Human papilloma virus–associated anal dysplastic lesions are prevalent in human immunodeficiency virus (HIV)–infected persons. In this study we compare patterns of lymphocytic infiltration in high-grade anal dysplastic lesions by HIV status and in association with treatment outcomes.
Keywords: human immunodeficiency virus (HIV), anal cancer precursors, immune microenviroment, mucosa-infiltrating lymphocytes
Abstract
Background
Anal high-grade squamous intraepithelial lesions (HSILs) are the precursors to anal cancer and frequently persist or recur following electrocautery ablation (EA). Impaired mucosal immunity may facilitate anal carcinogenesis. We characterized the immune microenvironment of anal HSILs in correlation with human immunodeficiency virus (HIV) serostatus and ablation outcomes.
Methods
Using immunohistochemistry, mucosa-infiltrating CD4+ and CD8+ lymphocytes were quantified in HSILs and benign mucosa from 70 HIV+ and 45 HIV− patients. Clinicopathological parameters were compared.
Results
Anal HSILs harbored more T lymphocytes than benign mucosa regardless of HIV status (P ≤ .03). Total T lymphocyte count and CD8+ subset were significantly higher in HIV+ HSILs versus HIV− HSILs (median cell count, 71 vs 47; 47 vs 22/high power field [HPF]; P < .001), whereas the CD4+ subset was comparable between groups (median, 24 vs. 25; P = .40). Post EA, HSILs persisted in 41% of HIV+ and 19% of HIV− patients (P = .04). Unadjusted analysis showed trends toward EA failures associated with HIV seropositivity (incidence rate ratio [IRR], 2.0; 95% CI, .8–4.9) and increased CD8+ cells (IRR, 2.3; 95% CI, .9–5.3).
Conclusions
. Human immunodeficiency virus is associated with alterations of the immune microenvironment of anal HSILs manifested by increased local lymphocytic infiltrates, predominately CD8+. Human immunodeficiency virus seropositivity and excess mucosa-infiltrating CD8+ cells may be associated with ablation resistance.
The incidence of squamous cell carcinoma of the anus (SCCA) has increased at a rate of 2.2% per year in the United States over the past decade [1, 2]. Anal high-grade squamous intraepithelial lesions (HSILs) are the immediate precursors for SCCA and are caused by persistent infection of high-risk human papillomavirus (HR-HPV) in the anal canal [3, 4]. Men who have sex with men (MSM) have a high prevalence of HPV infection in the anal canal regardless of human immunodeficiency virus (HIV) status [5, 6]. However, HIV-infected (HIV+) MSM are more likely to develop HSILs than their uninfected (HIV−) counterparts: the 4-year incidence of anal HSILs is estimated to be 49% in HIV+ and 17% in HIV− MSM [7]. Although anal HSILs are histologically identical among HIV− and HIV+ patients, they are more likely to persist and to resist treatment in the latter group. Even among those undergoing antiretroviral therapy, HIV+ MSM develop SCCA at a significantly higher rate than their HIV− counterparts [8, 9]. This suggests that despite systemic HIV virological suppression, disturbances in the local microenvironment may play a role in the progression of anal cancer precursors [10, 11].
The anal canal, part of the mucosa-associated lymphoid tissue system (MALT), is populated by immune cells such as Langerhans cells, CD4+ T-helper cells, and CD8+ T-cytotoxic cells [12]. The few existing studies pertaining to the anal MALT system have shown abnormal immune responses in HIV+ individuals, including the depletion of Langerhans cells and CD3+ lymphocytes and increases in Foxp3+ T-regulatory cells, along with multiple regulatory cytokines such as interleukin 8, interleukin 23, tumor necrosis factor α, interleukin 17A, and interferon γ [13–16]. Little is known about the immune microenvironment of anal cancer precursors or its alterations in the setting of HIV coinfection [17]. Similarly, only scant evidence is available regarding the influence of mucosa-infiltrating lymphocytes on the natural history and treatment response of anal HSILs.
This study aims to characterize the subpopulations of mucosa-infiltrating lymphocytes in anal HSILs, correlating them with HIV serostatus and responsiveness to electrocautery ablation (EA). Our results should expand current understanding of cell-mediated mucosal immunity in anal carcinogenesis and thereby facilitate the further development of diagnostic tests and targeted immunotherapeutic strategies.
METHODS
Patient Selection
After institutional review board approval was obtained from the Icahn School of Medicine at Mount Sinai, we searched the Mount Sinai high-resolution anoscopy (HRA) database from January 2013 to January 2016 for patients with their first episode of incident biopsy-proven anal HSILs. We included male patients self-reporting as MSM with known HIV status and available anal biopsy tissue. Information regarding age, race/ethnicity, smoking status, HPV vaccination history, HIV status, CD4+ T-cell count, and HIV-1 plasma viral load (VL) level was abstracted from the electronic medical record.
