany 92, 1911–1928. [DOI] [PubMed] [Google Scholar]
- Monson RK. 1989. The relative contributions of reduced photorespiration, and improved water-and nitrogen-use efficiencies, to the advantages of C3–C4 intermediate photosynthesis in Flaveria. Oecologia 80, 215–221. [DOI] [PubMed] [Google Scholar]
- Monson RK, Moore BD. 1989. On the significance of C3–C4 intermediate photosynthesis to the evolution of C4 photosynthesis. Plant, Cell and Environment 12, 689–699. [Google Scholar]
- Moore BD, Franceschi VR, Cheng SH, Wu J, Ku MS. 1987. Photosynthetic characteristics of the C3–C4 intermediate Parthenium hysterophorus. Plant Physiology 85, 978–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New M, Lister D, Hulme M, Makin I. 2002. A high-resolution data set of surface climate over global land areas. Climate Research 21, 1–25. [Google Scholar]
- Ocampo G, Columbus JT. 2010. Molecular phylogenetics of suborder Cactineae (Caryophyllales), including insights into photosynthetic diversification and historical biogeography. American Journal of Botany 97, 1827–1847. [DOI] [PubMed] [Google Scholar]
- Osborne CP, Freckleton RP. 2009. Ecological selection pressures for C4 photosynthesis in the grasses. Proceedings of the Royal Society of London. Series B, Biological Sciences 276, 1753–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne CP, Sack L. 2012. Evolution of C4 plants: a new hypothesis for an interaction of CO2 and water relations mediated by plant hydraulics. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 367, 583–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell AM. 1978. Systematics of Flaveria (Flaveriinae Asteraceae). Annals of the Missouri Botanical Garden 65, 590–636. [Google Scholar]
- Prendergast HDV, Hattersley PW. 1985. Distribution and cytology of Australian Neurachne and its allies (Poaceae), a group containing C3, C4 and C3–C4 intermediate species. Australian Journal of Botany 33, 317–336. [Google Scholar]
- Rajendrudu G, Prasad JS, Das VS. 1986. C3–C4 intermediate species in Alternanthera (Amaranthaceae): Leaf anatomy, CO2 compensation point, net CO2 exchange and activities of photosynthetic enzymes. Plant Physiology 80, 409–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklefs RE. 2008. The economy of nature, 6th edn. New York: W. H. Freeman. [Google Scholar]
- Roalson EH, Hinchliff CE, Trevisan R, da Silva CR. 2010. Phylogenetic relationships in Eleocharis (Cyperaceae): C4 photosynthesis origins and patterns of diversification in the spikerushes. Systematic Botany 35, 257–271. [Google Scholar]
- Rundel PW. 1980. The ecological distribution of C4 and C3 grasses in the Hawaiian-islands. Oecologia 45, 354–359. [DOI] [PubMed] [Google Scholar]
- Sage RF. 2004. The evolution of C4 photosynthesis. New Phytologist 161, 341–370. [DOI] [PubMed] [Google Scholar]
- Sage RF, Stata M. 2015. Photosynthetic diversity meets biodiversity: the C4 plant example. Journal of Plant Physiology 172, 104–119. [DOI] [PubMed] [Google Scholar]
- Sage RF, Sage TL, Kocacinar F. 2012. Photorespiration and the evolution of C4 photosynthesis. Annual Review of Plant Biology 63, 19–47. [DOI] [PubMed] [Google Scholar]
- Sage RF, Wedin DA, Li M. 1999. The biogeography of C4 photosynthesis: patterns and controlling factors. In: Sage RF, Monson RK, eds. C4 plant biology. San Diego: Academic Press, 313–373. [Google Scholar]
- Sage TL, Busch FA, Johnson DC, et al. 2013. Initial events during the evolution of C4 photosynthesis in C3 species of Flaveria. Plant Physiology 163, 1266–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage TL, Sage RF, Vogan PJ, Rahman B, Johnson DC, Oakley JC, Heckel MA. 2011. The occurrence of C2 photosynthesis in Euphorbia subgenus Chamaesyce (Euphorbiaceae). Journal of Experimental Botany 62, 3183–3195. [DOI] [PubMed] [Google Scholar]
- Schlüter U, Weber AP. 2016. The road to C4 photosynthesis: evolution of a complex trait via intermediary states. Plant and Cell Physiology 57, 881–889. [DOI] [PubMed] [Google Scholar]
