Abstract
Recent data have demonstrated the potential of sphingosine 1-phosphate (S1P) receptor (S1PR) agonism in the treatment of infectious diseases. A previous study used a murine model of Bordetella pertussis infection to demonstrate that treatment with the S1PR agonist AAL-R reduces pulmonary inflammation during infection. In the current study, we showed that this effect is mediated via the S1PR1 on LysM+ (myeloid) cells. Signaling via this receptor results in reduced lung inflammation and cellular recruitment as well as reduced morbidity and mortality in a neonatal mouse model of disease. Despite the fact that S1PRs are pertussis toxin–sensitive G protein-coupled receptors, the effects of AAL-R were pertussis toxin insensitive in our model. Furthermore, our data demonstrate that S1PR agonist administration may be effective at therapeutic time points. These results indicate a role for S1P signaling in B. pertussis–mediated pathology and highlight the possibility of host-targeted therapy for pertussis.
Keywords: Bordetella, sphingosine 1-phosphate, AAL-R, CYM-5442, host-directed therapy
Pertussis (whooping cough) is a respiratory disease caused by acute infection with the gram-negative bacterium Bordetella pertussis. Symptoms progress to episodes of paroxysmal coughing, often persisting for several weeks after initial onset [1]. In unvaccinated infants pertussis can be fatal, with pneumonia, pulmonary hypertension, and high-level circulating leukocytosis [2]. Pertussis is the only vaccine-preventable bacterial infectious disease currently on the rise in the United States. In 2012, there were 48 277 reported cases, the highest number since 1955 [3]. Pertussis is also prevalent in adults and adolescents and may be responsible for a significant proportion of adult cough disease [4, 5]. A major hurdle in the fight against pertussis is the lack of effective therapeutic treatments [6]. Because antibiotics rarely help affected individuals [7], development of novel host-directed therapies may be the only effective approach to treat pertussis.
Previous studies, using mice infected with a wild-type (WT) pertussis toxin (PT)–producing B. pertussis strain or an isogenic PT-deficient strain (ΔPT), had shown that PT activity was associated with up-regulated expression of proinflammatory cytokines and chemokines [8, 9]. Mice inoculated with the ΔPT strain had transient lung inflammation and pathology that was resolved within 1–2 weeks, whereas PT-producing WT bacteria exacerbated and prolonged inflammatory pathology [9]. Because PT does not directly damage host cells but inhibits signaling through G protein-coupled receptors (GPCRs) coupled to Gi proteins, we hypothesized that one or more of these GPCRs mediate attenuation and resolution of lung inflammation during infection and that PT inhibition of these receptors causes the exacerbating effect.
One possible host GPCR target for this PT effect is sphingosine 1-phosphate (S1P) receptors (S1PRs). S1P is a sphingolipid metabolite formed after phosphorylation of sphingosine by 2 sphingosine kinases, SphK1 and SphK2 [10]. S1P signals through 5 GPCRs (S1PR1–5), which couple to heterotrimeric G proteins of the Gi subclass [11]. S1P signaling is involved in regulation of many cellular processes important to health and disease [12]. S1P regulates T-lymphocyte differentiation and trafficking, as well as inflammatory and allergic responses in several systems [13, 14]. In the lung, S1P promotes endothelial barrier integrity [15] and can attenuate lipopolysaccharide-mediated inflammatory pathology [16]. Administration of S1PR ligands attenuates the pulmonary cytokine storm and inflammatory pathology associated with influenza virus infection [17–20]. Therefore, we hypothesized that PT inhibits S1PR-mediated attenuation of airway inflammatory responses exacerbating airway inflammatory pathology during B. pertussis infection.
The S1P signaling pathway represents an attractive target for development of host-directed treatments for pertussis. Skerry et al [21] previously showed that prophylactic treatment of B. pertussis–infected mice with the S1PR ligand AAL-R, which binds 4 of the 5 S1PRs [22], significantly reduces lung inflammatory pathology due to the infection. In the current study, we extended those findings to show that the S1PR1 on myeloid cells seems to be the relevant target for this effect, that therapeutic administration of S1PR ligands during infection is also effective in reducing lung inflammation, and surprisingly that this effect is not inhibited by PT. We also show that treatment of B. pertussis–infected neonatal mice with these ligands reduces lethality.
METHODS
Bacterial Strains
The B. pertussis strain used in the current study, WT, is a streptomycin-resistant derivative of Tohama I [23]. B. pertussis was grown on Bordet-Gengou agar plates supplemented with 10% defibrinated sheep blood and 200 µg/mL streptomycin.
Mouse Infections
C57BL/6 mice (Charles River Laboratories or bred in house) were used in accordance with the University of Maryland, Baltimore, Institutional Animal Care and Use Committee. Bacterial inoculum was prepared in a phosphate-buffered saline (PBS) suspension after 48 hours growth on Bordet-Gengou agar. Adult mice (6–8-week-old) were anesthetized with isoflurane, and the inoculum was administered intranasally. AAL-R and CYM-5442 [12] prepared in sterile water (0.5 and 2 mg/kg, respectively). Sterile water was used as a vehicle control. Lungs were removed for bacterial counts, histology, and RNA purification. For neonatal infections, litters of 7-day-old mice were allowed to inhale the bacterial suspension (or PBS as control) for 20 minutes in an aerosol chamber. PT pretreatment was involved intranasal administration of purified toxin (100 ng) 48 hours before infection.
RNA Isolation and Processing
Lung tissue was snap-frozen on harvest, using a dry ice-isopropanol bath. RNA was extracted using RNA Stat60 (TelTest), according to the manufacturer's instructions. In brief, samples were homogenized in RNA Stat60 using an Omni TH mixer (Omni), phase separated with chloroform, and precipitated with isopropanol. Quantitative real-time polymerase chain reaction (PCR) was performed with Maxima SYBR green/ROX quantitative PCR master mix (Thermo Scientific) in an Applied Biosystems 7500 Fast real-time PCR system. The hypoxanthine phosphoribosyltransferase gene (HPRT) was used as an internal housekeeping control gene, with all other genes normalized to HPRT and expression calculated as fold change compared with PBS-inoculated control (2[−ΔΔCT] method).
