Abstract
The global community, including the World Health Organization (WHO), has committed to ending the AIDS epidemic and to ensuring that 90% of people living with human immunodeficiency virus (HIV) are diagnosed, 90% start treatment, and 90% achieve and maintain virological suppression. The emergence of HIV drug resistance (HIVDR) as antiretroviral treatment programs expand could preclude the 90-90-90 targets adopted by the United Nations General Assembly at the High-Level Meeting on Ending AIDS from being achieved. The Global Action Plan on HIVDR is a call for collective action grounded on normative guidance providing a standardized and robust approach to monitoring, preventing, and responding to HIVDR over the next 5 years (2017–2021). WHO is committed to supporting country, global, regional, and national partners to implement and monitor the progress of the Global Action Plan. This article outlines the key components of WHO’s strategy to tackle HIVDR and the role the organization takes in leading the global response to HIVDR.
Keywords: human immunodeficiency virus (HIV), drug resistance, monitoring, prevention, response
In 2016 the United Nations’ High-Level Meeting on Ending AIDS [1] committed to “establishing an effective system to monitor, prevent and respond to the emergence of drug resistance strains of HIV in populations and antimicrobial resistance among people living with HIV.” As the world transitions to a “treat all” approach in the global response to the human immunodeficiency virus (HIV) epidemic, it is vital that population levels of HIV drug resistance (HIVDR) be routinely monitored and that countries respond appropriately when HIVDR reaches levels that can lead to unacceptably high rates of virological failure, a reversal of HIV-associated morbidity and mortality, increasing incidence of HIV, and higher overall costs of providing antiretroviral therapy (ART) [2].
Despite the progress made by the global HIV community in achieving the Millennium Development Goals [3], which resulted in 19.5 million people initiating ART by the end of 2016 and reductions in the morbidity and mortality of people living with HIV [4], the next 10 years will be a time of continued expansion in HIV programs, particularly in countries identified as “Fast Track” for focused support. The potential emergence and transmission of HIVDR must be considered a priority in conjunction with the development of quality HIV treatment and care services to ensure that highly effective drug regimens are available to all those who are diagnosed and that antiretroviral (ARV) medicines used for pre-exposure (PrEP) and post-exposure prophylaxis (PEP) remain effective.
At an individual level, the presence of pretreatment drug resistance when starting ART (or pretreatment HIV drug resistance [PDR]) increases the risk of virological failure in adults and children [5–23], the need to switch to a more costly regimen [24], treatment discontinuation [5,16,25], and accumulation of additional drug-resistance mutations [11, 12]. At a population level, the cost of inaction has both human and financial consequences. Modeling predicts that levels of PDR >10% will result in an additional 890000 deaths, 450000 new infections, and an ART program cost of $6.5 billion over the next 15 years in sub-Saharan Africa alone if current first-line ART remains unchanged [2]. The same model predicts increasing negative impact on mortality, HIV incidence, and viral load suppression (VLS) resulting from the rising prevalence of PDR and indicates that interventions such as the introduction of pretreatment HIVDR testing or, even more, a shift to dolutegravir-based ART in first-line regimens can result in more favorable health outcomes [26]. The 2017 World Health Organization (WHO) HIVDR report highlights that levels of PDR to efavirenz or nevirapine, the commonly used ARVs in first-line ART, exceed 10% in several low- and middle-income countries [27].
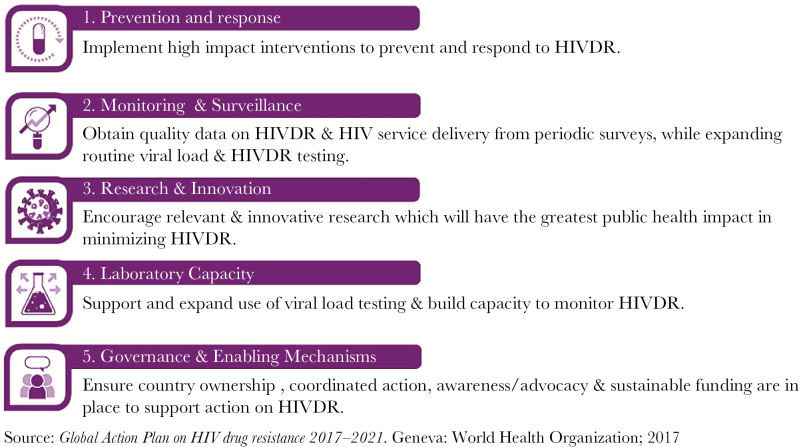
In its role as global convener, the WHO has led the development of a Global Action Plan (GAP) on HIV drug resistance (2017–2021) with the overarching goal to preventing HIVDR from undermining the attainment of global targets on health and HIV and enabling the most effective treatment to be provided to all people living with HIV [28]. The 5 strategic objectives of the action plan highlight the importance of effective prevention and response to HIVDR and the need to strengthen monitoring and surveillance of HIVDR, invest in innovative research, strengthen laboratory capacity, and establish enabling financial, governance, and advocacy mechanisms to achieve tangible results (Figure 1). All stakeholders with a role in the global response to HIV are called on to support implementation of the plan and to act collectively to respond to the potential threat that HIVDR poses to the global elimination of AIDS.