High-Resolution Anoscopy and Pathology Review
All patients underwent HRA and biopsy performed by a single provider (M. G.) following previously described techniques [18]. Anal mucosa with gross appearance suspicious for HSILs or cancer was biopsied, and the anatomic location within the anal canal was recorded. One pathologist (Y. L.) examined all hematoxylin and eosin–stained slides from the biopsies and diagnosed all biopsies using the terminology and morphological criteria published in the Lower Anogenital Squamous Terminology (LAST) project: benign, low-grade squamous intraepithelial lesion (LSIL), and HSIL [19]. P16 immunohistochemistry was used in a subset of cases, where strong/diffuse positive immunoreactivity supported the diagnosis of HSILs [20]. Benign mucosal samples and HSILs with sufficient tissue for further immunohistochemistry study were selected. Anorectal cytology (ARC) samples were collected within 3 months of or concomitantly with HRA, and results were reported using the 2001 Bethesda System terminology: negative for intraepithelial lesions or malignancy; atypical squamous cells of undetermined significance (ASC-US); LSIL; atypical squamous cells, cannot exclude HSIL (ASC-H); and HSIL [21]. Oncogenic HPV subtype analysis was performed from liquid cytology fluid using the Roche Cobas HPV kit capable of detecting 14 types of HR-HPV (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68).
Electrocautery Ablation and Follow-up
Anal HSILs were treated with EA using a hyfrecator (ConMed Corporation) by a single provider (M. G.). Target lesions were fulgurated and debrided with blunt and sharp dissection to healthy tissue, and submucosal vessels were coagulated. Following EA, patients underwent surveillance HRA at an average interval of 8.5 months (range, 1–20 mo). If HRA revealed suspicious lesions, a biopsy was performed. Random biopsies of healthy-appearing tissue were not pursued during this study.
Immunohistochemistry and Quantification of T-Lymphocyte Subsets
Immunohistochemistry was performed on 5-µm sections of formalin-fixed, paraffin-embedded tissue subjected to monoclonal antibodies against CD4 (VECTOR VP-C319, mouse clone 4B12, 1:200 dilution) and CD8 (DAKO M7103, mouse clone C8/144, 1:80 dilution) using the Dako EnVision+ horseradish peroxidase kit (Carpinteria, CA). Sections of tonsil tissue were used as positive controls. Mononuclear cells with dark brown cytoplasmic signal were recorded as positive cells. All cell counts were performed by a single pathologist (Y. L.) without knowledge of the subject’s HIV status. Lymphocytes within the squamous epithelium (ie, intraepithelial T cell) and underlying stroma (ie, stromal T cell) were counted separately using light microscopy at 400× magnification. Three independent high-power fields were assessed for each case, avoiding lymphoid follicles.
Statistical Analysis
Baseline characteristics were compared by HIV status using the Wilcoxon test for nonnormally distributed continuous variables (age, CD4+ T-cell count) and the χ2 test and Fisher’s exact test for categorical variables where appropriate. We then calculated median values for infiltrating CD4+ and CD8+ cells by location (intraepithelial, stromal, and total), testing for differences by HIV, HR-HPV status, and HPV vaccine history using the Wilcoxon test. In HIV+ subjects, we evaluated for an association between peripheral CD4 cell counts and mucosa-infiltrating cell counts by calculating correlation coefficients. For all subjects with follow-up HRA, we then calculated incidence rates for recurrent (at different anatomic sites of the anal canal) or persistent (at the site of the previously treated lesion) HSILs after ablation therapy and compared these rates by HIV status, smoking history, HPV vaccination history, and tertiles of mucosal CD4, CD8, and CD4/CD8 ratio infiltration using Poisson methods. All analyses were performed in STATA version 13 (Stata Corp, College Station, TX).
RESULTS
Patient Baseline Characteristics According to Human Immunodeficiency Virus Status
A total of 115 MSM (70 HIV+ and 45 HIV−) with biopsy-proven anal HSILs were studied. Table 1 illustrates patient baseline characteristics according to HIV status. The 2 groups shared a similar distribution for age range and smoking status (both P > .05). The HIV+ group included a higher proportion of black (20%) and Hispanic (27%) patients, whereas the HIV− group was predominantly white (71%; P = .01). Regarding HPV genotypes, both groups revealed similar rates of HPV-16 and HPV-18 infections (49% vs 47%). The HIV+ group had a higher rate of infection by other HR-HPV strains than the HIV− group (40% vs 24%; P = .007). The HIV+ group had significantly higher rates of ASC-H and HSIL cytology (24% vs 11%; P = .001) as well as multifocal HSILs (71% vs. 36%, P < .001) than their HIV− counterparts. All HIV+ individuals were undergoing antiretroviral therapy at the time of visit and had a median serum CD4+ cell count of 677 cells/mL (interquartile range, 469–865 cells/mL). HIV RNA was <50 copies/mL in 40 patients (57%), 50–1000 copies/mL in 18 (26%), and >1000 in 12 (17%).
Table 1.