Pathology
Lungs were intracardially perfused with PBS and removed into 10% (wt/vol) buffered formalin (Sigma). Hematoxylin-eosin staining was performed by the Pathology, EM and Histology Laboratory (University of Maryland, Baltimore). Histopathological findings were scored on a scale of 0–3, with 3 the most severe, for each of (1) the degree of inflammation at the site of the bronchovascular bundle, (2) the percentage of bronchovascular bundle involved, and (3) the degree of tissue consolidation observed.
Generation of LysM+S1PR1−/− Mice
Mice with S1PR1 deleted specifically in LysM+ cells (LysM+S1PR1−/– mice) were in the C57BL/6J background. LysM+S1PR1−/– mice were generated by crossing LysMCre mice [24] (Jackson Laboratory) with S1PR1-floxed mice [25] (kindly provided by Richard L. Proia, National Institutes of Health). Genotyping was done by PCR using primers 5′CCCAGAAATGCCAGATTACG3′, 5′CTTGGGCTGCCAGAATTTCTC3′, and 5′TTACAGTCGGCCAGGCTGAC3′ for the cre allele and 5′GAGCGGAGGAAGTTAAAAGTG3′ and 5′CCTCCTAAGAGATTGCAGCAA3′ for the floxed allele. S1PR1f/fCre+/+ and S1PR1f/fCre+/0 mice were used as LysM+S1PR1−/– mice, and their S1PR1f/fCre0/0 littermates were used as WT controls.
Leukocytosis
Whole blood was harvested by cardiac puncture into a chilled ethylenediaminetetraacetic acid–K2–coated tube (Milian). Red blood cells were then lysed in ammonium-chloride-potassium lysis buffer (Quality Biological). The white blood cell-containing pellet was resuspended in PBS before counting on a hemocytometer.
Statistical Analysis
Graphs were plotted and data analyzed using GraphPad Prism 6.0 software. Fold changes, for real-time PCR, were calculated per mouse compared with the average value obtained for the respective PBS/water-inoculated group. All plots represent the mean values with SDs. Significance was determined with Student t test, using GraphPad Prism software.
RESULTS
Effect of Early S1PR1-Specific Signaling on Lung Inflammatory Pathology
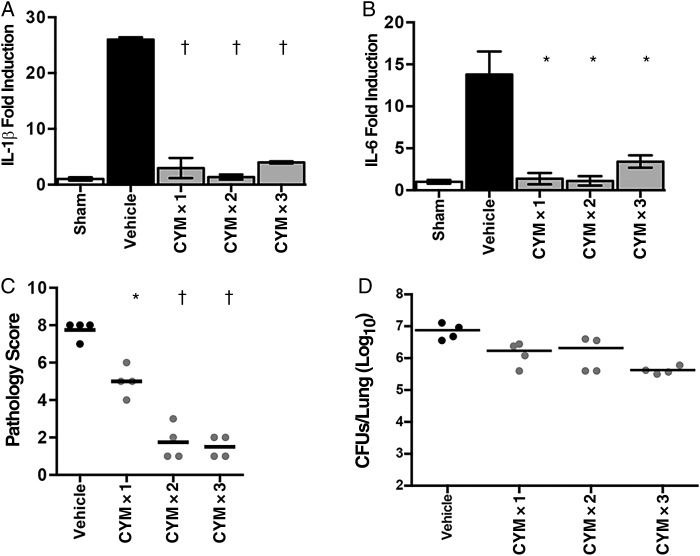
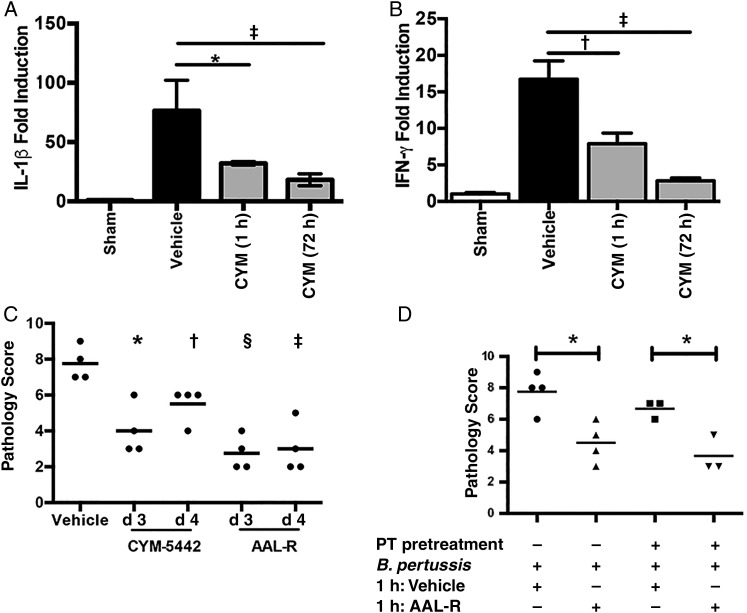
Skerry et al [21] showed previously that intranasal administration of the S1PR agonist AAL-R reduces inflammatory pathology in the murine model of B. pertussis. AAL-R signals via 4 of the 5 S1PRs (S1PR1, S1PR3, S1PR4, and S1PR5) [22]. Administration of S1PR1-specific agonists reduced pathology in a murine influenza model by suppressing the cytokine storm associated with infection [18]. To determine whether the reduced pathology observed after AAL-R treatment of B. pertussis–infected mice could be reproduced by signaling specifically via the S1PR1 receptor, animals were treated with S1PR1-specific agonist CYM-5442 as a single (1 hour after inoculation), double (1 and 6 hours after inoculation) or triple (1, 6, and 24 hours after inoculation) dose or with vehicle control (water). Treatment with even a single dose of CYM-5442 1 hour after inoculation resulted in significant reductions in lung inflammatory cytokine gene expression (interleukin 1β and interleukin 6; Figure 1A and 1B) 4 days after inoculation. From this, we hypothesized that S1PR1 agonism early in the course of infection could inhibit pulmonary pathology. Indeed treatment with single, double, or triple doses of CYM-5442 significantly reduced inflammatory pathology, as assessed by histological examination 7 days after inoculation (Figure 1C), even though lung bacterial loads were not significantly reduced by this treatment (Figure 1D). Unlike the effect of the nonspecific S1PR agonist AAL-R, of which a single dose was maximally effective at reducing lung pathology [21], the effect of CYM-5442 seemed to be dose dependent (Figure 1C), possibly owing to the reduced half-life of CYM-5442 [26].
