Ottelia alismoides grown at low CO2 operates CAM during the night and C4 metabolism during the day. Night-time accumulation of acid is controlled by CO2. Decarboxylation is controlled by light.
Keywords: Aquatic plant, C4, CAM, CCMs, PEPC, photosynthesis, PPDK, Rubisco, starch
Abstract
Ottelia alismoides is a constitutive C4 plant and bicarbonate user, and has facultative crassulacean acid metabolism (CAM) at low CO2. Acclimation to a factorial combination of light and CO2 showed that the ratio of phosphoenolpyruvate carboxylase (PEPC) to ribulose-bisphosphate carboxylase/oxygenase (Rubisco) (>5) is in the range of that of C4 plants. This and short-term response experiments showed that the activity of PEPC and pyruvate phosphate dikinase (PPDK) was high even at the end of the night, consistent with night-time acid accumulation and daytime carbon fixation. The diel acidity change was maximal at high light and low CO2 at 17–25 µequiv g−1 FW. Decarboxylation proceeded at ~2–3 µequiv g−1 FW h−1, starting at the beginning of the photoperiod, but did not occur at high CO2; the rate was greater at high, compared with low light. There was an inverse relationship between starch formation and acidity loss. Acidity changes account for up to 21% of starch production and stimulate early morning photosynthesis, but night-time accumulation of acid traps <6% of respiratory carbon release. Ottelia alismoides is the only known species to operate CAM and C4 in the same tissue, and one of only two known aquatic species to operate CAM and bicarbonate use.
Introduction
In terrestrial environments, some photoautotrophic plants have evolved carbon dioxide-concentrating mechanisms (CCMs), such as C4 and crassulacean acid metabolism (CAM), that allow them to maximize carbon uptake when temperature is high or water restricted, or both (Keeley and Rundel, 2003; Herrera, 2009; Silvera et al., 2010; Sage et al., 2012). In contrast, freshwater plants have CCMs that overcome the problem of limited inorganic carbon supply which arises from several factors (Madsen and Maberly, 1991; Vadstrup and Madsen, 1995; Maberly and Madsen, 2002). First, the rate of CO2 diffusion in water is ~10 000 times lower than in air, limiting the rate of transport of CO2 into freshwater plants through the external boundary layer (Raven, 1970; Black et al., 1981; Maberly, 2014). Consequently, the CO2 concentration required to half-saturate the net photosynthesis of freshwater plants is ~8–14 times greater than air equilibrium (Maberly and Madsen, 1998). Secondly, in productive systems, the concentration of CO2 can be depleted close to zero when the demand for inorganic carbon by community photosynthesis exceeds the supply from the atmosphere, the catchment, and heterotrophic regions such as sediments.
In addition, freshwater plants have evolved a diversity of strategies that counter CO2 limitation (Klavsen et al., 2011). Physiological or biochemical strategies involve CCMs because they increase the concentration of CO2 around the active site of ribulose-bisphosphate carboxylase/oxygenase (Rubisco) (Bowes and Salvucci, 1989; Maberly and Madsen, 2002; Raven et al., 2008). The most frequent CCM in freshwater plants is based on the biophysical uptake of bicarbonate, which is found in >50% of tested species (Maberly and Madsen, 2002). In addition, the two biochemical CCMs in terrestrial plants, C4 and CAM, are also found in freshwater plants. Both depend on carbon fixation via the enzyme phosphoenolpyruvate carboxylase (PEPC) that is active during either the day (C4) or the night (CAM) (Keeley, 1981; Bowes and Salvucci, 1989; Bowes et al., 2002; Keeley and Rundel, 2003).
The C4 pathway in freshwater plants is analogous to that in terrestrial C4 plants, and the percentage of species with this syndrome in both systems is ~3% (Zhang et al., 2014). In freshwater plants, C4 metabolism has been observed in Hydrilla verticillata, Egeria densa, and Ottelia alismoides from the Hydrocharitaceae, and in some other species including Sagittaria subulata from the Alismataceae, the grasses Orcuttia californica and O. viscida, and the sedge Eleocharis acicularis (Bowes and Salvucci, 1989; Madsen and Sand-Jensen, 1991; Keeley, 1998a; Casati et al., 2000; Zhang et al., 2014). In most terrestrial C4 plants, PEPC and Rubisco are located in different cells, but some species from the Chenopodiaceae, in saline semi-deserts, operate single-cell C4 photosynthesis (Voznesenskaya et al., 2001). In freshwater plants, C4 occurs within a single cell in the few plants studied (Bowes, 2011). Terrestrial C4 is generally constitutive, while it is facultative in H. verticillata and E. densa, being induced when inorganic carbon is limiting (Van et al., 1976; Reiskind et al., 1997;Casati et al., 2000). In contrast, it is constitutive in O. alismoides (Zhang et al., 2014).
The CAM pathway enables plants to fix CO2 at night via PEPC and store it as malic acid in the vacuole. During the day, malic acid is decarboxylated and the released CO2 is fixed by Rubisco, entering the Calvin–Benson cycle (Cushman and Bohnert, 1999; Nimmo, 2000). The frequency of species with CAM in terrestrial and freshwater environments is ~6% (Zhang et al., 2014). Freshwater CAM plants have been observed in the following genera: Crassula, Deinostema, Isoetes, Littorella, Ottelia, Sagittaria, and Vallisneria (Keeley, 1981, 1982, 1998b; Aulio, 1986; Klavsen et al., 2011; Pedersen et al., 2011; Zhang et al., 2014; Yang and Liu, 2015; Yin et al., 2017). Unlike terrestrial CAM plants where stomatal closure suppresses CO2 uptake during the day, daytime uptake of exogenous CO2 can occur in freshwater plants, allowing them to assimilate exogenous CO2 continuously (Keeley, 1998b; Madsen et al., 2002). The expanded time scale for inorganic carbon uptake enhances the daily carbon supply and facilitates the recapture of respired CO2 at night.
CAM metabolism is a plastic process in freshwater plants (Bowes and Salvucci, 1989). Regulation can involve long-term acclimation (over weeks or months) or short-term exposure (over a 24 h cycle) to environmental change, including variation in water, light, CO2, temperature, and nutrients (Keeley et al., 1983; Aulio, 1985; Madsen, 1987; Robe and Griffiths, 1990; Hostrup and Wiegleb, 1991; Klavsen and Maberly, 2010). CAM activity is dependent on the interactions among these environmental variables and particularly light (Robe and Griffiths, 1990; Rattray et al., 1992) and CO2 (Richardson et al., 1984; Zhang et al., 2014).
Ottelia alismoides is a member of the Hydrocharitaceae, and, perhaps uniquely, has three CCMs: use of bicarbonate and C4 metabolism that are constitutive, and CAM that is facultative (Zhang et al., 2014). This raises the question of how these three CCMs interact and are regulated over different time scales, especially in response to variable CO2 and light. The aims of this study were: (i) to investigate the long-term regulation of inorganic carbon uptake in O. alismoides, including the interactive effects on C4 and CAM, in response to variable light and CO2 concentration; and (ii) to assess the short-term effect of changes in light and CO2 on the daily CAM cycle.