Patient Baseline Characteristics According to Human Immunodeficiency Virus Status
| Characteristic | HIV+ (n = 70) | HIV− (n = 45) | P value |
|---|---|---|---|
| Age, median (IQR) | 41 (23–64) | 42 (32–52) | .70 |
| Race/ethnicity, no. (%) | |||
| White | 31 (44) | 32 (71) | .01 |
| Black | 14 (20) | 3 (7) | |
| Hispanic | 19 (27) | 5 (11) | |
| Other | 6 (9) | 5 (11) | |
| Smoking status, no. (%) | |||
| Current smoker | 17 (24) | 5 (11) | .10 |
| Former smoker | 16 (23) | 15 (33) | |
| Never smoker | 37 (53) | 25 (56) | |
| CD4 Count, median cells/mm3 (IQR) | 677 (469–865) | N/A | |
| HIV RNA (copies/mL), no. (%) | |||
| <50 | 40 (57) | N/A | |
| 50–1000 | 18 (26) | ||
| ≥1000 | 12 (17) | N/A | |
| High-risk HPV type, no. (%) | |||
| 16,18, or both | 34 (49) | 21 (47) | .007 |
| Others | 28 (40) | 11 (24) | |
| Negative | 3 (4) | 0 (0) | |
| Unknown | 5 (7) | 13 (29) | |
| Anorectal cytology, no. (%) | |||
| LSIL or less | 49 (70) | 26 (58) | .001 |
| HSIL or ASC-H | 17 (24) | 5 (11) | |
| Unknown | 4 (6) | 14 (31) | |
| HPV vaccination, no. (%) | 15 (21) | 16 (36) | .20 |
| Number of biopsy-proven HSILs, no. (%) | |||
| Unifocal | 20 (29) | 29 (64) | <.001 |
| Multifocal | 50 (71) | 16 (36) | |
Abbreviations: ASC-H, atypical squamous cells, cannot rule out high-grade lesion; HIV, human immunodeficiency virus; HPV, human papillomavirus; HSIL, high-grade squamous intraepithelial lesion; IQR, intraquartile range; LSIL, low-grade squamous intraepithelial lesion.
Quantitation of Mucosal CD4+ and CD8+ T Lymphocytes
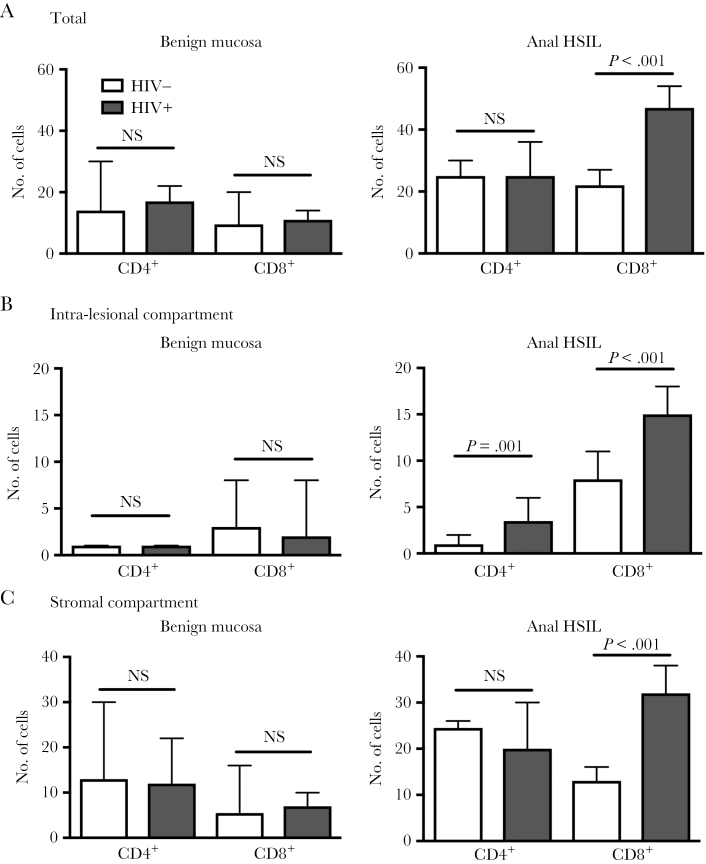
As shown in Table 2 and Figure 1, CD4+ and CD8+ T lymphocytes were quantified in 115 anal HSILs (70 HIV+, 45 HIV−) and 23 benign anal mucosa samples (11 HIV+, 12 HIV−). Benign anal mucosa from HIV+ and HIV− subjects showed similarly scant total mucosa-infiltrating CD4+ and CD8+ lymphocytes (17 vs 14, P = .50; 11 vs 10, P = .90), including the intraepithelial as well as stromal compartments.
Table 2.
Quantity of Mucosa-Infiltrating T Lymphocytes in Anal High-Grade Squamous Intraepithelial Lesions and Benign Anal Mucosa According to HIV Status and Compartment
| T-cell subset | Anal HSIL | P value | Benign anal mucosa | P value | ||
|---|---|---|---|---|---|---|
| HIV+ (n = 70) | HIV− (n = 45) | HIV+ (n = 11) | HIV− (n = 12) | |||
| Total a | ||||||
| CD4+ | 24b | 25 | .40 | 17b | 14 | .50 |
| CD8+ | 47b | 22c | <.001 | 11b | 10c | .90 |
| Intraepithelial compartment a | ||||||
| CD4+ | 3b | 1 | .001 | 1b | 1 | .90 |
| CD8+ | 15b | 8c | <.001 | 2b | 3c | .90 |
| Stromal compartment a | ||||||
| CD4+ | 20b | 25 | .80 | 12b | 13 | .40 |
| CD8+ | 32b | 14 | <.001 | 7b | 6 | .60 |
Abbreviations: HIV, human immunodeficiency virus; HSIL, high-grade squamous intraepithelial lesion.
aMedian cell count detected in 3 high-power fields (400×).
bStatistically significant comparisons between HIV+ HSILs and HIV+ benign mucosa (P ≤ .03).
cStatistically significant comparisons between HIV− HSILs and HIV− benign mucosa (P ≤ .01).