Figure 1.
Effect of sphingosine 1-phosphate receptor 1 (S1PR1) agonism on murine pertussis disease. A, B, Relative gene expression levels of interleukin 1β (IL-1β) (A) and interleukin 6 (IL-6) (B), as measured by quantitative polymerase chain reaction from lung RNA of animals treated with a sham inoculum of phosphate-buffered saline (white bars), Bordetella pertussis followed by administration of vehicle (black bars) or B. pertussis followed by administration of S1PR1 agonist CYM-5442 at 1 hour (CYM × 1), 1 and 6 hours (CYM × 2) or 1, 6, and 24 hours (CYM × 3) after inoculation (gray bars). C, Impact of CYM-5442 on pulmonary pathology, which was assessed after hematoxylin-eosin staining, by 2 independent researchers, based on the degree and breadth of bronchovascular bundle inflammation and tissue consolidation. D, Pulmonary bacterial burden after S1PR1 agonism. Results are presented as means and SDs. *P < .01; †P < .001 (vs vehicle control). Abbreviation: CFUs, colony-forming units.
Requirement of S1PR1 on LysM+ Cells for Suppression of B. pertussis-Induced Inflammation
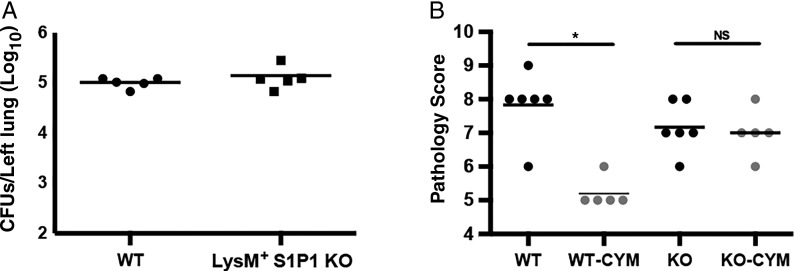
The S1PR1 receptor is found on the surface of a wide variety of cell types, influencing a broad array of pathways [27]. Murine S1PR1 knockout mutations are embryonically lethal [28], but conditional knockouts exist [29]. In the current study, we used a mouse strain lacking S1PR1 specifically on LysM+ cells (derived as described in Methods). Because LysM is expressed exclusively on cells of the myeloid lineage [24], myeloid cells in these mice lack S1PR1, but LysM-negative cells retain functional S1PR1. Lack of S1PR1 on myeloid cells had no impact on the ability of animals to control B. pertussis infection (Figure 2A; P = .41). However, treatment of animals lacking S1PR1 on LysM+ cells with S1PR1-specific agonist CYM-5442 failed to reduce lung inflammatory pathology (Figure 2B; P = .71), whereas treatment reduced pathology in WT control animals (Figure 2B; P < .01). From this, we conclude that myeloid cells are essential for CYM-5442-mediated reduction in B. pertussis pathology.
Figure 2.
Agonism of sphingosine 1-phosphate receptor 1 (S1PR1) on LysM+ cells is required for CYM-5442-mediated reduction in pathology. A, Bacterial colony-forming units (CFU) counts were determined from the lungs of C57BL/6 (wild-type [WT]) mice or mice lacking the S1PR1 receptor exclusively on LysM+ cells (LysM+ S1PR1 knockout [KO]) at 7 days after inoculation. B, Pulmonary pathology compared in WT or LysM+ S1PR1 KO mice (KO) with administration of vehicle or CYM-5442 (CYM) 1 hour after inoculation. Pathology was assessed 7 days after inoculation, after hematoxylin-eosin staining, by 2 independent researchers based on the degree and breadth of bronchovascular bundle inflammation and tissue consolidation. Results are presented as means with SDs. *P < .01. Abbreviation: NS, not significant (P > .05)
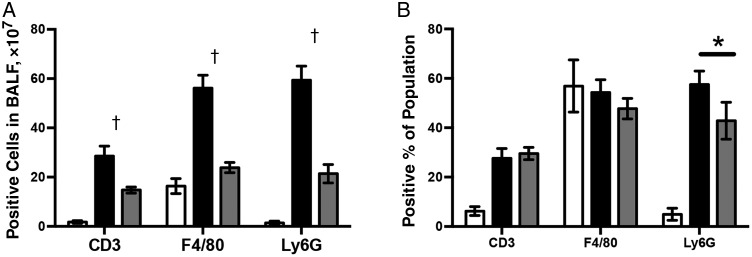
After observing that S1PR agonism reduced pulmonary pathology during B. pertussis infection, we sought to assess its impact on the recruitment of both specific cell types and total cell recruitment to the lungs. Analysis of the total numbers and types of cells present in bronchoalveolar lavage fluid by flow cytometry revealed that on day 7 of B. pertussis infection large numbers of inflammatory cells were recruited to the airways (Figure 3A). AAL-R administration early after inoculation (1 hour) reduced both the total number and the proportion of neutrophils recruited to the site of infection at 7 days after inoculation but had a minimal effect on the proportion of macrophages and T cells (Figure 3B).
Figure 3.
Sphingosine 1-phosphate receptor (S1PR) agonism reduces cellular recruitment to the lungs after Bordetella pertussis infection. A, Total cell numbers in bronchoalveolar lavage fluid (BALF) isolated from animals receiving a sham inoculum of phosphate-buffered saline (PBS) (white bars), B. pertussis inoculation followed by delivery of vehicle (black bars) 1 hour later, or B. pertussis infection followed by delivery of S1PR agonist AAL-R (gray bars) 1 hour later. B, Percentage of cells isolated from the BALF of animals receiving PBS (white bars), B. pertussis followed by vehicle (black bars), or B. pertussis followed by S1PR agonist AAL-R (gray bars) were stained for T-cell marker CD3, macrophage marker F4/80, or neutrophil marker Ly6G. Populations were assessed using the LSR-II flow cytometer. Results are presented as means with SDs. *P < .05; †P < .01.
