Abstract
Background.
The modalities of malaria transmission along the Thailand-Myanmar border are poorly understood. Here we address the relevance of using a specific Anopheles salivary biomarker to measure the risk among humans of exposure to Anopheles bites.
Methods.
Serologic surveys were conducted from May 2013 to December 2014 in 4 sentinel villages. More than 9400 blood specimens were collected in filter papers from all inhabitants at baseline and then every 3 months thereafter, for up to 18 months, for analysis by enzyme-linked immunosorbent assay. The relationship between the intensity of the human antibody response and entomological indicators of transmission (human biting rates and entomological inoculation rates [EIRs]) was studied using a multivariate 3-level mixed model analysis. Heat maps for human immunoglobulin G (IgG) responses for each village and survey time point were created using QGIS 2.4.
Results.
The levels of IgG response among participants varied significantly according to village, season, and age (P<.001) and were positively associated with the abundance of total Anopheles species and primary malaria vectors and the EIR (P<.001). Spatial clusters of high-IgG responders were identified across space and time within study villages.
Conclusions.
The gSG6-P1 biomarker has great potential to address the risk of transmission along the Thailand-Myanmar border and represents a promising tool to guide malaria interventions.
Keywords: Thailand-Myanmar border, malaria vectors, transmission, human antibody response, Salivary Biomarker, gSG6-P1.
In Thailand, malaria displays geographical heterogeneity and is exemplified by the so-called border malaria type, with most of the malaria cases concentrated along the borders with Myanmar [1]. Malaria transmission along the Thailand-Myanmar border is high because of extensive population movement across the border, especially mobile and forest workers, who make a substantial contribution to the regional malaria burden [2]. The forest area along the border presents very efficient vectors species, including Anopheles minimus sensu lato, Anopheles maculatus sensu lato, and Anopheles dirus sensu lato [3, 4]. The vectorial capacity and relative importance of these vector species in malaria transmission are, however, poorly understood, hence representing a threat to the success of malaria control and elimination in the region [2].
The emergence of artemisinin-resistant Plasmodium falciparum is a threat to malaria control. Given the paucity of new antimalarials, the only viable option is elimination of the parasite. Eliminating malaria requires accurate tools for monitoring local malaria transmission intensity [5]. The gold standard for estimating malaria transmission is the entomological inoculation rate (EIR), which is defined by the number of infected bites received per human per unit of time [6]. The EIR is estimated by human-landing collection events that are strongly dependent on the density of human-biting mosquitoes in a given time [5]. However, the density of vectors has been shown to greatly vary according to collection site and season and seems to be insensitive within small geographical areas [7–9]. Moreover, mosquito collections are time-consuming, costly, difficult to sustain for the long term, and pose ethical challenges in areas of endemicity for vector-borne diseases [10]. In settings of low malaria transmission, where people received generally <1 infected bite per person per year [11], the EIR may lack sensitivity because the number of Plasmodium-positive samples is inadequate to estimate of the sporozoite index [12–14]. Effectively using limited resources for malaria elimination and evaluating interventions require new measurements of the risk of being infected with Plasmodium at both population and individual levels [15, 16].
Recently, alternative serological methods for monitoring human-vector contact by measuring the intensity of antibody response to mosquito bites have been developed [17]. Positive correlation between the human exposure level to Anopheles bites and human anti–mosquito saliva antibody level has been extensively reviewed [18, 19]. The gSG6-P1 peptide, based on the Anopheles gambiae SG6 protein sequence, has been validated as a specific biomarker of Anopheles exposure in various settings, including Africa and the Americas [20–22]. Several studies in Africa showed that human antibody response to gSG6-P1 salivary peptide is a quantitative and specific biomarker to measure recent exposure of individuals to Anopheles bites [23–26], even in a context of a low level of exposure to malaria vector bites [20, 27], as well as to evaluate the human risk of malaria transmission [28–31]. The gSG6 protein and especially gSG6‐P1 peptide showed to be well conserved among major Anopheles species [32] hence representing a promising tool for estimating the risk of malaria transmission in Southeast Asia.
This study represents the first attempt to validate the gSG6-P1 peptide as an epidemiological tool for evaluating the direct exposure of human populations to Anopheles species in malaria hot spots along the Thailand-Myanmar border. Here we investigated the relationships between the anti–gSG6-P1 antibody response and entomological indicators of transmission—the human biting rate (HBR) and the EIR—through a cohort of approximately 2600 participants followed up every 3 months for 18 months. This study demonstrates that the Anopheles gSG6-P1 salivary biomarker has great potential to quantify human exposure to malaria vectors and to estimate the risk of malaria transmission along the Thailand-Myanmar border.
METHODS
Study Site
The study was conducted in 4 sentinel Myanmar villages located within 10 km of the Thailand border that are considered representative of the area in terms of environment, ecology, population, and behavior. Villages were Htoo Pyin Nyar (TPN; 17°14′N, 98°29′E), Tar Au Ta (TOT; 16°36′N, 98°57′E), Ka Nu Hta (KNH; 17°18′N, 98°24′E), and Htee Kaw Taw (HKT; 16°85′N, 98°47′E). These villages were selected because they showed the highest prevalence of P. falciparum (2%–12%) and Plasmodium vivax (7%–24%) submicroscopic infections in the area [33].
Study Design, Populations, and Sampling Methods
Seven serologic surveys were performed every 3 months from May 2013 to December 2014. In each village, a committee composed of village leaders, village malaria workers, and volunteers was formed to assist the Shoklo Malaria Research Unit (SMRU) staff in organizing the surveys and in engaging and mobilizing the community [33]. Informed consent was obtained directly from participating adults, and parental consent was obtained on behalf of participating children aged <16 years. Brief history of travels, professional activity, and insecticide-impregnated bed net use was also obtained. At each survey, blood specimens from inhabitants were collected on Whatman filter papers, using the dried blood spot technique, and properly labeled for analysis by enzyme-linked immunosorbent assay (ELISA).
