Abstract
Host-directed therapies that augment host immune effector mechanisms may serve as important adjunctive therapies for tuberculosis treatment. We evaluated the activity of denileukin diftitox in an acute mouse model of tuberculosis (TB) infection and analyzed the cellular composition and bacterial burden in lungs and spleens. These in vivo studies show that denileukin diftitox potentiates standard TB treatment in the mouse model, an effect which may be due to depletion of T-regulatory and myeloid-derived suppressor cells during TB infection. Our results indicate that denileukin diftitox and other suppressor cell–depleting therapies may be useful adjunctive, host-directed therapies for TB.
Keywords: Tuberculosis, Immunotherapy, Denileukin diftitox recombinant fusion protein toxin, Host-directed therapy.
Current therapies for tuberculosis (TB) are problematic due to emerging drug resistance, toxicity, and the need for prolonged treatment. Thus, there is a clear need for novel therapeutic approaches to treat patients infected with Mycobacterium tuberculosis. During initial infection by the bacterium, dendritic cells take up M. tuberculosis in lung and migrate to the draining mediastinal lymph nodes where they activate naive T-lymphocytes including CD4 and CD8 cells, which are essential for protective immunity against TB. Regulatory T cells (Treg, CD4+CD25+Foxp3+) limit potentially protective immune responses and facilitate bacterial replication and numbers during tuberculosis and other diseases [1, 2]. Depleting Tregs using specific antibodies has been shown to decrease the M. tuberculosis bacterial burden in the mouse model [3]. In addition to Tregs, which are of lymphoid origin, another immunosuppressive population of cells known as myeloid-derived suppressor cells (MDSCs, CD11b+Gr1HI) expands during TB infection and promotes T-cell dysfunction that in turn favors disease progression [4]. MDSCs accumulate within the inflamed lung during TB, interact with granuloma-residing cells, and contribute to exuberant inflammation [5]. Depleting MDSCs using Gr1-specific antibodies ameliorates pathology and restricts lung bacterial replication during acute and chronic murine TB [4].
Recent immunotherapy approaches for cancer include biologics for selective depletion of suppressor cell populations such as Tregs. Denileukin diftitox (DD) is one such agent. DD is a diphtheria toxin (DT)–related interleukin 2 (IL-2) fusion protein toxin that depletes cells expressing the high-affinity form of the IL-2 receptor (IL-2R), CD25 [6]. DD is a 3-domain fusion protein toxin comprised of the DT catalytic domain, the DT membrane translocation domain, and human IL-2 substituted in place of the native DT-receptor binding domain. The IL-2 moiety of the molecule retargets the fusion toxin to only those cells bearing the IL-2R. Once bound, the toxin is internalized and delivered into the cytosol where the catalytic domain inhibits protein synthesis via ADP-ribosylation of elongation factor 2, ultimately resulting in cell death via apoptosis. DD was approved by the US Food and Drug Administration for treating refractory cutaneous T-cell lymphoma (CTCL), and subsequent human studies confirmed that in addition to killing CD25+ T-cell lymphoma cells in CTCL, it also transiently depletes Tregs [7]. It has been recently shown that treating Leishmania-infected mice with DD suppressed lesional Treg abundance, enhanced lesion resolution, and decreased parasite burden in a mouse model [8].
Given the suppressive effects of Tregs and MDSCs in the tuberculous granuloma and the fact that both express IL-2R, we evaluated whether DD treatment might enhance the resolution of experimental TB infection in the mouse model.
METHODS
Animals and Treatment
We infected C57BL/6 mice, purchased from Charles River Laboratories (Wilmington, Massachusetts), by aerosol infection using M. tuberculosis H37Rv as previously described using an Institutional Animal Care and Use Committee–approved protocol [9]. One group of animals was treated with 2 cycles of DD (each cycle is comprised of 2 doses of drug at 35 μg/kg intraperitoneal injection separated by 2 days), the first starting on day –3 (preinfection) and the second starting on day 11 (postinfection). A second group of mice was treated with 1 cycle only starting on day 11 (postinfection) as per the experimental scheme in shown in Supplementary Figure 1. Mouse organs were homogenized, diluted, and plated for colony-forming unit (CFU) counts. For flow cytometry, organs were harvested on day 14 and single-cell suspensions were stained with CD4, CD25, FoxP3, CD11b, and Gr1 fluorescently labeled antibodies. The data were analyzed using BD FACSDiva software.