Reduction of Inflammatory Cytokine Expression in a Human Monocyte Cell Line by S1PR Agonism
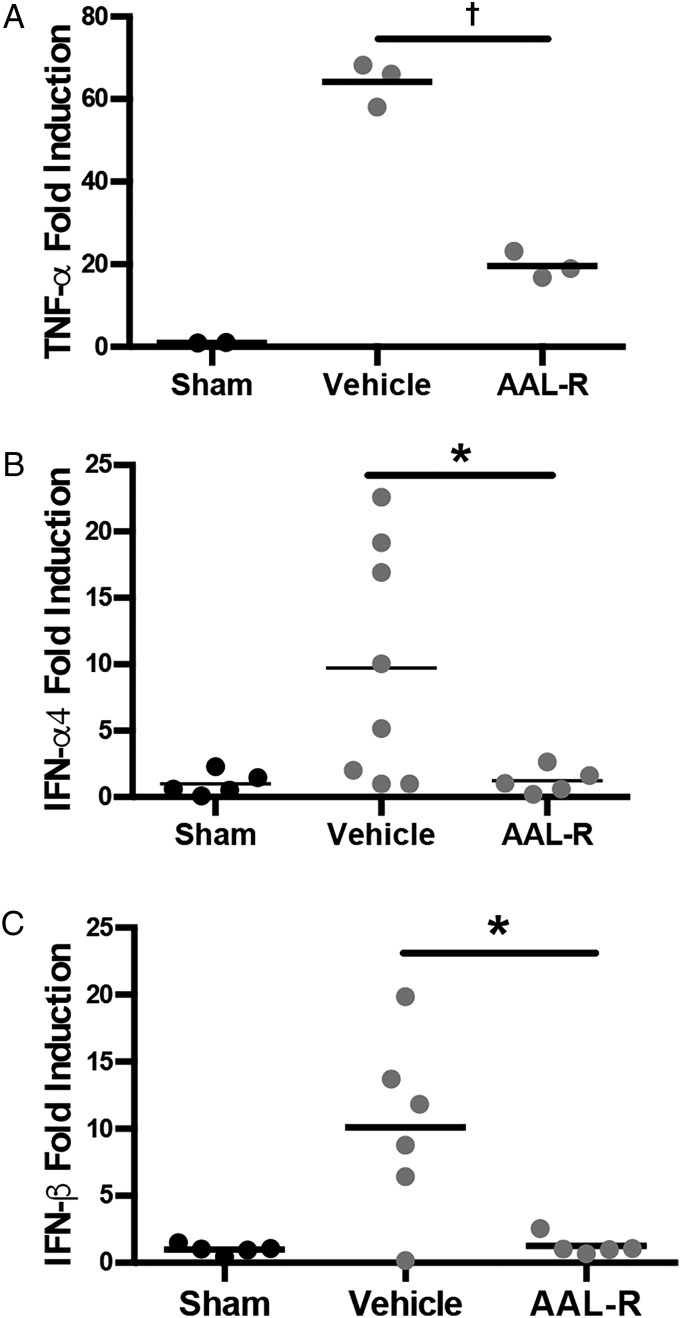
Next we sought to reproduce our in vivo data in an in vitro macrophage model of B. pertussis infection. In response to B. pertussis exposure, the human monocyte cell line THP-1 produced robust inflammatory gene expression responses (TNF-α is shown, Figure 4A). The addition of AAL-R at the time of infection significantly reduced these responses (P < .01). In an influenza virus infection model, S1PR agonists reduced inflammatory responses in myeloid cells by suppressing type I interferon (IFN) production [30]. To test the impact of type I IFNs in B. pertussis infection and subsequent S1PR agonist treatment, we challenged THP-1 cells with B. pertussis in the presence or absence of AAL-R. In response to infection, THP-1 cells demonstrated up-regulation of type I IFN genes IFN-α4 and IFN-β (Figure 4B and 4C). This induction of type I IFN genes was completely inhibited by AAL-R administration (Figure 4B and 4C). These data suggest that type I IFN production may play a role in the induction of B. pertussis–induced lung inflammatory pathology and its reduction mediated by AAL-R.
Figure 4.
Sphingosine 1-phosphate receptor (S1PR) agonism reduces inflammatory cytokine gene expression in a human monocyte cell-line exposed to Bordetella pertussis. THP-1 cells were challenged with B. pertussis followed by either vehicle control or S1PR agonist AAL-R. RNA was isolated from cells 6 hours after infection and transcriptional levels of tumor necrosis factor (TNF) α (A), interferon (IFN) α4 (B), or IFN-β (C) were assessed. Results are presented as means with SDs. *P < .05; †P < .001.
Effectiveness of Delayed Administration of S1PR Agonists
Treatment of mice with S1PR1-specific agonist CYM-5442 or nonspecific S1PR agonist AAL-R shortly after B. pertussis inoculation is effective in reducing lung pathology ([21]; Figure 1). However, because B. pertussis infection in humans is difficult to diagnose at early stages, early treatment is unlikely to occur. Therefore, we sought to determine whether S1P agonism can be delayed while maintaining effectiveness. In the murine model of B. pertussis infection, robust expression of inflammatory cytokines can be detected from 4 days after inoculation [9, 31]. When treatment of B. pertussis–infected mice with S1PR1 agonist CYM-5442 was delayed until day 3 after inoculation, blunted inflammatory cytokine expression at day 4 was still noted (Figure 5A and 5B). Furthermore, S1PR agonism was effective at reducing pulmonary inflammatory pathology at day 7 if delivered up to 4 days after inoculation (Figure 5C). This demonstrates that S1PR agonism can be effective when administered therapeutically later in the course of disease.
Figure 5.