In each village, mosquitoes were collected monthly, using the human-landing collection technique, to determine the vector abundance and composition [14]. Briefly, mosquitoes were collected in the same 5 catching sites (indoor and outdoor) from 6:00 pm to 6:00 am for 5 consecutive nights per month. Mosquitoes landing on humans, at the time of collection, were caught individually by glass tubes and brought back to the laboratory for morphological identification [34] and assessment of sporozoite rates, using a real-time polymerase chain reaction assay [35]. Anopheles minimus s.l., An. maculatus s.l., and An. dirus s.l. were considered primary vectors [4], whereas secondary vectors were Anopheles aconitus sensu lato, Anopheles barbirostris sensu lato, and Anopheles annularis sensu lato [36]. All houses and mosquito collection sites were georeferenced using Garmin etrex 20 global positioning system units. Temperature and relative hygrometry were recorded daily, using captors located in a central house of the village.
Sequence Alignment of gSG6‐P1 for Southeast Asian Anopheles Species
Ten samples of each Anopheles species collected were sequenced for clustal alignment of SG6-P1 salivary peptides. Alignments were done with ClustalW, which enabled comparison of the sequence of gSG6 peptide from local Anopheles species to that of the reference African (An. gambiae) vector [23]. The gSG6-P1–specific Anopheles peptide was synthesized and purified (95%) by Genepep (St-Clément de Riviere, France).
Measurement of Human Antibody Levels to Anopheles Saliva Antigens
Serologic testing of human exposure to gSG6-P1 saliva peptide was carried out by ELISA as described in [25] but with some modifications (Supplementary Materials). The intensity of the immunoglobulin G (IgG) response was measured at the individual level and was expressed as the ∆OD, calculated as ODx − ODn, where ODx and ODn represent the mean of the individual ODs in 2 antigen wells and the OD in 1 blank well containing no gSG6-P1 antigen, respectively. As a negative control, the specific anti–gSG6-P1 IgG response was also assayed in 16 non–Anopheles-exposed individuals from France and a Thai citizen who were living in Bangkok for >2 months, to quantify the nonspecific background antibody level and to calculate the cutoff (calculated as the mean ∆OD + 3 SDs). Based on our findings, a participant was classified as an immune responder if their ∆OD was >0.450.
Statistical Analysis
Covariates
Individual-level covariates included age group (sorted in 4 classes: <5 years, 5–15 years, 16–59 years, and ≥60 years) and sex. Household-level covariates included long-lasting insecticide-treated bed net (LLIN) use, based on a questionnaire conducted at the baseline visit (whether the participants had and used a LLIN “every night,” “some nights,” or “never”). At the village level, the population size at each survey, temperature, and relative humidity (2 time-dependent variables defined as the estimated mean and maximum humidity during the 2 weeks preceding mosquito collection events) were recorded. The mean HBR and EIR were estimated at each catching site 1 month before blood sample collection. Seasons were grouped as the hot season (mid February–mid May), the rainy season (mid May–mid October), and the cool season (mid October–mid February) according to the Thai Meteorological Department [37].
Statistical Approach
The relationship between the intensity of the human antibody response (∆OD) and entomological indicators of transmission (HBR and EIR) was studied using a multivariate 3-level (house, individual, and measurement) mixed model analysis. We considered (1) the HBR (or EIR) of total Anopheles mosquitoes, (2) the HBR (or EIR) of the primary vectors, and (3) the HBR (or EIR) of the secondary vectors, in 6 separated analyses. The potential adjustment factors were all of the covariates described above. In each analysis, the HBR variable was categorized in 4 classes (according to the quartiles) to avoid the assumption of a linear relationship between the HBR and the human antibody response. For the EIR model, data were categorized as a binary variable (0 and >0) because of the high number of data collected from uninfected mosquitoes. In all models, a univariate analysis was first performed, where we estimated the relationship between each adjustment factor and the antibody response through a univariate mixed model. In a second step, we entered in a multivariate mixed model all of the adjustment factors with a P value of <.2 from the univariate analysis and then removed sequentially all the adjustment factors with a P value of >.05 (backward selection). Statistical analyses were done with Stata, version 13.0 (StataCorp, College Station, TX). Graphs were constructed using GraphPad Prism 5 software (San Diego, CA).
Spatial Analysis
Heat map raster layers were created for IgG responses among individuals within each village and survey time point, using QGIS 2.4 (available at: http://www.qgis.org/). The raster layers give a smoothed representation of IgG intensity within study villages (Supplementary Materials). Mean HBRs for malaria vectors and EIR positives (meaning >0) were also plotted in the maps to indicate catch site location, vector abundance, and malaria transmission foci.
Spatial autocorrelation (clustering) of IgG values was calculated for each village and time point, using 2 approaches: the Moran I statistic (a global clustering method) and local indicators of spatial autocorrelation (LISAs) [38]. The Moran I tests give a single test statistic and associated P value for each village/month combination, while the LISAs give a test statistic and P value for each individual/month combination. We used a Benjamini-Hochberg correction to account for multiple testing. All results were mapped using ArcMap 10.2 (available at: http://www.esri.com/).
Ethical Statement
The Ethics Review Committee for Research Involving Human Research Subjects Health Science Group, Chulalongkorn University, Thailand, approved the study (096.1/56; 16 October 2014). The protocols for blood sample collection and the dried spot technique have been approved by the Oxford Tropical Research Ethics Committee (1015-13; 29 April 2013).