Figure 1.
Quantity of mucosa-infiltrating T lymphocytes in anal high-grade squamous intraepithelial lesions and benign anal mucosa according to human immunodeficiency virus status and compartment. Abbreviations: HSIL, high-grade squamous intraepithelial lesions; NS, not significant.
Anal HSILs harbored a significantly higher number of lymphocytes than benign mucosa in both HIV+ and HIV− groups (P ≤ .03 and P ≤ .01). The total CD8+ T-cell count was significantly higher in HIV+ HSILs than in HIV− HSILs (median 47 vs 22; P < .001). The total CD4+ T-cell count was similar between the 2 groups (median, 24 vs 25; P = .40). Within the dysplastic epithelial layer (ie, intraepithelial T cell), both CD4+ and CD8+ T-cell counts were higher in HIV+ HSILs than HIV− HSILs (3 vs 1; 15 vs 8; P ≤ .001). Within the stromal compartment, a significant increase of CD8+ T cells was observed in the HIV+ HSILs compared with the HIV− HSILs (32 vs 14; P < .001), whereas CD4+ T-cell counts were similar between the 2 groups (20 vs 25; P = .80). We also found a significant difference in stromal CD8 infiltration in subjects with HR-HPV infection compared with those without HR-HPV on concurrent cytology specimens (23 vs 14; P = .03), but otherwise there were no differences in infiltrating cell counts in any other tissue compartments by HPV status (all P > .08; results not otherwise shown). Similar analyses comparing lymphocyte infiltration amounts by HPV vaccination history also did not demonstrate any statistically significant differences (all P > .08; results not otherwise shown). There was no correlation noted between recent serum CD4 cell counts and mucosal T-lymphocyte counts (all R values ≤ 0.10, with corresponding P values > .40). An example of HIV+ anal HSILs with corresponding CD4+ and CD8+ mucosa-infiltrating lymphocytes is illustrated in Figure 2.
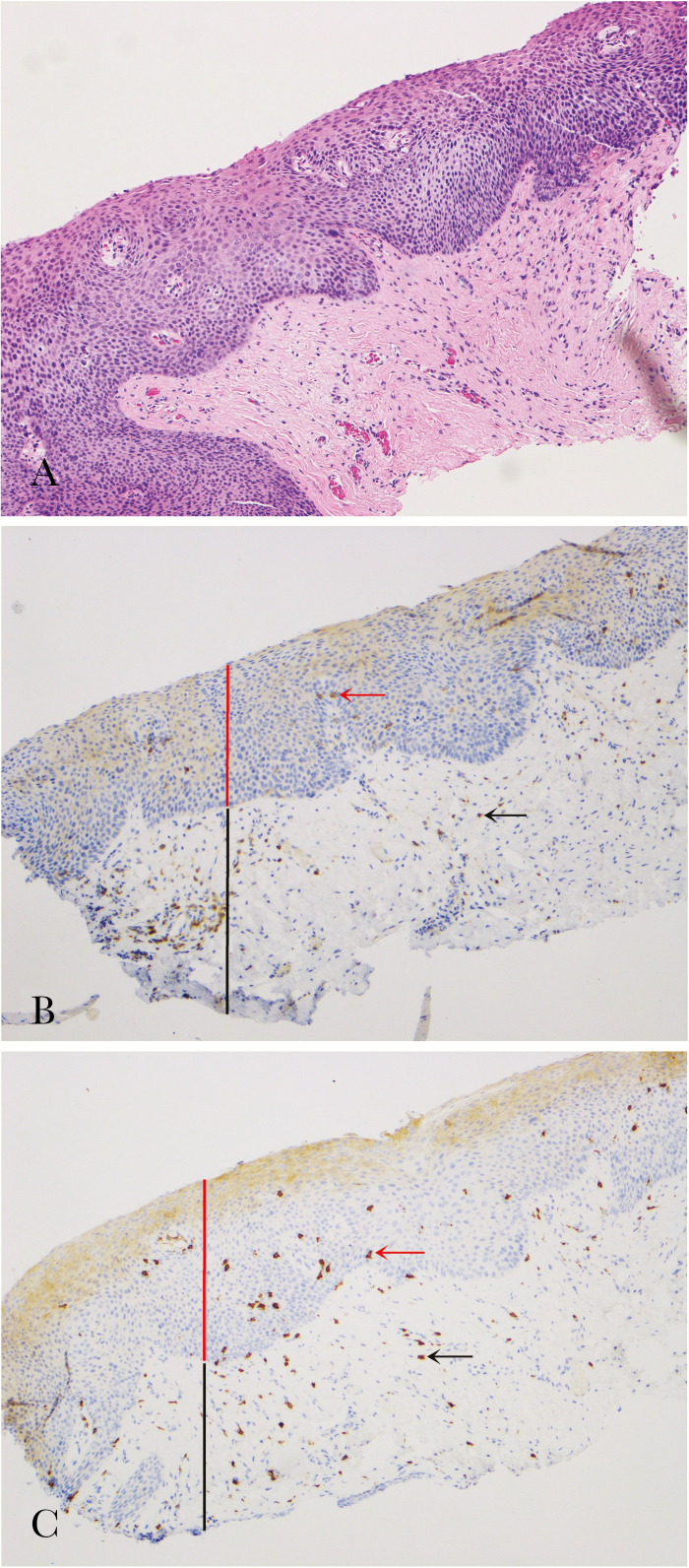
Figure 2.