Sphingosine 1-phosphate receptor (S1PR) agonism is effective at therapeutic time points and is pertussis toxin (PT) insensitive. C57BL/6 animals were inoculated with Bordetella pertussis or a sham inoculum of phosphate-buffered saline (PBS) (sham) before treatment with CYM-5442 1 hour after inoculation (CYM [1 h]), CYM-5442 at 72 hours after inoculation (CYM [72 h]), or vehicle at 72 hours after inoculation (vehicle). Lung RNA was isolated 4 days after inoculation for assessment of transcriptional levels of interleukin 1β (IL-1β) (A) or interferon (IFN) γ (B) with quantitative polymerase chain reaction. C, Lung pathology was assessed after hematoxylin-eosin staining, by 2 independent researchers based on the degree and breadth of bronchovascular bundle inflammation and tissue consolidation. D, S1PR agonist-mediated suppression of inflammatory pathology is not PT sensitive. At 48 hours before inoculation, animals received either PT pretreatment (100 ng) or PBS control. All animals were then inoculated with B. pertussis before treatment 1 hour later with S1PR agonist (AAL-R) or vehicle control. Results are presented as mean and SDs. *P < .05; †P < .01; ‡P < .005; §P < .001.
PT Insensitivity of Pathology-Reducing Effect of S1PR Agonism
Because PT is known to exacerbate and prolong B. pertussis lung inflammatory pathology in the mouse model [9], we hypothesized that PT inactivation of S1PR-mediated G protein signaling prevents endogenous S1P-mediated attenuation of pathology during the course of infection. However, agonist delivery remained effective at 96 hours after inoculation (Figure 5), despite the production of PT by B. pertussis during the infection. To determine whether the effect of S1PR agonist administration is PT, we pretreated animals with PT (48 hours before inoculation) to allow inactivation of G protein signaling, before infection and agonist delivery. PT pretreatment failed to prevent AAL-R-mediated reduction in pathology (Figure 5D), indicating that agonist signaling through S1PR1 may not be via the G protein pathway.
Reduction of Morbidity and Mortality in Neonatal Mice by S1PR Ligand Treatment
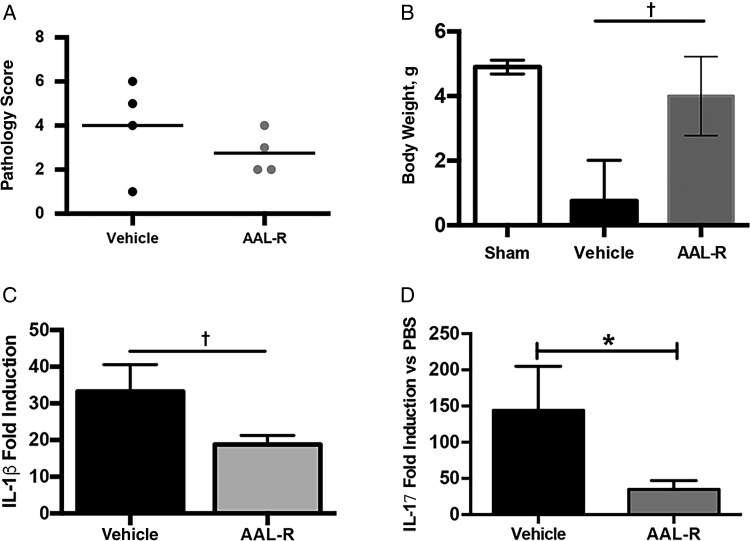
The neonatal mouse model of sublethal B. pertussis infection is characterized by robust proinflammatory cytokine expression, leukocytosis, and stunted growth [32, 33]. To determine the potential of S1PR agonism in neonatal disease, 7-day-old mice were challenged with B. pertussis and treated with a single dose of AAL-R 1 hour after inoculation. Lung bacterial burden, inflammatory cytokine gene expression, histopathology, and changes in body weight were monitored 14 days after inoculation. Despite no differences in bacterial burden (data not shown) or lung pathology, animals receiving S1PR agonist treatment gained significantly more weight than control animals (Figure 6; P < .01). AAL-R-mediated reduction in lung inflammatory cytokine gene expression was also noted (Figure 6).
Figure 6.
Sphingosine 1-phosphate receptor agonist AAL-R reduces morbidity in neonatal mice with sublethal Bordetella pertussis infection. Seven-day-old mice were infected with B. pertussis via aerosol and treated 1 hour after inoculation with AAL-R or vehicle. A, Lung pathology was assessed after hematoxylin-eosin staining, by 2 independent researchers based on the degree and breadth of bronchovascular bundle inflammation and tissue consolidation. Results are presented as means with SDs. B, Change in body weight between day 0 and 14 days after inoculation. C, D, Inflammatory cytokine gene expression was measured by means of quantitative polymerase chain reaction using RNA extracted from the lungs of animals receiving vehicle (water) or AAL-R 1 hour after inoculation. *P < .05; †P < .001. Abbreviations: IL-1β, interleukin 1β; IL-17, interleukin 17; PBS, phosphate-buffered saline.
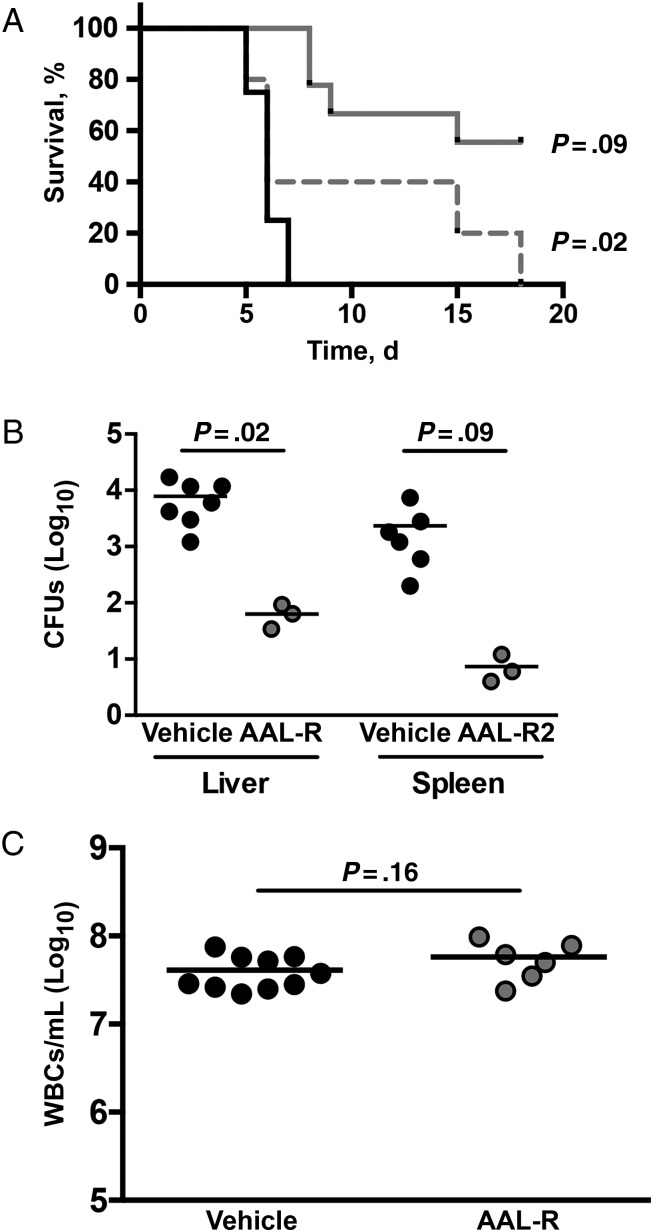
As in adult humans, adult mice clear B. pertussis infection without intervention, but high-dose inoculation of neonatal mice can be fatal. In our study, we used a model of lethal neonatal infection to assess the ability of S1PR agonism to increase survival after infection. In mouse pups receiving vehicle control after B. pertussis inoculation, there was 0% survival by 7 days after inoculation (Figure 7). A single dose of AAL-R administered 1 hour after inoculation significantly improved survival rates compared with vehicle-treated littermates (Figure 7; P < .01). Delaying the delivery of agonist by 3 days also seemed to improve survival, but this difference failed to reach significance (Figure 7; P = .20).