RESULTS
Characteristic of the Study Populations and Immunological Outcomes
Table 1 describes the population characteristics during the period of the study. Participants consisted in 2602 people followed up every 3 months over 18 months. Participants from the 4 study villages were comparable in age, and the sex ratio varied from 0.46 (in KNH) to 0.52 (in TOT). A total of 1906, 1970, 2046, and 3503 blood specimens were collected using the dried spot technique and analyzed at TPN, TOT, KNH, and HKT, respectively. The proportion of participants with an immune response to Anopheles salivary antigen ranged from 59% at TPN to 86% at HKT (Supplementary Materials).
Table 1.
Descriptive Statistics of Participants, by Study Sites
| Characteristics | Htoo Pyin Nyar (n = 452) | Tar Au Ta (n = 659) | Ka Nu Hta (n = 459) | Htee Kaw Taw (n = 1032) |
|---|---|---|---|---|
| Age, y, median (range) | 21 (0–66) | 19 (0–80) | 22 (0–73) | 19 (0–94) |
| Female sex, % | 47 | 52 | 46 | 50 |
| Antibody prevalence, visits, % (proportion) | ||||
| All ages | 59.3 (1131/1906) | 68.8 (1356/1970) | 61.4 (1256/2046) | 86.3 (3024/3503) |
| Ages 0–4 y | 57.4 (139/242) | 57.6 (175/304) | 52.5 (117/223) | 82.4 (375/455) |
| Ages 5–15 y | 59.9 (332/554) | 68.2 (433/635) | 56.8 (303/533) | 86.6 (1101/1272) |
| Ages 16–59 y | 59.2 (629/1062) | 72.6 (682/939) | 64.5 (786/1218) | 87.2 (1467/1683) |
| Ages >60 y | 64.6 (31/48) | 71.7 (66/92) | 69.4 (50/72) | 87.1 (81/93) |
Entomology Outcomes
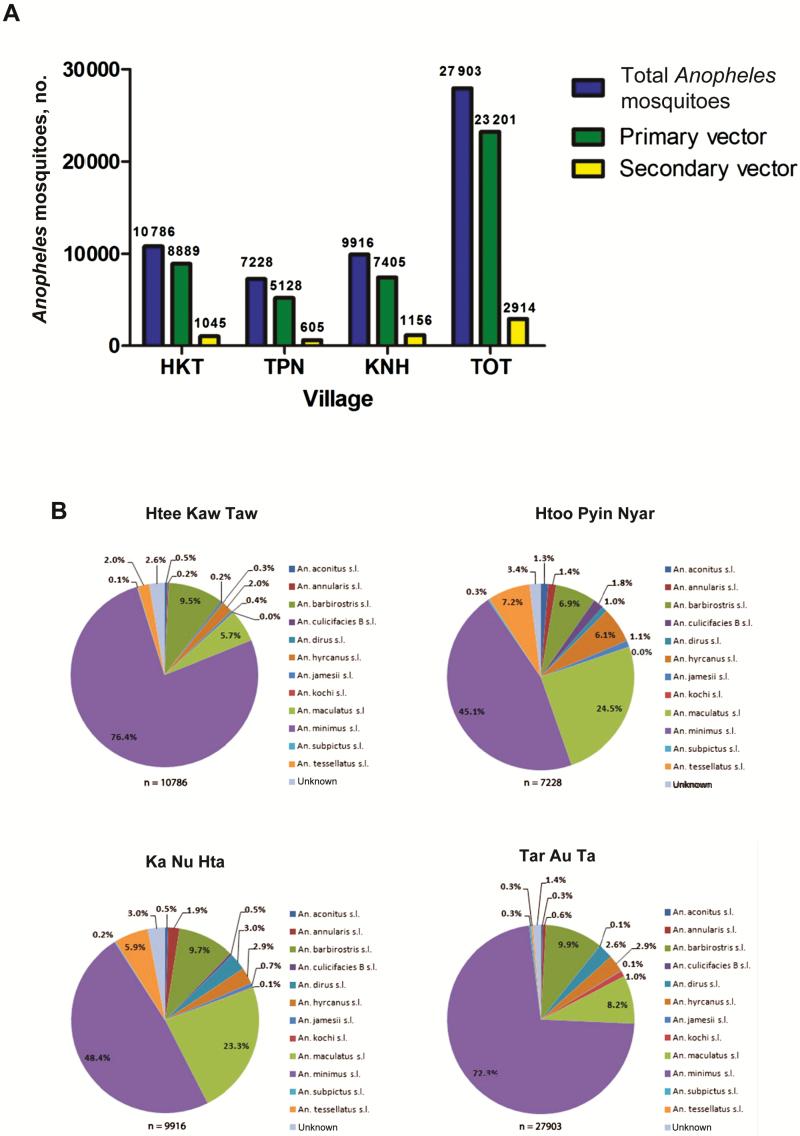
A total of 58 833 Anopheles mosquitoes were collected on human volunteers over 18 months. The overall abundance of Anopheles species was higher in TOT (n = 27 903) as compared to other villages, where it ranged from 7228 in TPN to 10 786 in HKT (Figure 1A). Twelve Anopheles species were identified, including An. minimus s.l., An. maculatus s.l., An. aconitus s.l., An. dirus s.l. An. annularis s.l., An. barbirostris s.l., Anopheles hyrcanus sensu lato, Anopheles jamesi, Anopheles kochi, Anopheles subpictus, Anopheles culicifacies species B, and Anopheles tessellatus (Figure 1B). The malaria vectors An. minimus s.l. and An. maculatus s.l. were by far the 2 dominant species, representing >70% of the total Anopheles collected. A total of 123 Plasmodium-positive Anopheles mosquitoes (sporozoite index, 0.23%; n = 47 914) were identified through 18 surveys, including 104 An. minimus s.l. (n = 35 177), 12 An. maculatus s.l. (n = 7251), 5 An. dirus s.l. (n = 1071), and 2 An. barbirostris s.l. (n = 4415).