- Schuster WS, Monson RK. 1990. An examination of the advantages of C3‐C4 intermediate photosynthesis in warm environments. Plant, Cell and Environment 13, 903–912. [Google Scholar]
- Simpson A, Simpson R. 2015. Nature guide to Yellowstone National Park. Lanham, MD, USA: Rowman & Littlefield. [Google Scholar]
- Spriggs EL, Christin PA, Edwards EJ. 2014. C4 photosynthesis promoted species diversification during the Miocene grassland expansion. PLoS One 9, e97722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowe LG, Teeri JA. 1978. The geographic distribution of C4 species of the dicotyledonae in relation to climate. American Naturalist 985, 609–623. [Google Scholar]
- Sudderth EA, Espinosa-García FJ, Holbrook NM. 2009. Geographic distributions and physiological characteristics of co-existing Flaveria species in south-central Mexico. Flora 204, 89–98. [Google Scholar]
- Svensson P, Bläsing OE, Westhoff P. 2003. Evolution of C4 phosphoenolpyruvate carboxylase. Archives of Biochemistry and Biophysics 414, 180–188. [DOI] [PubMed] [Google Scholar]
- Taub DR. 2000. Climate and the U.S. distribution of C4 grass subfamilies and decarboxylation variants of C4 photosynthesis. American Journal of Botany 87, 1211–1215. [PubMed] [Google Scholar]
- Teeri JA, Stowe LG. 1976. Climatic patterns and the distribution of C4 grasses in North America. Oecologica 23, 1–12. [DOI] [PubMed] [Google Scholar]
- USDA/NRCS 2016. The PLANTS Database. Greensboro, NC, USA: National Plant Data Team; http://plants.usda.gov (accessed 22 July 2016). [Google Scholar]
- Vamosi SM, Vamosi JC. 2005. Endless tests: guidelines for analysing non-nested sister-group comparisons. Evolutionary Ecology Research 7, 567–579. [Google Scholar]
- Vogan PJ, Frohlich MW, Sage RF. 2007. The functional significance of C3–C4 intermediate traits in Heliotropium L. (Boraginaceae): gas exchange perspectives. Plant, Cell and Environment 30, 1337–1345. [DOI] [PubMed] [Google Scholar]
- Vogan PJ, Sage RF. 2011. Water-use efficiency and nitrogen-use efficiency of C3–C4 intermediate species of Flaveria Juss. (Asteraceae). Plant, Cell and Environment 34, 1415–1430. [DOI] [PubMed] [Google Scholar]
- Voznesenskaya EV, Koteyeva NK, Chuong SD, Ivanova AN, Barroca J, Craven LA, Edwards GE. 2007. Physiological, anatomical and biochemical characterisation of photosynthetic types in genus Cleome (Cleomaceae). Functional Plant Biology 34, 247–267. [DOI] [PubMed] [Google Scholar]
- Voznesenskaya EV, Koteyeva NK, Edwards GE, Ocampo G. 2010. Revealing diversity in structural and biochemical forms of C4 photosynthesis and a C3–C4 intermediate in genus Portulaca L. (Portulacaceae). Journal of Experimental Botany 61, 3647–3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voznesenskaya EV, Koteyeva NK, Akhani H, Roalson EH, Edwards GE. 2013. Structural and physiological analyses in Salsoleae (Chenopodiaceae) indicate multiple transitions among C3, intermediate, and C4 photosynthesis. Journal of Experimental Botany 64, 3583–3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way DA, Katul GG, Manzoni S, Vico G. 2014. Increasing water use efficiency along the C3 to C4 evolutionary pathway: a stomatal optimization perspective. Journal of Experimental Botany 65, 3683–3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BP, Johnston IG, Covshoff S, Hibberd JM. 2013. Phenotypic landscape inference reveals multiple evolutionary paths to C4 photosynthesis. eLife 2, e00961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DG, Mack RN, Black RA. 1995. Ecophysiology of introduced Pennisetum setaceum on Hawaii: the role of phenotypic plasticity. Ecology 76, 1569–1580. [Google Scholar]
- Zanne AE, Tank DC, Cornwell WK, et al. 2014. Three keys to the radiation of angiosperms into freezing environments. Nature 506, 89–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.