Example of human immunodeficiency virus–positive anal high-grade squamous intraepithelial lesions (HSILs) and corresponding mucosa-infiltrating T lymphocytes. (A), The lesion shows severe cytological atypia and numerous mitoses in the squamous epithelium, consistent with HSILs. Hematoxylin and eosin, original magnification 100×. Scattered CD4+ T lymphocytes (red and black arrows) (B) and numerous CD8+ T lymphocytes (red and black arrows) (C) are present in the dysplastic epithelium (intraepithelial compartment, red line) and underlying lamina propria (stromal compartment, black line). Immunohistochemistry using antibodies against CD4 and CD8, original magnification 100×.
Efficacy of Electrocautery Ablation
A total of 78 patients (51 HIV+ and 27 HIV−) with biopsy-proven HSILs were treated with EA and underwent surveillance HRA. The average interval between diagnosis and ablation was 2 months (range, 1–10 mo). After EA, 21 HIV+ and 5 HIV− patients had biopsy-proven HSILs at the previously treated site (41% vs 19%; P = .04) (Table 3), with mean time-to-recurrence of 9 (range, 5–22) months and 9 (range, 6–16) months, respectively. The remaining 30 HIV+ and 22 HIV− patients had normal mucosa or LSILs, with mean follow-up time of 14 (range, 3–36) months and 10 (range, 4–23) months, respectively. The success of treatment was 67% (n = 52/78) overall, 59% (n = 30/51) for the HIV+ group, and 81% (n = 22/27) for the HIV− group.
Table 3.
Recurrence or Persistence of Anal High-Grade Squamous Intraepithelial Lesions After Ablation According to Human Immunodeficiency Virus Status
| Outcome | HIV+ HSIL (n = 51) | HIV− HSIL (n = 27) | P value |
|---|---|---|---|
| Persistence/recurrence of HSILs, no. (%) | 21 (41) | 5 (19) | .04 |
| HSIL incidence rate after ablation, per 100 person-months (95% CI) | 3.8 (2.4–5.9) | 2.0 (.6–4.6) | .20 |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; HSIL, high-grade squamous intraepithelial lesion.
There was no statistical difference in postablation HSIL incidence between the HIV+ and HIV− group (3.8 vs.2.0 per 100 person-months; P = .20). In the unadjusted analysis, none of the predictors (HIV status, current smoking, HPV vaccination, total CD4+ or CD8+ T cells, and CD4/CD8 ratio) (Table 4) revealed any significant associations with persistence or incidence of postablation HSILs. However, we observed a trend toward more ablation failures among HIV+ subjects (incidence rate ratio (IRR), 2.0; 95% confidence interval (CI), .8–4.9), as well as increased total CD8+ T cells (IRR, 2.3; 95% CI, .9–5.3).
Table 4.
Unadjusted Poisson Regression Models Evaluating Predictors of High-Grade Squamous Intraepithelial Lesions Recurrence or Persistence Rates After Ablation
| Predictor | Unadjusted incidence rate ratio (95% CI) |
|---|---|
| HIV status | 2.0 (.80–4.9) |
| Current smoking | 0.9 (.4–2.3) |
| HPV vaccination | 0.9 (.4–2.1) |
| Total CD4 T cell | |
| <19 cells/HPF | Referent |
| 19–29 cells/HPF | 0.2 (.07–.8) |
| >29 cells/HPF | 0.9 (.5–1.9) |
| Total CD8 T cell | |
| <26 cells/HPF | Referent |
| 26–45 cells/HPF | 1.5 (.6–4.2) |
| >45 cells/HPF | 2.3 (.9–5.3) |
| CD4/CD8 ratio | |
| <0.46 | Referent |
| 0.46–0.96 | 1.2 (.6–2.5) |
| >0.96 | 0.5 (.2–1.3) |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; HPF, high-power field.
DISCUSSION
Anal HSILs, the HPV-associated anal cancer precursors, follow a more virulent course in HIV+ MSM than in their HIV− counterparts [22, 23]. The underlying mechanisms for the progression of anal HSIL to cancer are yet to be fully established. Growing evidence indicates that impaired local, rather than systemic, immunity plays a critical role in anal carcinogenesis [24, 25].