Statistical Analysis
CFU counts were log10 transformed before analysis. Mean CFU counts were compared using Student t test. All measures of statistical variation are expressed as ± standard error of the mean (SEM).
RESULTS
DD Monotherapy Inhibits Mycobacterial Replication in Mouse Model
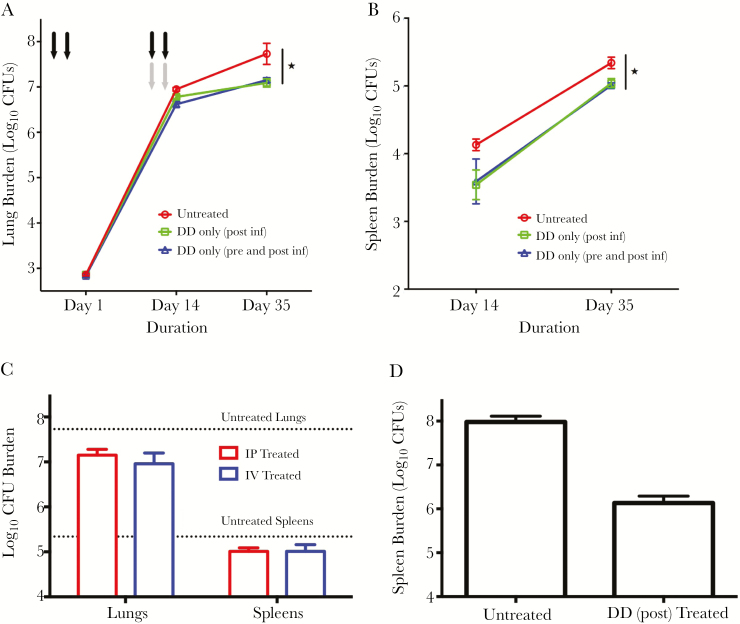
To evaluate the effect of DD treatment during tuberculosis, we determined quantitative lung and spleen CFUs at days 1, 14, and 35 after infection and treatment (Figure 1A and 1B). The initial implantation CFU (day 1) was 2.86 (±0.06) log, and the CFUs in the untreated mice lungs at day 14 were 6.95 (±0.09) log units. The DD treated pre- and postinfection group had quantitative lung CFU counts that were 0.33 and 0.58 log units lower than the untreated control at days 14 and 35, respectively (P < .01 at day 14 and P < .05 at day 35), while the group that was treated postinfection only had quantitative CFU counts that were 0.18 and 0.65 log units lower than the untreated control at days 14 and 35, respectively (P < .05 at days 14 and 35) (Figure 1A). We observed similar effect of DD treatment on spleen burden (Figure 1B). These data show that both monotherapy treatments with DD were efficacious in reducing M. tuberculosis organ burdens. Additionally, one postinfection treatment cycle was sufficient to reduce the CFU numbers in lungs and spleens, and hence treating prior to infection essentially had no effect (P = not significant).
Figure 1.
Denileukin diftitox (DD) treatment decreases tuberculosis disease progression and lung-to-spleen dissemination. A and B, Groups of C57BL/6 mice (5 mice per time point) were infected with 2.9 log10 units of Mycobacterium tuberculosis H37Rv by the aerosol route on day 0. Pre- and postinfection–treated mice (black arrows) received DD (35 μg/kg intraperitoneally [IP]) on days –3 and –1 preinfection (first cycle) and on days 11 and 13 postinfection (second cycle), while a separate postinfection-only group (gray arrows) received only 1 cycle of treatment on days 11 and 13. Mouse lungs (A) and spleens (B) were homogenized, diluted, and plated for colony-forming unit (CFU) counts. C, Groups of mice were treated with DD either by the IP or intravenous (IV) routes with 2 drug cycles (1 pre- and 1 postinfection cycle as in A), and lungs and spleens were plated for CFU counts on day 35. The dotted lines represent the day 35 CFU counts for untreated animals. D, Mice were infected with 3.84 log10 units of M. tuberculosis H37Rv by the aerosol route, and were given DD (35 μg/kg IP) for 1 cycle starting on day 11 postinfection. The mice were killed at day 28, and spleens were homogenized, diluted, and plated for CFU counts. *P < .05.
To assess the effect of route of administration of DD, we designed another arm in the experiment where the animals received 2 cycles of drug via the intravenous route pre- and postinfection. The extent of decrease in the lung and spleen CFU counts compared to untreated mice were similar for the 2 groups at day 35 (P = not significant) (Figure 1C). Thus, the route of administration had little or no effect on the reduction of organ bacterial burden.