Figure 7.
Sphingosine 1-phosphate receptor (S1PR) agonism reduces the mortality rate in a lethal model of neonatal pertussis infection. Seven-day-old mice were challenged via aerosol with a lethal dose of Bordetella pertussis before receiving either S1PR agonist AAL-R or vehicle intranasally 1 or 72 hours after inoculation. A, Animals receiving vehicle (black line), AAL-R at 1 hour (solid gray line) or 72 hours (dashed gray line) after inoculation were monitored daily for survival. B, On day 6 after inoculation, bacterial burden was assessed from liver and spleen to measure disseminated infection. C, To determine whether AAL-R affected leukocytosis, whole blood was collected on day 6 after inoculation before white blood cell (WBC) counts. Abbreviation: CFUs, colony-forming units.
These data suggest that S1PR agonism may be sufficient to rescue mice from a potentially lethal infection. Pertussis death in human infants has been linked to high levels of leukocytosis, leading to pulmonary hypertension [34]. To determine whether S1PR agonism affected leukocytosis, white blood cells counts were determined in agonist- or vehicle-treated neonatal mice. Surprisingly, S1PR agonism had little impact of the numbers of leukocytes circulating during infection (Figure 7B), demonstrating that leukocytosis is not the sole cause of death in these mice. We have also seen dissemination of B. pertussis to other organs after aerosol inoculation of neonatal mice (K. S., Yael Snyder, C. S., N. H. C., manuscript in preparation). We noted a significant reduction in the number of bacteria in the spleen and liver when comparing animals receiving S1PR agonism with vehicle controls (Figure 7C), indicating that S1PR agonism affect bacterial dissemination in neonatal mice.
DISCUSSION
Despite mass vaccination campaigns, whooping cough incidence in the United States has reached a level not observed since the 1950s [35]. Further complicating this problem, antibiotic treatment is beneficial only when disease is diagnosed early [7, 36]. Despite this, little headway has been made in the discovery of novel therapeutics for pertussis. In the current study, we continued our work examining the potential of S1PR signaling in quelling pertussis pathogenesis. We showed that the S1PR1 receptor on cells of the myeloid lineage mediates reduction in lung pathology observed in agonist-treated animals during B. pertussis infection. After stimulation of S1PR1 on myeloid cells, neutrophil recruitment to the site of infection is dampened. S1PR1 agonism is effective at reducing pathology when administered at potentially therapeutic time points and this effect does not appear to be PT-sensitive. B. pertussis–infected neonatal mice treated with S1PR agonist gained more weight and expressed lower levels of inflammatory cytokines than vehicle-treated counterparts. Importantly, S1PR agonist treatment reduced mortality rates in neonatal survival studies.
S1P mediates its diverse biological functions via 5 GPCRs (S1PR1–5). Previously, Skerry et al [37] showed that early administration of S1PR agonist AAL-R, which signals through 4 of the 5 S1PRs, reduces inflammatory cytokine expression and pulmonary pathology in response to B. pertussis infection. In the current study, we used S1PR1-specific agonist CYM-5442 to demonstrated that signaling via S1PR1 is sufficient to reduce pathology and inflammatory cytokine expression in B. pertussis–infected mice without altering bacterial burden. Because S1P uses 5 different receptors with varying expression patterns throughout the body to produce a broad range of effects [12], limiting the number of receptors whose agonism is required to treat pertussis may limit the potential for adverse effects. This allows for the more targeted design of host-directed therapeutics specific for the S1PR1 pathway for pertussis treatment.
This is illustrated by effects of the S1PR agonist FTY720 (Fingolimod), which is used clinically to treat relapsing-remitting multiple sclerosis. Like AAL-R, FTY720 signals through 4 of the 5 S1PRs [38], but some studies show that its beneficial effects are mediated through S1PR1 while adverse effects are mediated through S1P3 [39, 40]. Furthermore, we observed no differences in ability to clear infection between agonist- or vehicle-treated animals. This is an important finding considering the potential immune dampening effect of these agonists [41].
S1PR agonism has been used successfully to improve disease outcome in a murine model of influenza [18]. This was found to be via suppression of the type I IFN autoamplification loop after S1PR1 signaling on plasmacytoid dendritic cells [30]. Null mutations to the S1PR1 gene are embryonically lethal, complicating studies on its role in disease. In our current study, we took advantage of a mouse model lacking S1PR1 specifically on LysM+ cells to demonstrate that S1PR1 signaling on myeloid cells is responsible for CYM-5442 mediated reduction in pathology in B. pertussis–infected mice. Previous data implicated pulmonary endothelial cells in the S1PR agonist response in the influenza model [18]. However, our finding indicates that the endothelial response is less important than that of LysM+ immune cells, consistent with the previous data indicating a role for plasmacytoid dendritic cells in the agonist-mediated response [30].