In our cohort of 115 MSM, we found that anal HSIL immune microenvironments differed significantly by HIV status. The total number of mucosa-infiltrating lymphocytes, predominately CD8+ T cells, was more than double in HIV+ anal HSIL cases. The excess CD8+ T cells were distributed unevenly, tending to scatter in the basal portion of the dysplastic squamous epithelium and to concentrate in the mucosal lamina propria immediately underneath. In contrast, CD4+ T-cell density was comparable between the 2 groups and not associated with HIV status. Our findings suggest there is a distinct cell-mediated immune response potentially affecting anal precancerous lesions in the setting of HIV infection.
Human immunodeficiency virus infection is believed to create a more conducive microenvironment for HPV-induced precancerous lesions to persist and progress [26, 27]. It is puzzling why HIV selectively increases mucosal CD8+ cytotoxic T cells, major players in cellular immune defense and the clearance of viral infection. Several studies on cervical immunity have described similar phenomena, noting a marked increase of primarily CD8+ infiltrates in HIV+ cervical mucosa, particularly in cervical HSILs [28, 29]. Furthermore, CD8+ T cells predominate in cervical HSILs that progress to invasive cancer, whereas CD4+ T cells tend to occupy lesions that spontaneously regress [30]. Taken together, increased mucosal CD8+ infiltration appears to be a consistent finding; further investigation is needed to explain this counterintuitive phenomenon.
Treatment options for anal HSIL include local destruction of individual lesions using various ablative techniques [31]. Reported success rates of local ablation range widely from 35% to 88% depending on patient cohort, provider experience, and surveillance method and duration [32–34]. Nevertheless, postablation persistence and/or recurrence are common and significant limitations that require patients to undergo repeat treatment and long-term follow-up [35, 36]. In our cohort, postablation surveillance was pursued with HRA and biopsy of the previously treated site. A single, experienced provider (M. G.) performed all HRA and ablative procedures. The overall EA success rate was 67% (mean, 12-month follow-up; range, 3–36): 59% for the HIV+ group and 81% for the HIV− group. In unadjusted analyses, the HIV+ group trended toward a 2-fold higher risk for postablation persistence or recurrence. Although the associated risk is notable, it did not reach statistical significance, presumably because of our small sample size.
These results are similar to the findings Goldstone et al published in previous studies using infrared coagulation for the treatment of anal HSILs [37, 38]. The success rate in their series for HIV+ MSM was 65% versus 81% for HIV− MSM (median, 575 days follow-up). Analogous to our cohort, the risk of persistent HSIL among HIV+ MSM was twice as high as among HIV− MSM, but it reached statistical significance in their cohort. The authors speculated that the increased risk was due to a combination of impaired host immune function, HIV infection increasing the oncogenic potential of HPV, and/or a greater burden of disease.
Among the potential predictors in our study (HIV status, smoking history, total CD4+ and CD8+ T cells, CD4/CD8 ratio), longitudinal analysis showed that the quantity of mucosal CD8+ infiltration was associated with the greatest increased risk for HSIL persistence following ablation, although this increase was not statistically significant. It is possible that these excess CD8+ cells are a local manifestation of the dysfunctional immune activation that has been associated with adverse complications of HIV, including non-AIDS cancer, chronic obstructive pulmonary disease, and overall mortality [39, 40]. Contrary to the current view that tumor-infiltrating lymphocytes are associated with favorable outcomes in malignancies, Grabenbauer et al reported that large numbers of granzyme B+ CD8+ T cells had a significantly negative prognostic effect on the clinical outcomes of anal carcinoma, possibly attributable to the selection of therapy-resistant tumor cell clones [41–44].
In summary, our study suggests that HIV alters the immune microenvironment of anal cancer precursors as demonstrated by the significant increase of local lymphocytic infiltrates, predominately CD8+. Ablation resistance appears to be associated with HIV seropositivity and an excess of mucosa-infiltrating CD8+ T lymphocytes. Further characterization of the function and interaction of these immune cells should assist in the development of preventive and immunotherapeutic treatments for this major source of morbidity in the HIV-infected population.