Materials and methods
Plant material
Ottelia alismoides that had been collected from Yunnan Province, China, was cultivated in a greenhouse at the Wuhan Botanical Garden for several years. Seeds from these plants were sown in six plastic containers (10 × 10 × 10 cm) filled with 5 cm of soil from nearby Donghu Lake and covered with 3 cm of sterile tap water. The containers were placed in a growth chamber at 25 °C and the water was replaced completely each day to maintain constant conditions. After several days, the seeds began to germinate and, after 6 weeks, when the seedlings were ~8 cm tall, two or three plants were transplanted into a plant pot (15 cm diameter, 12 cm high) containing the same soil. Nineteen pots were placed in a tank containing ~400 litres of tap water located in a glasshouse on the flat roof of the laboratory. The tap water was replaced every 2 d and the water surface gradually increased as the plants grew. Snails and moribund leaves were removed daily. The plants were grown at ambient (high) light without shading and, because of their high biomass, they generated high pH values and low concentrations of CO2. After ~4–5 weeks, plants of similar height (~25–30 cm) were selected for the experiments, in July and August 2015.
Acclimation to light and CO2
Pots of O. alismoides plants from the glasshouse were placed in white plastic buckets (25 × 25 × 35 cm) containing ~20 litres of tap water; two pots per bucket. Sixteen buckets were placed in a constant temperature room at 25 ± 2 °C and were illuminated with FSL T5/865 28 W fluorescence tubes on a 14 h light (08.00–22.00 h), 10 h dark photoperiod. The tap water was renewed twice a week. The plants were grown at two CO2 concentrations. A low CO2 concentration was produced by the natural photosynthetic activity of the plants which depleted the inorganic carbon concentration of the water, and increased the pH (from 8.32 to >9.0), with a CO2 concentration range of ~0.3–85 µmol l−1 and a mean of 11 µmol l−1. The high CO2 concentration was produced by adding CO2-saturated tap water to the buckets twice each day to reduce the pH to between 6.8 and 7.0, which generated on average 405 µmol l−1. The corresponding CO2 concentration over the whole growth period was between 136 µmol l−1 and 455 µmol l−1, with a mean of 286 µmol l−1. The buckets were gently stirred to mix the water before measurement and after each addition of CO2 solution. The buckets were covered with neutral-density shading material that produced a low level of light 25–30 µmol photon m−2 s−1, photosynthetically active radiation [PAR; Li-Cor underwater sensor (UWQ) connected to a Li-Cor LI-1400 data logger]. Alkalinity was measured every 2 d, and pH was measured every day (see methods below). Four days before the measurements, half the plants at high and low CO2 were exposed to a moderately high light at 150–165 μmol photon m−2 s−1 by removing the neutral-density shading material. There were therefore four treatments: high light and high CO2 (HLHC), high light and low CO2 (HLLC), low light and high CO2 (LLHC), and low light and low CO2 (LLLC), with the CO2 treatment lasting for 18 d and the high light treatment lasting for 4 d.
Whole leaves were collected at 07.30 h (just before the start of the photoperiod), 14.00 h, and 21.00 h (at the end of the photoperiod). Leaves grown at low concentrations of CO2 tended to have a layer of marl on the upper surface which was gently removed by rubbing. Leaves were immediately placed on aluminium foil on top of ice in a polystyrene box and kept in the dark to reduce metabolic changes. Three leaves per treatment were collected on each occasion from different plants in two or three different tanks. Each whole leaf was photographed, blotted gently with paper towels, and quickly weighed to determine the fresh weight. Each leaf was then cut in half down the mid-rib; one half was used for the determination of starch and photosynthesis enzymes, the other half for the determination of acidity. Each part of the leaf was photographed, placed in a pre-weighed foil envelope, weighed, and placed on ice. The procedure for processing 48 leaves took ~50 min. The foil envelopes containing the samples were stored in liquid nitrogen for later determination of acidity, starch, and enzyme activities.
Response to short-term exposure to high CO2
Sixteen plants from the greenhouse, grown at high light and low CO2, were transferred in the early evening to the buckets and placed in the constant temperature room in tap water at high light (180 µmol photon m−2 s−1). Of the eight buckets, CO2-saturated tap water was added to four to produce high concentrations of CO2 (HC) as described above, while four tanks were untreated to retain a low concentration of CO2 (LC). The next day, whole leaves were collected just before 08.00 h (the start of the photoperiod), 10.00, 12.00, 14.00, 17.00, and 20.00 h (1 h before the end of the photoperiod). Three leaves per treatment were kept on ice and treated as above, except that a piece of the leaf was also used to measure rates of oxygen exchange. Chlorophyll content and fluorescence yield were also measured as described below.
Response to short-term exposure to light and to different CO2 concentrations at night
Plants of O. alismoides, collected from the glasshouse at high light and low carbon, were placed in the growth room overnight and then exposed during the day to a photon irradiance of 150 µmol photon m−2 s−1 (HL treatment) and 15 µmol photon m−2 s−1 (LL treatment), both at low carbon. Leaves were collected as before in triplicate at 08.00, 12.00, 16.00, and 20.00 h, and stored in liquid nitrogen. During the subsequent night, the remaining plants from the two light treatments were incubated at two different CO2 concentrations (low or high CO2, produced as before) in the dark for 10 h. Three leaves per treatment were harvested at 07.30 h, before the onset of light, and stored in liquid nitrogen. Leaves were analysed for acidity, starch, malic acid, and enzyme activities.
Measurement of acidity
Acidity was detected as described previously (Zhang et al., 2014). Briefly, 10 ml of CO2-free milliQ water was added to a known fresh weight of leaves (0.2–0.5 g) that had been stored in liquid nitrogen and then boiled for 30 min. Acidity was measured by titration of the sample with 0.01 N NaOH to an endpoint of pH 8.3.
Measurement of malic acid
A known fresh weight of frozen leaves (~0.4 g) was homogenized in 3 ml of ice-cold 0.6 N perchloric acid. The extract was centrifuged at 12 000 g for 10 min at 4 °C. The supernatant was neutralized with 5 mol l−1 K2CO3, centrifuged again, and assayed for malic acid following Delhaize et al. (1993).
Measurement of starch
A known fresh weight of frozen leaves (~0.5 g) was homogenized in 5 ml of 80% ethanol. The homogenate was boiled for 3 min and centrifuged at 6000 g for 10 min at room temperature. The pellet was washed with 80% ethanol and this was repeated until the solution was colourless. The pellet was boiled for 10 min and, after cooling, 0.5 ml of 0.2 M Na acetate (pH 5.5) was added and the starch concentration was determined by the amyloglucosidase assay (Smith and Zeeman, 2006).
Measurement of enzyme activities
The extraction and assay of PEPC, Rubisco, and pyruvate phosphate dikinase (PPDK) were based on the methods described previously (Zhang et al., 2014). All these enzyme activities were assessed from the rate of appearance or disappearance of NADH at 340 nm at 25 °C measured using a microplate reader (Tecan M200 PRO, Austria).
Measurement of chlorophyll, dry weight, and leaf area
About 0.1 g FW of leaf was extracted in 5 ml or 10 ml of ethanol and left overnight at 4 °C or boiled until the leaves were colourless, before measuring absorbance with a spectrophotometer at 649, 665, and 750 nm. Concentrations of Chl a and b were calculated using equations in Brain and Solomon (2007). Dry weight was measured after the leaves from HLLC in the glasshouse were dried for 48 h at 80 °C. Projected (one-sided) leaf area was calculated from digital photographs using AreaAna software (Huazhong University of Sciences and Technology, China), using squares of known area as reference.
Measurement of pH and alkalinity
The alkalinity in the solution was measured by Gran titration with a standard solution of HCl. pH was measured with a combination pH electrode (E-201F, Shanghai Electronics Science Instrument Co., China) connected to a Thermo Orion Dual Star Benchtop pH/ISE Meter.