Our work so far has highlighted the potential for S1PR1 agonists as pertussis therapeutics. However, drug administration at time points early after inoculation does not represent therapeutic treatment. Our data in this study suggest that S1PR agonist treatment as late as 4 days after inoculation significantly reduced pulmonary histopathology demonstrates the therapeutic potential of this approach. It is difficult to compare the timing between human and mouse disease, especially because mice do not cough, but inflammatory events in the lungs of infected mice are well underway by day 4 after inoculation [9]. A better test of the therapeutic potential of this treatment for pertussis would be the recently developed baboon model, in animals that cough and develop inflammation in the lungs [42].
We initially hypothesized that PT exacerbates inflammation during B. pertussis infection by preventing endogenous S1P signaling through a G protein pathway. However, administration of purified PT to animals before S1PR1 agonism, to inhibit G protein signaling, was found to have no inhibitory effects on S1PR agonist treatment. This is supported by work from Teijaro et al [30] demonstrating that S1P agonist–mediated anti-inflammatory effects after influenza virus infection were not PT-sensitive. S1PR1, a GPCR, can interact with cytoplasmic signaling proteins via the third intracellular loop (Gi/Go proteins) or through its C-terminal tail (GRK2, β-arrestin) [43]. In the studies on influenza virus wherein S1PR agonism reduced inflammatory responses in a PT-insensitive manner, Teijaro et al [30] used an inhibitory peptide mimic of the S1PR1 C-terminus to demonstrate that the effect of CYM-5442 occurs via the C-terminal tail, presumably through β-arrestin signaling. If S1PR1 signaling after agonist treatment in our model is via β-arrestin as opposed to the G protein, it may explain the PT-insensitive nature of our findings. Another possibility is that S1PR agonists as lipids may gain access to cells or locations that are inaccessible to the protein toxin PT. In either case, the fact that PT does not inhibit the beneficial effect of S1PR agonist treatment during B. pertussis infection is encouraging for its therapeutic potential.
Pertussis disease is most severe in infants. Neonatal mice inoculated with relatively low doses of B. pertussis survive infection, but higher doses are fatal [44]. In our sublethal neonatal mouse model, S1PR agonism resulted in reduced inflammatory cytokine gene expression and increased weight gain compared with controls. These results demonstrate improved health after infection in S1PR agonist-treated pups versus controls despite a lack of significant differences in pulmonary pathology. In neonatal mice inoculated with a lethal dose of B. pertussis, treatment with a single dose of S1PR agonist 1 hour after inoculation significantly extended survival, and there was an indication of improved survival when treatment was delayed until day 3 after inoculation. Current treatment options for severe pertussis in infants are limited [45], and our results suggest that S1PR agonism has the potential to improve severe disease outcome or possibly even prevent pertussis fatalities.
Our work is intended to highlight a novel pathway's potential as a host-directed antipertussis therapeutic. Taken together, these results further support the potential use of S1PR agonists as a pertussis treatment. We identified S1PR1 as a key receptor and cells of the myeloid lineage as key targets in the therapeutic dampening of pertussis inflammation. Agonists of these receptors can be administered at clinically relevant time points with beneficial effects. The further elucidation of relevant mechanisms of action of these drugs will provide an increased ability to manipulate the host response to infection for therapeutic gain, to identify effective postexposure antipertussis therapeutics.
Notes
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (Public Health Service grants AI-119566 and AI-117095.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Heininger U, Klich K, Stehr K, Cherry JD. Clinical findings in Bordetella pertussis infections: results of a prospective multicenter surveillance study. Pediatrics 1997; 100:E10. [DOI] [PubMed] [Google Scholar]
- 2. Paddock CD, Sanden GN, Cherry JD et al. . Pathology and pathogenesis of fatal Bordetella pertussis infection in infants. Clin Infect Dis 2008; 47:328–38. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Pertussis outbreaks. https://www.cdc.gov/pertussis/outbreaks/trends.html. Accessed 29 August 2016.
- 4. Birkebaek NH, Kristiansen M, Seefeldt T et al. . Bordetella pertussis and chronic cough in adults. Clin Infect Dis 1999; 29:1239–42. [DOI] [PubMed] [Google Scholar]
- 5. De Serres G, Shadmani R, Duval B et al. . Morbidity of pertussis in adolescents and adults. J Infect Dis 2000; 182:174–9. [DOI] [PubMed] [Google Scholar]
- 6. Wang K, Bettiol S, Thompson MJ et al. . Symptomatic treatment of the cough in whooping cough. Cochrane Database Syst Rev 2014; 9:CD003257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Altunaiji S, Kukuruzovic R, Curtis N, Massie J. Antibiotics for whooping cough (pertussis). Cochrane Database Syst Rev 2007:CD004404 doi:10.1002/14651858.CD004404.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Andreasen C, Powell DA, Carbonetti NH. Pertussis toxin stimulates IL-17 production in response to Bordetella pertussis infection in mice. PLoS One 2009; 4:e7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Connelly CE, Sun Y, Carbonetti NH. Pertussis toxin exacerbates and prolongs airway inflammatory responses during Bordetella pertussis infection. Infect Immun 2012; 80:4317–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu H, Chakravarty D, Maceyka M, Milstien S, Spiegel S. Sphingosine kinases: a novel family of lipid kinases. Prog Nucleic Acid Res Mol Biol 2002; 71:493–511. [DOI] [PubMed] [Google Scholar]
- 11. Sanchez T, Hla T. Structural and functional characteristics of S1P receptors. J Cell Biochem 2004; 92:913–22. [DOI] [PubMed] [Google Scholar]