Notes
Financial support. This work was supported by the National Cancer Institute (K07CA180782 to K. S., P30CA008748 to C. S.) and the Junior Faculty Translational Collaborative Research Initiative, Department of Medicine, Icahn School of Medicine at Mount Sinai.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Johnson LG, Madeleine MM, Newcomer LM et al. . Anal cancer incidence and survival: the surveillance, epidemiology, and end results experience, 1973–2000. Cancer 2004; 101:281–8. [DOI] [PubMed] [Google Scholar]
- 2. National Cancer Institute. SEER cancer statistics factsheets: anal cancer. http://seer.cancer.gov/statfacts/html/anus.html. [Google Scholar]
- 3. Frisch M, Fenger C, van den Brule AJ et al. . Variants of squamous cell carcinoma of the anal canal and perianal skin and their relation to human papillomaviruses. Cancer Res 1999; 59:753–7. [PubMed] [Google Scholar]
- 4. Hoots BE, Palefsky JM, Pimenta JM, Smith JS. Human papillomavirus type distribution in anal cancer and anal intraepithelial lesions. Int J Cancer 2009; 124:2375–83. [DOI] [PubMed] [Google Scholar]
- 5. Palefsky JM, Holly EA, Ralston ML, Jay N. Prevalence and risk factors for human papillomavirus infection of the anal canal in human immunodeficiency virus (HIV)–positive and HIV-negative homosexual men. J Infect Dis 1998; 177:361–7. [DOI] [PubMed] [Google Scholar]
- 6. Machalek DA, Poynten M, Jin F et al. . Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: a systematic review and meta-analysis. Lancet Oncol 2012; 13:487–500. [DOI] [PubMed] [Google Scholar]
- 7. Palefsky JM, Holly EA, Ralston ML, Jay N, Berry JM, Darragh TM. High incidence of anal high-grade squamous intra-epithelial lesions among HIV-positive and HIV-negative homosexual and bisexual men. AIDS 1998; 12:495–503. [DOI] [PubMed] [Google Scholar]
- 8. Shiels MS, Pfeiffer RM, Gail MH et al. . Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst 2011; 103:753–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brickman C, Palefsky JM. Human papillomavirus in the HIV-infected host: epidemiology and pathogenesis in the antiretroviral era. Curr HIV/AIDS Rep 2015; 12:6–15. [DOI] [PubMed] [Google Scholar]
- 10. Palefsky JM, Holly EA, Efirdc JT et al. . Anal intraepithelial neoplasia in the highly active antiretroviral therapy era among HIV-positive men who have sex with men. AIDS 2005; 19:1407–14. [DOI] [PubMed] [Google Scholar]
- 11. Seaberg EC, Wiley D, Martínez-Maza O et al. . Cancer incidence in the multicenter AIDS Cohort Study before and during the HAART era: 1984 to 2007. Cancer 2010; 116:5507–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gervaz E, Dauge-Geffroy MD, Sobhani I et al. . Quantitative analysis of the immune cells in the anal mucosa. Pathol Res Pract 1995; 191:1067–71. [DOI] [PubMed] [Google Scholar]
- 13. Paiardini M, Frank I, Pandrea I et al. . Mucosal immune dysfunction in AIDS pathogenesis. AIDS Rev 2008; 10:36–46. [PubMed] [Google Scholar]
- 14. Yaghoobi M, Le Gouvello S, Aloulou N, Duprez-Dutreuil C, Walker F, Sobhani I. FoxP3 overexpression and CD1a+ and CD3+ depletion in anal tissue as possible mechanisms for increased risk of human papillomavirus-related anal carcinoma in HIV infection. Colorectal Dis 2011; 13:768–73. [DOI] [PubMed] [Google Scholar]
- 15. Guimarães AG, Silva Junior RM, Costa OT et al. . Morphometric analysis of dendritic cells from anal mucosa of HIV-positive patients and the relation to intraepithelial lesions and cancer seen at a tertiary health institution in Brazil. Acta Cir Bras 2011; 26:521–9. [DOI] [PubMed] [Google Scholar]
- 16. Guimarães AG, da Costa AG, Martins-Filho OA et al. . CD11c+CD123Low dendritic cell subset and the triad TNF-α/IL-17A/IFN-γ integrate mucosal and peripheral cellular responses in HIV patients with high-grade anal intraepithelial neoplasia: a systems biology approach. J Acquir Immune Defic Syndr 2015; 68:112–22. [DOI] [PubMed] [Google Scholar]
- 17. Haase AT. Perils at mucosal front lines for HIV and SIV and their hosts. Nat Rev Immunol 2005; 5:783–92. [DOI] [PubMed] [Google Scholar]
- 18. Jay N, Berry JM, Hogeboom CJ, Holly EA, Darragh TM, Palefsky JM. Colposcopic appearance of anal squamous intraepithelial lesions: relationship to histopathology. Dis Colon Rectum 1997; 40:919–28. [DOI] [PubMed] [Google Scholar]
- 19. Darragh TM, Colgan T, Cox JT et al. . The lower anogenital squamous terminology standardization project for HPV-associated lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. J Low Genit Tract Dis 2012; 16:205–42. [DOI] [PubMed] [Google Scholar]
- 20. Yang EJ, Kong CS, Longacre TA. Vulvar and anal intraepithelial neoplasia: terminology, diagnosis, and ancillary studies. Adv Anat Pathol 2017; 24:136–50. [DOI] [PubMed] [Google Scholar]
- 21. Solomon D, Davey D, Kurman R et al. ; Forum Group Members; Bethesda 2001 Workshop The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA 2002; 287:2114–9. [DOI] [PubMed] [Google Scholar]
- 22. Tong WW, Jin F, McHugh LC et al. . Progression to and spontaneous regression of high-grade anal squamous intraepithelial lesions in HIV-infected and uninfected men. AIDS 2013; 27:2233–43. [DOI] [PubMed] [Google Scholar]