Figure 1.
Abundance and diversity of Anopheles mosquitoes, according to village. A, Total Anopheles represents the numbers of Anopheles mosquitoes collected in each village, based on monthly collections over 18 months. B, Anopheles composition in the study area. Anopheles minimus sensu lato, Anopheles maculatus sensu lato, and Anopheles dirus sensu lato were considered as primary vectors [4], whereas secondary vectors were Anopheles aconitus sensu lato, Anopheles barbirostris sensu lato, and Anopheles annularis sensu lato [36]. Five sites were used for human landing collections (HLCs) to cover all subareas of the village. Human catch sites were separated by a minimum 50 m from each other to avoid potential bias in attracting mosquitoes. Five teams of 2 volunteers were rotated between catching sites for 5 successive nights (equivalent to 50 human-nights of collection) to mitigate potential collector bias. HLCs lasted for 45 minutes per hour, followed by a 15-minute break for collectors.
Sequence Alignment of gSG6‐P1 for Local Anopheles Species
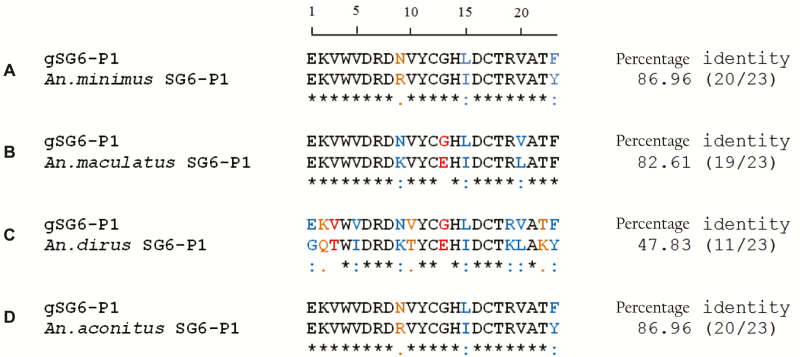
The homology of gSG6‐P1 peptide sequence with that of An. gambiae was high for An. minimus, An. aconitus, and An. maculatus (Figure 2). A lower score was found for An. dirus. The peptide sequence for these malaria vector species were antigenic as determined by computerized predictions of antigenicity based on physicochemical properties of the amino acid sequences by different programs (BCEPred, ABCPred, and BepiPred). Sequencing of gSG6‐P1 peptide for all other Anopheles species was unsuccessful.
Figure 2.
Clustal alignment and sequence identity of the gSG6-P1 salivary peptide for Anopheles minimus, Anopheles maculatus, Anopheles dirus, and Anopheles aconitus. The amino acid sequence of the gSG6-P1 peptide of Anopheles gambiae (gi:13537666) is presented as reference. Sequence identities are marked with an asterisk, strong amino acid conservations are marked with a colon, and weak amino acid conservations are marked with a period. Sequence alignment showed 87% identity (20 of 23 amino acids) for An. minimus and An. aconitus, 83% identity (19 of 23 amino acids) for An. maculates, and 48% identity (11 of 23 amino acids) for An. dirus.
Human Antibody Response to the gSG6‐P1 to Quantify Anopheles Exposure and Estimate Malaria Transmission Risk
Multivariate analyses were performed on 2602 participants and the mean number of visits per individual was 3.8 (range, 1–7 visits). Multivariate analyses showed a highly significant and positive dose-response relationship between the intensity of antibody responses to gSG6‐P1 and the HBR of both the total Anopheles population recovered (P <.001) and the primary malaria vectors (P<.001; Table 2). Post hoc analyses showed that adjusted mean IgG response intensities were significantly different between all HBR classes (Supplementary Materials). Interestingly, we also found a significant and positive relationship between the intensity of antibody responses and the EIR for total Anopheles (P <.001) and primary vectors (P <.001; Table 3). The HBR and EIR models were, however, not significant for secondary vectors, probably because of the limited sample size (P >.05; data not shown).
Table 2.
Multivariate Linear Mixed Model Showing the Relationship Between the Intensity of Antibody Responses to gSG6‐P1 and the Human Biting Rate (HBR) and Other Covariates
| Characteristic | Intensity for All Anopheles | Intensity for Primary Vectors | ||
|---|---|---|---|---|
| Mean Differencea | P | Mean Differencea | P | |
| HBR class | <.001c | <.001c | ||
| Low | Reference | Reference | ||
| Medium | 0.06 | <.001 | 0.06 | <.001 |
| High | 0.10 | <.001 | 0.09 | <.001 |
| Very high | 0.19 | <.001 | 0.18 | <.001 |
| Age, y | <.001c | <.001c | ||
| <5 | Reference | Reference | ||
| 5–15 | 0.09 | <.001 | 0.09 | <.001 |
| 15–59 | 0.13 | <.001 | 0.13 | <.001 |
| ≥59 | 0.16 | <.001 | 0.16 | <.001 |
| Village | <.001c | <.001c | ||
| Htee Kaw Taw | Reference | Reference | ||
| Htoo Pyin Nyar | 0.05 | 0.04 | ||
| Ka Nu Hta | 0.15 | <.001 | 0.15 | <.001 |
| Tar Au Ta | −0.08 | <.001 | −0.09 | <.001 |
| Season | <.001c | <.001c | ||
| Cool | Reference | Reference | ||
| Hot | 0.06 | <.001 | 0.08 | <.001 |
| Rainy | 0.08 | <.001 | 0.05 | <.001 |
Analyses were adjusted for temperature and humidity variables, in addition to the specified variables.
aDefined as the difference between each class and the reference class.
bHBR classes for total Anopheles were <96 for low HBR, 96–204 for medium HBR, 204–531 for high HBR, and ≥531 for very high HBR. HBR classes for primary malaria vectors were <46.5 for low HBR, 46.5–159 for medium HBR, 159–468 for high HBR, and ≥468 for very high HBR.
cBy the likelihood ratio test, for analysis of the global effect of the variable.