- 12. Maceyka M, Harikumar KB, Milstien S, Spiegel S. Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol 2012; 22:50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chi H. Sphingosine-1-phosphate and immune regulation: trafficking and beyond. Trends Pharmacol Sci 2011; 32:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Spiegel S, Milstien S. The outs and the ins of sphingosine-1-phosphate in immunity. Nat Rev Immunol 2011; 11:403–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Garcia JG, Liu F, Verin AD et al. . Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest 2001; 108:689–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Peng X, Hassoun PM, Sammani S et al. . Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. Am J Respir Crit Care Med 2004; 169:1245–51. [DOI] [PubMed] [Google Scholar]
- 17. Marsolais D, Hahm B, Walsh KB et al. . A critical role for the sphingosine analog AAL-R in dampening the cytokine response during influenza virus infection. Proc Natl Acad Sci U S A 2009; 106:1560–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Teijaro JR, Walsh KB, Cahalan S et al. . Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell 2011; 146:980–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Walsh KB, Teijaro JR, Brock LG et al. . Animal model of respiratory syncytial virus: CD8+ T cells cause a cytokine storm that is chemically tractable by sphingosine-1-phosphate 1 receptor agonist therapy. J Virol 2014; 88:6281–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Teijaro JR, Walsh KB, Long JP et al. . Protection of ferrets from pulmonary injury due to H1N1 2009 influenza virus infection: immunopathology tractable by sphingosine-1-phosphate 1 receptor agonist therapy. Virology 2014; 452-453:152–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Skerry C, Scanlon K, Rosen H, Carbonetti NH. Sphingosine-1-phosphate receptor agonism reduces Bordetella pertussis-mediated lung pathology. J Infect Dis 2015; 211:1883–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marsolais D, Hahm B, Edelmann KH et al. . Local not systemic modulation of dendritic cell S1P receptors in lung blunts virus-specific immune responses to influenza. Mol Pharmacol 2008; 74:896–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Carbonetti NH, Artamonova GV, Mays RM, Worthington ZE. Pertussis toxin plays an early role in respiratory tract colonization by Bordetella pertussis. Infect Immun 2003; 71:6358–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res 1999; 8:265–77. [DOI] [PubMed] [Google Scholar]
- 25. Allende ML, Yamashita T, Proia RL. G-protein-coupled receptor S1P1 acts within endothelial cells to regulate vascular maturation. Blood 2003; 102:3665–7. [DOI] [PubMed] [Google Scholar]
- 26. Gonzalez-Cabrera PJ, Jo E, Sanna MG et al. . Full pharmacological efficacy of a novel S1P1 agonist that does not require S1P-like headgroup interactions. Mol Pharmacol 2008; 74:1308–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. O'Sullivan C, Dev KK. The structure and function of the S1P1 receptor. Trends Pharmacol Sci 2013; 34:401–12. [DOI] [PubMed] [Google Scholar]
- 28. Liu Y, Wada R, Yamashita T et al. . Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J Clin Invest 2000; 106:951–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Clay H, Wilsbacher LD, Wilson SJ et al. . Sphingosine 1-phosphate receptor-1 in cardiomyocytes is required for normal cardiac development. Dev Biol 2016; 418:157–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Teijaro JR, Studer S, Leaf N et al. . S1PR1-mediated IFNAR1 degradation modulates plasmacytoid dendritic cell interferon-alpha autoamplification. Proc Natl Acad Sci U S A 2016; 113:1351–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Scanlon KM, Gau Y, Zhu J et al. . Epithelial anion transporter pendrin contributes to inflammatory lung pathology in mouse models of Bordetella pertussis infection. Infect Immun 2014; 82:4212–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pittman M, Furman BL, Wardlaw AC. Bordetella pertussis respiratory tract infection in the mouse: pathophysiological responses. J Infect Dis 1980; 142:56–66. [DOI] [PubMed] [Google Scholar]
- 33. Shahin RD, Witvliet MH, Manclark CR. Mechanism of pertussis toxin B oligomer-mediated protection against Bordetella pertussis respiratory infection. Infect Immun 1990; 58:4063–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Carbonetti NH. Pertussis leukocytosis: mechanisms, clinical relevance and treatment. Pathog Dis 2016. pii:ftw087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Clark TA. Changing pertussis epidemiology: everything old is new again. J Infect Dis 2014; 209:978–81. [DOI] [PubMed] [Google Scholar]
- 36. Carlsson RM, von Segebaden K, Bergstrom J, Kling AM, Nilsson L. Surveillance of infant pertussis in Sweden 1998-2012; severity of disease in relation to the national vaccination programme. Euro Surveill 2015; 20 pii:21032. [DOI] [PubMed] [Google Scholar]
- 37. Skerry C, Scanlon K, Rosen H, Carbonetti NH. Sphingosine-1-phosphate receptor agonism reduces Bordetella pertussis-mediated lung pathology. J Infect Dis 2015; 211:1883–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brinkmann V, Davis MD, Heise CE et al. . The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem 2002; 277:21453–7. [DOI] [PubMed] [Google Scholar]
- 39. Forrest M, Sun SY, Hajdu R et al. . Immune cell regulation and cardiovascular effects of sphingosine 1-phosphate receptor agonists in rodents are mediated via distinct receptor subtypes. J Pharmacol Exp Ther 2004; 309:758–68. [DOI] [PubMed] [Google Scholar]
- 40. Sanna MG, Liao J, Jo E et al. . Sphingosine 1-phosphate (S1P) receptor subtypes S1P1 and S1P3, respectively, regulate lymphocyte recirculation and heart rate. J Biol Chem 2004; 279:13839–48. [DOI] [PubMed] [Google Scholar]
- 41. Luessi F, Kraus S, Trinschek B et al. . FTY720 (fingolimod) treatment tips the balance towards less immunogenic antigen-presenting cells in patients with multiple sclerosis. Mult Scler 2015; 21:1811–22. [DOI] [PubMed] [Google Scholar]
- 42. Warfel JM, Beren J, Kelly VK, Lee G, Merkel TJ. Nonhuman primate model of pertussis. Infect Immun 2012; 80:1530–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rosen H, Stevens RC, Hanson M, Roberts E, Oldstone MB. Sphingosine-1-phosphate and its receptors: structure, signaling, and influence. Annu Rev Biochem 2013; 82:637–62. [DOI] [PubMed] [Google Scholar]
- 44. Weiss AA, Goodwin MS. Lethal infection by Bordetella pertussis mutants in the infant mouse model. Infect Immun 1989; 57:3757–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Scanlon KM, Skerry C, Carbonetti NH. Novel therapies for the treatment of pertussis disease. Pathog Dis 2015; 73:ftv074. [DOI] [PMC free article] [PubMed] [Google Scholar]