It has been reported that dissemination of M. tuberculosis to extrapulmonary organs is governed immunologically in the early phases of infection [10]. To test whether DD could augment pulmonary containment of M. tuberculosis, we conducted a dissemination study with a high-burden aerosol infection of C57BL/6 mice (3.84 log10 CFU counts on day 1). A group of animals received 1 cycle of DD intraperitoneally starting on day 11 postinfection (750 ng per mouse). After 4 weeks of infection, the animals were sacrificed and spleens plated for log CFU counts. The group of mice receiving DD showed a 72-fold (1.86 log units) lower CFU count in spleens than the untreated animals (Figure 1D). Hence, administration of 1 cycle of DD starting on day 11 significantly reduced dissemination of M. tuberculosis from lungs to spleen in treated mice.
DD Potentiates Standard TB Treatment
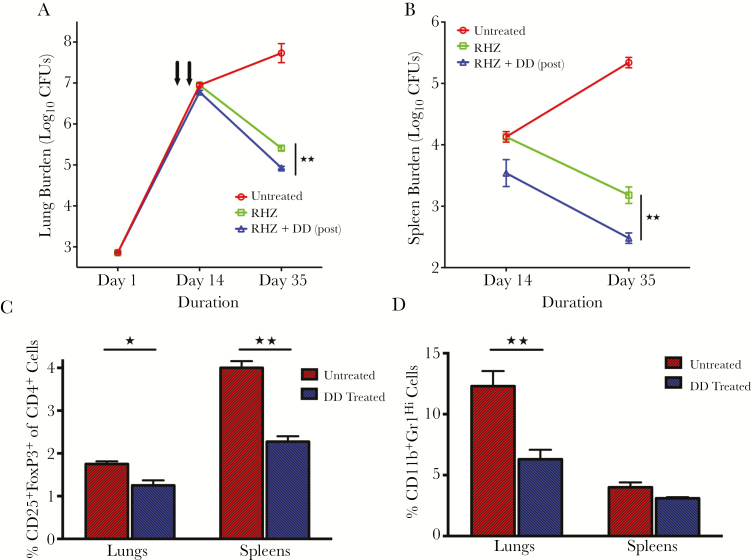
To determine the efficacy of DD as an adjunctive therapeutic when given with standard TB treatment, we administered the drugs rifampin (R), isoniazid (H), and pyrazinamide (Z) with or without the protein toxin. Mice were infected with M. tuberculosis H37Rv and treated daily with RHZ starting on day 14 postinfection, and the standard therapy-plus-DD group of mice received 1 cycle of protein toxin immunotherapy intraperitoneally starting on day 11 postinfection. At day 35 after infection and treatment, gross lung pathology revealed that mice receiving DD-plus-RHZ treatment had fewer visible lesions compared with those receiving RHZ alone (Supplementary Figure 2). In C57BL/6 mouse lungs (Figure 2A), standard RHZ treatment yielded a 2.32 log10 unit CFU decrease at day 35 compared with the untreated controls, whereas the RHZ-plus-DD group gave quantitative CFU counts that were 2.80 log10 units lower (P = .0002). We observed similar effect of DD treatment in spleens (Figure 2B; P = .003). Taken together, these results reveal that DD immunotherapy adds significantly to the bactericidal effectiveness of standard RHZ therapy (Figure 2A and 2B).
Figure 2.
Denileukin diftitox (DD) has an additive effect with the antimicrobial standard treatment regimen and decreases the frequency of T-regulatory cells and myeloid-derived suppressor cells (MDSCs) in lungs and spleens of Mycobacterium tuberculosis–infected animals. Groups of mice (5 mice per time point) infected with M. tuberculosis H37Rv were treated with rifampin (R; 10 mg/kg), isoniazid (H; 10 mg/kg), and pyrazinamide (Z; 150 mg/kg) daily for 5 days/week, with or without DD (DD; 35 μg/kg intraperitoneally) given as 1 cycle postinfection starting on day 11. The lungs (A) and spleens (B) were homogenized, diluted, and plated for colony-forming unit (CFU) counts. Single-cell suspension from lungs and spleens of infected mice at 14 days postinfection were analyzed by flow cytometry for frequency of Tregs (C) and MDSCs (D). *P < .05 and **P < .01.