Measurement of fluorescence yield
Fluorescence was recorded on six replicate leaves with a chlorophyll fluorometer (PAM-win, Walz, Germany) with a leaf clip attachment. The maximal photochemical efficiency of PSII (Fv/Fm) was measured 10 min before the start of the photoperiod, and subsequently yield (the actual photochemical efficiency of PSII) was measured immediately after illumination and every 5 min thereafter for the first hour. Fv/Fm and the yield were obtained using the Win Control (3.25) software.
Oxygen exchange
Oxygen exchange was measured with an optical oxygen electrode (Unisense OX-13298 and a Unisense microsensor multimeter Version 2.01) in a glass and Perspex chamber with a volume of 62 ml. A magnetic stirring bar at the base of the chamber, activated by an external motor, produced a steady flow of water around the chamber. The chamber was placed in a constant temperature water bath at 25 °C and illuminated from the side by fluorescent tubes (36 W, 6500 K colour temperature) that produced a photon irradiance (PAR) of 115 μmol photon m−2 s−1. The oxygen electrode was calibrated in the chamber with tap water (alkalinity ~1.8 mequiv l−1) that had been vigorously bubbled with air from outside the laboratory (100% saturation) and in tap water that had been vigorously bubbled with nitrogen (0% saturation). Leaves with an average FW of ~0.4 g and a projected area of ~28 cm2 were rinsed in tap water and placed in the chamber with tap water that had been bubbled with outside air and then bubbled with nitrogen for a few seconds to reduce the oxygen concentration. The concentration of CO2 at air equilibrium was calculated to be 14 µmol l−1 for an assumed outside air CO2 of 400 ppm. Tap water that had been bubbled vigorously with pure CO2 at 25 °C was calculated to have a CO2 concentration of 34.13 mmol l−1. Small volumes of this solution were added to produce 100 µmol l−1 CO2 (0.16 ml), followed by 625 µmol l−1 CO2 (0.97 ml). Rates of oxygen change were recorded for 5–10 min for each CO2 concentration. At the end of the measurement of photosynthesis, the chamber was placed in the dark and the decline in oxygen concentration was followed for ~15 min. O2 concentrations were recorded on a laptop connected to the Unisense meter, and rates of photosynthesis were calculated by linear regression in Microsoft Excel.
Statistical analysis
All data presented in this study are the average ±SD. The data were analysed using SPSS 16.0 (SPSS Inc., Chicago, IL, USA). Treatment and time significance were determined with two-way and one-way ANOVA, and means denoted by different letters were significantly different at P<0.05 based on Duncan’s and Tukey’s post-hoc tests.
Results
CO2 concentrations based on pH and alkalinity
The high and low carbon treatments produced very different CO2 concentrations. Over the whole set of experiments, the average CO2 concentration was 286 µmol l−1 in the HC and 11 µmol l−1 in the LC treatment. There was no consistent diurnal pattern at HC, but at LC in the frequent measurements involved in the short-term responses to CO2 (below) the concentration of CO2 was 3.1 µmol l−1 at the start of the photoperiod and 0.6 µmol l−1 at the end. The concentration of HCO3− in this experiment was 1.81 mmol l−1 in the HC treatment and fell from 0.47 mmol l−1 to 0.39 mmol l−1 during the day in the LC treatment.
Acclimation to light and CO2
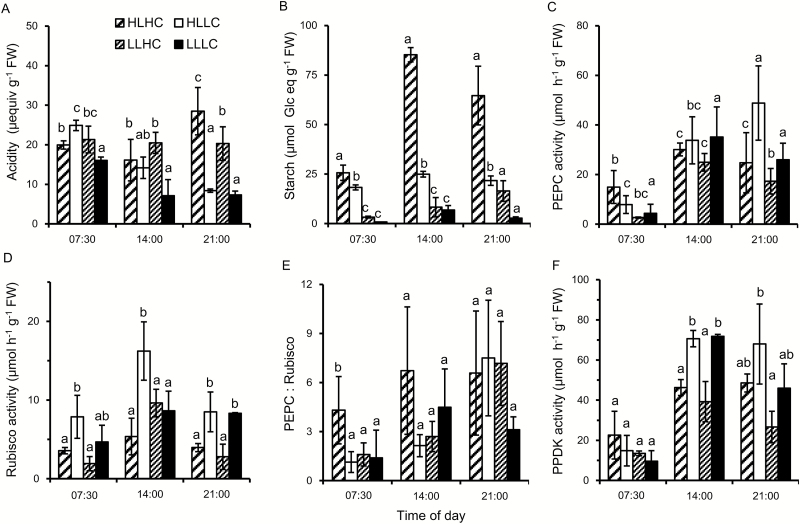
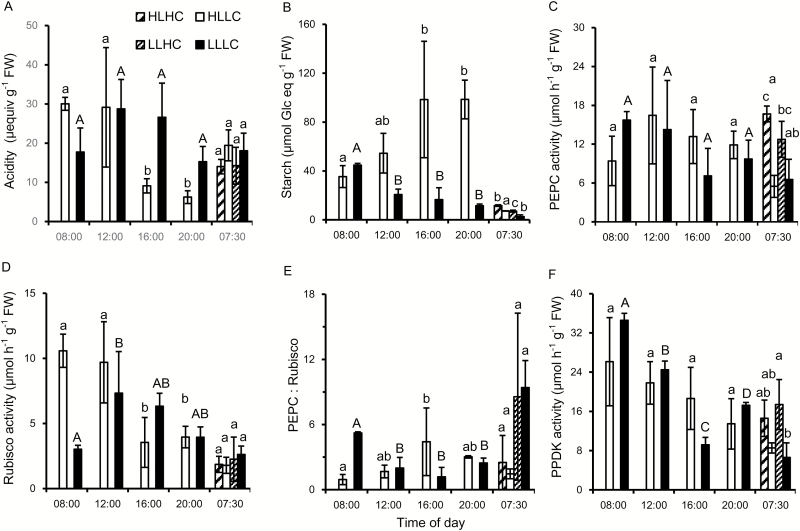
Biochemical and physiological properties of O. alismoides were compared after acclimation to low and high carbon (LC and HC) for 18 d and to low and high light (LL and HL) for the final 4 d. Night-time levels of acidity were similar across the treatments, varying between 17 µequiv g−1 FW and 25 µequiv g−1 FW (Fig. 1A). The average ratio of dry to fresh weight was 0.062 (SD is 0.007, n=25) so the acidity is equivalent to 1 µequiv g−1 DW and 1.55 µequiv g−1 DW. In the LC plants, there was a diel change in acidity of 17 µequiv g−1 FW at HL and 9 µequiv g−1 FW at LL, suggesting that CAM activity was present. In contrast, there was no diel change in acidity in the HC plants at HL or LL, because of a lack of decarboxylation, signifying the absence of CAM. High or low light did not affect the pattern of change, but the magnitude of change was lower in the LLLC plants than in the HLLC plants. An ANOVA consequently showed highly significant effects on acidity content of treatment and time of day, and there was an interaction between the two factors (see Supplementary Table S1 at JXB online). Starch content was lowest at the end of the night and increased during the day, and on average was statistically highest in the HLHC plants and statistically lowest in the LLLC plants (Fig. 1B; Supplementary Table S1). The activity of PEPC was lowest at the end of the night and highest during the day (Fig. 1C). Daytime PEPC activities were between 20 µmol g−1 FW h−1 and 50 µmol g−1 FW h−1, and similar across treatments (Supplemetnary Table S1). Rubisco activity tended to be higher in the middle than at the start and the end of the day (Fig. 1D).
Fig. 1.