Table 3.
Multivariate Linear Mixed Model Showing the Relationship Between the Intensity of Antibody Responses to gSG6‐P1 and Entomological Inoculation Rates (EIRs)
| Characteristics | Intensity for All Anopheles | Intensity for Primary Vectors | ||
|---|---|---|---|---|
| Mean Difference | P | Mean Difference | P | |
| EIRa | <.001b | <.001b | ||
| 0 | Reference | Reference | ||
| >0 | 0.14 | <.001 | 0.15 | <.001 |
Analyses were adjusted for temperature, humidity, age, season, and village.
aBy the likelihood ratio test, for analysis of the global effect of the variable.
bA value of 0 indicates no transmission, and a value of >0 indicates transmission.
Demographic, Social, and Environmental Factors Associated with Human Vector Contact
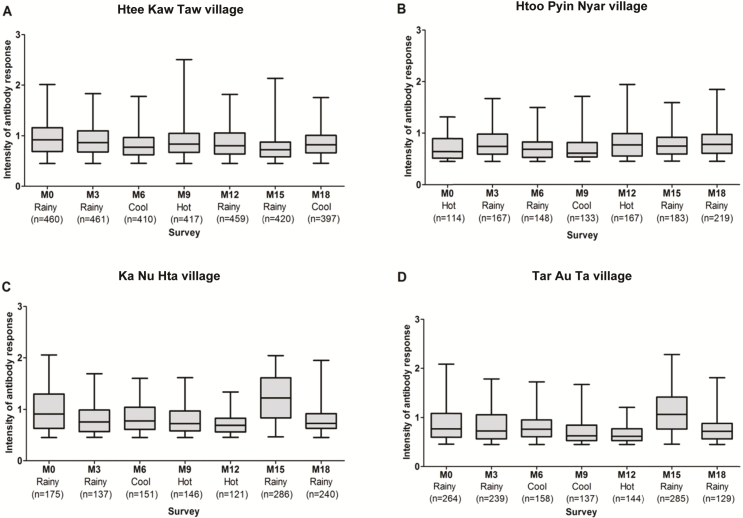
For all models, after the univariate analysis, all covariates (except the sex) were selected for the multivariate analyses. Spatial (villages) and temporal (surveys) heterogeneity in IgG intensity was apparent across and within the study villages (Figure 3). The multivariate analysis showed that the IgG response to malaria vector bites differed according to village (P<.001); the mean antibody response was higher at KNH than at other villages when adjusted for HBR (both for malaria vectors and total Anopheles) and other covariates (Table 2). A higher intensity of the antibody response was recorded during the rainy season, compared with the cool season (P <.001) and the hot season (P<.001). A positive monotonic relationship between the age and the intensity of antibody response was noted (P < 0.001). A positive relationship was found between the population size and the intensity of the antibody response (P < 0.001). Conversely, bed net use was not significant in any multivariate models.
Figure 3.
Changes in immunoglobulin (IgG) response intensity to gSG6-P1 peptide, according to surveys and village. Boxes display the median ΔOD for IgG responders (ΔOD >0.450) at each survey (at month 0 [M0], M3, M6, M9, M12, M15, and M18) with 25th and 75th percentiles. The whiskers show the 5th/95th percentiles.
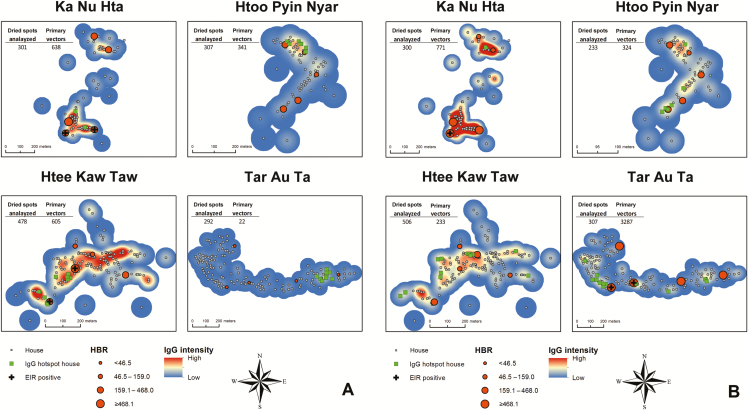
Spatial Clustering of Human Antibody Response To Malaria Vector Bites Within Villages
Heat maps of the IgG antibody response to gSG6-P1 indicated variation in the spatial distribution of the IgG antibody across space and time within villages and surveys. In all villages, areas of mid-to-high IgG intensity were detected in almost every survey in the same place. For example, in TPN the highest IgG intensity during each month occurred in a patch in the northern part of the village and was evident in both rainy and dry season (Figure 4A and 4B). Conversely, HKT had high-intensity patches in each survey month. Spatial clustering of high-IgG responders occurred within all 4 villages but varied over time (Supplementary Materials). Furthermore, LISAs indicated statistically significant clusters of individuals and houses with high antibody values near other high antibody values (green squares) during most surveys. High IgG intensity appeared more dispersed in the villages during the rainy season (Figure 4B) and patchier during the dry season (Figure 4A). This was well illustrated in TOT, where houses with residents in whom a high IgG intensity was detected occurred in a single location in the eastern portion of the village during the dry and hot seasons but were dispersed throughout much of the village during the rainy season.
Figure 4.