DD Immunotherapy Reduces Treg and MDSC Frequencies in Lungs and Spleens During TB Infection
It has been demonstrated that administering DD can transiently deplete Tregs in humans with melanoma and that the drug enhances antitumor immune responses in these patients [11]. Human IL2 has been shown to bind to the murine IL-2R, and studies of DD in mice have confirmed transient depletion of murine Tregs with maximal depletion in C57BL/6 mice occurring on day1 posttreatment [7]. To better understand the cellular populations involved during DD treatment in TB-infected animals, we analyzed the number of Tregs and MDSCs in treated lungs and spleens. The infected animals were treated with 1 cycle of DD IP starting on day 11 postinfection. The percentages of CD25+FoxP3+ of total CD4+ cells (Tregs) in lungs were found to be 1.75% for the untreated animals and 1.25% for the treated group (P = .01), indicating a modest but significant decrease in the numbers of Tregs after treatment with DD (Figure 2C). We detected a similar reduction (34%; P = .002) in the Treg frequencies and cell counts in spleens from the treated group compared with the untreated animals (Figure 2C and Supplementary Figure 3).
We further investigated the numbers of MDSCs in the organs of infected animals that were treated with the fusion protein toxin immunotherapy at the same time point. The percentages of CD11b+Gr1Hi cells of total leukocytes in lungs were found to be 12.3% for the untreated animals and 6.3% for the treated group (P = .0064), indicating a significant decrease in the numbers of MDSCs in lungs after DD treatment (Figure 2D). A similar degree of MDSC reduction (23%; P = .07) was observed in the spleens of treated mice when compared to the untreated group. We examined the surface expression of CD25 on MDSCs and found that MDSCs infected with M. tuberculosis H37Rv have higher CD25 levels compared to the uninfected controls (Supplementary Figure 4).
DISCUSSION
Our data reveal that during M. tuberculosis infection in mice, DD successfully reduces the frequencies of both Treg cells and MDSCs in whole organs. It has been recently shown that Tregs and MDSCs can functionally crosstalk during murine melanoma development [12]. The reduction in the frequency of 2 different immune suppressive cell types from adaptive and innate responses during tuberculosis infection and subsequent treatment raises possibility of an interaction between Tregs and MDSCs during TB disease. Moreover, a single cycle of this immunotherapy postinfection had a beneficial effect for the host with at least a 7-fold decrease of M. tuberculosis CFU counts in the treated animals 3 weeks after therapy. We found that use of the drug preinfection did not add to the efficacy of postinfection therapy, indicating that the drug’s effectiveness is related to killing of cell types that expand during the infection process. Importantly, the activity of DD significantly augmented the bacterial killing of RHZ standard therapy, illustrating that immunotherapeutic TB control was exerted by a mechanism independent of antimicrobial-mediated bacterial death. We also demonstrated that immunotherapeutic efficacy is independent of whether DD is administered intraperitoneally or intravenously.
Mycobacterium tuberculosis disseminates from the lungs to other organs such as liver and spleen within 11–14 days after infection [10]. We considered the possibility that DD may inhibit dissemination to other organs during treatment. Using a high-burden M. tuberculosis aerosol inoculation model, we found that administration of 1 cycle of the protein toxin on day 11 postinfection reduces lung-to-spleen dissemination by 70-fold when assessed at day 28 postinfection. Hence, our study revealed that DD treatment enhances immunologic containment of M. tuberculosis in mice lungs and as such alters deleterious pathological sequelae of pulmonary tuberculosis.
DD (Ontak) had been used extensively in the clinic for CTCL and has been studied for peripheral T-cell lymphoma, renal cell carcinoma, and melanoma [13–15]. DD treatment in stage IV melanoma patients resulted in transient depletion in CD25+FoxP3+ Tregs [11]. In this study, we demonstrate that DD treatment during TB infection reduces the frequencies of immune suppressor cell types such as Tregs and MDSCs and that treatment leads to reduced bacterial burdens either as monotherapy or when used together with standard therapy. Nonhuman primates have granulomatous lung pathology with caseous necrosis resembling the human disease; hence, a study in M. tuberculosis–infected nonhuman primates treated with DD is warranted before proceeding to test DD in patients.