Diurnal changes in acidity, starch content, and enzyme activities in O. alismoides acclimated to a factorial combination of high light (HL), low light (LL), high CO2 (HC), and low CO2 (LC). (A) Acidity, (B) starch content as glucose equivalents, (C) PEPC activity, (D) Rubisco activity, (E) the PEPC:Rubisco ratio, and (F) PPDK activity. Bars show the mean with 1 SD for three replicates. Data with different letters are significantly different within a specific time (P<0.05; one-way ANOVA).
Surprisingly, in plants acclimated to HLHC, the PEPC:Rubisco ratios were always >4 (Fig. 1E). In plants from the LC treatment, the PEPC:Rubisco ratio was 1 at the end of the night and increased to 6 by the end of the day for HL and to 3 for LL. In all the treatments, the activity of PPDK increased during the day, but the activity was lower in the HC than in the LC plants (Fig. 1F). There was a close relationship between the activity of PPDK and PEPC: on average, the activity of PPDK was 1.2 times greater than that of PEPC [linear regression: PPDK=1.23×(PEPC)+12.1; R2=0.62, P<0.001].
Response to short-term exposure to high CO2
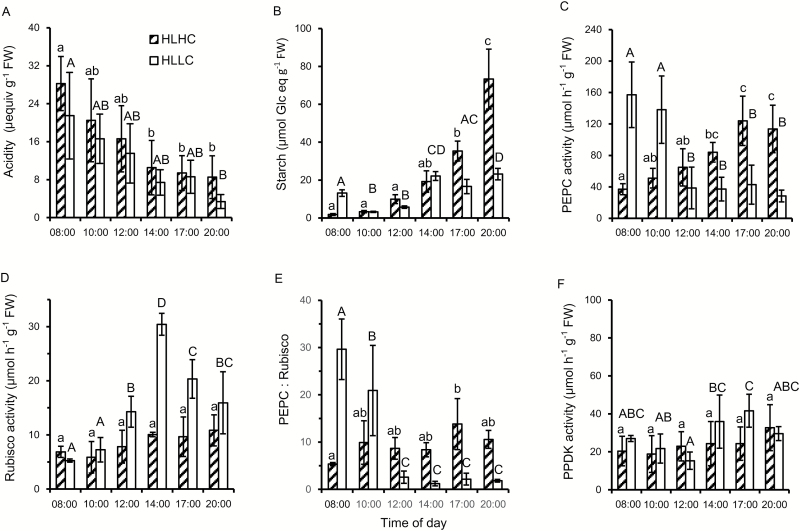
Plants that were acclimated to high light and low CO2 were then exposed overnight to high and low CO2 at high light giving two treatments, HLLC and HLHC. The diel change in acidity was ~20 µequiv g−1 FW h−1 in plants kept either at LC or at HC (Fig. 2A). In both treatments, decarboxylation began immediately at the start of the photoperiod at a rate of 2–3 µequiv g−1 FW h−1, and was largely complete by 14.00 h. These results suggest that decarboxylation of the organic acid is not abolished by a high concentration of external CO2. Plants from the two treatments had similar starch contents until 14.00 h, which coincided with the time when the reduction in acidity had ceased (Fig. 2B). Subsequently, the starch content of plants in the HLLC treatment varied between 17 µmol and 23 µmol glucose equivalents g−1 FW, unlike that of plants in the HLHC treatment that increased markedly, up to 73 µmol glucose equivalents g−1 FW, by the end of the day. At high light, the activity of Rubisco was similar at HLLC and HLHC at the start of the day, but at 14.00 h the activity of Rubisco from plants at HLLC was 3-fold higher than that from plants at HLHC. The pattern of Rubisco activity at HLLC and HLHC was similar to that observed in the acclimation experiment, with 3-fold higher activity at 14.00 h in plants treated at HLLC (Fig. 2D). The PEPC activity of plants at HLLC was very high at the end of the night, nearly 160 µmol g−1 FW h−1, and the PEPC:Rubisco ratio was nearly 30 (Fig. 2C, E). In the HLLC treatment, the activity of PEPC and the PEPC:Rubisco ratio decreased in the morning and from 12.00 h onwards. In plants acclimated to HLHC, the activity of PEPC was lower than in the HLLC plants at the start of the day but increased significantly during the day, and reached 120 µmol g−1 FW h−1 and exceeded the activity in the HLLC plants from 12.00 h onwards. PPDK activity did not change significantly with treatment or time of day for the HLHC treatment and did not change markedly for the HLLC treatment (Fig. 2F; Supplementary Table S2).
Fig. 2.
Effect of a short-term exposure to high CO2 on diurnal changes in acidity, starch content, and enzyme activities in O. alismoides. Plants acclimated to high light (HL) and low CO2 (LC) were exposed overnight to high CO2 (HC) and then kept at HLHC during the day. (A) Acidity, (B) starch content as glucose equivalents, (C) PEPC activity, (D) Rubisco activity, (E) the PEPC:Rubisco ratio, and (F) PPDK activity. Bars show the mean with 1 SD for three replicates. Data with different letters are significantly different within a specific treatment (P<0.05; one-way ANOVA).
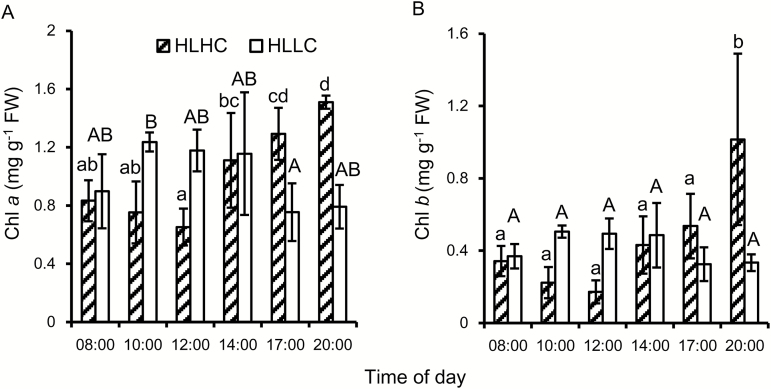
The contents of Chl a and Chl b (Fig. 3) were not significantly different for HLLC and HLHC during the day (Supplementary Table S2) but for HLHC they increased at the end of the day. Chl a content was always >2-fold higher than that of Chl b.
Fig. 3.
Effect of a short-term exposure to high CO2 on diurnal changes in chlorophyll content in O. alismoides. Plants acclimated to high light (HL) and low CO2 (LC) were exposed overnight to high CO2 (HC) and then kept at HLHC during the day. (A) Chl a, (B) Chl b. Bars show the mean with 1 SD for three replicates. Data with different letters are significantly different within a specific treatment (P<0.05; one-way ANOVA).