Heat maps of human immunoglobulin G (IgG) responses to mosquito saliva for each village in dry (A) and rainy (B) seasons (month 15 for the rainy season and month 9 for the dry season). The smoothed maps indicate relative intensities of IgG values, with dark blue denoting low intensity, yellow denoting medium intensity, and dark red denoting high. Houses are represented by gray circles, and clusters of neighbors with higher than expected IgG values (from local indicators of spatial autocorrelation statistics) are indicated by bright green squares. The human biting rates (HBRs) for each survey time are indicated by dark orange graduated cylinders, whereas foci of malaria transmission (positive entomological inoculation rates [EIRs]) are indicated by a black cross.
DISCUSSION
In this study, we demonstrated the usefulness of an innovative serological marker for quantifying human-vector contact and estimating malaria transmission risk in areas exhibiting a high prevalence of subclinical malaria infections [33]. The serological evaluation of the antibody response to mosquito saliva and its association with the exposure to malaria vectors has received increasing attention because of the limitations of current techniques in estimating malaria transmission [39]. The relevance of the gSG6-P1 biomarker for malaria epidemiologic studies has been validated in various settings worldwide [19, 40], with the exception of Southeast Asia. Here, we first demonstrated high identities of An. gambiae gSG6-P1 sequences with the dominant malaria vector species An. minimus s.l., An. aconitus (87%), and An. maculatus s.l. (83%), hence confirming that the gSG6-P1 antigen is highly conserved among malaria vectors worldwide [32]. The lower match observed with An. dirus (48%) does not indicates an absence of antibody response to this species, because An. dirus salivary proteins were detected in patient with malaria in Thailand, where An. dirus s.l. was the main vector [41]. We were unable, however, to demonstrate whether secondary vectors and nonvectors can efficiently induce an antibody response, considering the unsuccessful alignment of SG6‐P1 peptide sequences for those Anopheles species.
Our study also revealed a high gSG6-P1 seroprevalence (approximately 70%) among the populations, which is consistent with previous findings in West Africa [17] and the Americas [21]. Our study first demonstrated a dose-response relationship between the intensity of antibody responses to gSG6‐P1 and the degree of exposure to Anopheles bites. The fact that the 2 HBR models (ie, primary vector versus total Anopheles) showed a similar trend is consistent with the fact that An. minimus and An. maculatus are the 2 dominant species in the study villages. Strikingly, our findings highlighted a strong association between the gSG6‐P1 antibody response and the EIR, indicating that heterogeneity in malaria transmission is associated with heterogeneous biting behavior. The salivary biomarker looks promising for identifying malaria hot spots and measuring small-scale variation in malaria exposure rates in an area of low transmission intensity.
Our findings showed that the antibody response to Anopheles salivary peptide varies according to age, season, and village. The intensity of the response was higher during the rainy season than during the cool and hot seasons, when adjusted for other covariates. This indicates that seasonal changes in biting patterns can reflect similar changes in antibody responses. Similarly, age was positively correlated with the intensity of antibody responses in all villages. The increase in the IgG response with age is generally consistent with the gradual acquisition of immunity against Anopheles mosquito saliva [30] following the development of individual factors and behaviors that increase the probability of human-vector contact. Human behaviors and agricultural practices are expected to modulate the human-vector contact in the study area. The population is essentially made up of local and temporary farmers working in rice paddies and cornfields around the villages during the rainy season, when vector density is the highest. During harvest time, men and women will, quite frequently, spend nights in the farms and may be particularly exposed to malaria vector bites. This behavior probably explains the absence of a sex effect on the intensity of human antibody responses to Anopheles bites. Regarding village, participants from KNH exhibited a higher specific IgG response than those from other sites, when analysis adjusted for HBR and other covariates. The reason for a higher vector exposure in this population is unknown, but we assume that this may reflect different human behavior, agricultural and vector control practices, population movement, and/or immunogenicity characteristics. More information on vector ecology, demographic characteristics, and socioeconomic structure in the study villages are needed to better understand the factors associated with human-vector contact and malaria transmission [42].
Interestingly, bed net use was not significant in univariate analysis, despite the fact that 79% of people declared sleeping under bed nets every night. Although this result has to be taken with caution, considering potential biases in measuring LLIN use, we suspect that insecticide-treated bed nets might offer limited personal protection against mosquito bites. In the study area, malaria vectors exhibit strong behavioral plasticity [43] and are known to feed preferentially outdoors and in the early evening, when people are not protected by bed nets [44]. The salivary biomarker may then be particularly relevant for national malaria control programs willing to evaluate the efficacy of new malaria vector control tools, such as insecticide-treated materials and repellents Finally, we identified spatial clusters of individuals with high immune responses to vector bites in all villages that correlated well with vector abundance and transmission risk. Our results showed that locations of hot spots varied according to season and tended to be more dispersed during the rainy season and tightly clustered in small pockets during the dry season. This is consistent with malaria epidemiology along the Thailand-Myanmar border, where spatial clustering of P. vivax infections was also observed during the dry season [45]. Clustering of anti-gSG6 IgG responders is less obvious in the rainy season, most probably because vectors tend to be dispersed throughout the village, owing to multiplication of larval breeding habitats. Interestingly, TOT seems to differ from other villages (especially in the dry season), because several hot spots of immune responders occurred without clear indication of high IgG responders and vector abundance. In this case, we suspect that those people may have been extensively exposed to Anopheles bites outside the village.
In conclusion, our results showed that the gSG6-P1 serologic biomarker is capable of providing accurate estimates of the malaria transmission risk along the Thailand-Myanmar border and has great potential for malaria epidemiologic studies. Timely identification of population subsets at high risk of exposure to malaria vectors could help national malaria control programs implement hot spot–targeted interventions with the aim to eliminate potential transmission sources and achieve malaria elimination.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. We thank villagers of KNH, TOT, HKT, and TPN, for their kind support and collaboration; and field and laboratory staff from the SMRU-TCE research project (Chiara Andolina, Prapan, Billion, Khin Maung Lwin, Myo Chit Min, Taw Nay Soh, and Poe Wah) and STOP-VEC (B. Fustec and V. Chaumeau), for their commitment to this project.