- 23. Roberts JR, Siekas LL, Kaz AM. Anal intraepithelial neoplasia: a review of diagnosis and management. World J Gastrointest Oncol 2017; 9:50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Piketty C, Selinger-Leneman H, Grabar S et al. ; FHDH-ANRS CO 4 Marked increase in the incidence of invasive anal cancer among HIV-infected patients despite treatment with combination antiretroviral therapy. AIDS 2008; 22:1203–11. [DOI] [PubMed] [Google Scholar]
- 25. Chaturvedi AK, Madeleine MM, Biggar RJ, Engels EA. Risk of human papillomavirus-associated cancers among persons with AIDS. J Natl Cancer Inst 2009; 101:1120–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Critchlow CW, Hawes SE, Kuypers JM et al. . Effect of HIV infection on the natural history of anal human papillomavirus infection. AIDS 1998; 12:1177–84. [DOI] [PubMed] [Google Scholar]
- 27. Clarke B, Chetty R. Postmodern cancer: the role of human immunodeficiency virus in uterine cervical cancer. Mol Pathol 2002; 55:19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bell MC, Schmidt-Grimminger D, Turbat-Herrera E et al. . HIV+ patients have increased lymphocyte infiltrates in CIN lesions. Gynecol Oncol 2000; 76:315–9. [DOI] [PubMed] [Google Scholar]
- 29. Monnier-Benoit S, Mauny F, Riethmuller D et al. . Immunohistochemical analysis of CD4+ and CD8+ T-cell subsets in high risk human papillomavirus-associated pre-malignant and malignant lesions of the uterine cervix. Gynecol Oncol 2006; 102:22–31. [DOI] [PubMed] [Google Scholar]
- 30. Edwards RP, Kuykendall K, Crowley-Nowick P, Partridge EE, Shingleton HM, Mestecky J. T lymphocytes infiltrating advanced grades of cervical neoplasia. CD8-positive cells are recruited to invasion. Cancer 1995; 76:1411–5. [DOI] [PubMed] [Google Scholar]
- 31. Alam NN, White DA, Narang SK, Daniels IR, Smart NJ. Systematic review of guidelines for the assessment and management of high-grade anal intraepithelial neoplasia (AIN II/III). Colorectal Dis 2016; 18:135–46. [DOI] [PubMed] [Google Scholar]
- 32. Goldstone SE, Kawalek AZ, Huyett JW. Infrared coagulator: a useful tool for treating anal squamous intraepithelial lesions. Dis Colon Rectum 2005; 48:1042–54. [DOI] [PubMed] [Google Scholar]
- 33. Cranston RD, Hirschowitz SL, Cortina G, Moe AA. A retrospective clinical study of the treatment of high-grade anal dysplasia by infrared coagulation in a population of HIV-positive men who have sex with men. Int J STD AIDS 2008; 19:118–20. [DOI] [PubMed] [Google Scholar]
- 34. Videla S, Sirera G, Pinol M et al. . Long-term effectiveness of infrared coagulation for anal intraepithelial neoplasia 2 and 3 in HIV-infected males and females. AIDS 2013; 27:951–959. [DOI] [PubMed] [Google Scholar]
- 35. Marks DK, Goldstone SE. Electrocautery ablation of high-grade anal squamous intraepithelial lesions in HIV-negative and HIV-positive men who have sex with men. J Acquir Immune Defic Syndr 2012; 59:259–65. [DOI] [PubMed] [Google Scholar]
- 36. Goldstone RN, Goldstone AB, Russ J, Goldstone SE. Long-term follow-up of infrared coagulator ablation of anal high-grade dysplasia in men who have sex with men. Dis Colon Rectum 2011; 54:1284–92. [DOI] [PubMed] [Google Scholar]
- 37. Goldstone SE, Hundert JS, Huyett JW. Infrared coagulator ablation of high-grade anal squamous intraepithelial lesions in HIV-negative males who have sex with males. Dis Colon Rectum 2007; 50:565–75. [DOI] [PubMed] [Google Scholar]
- 38. Stier EA, Goldstone SE, Berry JM et al. . Infrared coagulator treatment of high-grade anal dysplasia in HIV-infected individuals: an AIDS malignancy consortium pilot study. J Acquir Immune Defic Syndr 2008; 47:56–61. [DOI] [PubMed] [Google Scholar]
- 39. Serrano-Villar S, Sainz T, Lee SA et al. . HIV-infected individuals with low CD4/CD8 ratio despite effective antiretroviral therapy exhibit altered T cell subsets, heightened CD8+ T cell activation, and increased risk of non-AIDS morbidity and mortality. PLoS Pathog 2014; 10:e1004078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Triplette M, Attia EF, Akgün KM et al. . A low peripheral blood CD4/CD8 ratio is associated with pulmonary emphysema in HIV. PLoS One 2017; 12:e0170857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang L, Conejo-Garcia JR, Katsaros D et al. . Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med 2003; 348:203–13. [DOI] [PubMed] [Google Scholar]
- 42. Cho Y, Miyamoto M, Kato K et al. . CD4+ and CD8+ T cells cooperate to improve prognosis of patients with esophageal squamous cell carcinoma. Cancer Res 2003; 63:1555–9. [PubMed] [Google Scholar]
- 43. Schumacher K, Haensch W, Roefzaad C et al. . Prognostic significance of activated CD8(+) T cell infiltrations within esophageal carcinomas. Cancer Res 2004; 61:3932–6. [PubMed] [Google Scholar]
- 44. Grabenbauer GG, Lahmer G, Distel L, Niedobitek G. Tumor-infiltrating cytotoxic T cells but not regulatory T cells predict outcome in anal squamous cell carcinoma. Clin Cancer Res 2006; 12:3355–60. [DOI] [PubMed] [Google Scholar]