Photosynthesis
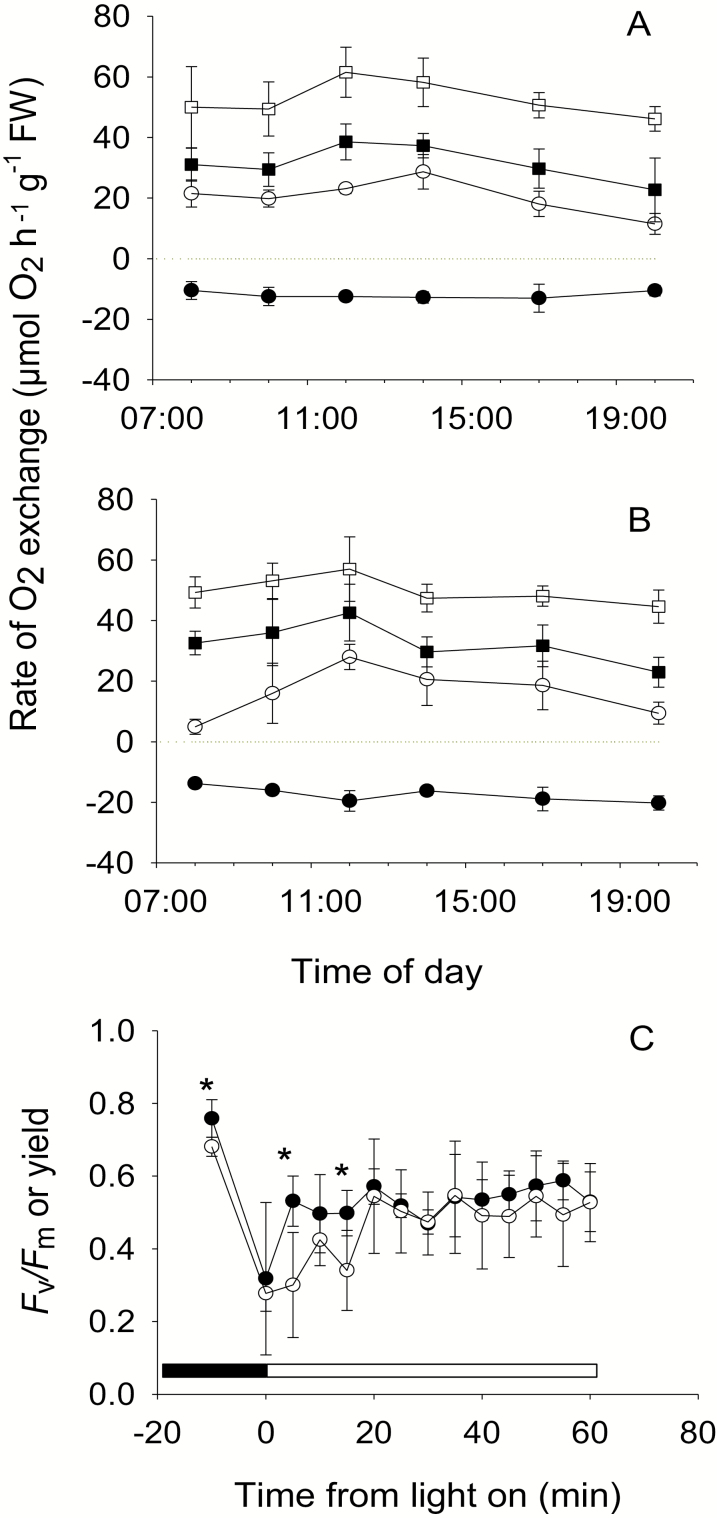
Rates of O2 exchange for leaves from HLHC and HLLC treatments had different diurnal patterns. Rates at CO2 concentrations that were at, or close to, saturating changed little over the day for the HLLC treatment (Fig. 4B), but declined slightly after mid-day in the HLHC treatment as the starch content increased (Figs 2B, 4A). The rate of photosynthesis for HLHC at 14 µmol l−1 CO2 varied between 18 µmol O2 h−1 g−1 FW and 29 µmol O2 h−1 g−1 FW over the day, apart from at 20.00 h when it declined in line with the decline in the rate at saturating CO2. In contrast, the rate of photosynthesis for HLLC, although it followed a similar pattern after mid-day, was much lower at the start of the day and increased during the period when internal CO2 was being produced, reaching maximal rates at 12.00 h, and then declined. Rates of dark respiration measured during the day were, on average, 12 µmol O2 h−1 g−1 FW for HLHC leaves and did not vary markedly during the day. In contrast, dark respiration rates were higher for HLLC leaves, on average 17 µmol O2 h−1 g−1 FW, and they tended to increase during the day in absolute values and significantly as a proportion of rates at saturating CO2 (linear regression; R2=0.97, P<0.001).
Fig. 4.
Diurnal changes in photosynthesis during the day and fluorescence at the start of the photoperiod in O. alismoides acclimated to high light and low CO2 (HLLC) and after a short exposure to high CO2 (HLHC). (A) Rate of net photosynthesis at 14 (open circles), 100 (filled squares), and 625 µmol l−1 CO2 (open squares) and respiration (filled circles) for HLHC leaves; (B) as for (A) but for HLLC leaves; (C) maximal and actual photochemical efficiency of PSII (Fv/Fm and yield). Values are the mean with 1 SD for three replicates (A and B) or six replicates (C). In (C), a significant difference between treatments is indicated by an asterisk (t-test), and the dark and light periods are shown by horizontal bars.
Chlorophyll fluorescence analysis showed that Fv/Fm was high at night and the yield decreased markedly at the onset of light (t-test, t=3.29, P<0.01). After this decrease, at HLHC, PSII yield increased rapidly to a steady-state value of ~0.54, within ~20 min. However, at HLLC, the rate of recovery was lower (Fig. 4C) and there were significant differences between HLLC and HLHC at 08.05 h and 08.15 h (t-test, t=3.52, P<0.01 and t=3.05, P<0.05, respectively).
Response to short-term exposure to light and to different CO2 concentrations at night
When plants acclimated to HLLC were transferred to LLLC, the acidity content during the day did not change significantly, whereas it decreased significantly in the HLLC plants (Fig. 5A).These plants also displayed very different patterns of starch content during the day (Fig. 5B). In plants at HLLC, the starch content increased significantly, while in plants at LLLC, the starch content decreased monotonically to 11.5 µmol glucose equivalents g−1 FW by the end of the day. At the end of the next night, the starch content had decreased to 7.2 and 2.6 µmol glucose equivalents g−1 FW for HLLC and LLLC treatments, respectively, but plants supplemented with CO2 overnight had between 4.4 and 4.5 µmol glucose equivalents g−1 FW more starch than plants treated with low CO2 overnight.
Fig. 5.
Effect of a short-term exposure to low light on diurnal changes in acidity, starch content, and enzyme activities in O. alismoides. Plants acclimated to high light (HL) and low CO2 (LC) were kept at low CO2 and exposed to low light (LLLC). At the end of the photoperiod, plants were treated overnight at high CO2 (HC) or kept at low CO2, and leaves were harvested at the end of the scotoperiod. (A) Acidity, (B) starch content as glucose equivalents, (C) PEPC activity, (D) Rubisco activity, (E) the PEPC:Rubisco ratio, and (F) PPDK activity. Bars show the mean with 1 SD for three replicates. Data with different letters are significantly different within a specific treatment. At the end of the scotoperiod (07.30 h), different letters designate treatments whose results are significantly different (P<0.05; one-way ANOVA).
There were no significant effects of the short-term light treatment on the activity of PEPC during the day (Fig. 5C). Rubisco activity was higher during the middle of the day for the LLLC treatment and declined during the day for the HLLC treatment (Fig. 5D). The PEPC:Rubisco ratio was highest at the start of the day for the LLLC treatment and increased during the day in the HLLC treatment (Fig. 5E).The PEPC activity in plants exposed overnight to high CO2 was greater at the end of the night than that in plants at low CO2. There was no significant effect of the light treatment on the activity of PPDK, and activity did not decrease significantly at HL but decreased significantly at LL during the day (Fig. 5F; Supplementary Table S3).
Contribution of CAM
Generally there was an inverse relationship between acidity and starch content. The expected linear relationship was found in the morning between malate expressed as 2H+ equivalents, and acidity (malate=1.086×acidity+4.14, R2=0.83). We assumed that 1 equivalent of acidity represents 0.5 mol of malate, that for every mole of malate (C4) decarboxylated 1 mol of carbon (C+C3) is produced, that every mole of glucose (C6) equivalent represents 6 mol of carbon, and therefore that every acidity equivalent removed represents a 12th of a mole of glucose. Assuming also that no other processes are involved in changes in starch and acidity apart from non-CAM photosynthesis, diurnal changes in acidity and starch were used to estimate the contribution of malate decarboxylation to starch formation. On this basis, at low carbon, malate decarboxylation contributes up to 21% of the carbon stored in starch, but <2.5% for plants at high carbon (Table 1).