In conclusion, the results of our present study conducted in the mouse model of pulmonary TB provides a proof of concept that recombinant protein toxin can significantly improve antibiotic-mediated killing and suggests novel approaches for treatment and prevention of tuberculosis. Future studies in other models, such as nonhuman primates, may further elucidate the effector cell responses and signaling mechanisms during this Treg-depleting immunotherapy in TB.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. We are grateful to Dr R. Harrison and Dr R. Ratts for recombinant denileukin diftitox and Dr Z. Chen for helpful discussions.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (grant numbers AI079590, 037856, and 036973) and the Howard Hughes Medical Institute.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Scott-Browne JP, Shafiani S, Tucker-Heard G, et al. Expansion and function of Foxp3-expressing T regulatory cells during tuberculosis. J Exp Med 2007; 204:2159–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kursar M, Koch M, Mittrücker HW, et al. Cutting edge: regulatory T cells prevent efficient clearance of Mycobacterium tuberculosis. J Immunol 2007; 178:2661–5. [DOI] [PubMed] [Google Scholar]
- 3. Shafiani S, Dinh C, Ertelt JM, et al. Pathogen-specific Treg cells expand early during Mycobacterium tuberculosis infection but are later eliminated in response to interleukin-12. Immunity 2013; 38:1261–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Knaul JK, Jörg S, Oberbeck-Mueller D, et al. Lung-residing myeloid-derived suppressors display dual functionality in murine pulmonary tuberculosis. Am J Respir Crit Care Med 2014; 190:1053–66. [DOI] [PubMed] [Google Scholar]
- 5. Dorhoi A, Kaufmann SH. Versatile myeloid cell subsets contribute to tuberculosis-associated inflammation. Eur J Immunol 2015; 45:2191–202. [DOI] [PubMed] [Google Scholar]
- 6. Kiyokawa T, Williams DP, Snider CE, Strom TB, Murphy JR. Protein engineering of diphtheria-toxin-related interleukin-2 fusion toxins to increase cytotoxic potency for high-affinity IL-2-receptor-bearing target cells. Protein Eng 1991; 4:463–8. [DOI] [PubMed] [Google Scholar]
- 7. Litzinger MT, Fernando R, Curiel TJ, Grosenbach DW, Schlom J, Palena C. IL-2 immunotoxin denileukin diftitox reduces regulatory T cells and enhances vaccine-mediated T-cell immunity. Blood 2007; 110:3192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Divanovic S, Trompette A, Ashworth JI, Rao MB, Karp CL. Therapeutic enhancement of protective immunity during experimental leishmaniasis. PLoS Negl Trop Dis 2011; 5:e1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gupta S, Tyagi S, Almeida DV, Maiga MC, Ammerman NC, Bishai WR. Acceleration of tuberculosis treatment by adjunctive therapy with verapamil as an efflux inhibitor. Am J Respir Crit Care Med 2013; 188:600–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chackerian AA, Alt JM, Perera TV, Dascher CC, Behar SM. Dissemination of Mycobacterium tuberculosis is influenced by host factors and precedes the initiation of T-cell immunity. Infect Immun 2002; 70:4501–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mahnke K, Schönfeld K, Fondel S, et al. Depletion of CD4+CD25+ human regulatory T cells in vivo: kinetics of Treg depletion and alterations in immune functions in vivo and in vitro. Int J Cancer 2007; 120:2723–33. [DOI] [PubMed] [Google Scholar]
- 12. Fujimura T, Ring S, Umansky V, Mahnke K, Enk AH. Regulatory T cells stimulate B7-H1 expression in myeloid-derived suppressor cells in ret melanomas. J Invest Dermatol 2012; 132:1239–46. [DOI] [PubMed] [Google Scholar]
- 13. Foss FM, Sjak-Shie N, Goy A, et al. A multicenter phase II trial to determine the safety and efficacy of combination therapy with denileukin diftitox and cyclophosphamide, doxorubicin, vincristine and prednisone in untreated peripheral T-cell lymphoma: the CONCEPT study. Leuk Lymphoma 2013; 54:1373–9. [DOI] [PubMed] [Google Scholar]
- 14. Atchison E, Eklund J, Martone B, et al. A pilot study of denileukin diftitox (DD) in combination with high-dose interleukin-2 (IL-2) for patients with metastatic renal cell carcinoma (RCC). J Immunother 2010; 33:716–22. [DOI] [PubMed] [Google Scholar]
- 15. Telang S, Rasku MA, Clem AL, et al. Phase II trial of the regulatory T cell-depleting agent, denileukin diftitox, in patients with unresectable stage IV melanoma. BMC Cancer 2011; 11:515. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.