V. C., T. C., and F. N. designed the study. P. Y. and D. C. performed all ELISAs and jointly conduct data analysis. F. R., A. P., and C. B. conducted sequence alignment and provided useful comments on the manuscript. G. C. and P. Y. worked on the statistical analysis. D. P. performed spatial mapping and the GIS study. P. Y., D. C., and V. C. managed the data. All authors contributed to the writing of versions of the manuscript. All authors read and approved the final manuscript.
Financial support. This work was supported by The Global Fund Thailand, through the MAEL research project; Fondation Méditerranée Infection (PhD scholarship to P. Y.); the Thailand International Development Cooperation Agency, Thai Ministry of Foreign Affairs (support to the IRD-KU international research program STOPVEC); and the Wellcome Trust of Great Britain (to the Mahidol Oxford University Research Unit, of which the Shoklo Malaria Research Unit is a part).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Carrara VI, Lwin KM, Phyo AP, et al. Malaria burden and artemisinin resistance in the mobile and migrant population on the Thai-Myanmar border, 1999–2011: an observational study. PLoS Med 2013; 10:e1001398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Corbel V, Nosten F, Thanispong K, Luxemburger C, Kongmee M, Chareonviriyaphap T. Challenges and prospects for dengue and malaria control in Thailand, Southeast Asia. Trends Parasitol 2013; 29:623–33. [DOI] [PubMed] [Google Scholar]
- 3. Manguin S, Bangs MJ, Pothikasikorn J, Chareonviriyaphap T. Review on global co-transmission of human Plasmodium species and Wuchereria bancrofti by Anopheles mosquitoes. Infect Genet Evol 2010; 10:159–77. [DOI] [PubMed] [Google Scholar]
- 4. Tainchum K, Kongmee M, Manguin S, Bangs MJ, Chareonviriyaphap T. Anopheles species diversity and distribution of the malaria vectors of Thailand. Trends Parasitol 2015; 31:109–19. [DOI] [PubMed] [Google Scholar]
- 5. Hay SI, Rogers DJ, Toomer JF, Snow RW. Annual Plasmodium falciparum entomological inoculation rates (EIR) across Africa: literature survey, Internet access and review. Trans R Soc Trop Med Hyg 2000; 94:113–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Birley MH, Charlewood JD. Sporozoite rate and malaria prevalence. Parasitol Today 1987; 3:231–2. [DOI] [PubMed] [Google Scholar]
- 7. Smith DL, Dushoff J, Snow RW, Hay SI. The entomological inoculation rate and Plasmodium falciparum infection in African children. Nature 2005; 438:492–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Orlandi-Pradines E, Rogier C, Koffi B, et al. Major variations in malaria exposure of travellers in rural areas: an entomological cohort study in western Cote d’Ivoire. Malaria Journal 2009; 8:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mbogo CM, Mwangangi JM, Nzovu J, et al. Spatial and temporal heterogeneity of Anopheles mosquitoes and Plasmodium falciparum transmission along the Kenyan coast. Am J Trop Med Hyg 2003; 68:734–42. [PubMed] [Google Scholar]
- 10. Service MW. A critical review of procedures for sampling populations of adult mosquitoes. Bulletin of Entomological Research 1977; 67:43–382. [Google Scholar]
- 11. Imwong M, Nakeesathit S, Day NP, White NJ. A review of mixed malaria species infections in anopheline mosquitoes. Malar J 2011; 10:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cook J, Reid H, Iavro J, et al. Using serological measures to monitor changes in malaria transmission in Vanuatu. Malar J 2010; 9:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chaumeau V, Andolina C, Fustec B, et al. Comparison of the performances of five primer sets for the detection and quantification of Plasmodium in anopheline vectors by real-time PCR. PLoS One 2016; 11:253e0159160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kwansomboon N, Chaumeau V, Fustec B, et al. Vector Bionomics and malaria transmission along the Thailand-Myanmar border: a baseline entomological survey. Malar J 2016; submitted. [DOI] [PubMed] [Google Scholar]
- 15. Bousema T, Stevenson J, Baidjoe A, et al. The impact of hotspot-targeted interventions on malaria transmission: study protocol for a cluster-randomized controlled trial. Trials 2013; 14:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bousema T, Griffin JT, Sauerwein RW, et al. Hitting hotspots: spatial targeting of malaria for control and elimination. PLoS Med 2012; 9:e1001165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rizzo C, Lombardo F, Ronca R, et al. Differential antibody response to the Anopheles gambiae gSG6 and cE5 salivary proteins in individuals naturally exposed to bites of malaria vectors. Parasit Vectors 2014; 7:549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Doucoure S, Drame PM. Salivary Biomarkers in the Control of Mosquito-Borne Diseases. Insects 2015; 6:961–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Drame PM, Poinsignon A, Marie A, et al. New salivary biomarkers of human exposure to malaria vector bites. In: Manguin S, ed. Anopheles mosquitoes: New insights into malaria vectors. Rijeka, Croatia: In Tech, 2013. [Google Scholar]