Table 1.
Contribution of diel acidity change to starch production in O. alismoides leaves during the day
| Experiment | Treatment | Mean diel change in acidity (µequiv g−1 FW) | Percentage contribution of acid change to starch |
|---|---|---|---|
| Acclimation | HLHC | 1.0 (3.1) | 0.0 |
| HLLC | –8.5 (3.5) | 20.7 | |
| LLHC | 8.8 (0.7) | 0.6 | |
| LLLC | 16.5 (0.8) | 12.1 | |
| Response to CO2 | HLHC | 19.7 (4.2) | 2.3 |
| HLLC | 18.1 (5.4) | 7.6 | |
| Response to light | HLLC | 23.8 (1.3) | 2.2 |
| LLLC | 2.5 (4.2) | 0.5 |
Data represent the mean with the SD in parentheses.
The percentage of night-time respiration of carbon that could be conserved by the overnight accumulation of acid was calculated as in Klavsen and Maberly (2010). This was 1.5% for HLHC and 6% for HLLC plants. Both are lower than the equivalent values for C. helmsii of 11% and 32%, respectively.
Discussion
Three CCMs in O. alismoides
Ottelia alismoides has been shown to use bicarbonate and to have C4 metabolism, both constitutively, and to possess CAM facultatively, being induced by low CO2 (Zhang et al., 2014; Yin et al., 2017). The results presented here confirmed that C4 is present regardless of the CO2 concentration or light level. At HLLC, PEPC activity declined during the day but the PEPC:Rubisco ratio was between 1.2 and 2.6, in agreement with a previous report and in a range typical of terrestrial C4 plants (Zhang et al., 2014). Moreover, the activity of other enzymes required for the operation of the C4 cycle, such as PPDK that regenerates PEP to ensure the supply of substrate for PEPC, was also high and greater at low versus high CO2.
In CAM plants, a high PEPC activity at night allows malic acid to be produced in the dark. In O. alismoides, the activity of PEPC in LC plants was very high at dawn, and the PEPC:Rubisco ratio was ~30, consistent with nocturnal carboxylation as a consequence of CAM activity. PEPC activity in plants exposed to HC at night was greater than in plants maintained at LC, consistent with greater CAM activity exploiting the nocturnal carbon reserves. The diel fluctuations in acidity found here, between 17 µequiv g−1 FW and 25 µequiv g−1 FW for plants at HLLC in the acclimation and short-term experiments, respectively, were slightly lower than those (up to 34 µequiv g−1 FW) found in Zhang et al. (2014). They are also lower than those reported in the literature for some species (Keeley, 1981, 1998b) but higher or similar to those reported in a number of other putative CAM species (Webb et al., 1988; Keeley, 1998b; Yin et al., 2017). Overall, these enzyme activities and their patterns of change confirm that O. alismoides grown at LC can operate CAM during the night and C4-like metabolism during the day.
Regulation of CAM
In aquatic CAM species, such as Littorella uniflora and Isoetes kirkii, both light and CO2 affect CAM activity (Madsen, 1987; Robe and Griffiths, 1990; Hostrup and Wiegleb, 1991; Rattray et al., 1992). Although O. alismoides was apparently co-limited by light and inorganic carbon when grown at low light and low CO2, CO2 seems to be a very effective regulator of CAM, because CAM was absent at high CO2. The importance of CO2 in controlling and inducing changes in CAM activity in O. alismoides is consistent with the hypothesis of Keeley (1981) suggesting that CAM has been selected as a mechanism for enhancing net carbon gain in inorganic carbon-limited aquatic environments. Investment in CAM enzymatic apparatus and energy is beneficial when the inorganic carbon supply is limiting but not when other environmental variables, such as light or nutrients, are limiting (Madsen, 1987; Baattrup-Pedersen and Madsen, 1999). In O. alismoides, CAM was down-regulated when grown at ~25 µmol photon m−2 s−1 compared with ~150 µmol photon m−2 s−1, similarly to L. uniflora (Madsen, 1987; Baattrup-Pedersen and Madsen, 1999) and C. helmsii (Klavsen and Maberly, 2010). Similarly, CAM in terrestrial plants is down-regulated when grown at low light (Borland et al., 1996; Taybi et al., 2002).
In aquatic CAM plants, a high concentration of CO2 during the day reduces the rate of decarboxylation, and therefore the acidity at the beginning and end of the day are similar (Keeley, 1982; Rattray et al., 1992). For C. helmsii, the rates of decarboxylation are similar at different concentrations of CO2 (Klavsen and Maberly, 2010), and this is also the case in O. alismoides where it was 2–3 µequiv g−1 FW h−1 (equal to 32–48 µequiv g−1 DW h−1), but lower than that of C. helmsii (Klavsen and Maberly, 2010). In C. helmsii, the start of decarboxylation varied with CO2 (Klavsen and Maberly, 2010). In contrast, in O. alismoides, decarboxylation began immediately at the start of the day, even at high CO2, suggesting that decarboxylation of malic acid was unaffected by high CO2. Therefore, in O. alismoides and C. helmsii, the decarboxylation of malic acid is not influenced by CO2 but the effect of CO2 on timing during the light period differs between the two species.
Light intensity affects the decarboxylation of malic acid in O. alismoides as expected from other CAM studies (Osmond, 1978). When O. alismoides grown at HLLC were transferred to LLLC, the acidity content did not change at the start of the photoperiod, suggesting that decarboxylation of acid is not under circadian control but only occurs if sufficient light energy is available, as a direct effect or an indirect effect on carbon demand. As starch degradation supplies PEP for malic acid synthesis, one might expect an inverse relationship between starch accumulation and malic acid concentration, and therefore acidity. This is indeed a key characteristic in CAM plants (Borland and Taybi, 2004). In O. alismoides, the content of starch showed an inverse diel relationship to titratable acidity. When plants from HLLC were transferred to LL for a short period of time, the acidity content was high and did not change during the day, and the diel cycle of starch was abolished as in another CAM plant, Aechmea ‘Maya’ (Ceusters et al., 2008).
The contribution of acidity change to starch production in O. alismoides was estimated from concomitant changes in these variables. When CAM was active, it contributed up to 21% of the starch produced. The contribution from CAM to the carbon balance in O. alismoides is similar to that in C. helmsii (Klavsen and Maberly, 2010) but is less than in Isoetes and L. uniflora (Robe and Griffiths, 1990; Madsen et al., 2002). In O. alismoides, CAM traps a relatively low proportion of night-time respiration but it does appear to have a beneficial effect on the rate of photosynthesis. At the start of the photoperiod, the rate of photosynthesis in plants at HLLC was low at air equilibrium CO2 concentrations but subsequently increased ~5-fold during the period of decarboxylation and internal production of CO2, and decreased again in the afternoon as the decarboxylation rate decreased. Furthermore, analysis of fluorescence kinetics confirmed that while photosynthesis by the plants was activated rapidly at HC, the plants at LC took ~20 min to reach their full activity, which is consistent with the activation of a CCM.
Compatibility between C4, CAM activity, and bicarbonate use
Many aquatic macrophytes can supplement normal C3 photosynthesis by additional strategies for C gain such as CAM, C4 metabolism, or bicarbonate use (Klavsen et al., 2011). Ottelia alismoides, perhaps uniquely, appears to have all three of the above-mentioned strategies (Zhang et al., 2014). The compilation of macrophyte CCMs in Maberly and Madsen (2002) suggested that no CAM plants can use bicarbonate. In addition to O. alismoides, a possible additional exception is another species from the Hydrocharitaceae, Vallisneria spinulosa, which can use bicarbonate and perform low-level CAM (Yin et al., 2017). The explanation for the rarity of this combination of CCMs is not known, but might relate to habitat. For example, CAM is common in isoetids which tend to grow at sites with low concentrations of bicarbonate and have exploitation strategies involving uptake of CO2 from the sediment via continuous lacunae between roots and leaves. The two elodeids O. alismoides and V. spinulosa do not have this option and typically are found at sites with high bicarbonate concentrations. More work is needed to understand why bicarbonate use and CAM is a rare combination of CCMs in aquatic plants. Ottelia alismoides is an annual aquatic plant and is invasive in several regions of the world (e.g. it is on the USA’s list of introduced, invasive, and noxious plants; https://plants.usda.gov/core/profile?symbol=OTAL, last accessed 21 February 2017). It generally inhabits still or slow-flowing water, and can produce a high plant biomass causing large diurnal fluctuations and low concentrations of dissolved CO2. The flexibility conferred by possessing three CCMs (bicarbonate and CO2 use, C4 and CAM metabolism) may help explain its ecological success and allow it to complete its growth cycle within a year.
CAM and C4 do not usually co-exist in terrestrial plants, despite the similarity of their biochemical processes. Some species in the genus Portulaca operate C4 photosynthesis but also CAM under drought conditions (Koch and Kennedy, 1982; Guralnick et al., 2002). However, in the best studied species, P. grandiflora, CAM and C4 do not occur in the same cells (Guralnick et al., 2002). Sage (2002) suggests that while low-level CAM can co-exist with C4 photosynthesis, albeit in different parts of the leaf based on the Portulaca example, there are a number of morphological and biochemical incompatibilities that prevent these two photosynthesis mechanisms from co-existing. One key difference between aquatic and terrestrial CAM plants is the role that stomata play in controlling carbon uptake from air. In water, if PEPC is not down-regulated during the night, and if biochemical intermediates are available, continued PEPC activity will lead to the accumulation of malic acid. Ottelia alismoides is currently the only known species where C4 and CAM appear to be present in the same tissue, given that its leaf is only two cells thick, although further studies are needed to establish the precise location of these two biochemical pathways, determine how futile cycling is prevented during C4 carbon fixation, and understand how the carboxylation and decarboxylation enzymes are regulated.
Supplementary data
Supplementary data are available at JXB online.
Table S1. Results of ANOVA for physiological parameters in O. alismoides grown under four combinations of light and CO2, with treatment and time as factors.
Table S2. Results of ANOVA for physiological parameters in O. alismoides treated with short-term variable CO2 during the day, with treatment and time as factors.
Table S3. Results of ANOVA for physiological parameters in O. alismoides treated with short-term variable light at day and variable CO2 concentration at night, with treatment and time as factors.
Supplementary Material
Acknowledgements
This research was supported by the Chinese Academy of Sciences President’s International Fellowship Initiative to SCM and BG (2015VBA023, 2016VBA006), the National Key Research and Development Program of China (2016YFA0601000), and the National Scientific Foundation of China (31460089).
References
- Aulio K. 1985. Differential expression of diel acid metabolism in two life forms of Littorella uniflora (L.) Aschers. New Phytologist 100, 533–536. [Google Scholar]
- Aulio K. 1986. CAM-like photosynthesis in Littorella uniflora (L.) Aschers—the role of humidity. Annals of Botany 58, 273–275. [Google Scholar]
- Baattrup-Pedersen A, Madsen TV. 1999. Interdependence of CO2 and inorganic nitrogen on crassulacean acid metabolism and efficiency of nitrogen use by Littorella uniflora (L.) Aschers. Plant, Cell and Environment 22, 535–542. [Google Scholar]
- Black MA, Maberly SC, Spence DHN. 1981. Resistances to carbon dioxide fixation in four submerged freshwater macrophytes. New Phytologist 89, 557–568. [Google Scholar]
- Borland AM, Griffiths H, Maxwell C, Fordham MC, Broadmeadow MSJ. 1996. CAM induction in Clusia minor L. during the transition from wet to dry season in Trinidad: the role of organic acid speciation and decarboxylation. Plant, Cell and Environment 19, 655–664. [Google Scholar]
- Borland AM, Taybi T. 2004. Synchronization of metabolic processes in plants with Crassulacean acid metabolism. Journal of Experimental Botany 55, 1255–1265. [DOI] [PubMed] [Google Scholar]
- Bowes G. 2011. Single-cell C4 photosynthesis in aquatic plants. In: Raghavendra AS, Sage RF, eds, C4 photosynthesis and related CO2 concentrating mechanisms. Dordrecht, The Netherlands: Springer, 63–80. [Google Scholar]
- Bowes G, Rao SK, Estavillo GM, Reiskind JB. 2002. C4 mechanisms in aquatic angiosperms: comparisons with terrestrial C4 systems. Functional Plant Biology 29, 379–392. [DOI] [PubMed] [Google Scholar]
- Bowes G, Salvucci ME. 1989. Plasticity in the photosynthetic carbon metabolism of submerged aquatic macrophytes. Aquatic Botany 34, 233–266. [Google Scholar]
- Brain RA, Solomon KR. 2007. A protocol for conducting 7-day daily renewal tests with Lemna gibba. Nature Protocols 2, 979–987. [DOI] [PubMed] [Google Scholar]
- Casati P, Lara MV, Andreo CS. 2000. Induction of a C(4)-like mechanism of CO(2) fixation in Egeria densa, a submersed aquatic species. Plant Physiology 123, 1611–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceusters J, Borland AM, Londers E, Verdoodt V, Godts C, De Proft MP. 2008. Diel shifts in carboxylation pathway and metabolite dynamics in the CAM bromeliad Aechmea ‘Maya’ in response to elevated CO2. Annals of Botany 102, 389–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman JC, Bohnert HJ. 1999. Crassulacean acid metabolism: molecular genetics. Annual Review of Plant Physiology and Plant Molecular Biology 50, 305–332. [DOI] [PubMed] [Google Scholar]
- Delhaize E, Ryan PR, Randall PJ. 1993. Aluminum tolerance in wheat (Triticum aestivum L.) (II. Aluminum-stimulated excretion of malic acid from root apices). Plant Physiology 103, 695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guralnick LJ, Edwards G, Ku MSB, Hockema B, Franceschi VR. 2002. Photosynthetic and anatomical characteristics in the C4-crassulacean acid metabolism-cycling plant, Portulaca grandiflora. Functional Plant Biology 29, 763–773. [DOI] [PubMed] [Google Scholar]