- 20. Poinsignon A, Cornelie S, Ba F, et al. Human IgG response to a salivary peptide, gSG6-P1, as a new immuno-epidemiological tool for evaluating low-level exposure to Anopheles bites. Malar J 2009; 8:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Londono-Renteria B, Drame PM, Weitzel T, et al. An. gambiae gSG6-P1 evaluation as a proxy for human-vector contact in the Americas: a pilot study. Parasit Vectors 2015; 8:533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Drame PM, Poinsignon A, Besnard P, et al. Human antibody response to Anopheles gambiae saliva: an immuno-epidemiological biomarker to evaluate the efficacy of insecticide-treated nets in malaria vector control. Am J Trop Med Hyg 2010; 83:115–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Poinsignon A, Cornelie S, Mestres-Simon M, et al. Novel peptide marker corresponding to salivary protein gSG6 potentially identifies exposure to Anopheles bites. PLoS One 2008; 3:e2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Poinsignon A, Samb B, Doucoure S, et al. First attempt to validate the gSG6-P1 salivary peptide as an immuno-epidemiological tool for evaluating human exposure to Anopheles funestus bites. Trop Med Int Health 2010; 15:1198–203. [DOI] [PubMed] [Google Scholar]
- 25. Drame PM, Machault V, Diallo A, et al. IgG responses to the gSG6-P1 salivary peptide for evaluating human exposure to Anopheles bites in urban areas of Dakar region, Senegal. Malar J 2012; 11:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Drame PM, Poinsignon A, Dechavanne C, et al. Specific antibodies to Anopheles gSG6-P1 salivary peptide to assess early childhood exposure to malaria vector bites. Malar J 2015; 14:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sagna AB, Sarr JB, Gaayeb L, et al. gSG6-P1 salivary biomarker discriminates micro-geographical heterogeneity of human exposure to Anopheles bites in low and seasonal malaria areas. Parasit Vectors 2013; 6:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sagna AB, Gaayeb L, Sarr JB, et al. Plasmodium falciparum infection during dry season: IgG responses to Anopheles gambiae salivary gSG6-P1 peptide as sensitive biomarker for malaria risk in Northern Senegal. Malar J 2013; 12:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brosseau L, Drame PM, Besnard P, et al. Human antibody response to Anopheles saliva for comparing the efficacy of three malaria vector control methods in Balombo, Angola. PLoS One 2012; 7:e44189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Drame PM, Poinsignon A, Besnard P, et al. Human antibody responses to the Anopheles salivary gSG6-P1 peptide: a novel tool for evaluating the efficacy of ITNs in malaria vector control. PLoS One 2010; 5:e15596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Remoue F, Cisse B, Ba F, et al. Evaluation of the antibody response to Anopheles salivary antigens as a potential marker of risk of malaria. Trans R Soc Trop Med Hyg 2006; 100:363–70. [DOI] [PubMed] [Google Scholar]
- 32. Calvo E, Pham VM, Marinotti O, Andersen JF, Ribeiro JM. The salivary gland transcriptome of the neotropical malaria vector Anopheles darlingi reveals accelerated evolution of genes relevant to hematophagy. BMC Genomics 2009; 10:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Imwong M, Nguyen TN, Tripura R, et al. The epidemiology of subclinical malaria infections in South-East Asia: findings from cross-sectional surveys in Thailand-Myanmar border areas, Cambodia, and Vietnam. Malar J 2015; 14:381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rattanarithikul R, Harrison BA, Harbach RE, Panthusiri P, Coleman RE, Panthusiri P. Illustrated keys to the mosquitoes of Thailand. IV. Anopheles. Southeast Asian J Trop Med Public Health 2006; 37 (Suppl 2):1–128. [PubMed] [Google Scholar]
- 35. Chaumeau V, Andolina C, Fustec B, et al. Comparison of the performances of five primer sets for the detection and quantification of Plasmodium in anopheline vectors by real-time PCR. PLoS One 2016; 11:e0159160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sriwichai P, Samung Y, Sumruayphol S, et al. Natural human Plasmodium infections in major Anopheles mosquitoes in western Thailand. Parasit Vectors 2016; 9:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Thai Meteorological Department. The climate of Thailand http://www.tmd.go.th/en/archive/thailand_climate.pdf.
- 38. Anselin L. Local indicators of spatial association—LISA. Geogr Anal 1995; 27: 93–115. [Google Scholar]
- 39. Drakeley CJ, Corran PH, Coleman PG, et al. Estimating medium- and long-term trends in malaria transmission by using serological markers of malaria exposure. Proc Natl Acad Sci U S A 2005; 102:5108–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stone W, Bousema T, Jones S, et al. IgG responses to Anopheles gambiae salivary antigen gSG6 detect variation in exposure to malaria vectors and disease risk. PLoS One 2012; 7:e40170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Waitayakul A, Somsri S, Sattabongkot J, Looareesuwan S, Cui L, Udomsangpetch R. Natural human humoral response to salivary gland proteins of Anopheles mosquitoes in Thailand. Acta Trop 2006; 98:66–73. [DOI] [PubMed] [Google Scholar]
- 42. Parker DM, Carrara VI, Pukrittayakamee S, McGready R, Nosten FH. Malaria ecology along the Thailand-Myanmar border. Malar J 2015; 14:388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Trung HD, Bortel WV, Sochantha T, Keokenchanh K, Briet OJ, Coosemans M. Behavioural heterogeneity of Anopheles species in ecologically different localities in Southeast Asia: a challenge for vector control. Trop Med Int Health 2005; 10:251–62. [DOI] [PubMed] [Google Scholar]
- 44. Tainchum K, Ritthison W, Chuaycharoensuk T, Bangs MJ, Manguin S, Chareonviriyaphap T. Diversity of Anopheles species and trophic behavior of putative malaria vectors in two malaria endemic areas of northwestern Thailand. J Vector Ecol 2014; 39:424–36. [DOI] [PubMed] [Google Scholar]
- 45. Parker DM, Matthews SA, Yan G, et al. Microgeography and molecular epidemiology of malaria at the Thailand-Myanmar border in the malaria pre-elimination phase. Malar J 2015; 14:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.