- Herrera A. 2009. Crassulacean acid metabolism and fitness under water deficit stress: if not for carbon gain, what is facultative CAM good for?Annals of Botany 103, 645–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostrup O, Wiegleb G. 1991. The influence of different CO2 concentrations in the light and the dark on diurnal malate rhythm and phosphoenolpyruvate carboxylase activities in leaves of Littorella uniflora (L.) Aschers. Aquatic Botany 40, 91–100. [Google Scholar]
- Keeley JE. 1981. Isoetes howelli—a submerged aquatic CAM plant. American Journal of Botany 68, 420–424. [Google Scholar]
- Keeley JE. 1982. Distribution of diurnal acid metabolism in the genus Isoetes. American Journal of Botany 69, 254–257. [Google Scholar]
- Keeley JE. 1998a C4 photosynthetic modifications in the evolutionary transition from land to water in aquatic grasses. Oecologia 116, 85–97. [DOI] [PubMed] [Google Scholar]
- Keeley JE. 1998b CAM photosynthesis in submerged aquatic plants. Botanical Review 64, 121–175. [Google Scholar]
- Keeley JE, Rundel PW. 2003. Evolution of CAM and C4 carbon concentrating mechanisms. International Journal of Plant Sciences 164, S55–S77. [Google Scholar]
- Keeley JE, Walker CM, Mathews RP. 1983. Crassulacean acid metabolism in Isoetes bolanderi in high elevation oligotrophic lakes. Oecologia 58, 63–69. [DOI] [PubMed] [Google Scholar]
- Klavsen SK, Maberly SC. 2010. Effect of light and CO2 on inorganic carbon uptake in the invasive aquatic CAM plant Crassula helmsii. Functional Plant Biology 37, 737–747. [Google Scholar]
- Klavsen SK, Madsen TV, Maberly SC. 2011. Crassulacean acid metabolism in the context of other carbon-concentrating mechanisms in freshwater plants: a review. Photosynthesis Research 109, 269–279. [DOI] [PubMed] [Google Scholar]
- Koch KE, Kennedy RA. 1982. Crassulacean acid metabolism in the succulent C(4) dicot, Portulaca oleracea L under natural environmental conditions. Plant Physiology 69, 757–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maberly SC. 2014. The fitness of the environments of air and water for photosynthesis, growth, reproduction and dispersal of photoautotrophs: an evolutionary and biogeochemical perspective. Aquatic Botany 118, 4–13. [Google Scholar]
- Maberly SC, Madsen TV. 1998. Affinity for CO2 in relation to the ability of freshwater macrophytes to use HCO3. Functional Ecology 12, 99–106. [Google Scholar]
- Maberly SC, Madsen TV. 2002. Freshwater angiosperm carbon concentrating mechanisms: processes and patterns. Functional Plant Biology 29, 393–405. [DOI] [PubMed] [Google Scholar]
- Madsen TV. 1987. The effect of different growth conditions on dark and light carbon assimilation in Littorella uniflora. Physiologia Plantarum 70, 183–188. [Google Scholar]
- Madsen TV, Maberly SC. 1991. Diurnal variation in light and carbon limitation of photosynthesis by two species of submerged freshwater macrophyte with a differential ability to use bicarbonate. Freshwater Biology 26, 175–187. [Google Scholar]
- Madsen TV, Olesen B, Bagger J. 2002. Carbon acquisition and carbon dynamics by aquatic isoetids. Aquatic Botany 73, 351–371. [Google Scholar]
- Madsen TV, Sand-Jensen K. 1991. Photosynthetic carbon assimilation in aquatic macrophytes. Aquatic Botany 41, 5–40. [Google Scholar]
- Nimmo HG. 2000. The regulation of phosphoenolpyruvate carboxylase in CAM plants. Trends in Plant Science 5, 75–80. [DOI] [PubMed] [Google Scholar]
- Osmond CB. 1978. Crassulacean acid metabolism—curiosity in context. Annual Review of Plant Physiology and Plant Molecular Biology 29, 379–414. [Google Scholar]
- Pedersen O, Rich SM, Pulido C, Cawthray GR, Colmer TD. 2011. Crassulacean acid metabolism enhances underwater photosynthesis and diminishes photorespiration in the aquatic plant Isoetes australis. New Phytologist 190, 332–339. [DOI] [PubMed] [Google Scholar]
- Rattray MR, Webb DR, Brown JMA. 1992. Light effects on crassulacean acid metabolism in the submerged aquatic plant Isoetes kirkii A. Braun. New Zealand Journal of Marine and Freshwater Research 26, 465–470. [Google Scholar]
- Raven JA. 1970. Exogenous inorganic carbon sources in plant photosynthesis. Biological Reviews of the Cambridge Philosophical Society 45, 167–221. [Google Scholar]
- Raven JA, Cockell CS, De La Rocha CL. 2008. The evolution of inorganic carbon concentrating mechanisms in photosynthesis. Philosophical Transactions of the Royal Society B: Biological Sciences 363, 2641–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiskind JB, Madsen TV, VanGinkel LC, Bowes G. 1997. Evidence that inducible C4-type photosynthesis is a chloroplastic CO2-concentrating mechanism in Hydrilla, a submersed monocot. Plant, Cell and Environment 20, 211–220. [Google Scholar]
- Richardson K, Griffiths H, Reed ML, Raven JA, Griffiths NM. 1984. Inorganic carbon assimilation in the isoetids, Isoetes lacustris L. and Lobelia dortmanna L. Oecologia 61, 115–121. [DOI] [PubMed] [Google Scholar]
- Robe WE, Griffiths H. 1990. Photosynthesis of Littorella uniflora grown under two PAR regimes—C3 and CAM gas exchange and the regulation of internal CO2 and O2 concentrations. Oecologia 85, 128–136. [DOI] [PubMed] [Google Scholar]
- Sage RF. 2002. Are crassulacean acid metabolism and C4 photosynthesis incompatible?Functional Plant Biology 29, 775–785. [DOI] [PubMed] [Google Scholar]
- Sage RF, Sage TL, Kocacinar F. 2012. Photorespiration and the evolution of C4 photosynthesis. Annual Review of Plant Biology 63, 19–47. [DOI] [PubMed] [Google Scholar]
- Silvera K, Neubig KM, Whitten WM, Williams NH, Winter K, Cushman JC. 2010. Evolution along the crassulacean acid metabolism continuum. Functional Plant Biology 37, 995–1010. [Google Scholar]
- Smith AM, Zeeman SC. 2006. Quantification of starch in plant tissues. Nature Protocols 1, 1342–1345. [DOI] [PubMed] [Google Scholar]
- Taybi T, Cushman JC, Borland AM. 2002. Environmental, hormonal and circadian regulation of crassulacean acid metabolism expression. Functional Plant Biology 29, 669–678. [DOI] [PubMed] [Google Scholar]
- Vadstrup M, Madsen TV. 1995. Growth limitation of submerged aquatic macrophytes by inorganic carbon. Freshwater Biology 34, 411–419. [Google Scholar]
- Van TK, Haller WT, Bowes G. 1976. Comparison of the photosynthetic characteristics of three submersed aquatic plants. Plant Physiology 58, 761–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voznesenskaya EV, Franceschi VR, Kiirats O, Freitag H, Edwards GE. 2001. Kranz anatomy is not essential for terrestrial C4 plant photosynthesis. Nature 414, 543–546. [DOI] [PubMed] [Google Scholar]
- Webb DR, Rattray MR, Brown JMA. 1988. A preliminary survey for Crassulacean acid metabolism (CAM) in submerged aquatic macrophytes in New Zealand. New Zealand Journal of Marine and Freshwater Research 22, 231–235. [Google Scholar]
- Yang T, Liu X. 2015. Comparing photosynthetic characteristics of Isoetes sinensis Palmer under submerged and terrestrial conditions. Scientific Reports 5, 17783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L, Li W, Madsen TV, Maberly SC, Bowes G. 2017. Photosynthetic inorganic carbon acquisition in 30 freshwater macrophytes. Aquatic Botany (in press). [Google Scholar]
- Zhang Y, Yin L, Jiang HS, Li W, Gontero B, Maberly SC. 2014. Biochemical and biophysical CO2 concentrating mechanisms in two species of freshwater macrophyte within the genus Ottelia (Hydrocharitaceae). Photosynthesis Research 121, 285–297. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.