Figure 1.
Five strategic objectives of the Global Action Plan (GAP) on HIV Drug Resistance (2017–2021). Abbreviation: HIVDR, human immunodeficiency virus drug resistance.
The Global Action Plan on HIVDR aligns itself with the broader Global Action Plan on antimicrobial resistance and calls for a comprehensive, coordinated, innovative, and integrated action to tackle HIVDR and broader antimicrobial challenges [29, 30], therefore providing an opportunity for strengthening synergy and collaboration between all allied programs at country, regional, and global levels. Collective action, including opportunities to leverage resources, can combat the threat of resistant microbes and viruses to population health in diseases of significant public health burden.
Success in monitoring, preventing, and responding to HIVDR will enable the global targets of 90% of people on ART to achieve VLS by 2020 and 95% by 2030 [1]. These targets have been shown to be attainable by the population-based HIV impact assessments supported by the US President’s Emergency Plan for AIDS Relief in 2016–2017 [31–34] with high levels of VLS in adults who self-report current use of ART. Despite these encouraging results, prevalence of VLS among all individuals living with HIV, and particularly in younger people, is suboptimal and is below desirable levels in certain countries population initiating ART (as opposed to people retained or ART and with viral load results available) is used as a denominator [27]. The ability to disaggregate the cascade of care by age and sex to identify differences in outcomes between subpopulations will inform ways to close gaps in the delivery of effective diagnosis, treatment, and care services and will assist countries in improving the outcomes of vulnerable populations who are not easily accessed or who are at higher risk of defaulting from care [35].
Standardized methods for HIVDR surveillance [36] developed by WHO guide countries and the global community in understanding levels of PDR in people starting first-line ART who are naive to ARVs and in ART starters reporting prior ARV drug exposure and prevalence and patterns of HIVDR in individuals failing ART. Analysis and interpretation of data generated from surveillance and monitoring activities, including a subset of quality-of-care indicators associated with HIVDR and known as early warning indicators of HIVDR [37–39], enable countries to identify factors associated with resistance and to plan appropriate actions, including adopting treatment regimens that will be most effective for the population [26].
The commitment from WHO to compile and release regular global reports on HIVDR providing timely estimates of global epidemiological data is essential for understanding the changing epidemic in all regions, particularly at a time when new HIV medicines are being introduced in low- and middle-income countries and PrEP programs are being expanded. Timely collection and dissemination of results allows greater clarity on population levels of PDR, acquired drug resistance, and resistance in young ART-naive children and facilitates analysis of trends of HIVDR over time. Following the first WHO HIVDR Report in 2012 [40], the 2017 report encouragingly demonstrates that countries supported by WHO and implementing partners have conducted surveys using WHO-recommended standardized survey methods. Since 2014, 20 countries have either completed or are implementing national PDR surveys, and 16 additional surveys are planned in 2017; 12 countries have national acquired drug resistance surveys completed or ongoing, and an additional 11 are planned to start in 2017. Between 2014 and 2017, 3 countries implemented HIVDR surveys among ART-naive children, and 4 surveys are planned for 2017 [27]. Despite accelerated implementation of HIVDR surveys by many countries, greater support is required to achieve broader and ongoing routine implementation. The 2017 WHO guidelines on the public health response to pretreatment HIV drug resistance [26] is a key resource in the timely response to national levels of PDR above the defined threshold of 10%.
To adequately capture HIVDR trends, WHO has developed a global repository of HIVDR survey data, which includes deidentified individual-level genotypic information linked to minimal epidemiological information. The repository has a country user interface and is designed to assist countries in managing and interpreting their HIVDR data for effective public health action. In the future, WHO plans to expand the database to capture routine programmatic data and findings coming from other research activities to depict the evolving epidemiology of HIVDR.
Prevention of HIVDR remains a pivotal element of the global response. Annual monitoring of clinic-level and program quality-of-care indicators that are predictive of and associated with HIVDR [37–39] is critical to characterize ART clinic and program performance with regard to patient adherence to ART, drug supply continuity, retention on ART, coverage of viral-load testing, viral-load testing outcomes, and appropriate switch to second-line ART. It also enables clinics and programs to identify gaps in the quality of HIV service delivery and implement targeted actions to optimize care and minimize emergence of HIVDR. WHO recommends that early warning indicators be fully integrated into the country monitoring system for HIV programs.
In addition to providing support and assistance with surveillance activities, WHO provides technical guidance to countries seeking designation of a national reference laboratory for HIVDR testing and coordinates the global WHO HIVResNet laboratory network [41], which currently includes 31 laboratories designated by WHO for HIVDR testing on plasma or dried blood spots. Countries monitoring levels of HIVDR but without in-country HIVDR testing capacity can receive assistance from this network.
Laboratory strengthening predicted by the Global Action Plan on HIVDR will also enable countries to scale up viral-load monitoring and build capacity around HIVDR testing and ensure that VLS be regularly monitored at individual and population levels.
Research and innovation on HIVDR testing, with the incorporation of newer techniques (ie, less costly point-of-care testing for HIVDR and next-generation sequencing assays) will support more cost-efficient approaches to monitor HIVDR. Future advances will need to include standardized bioinformatic algorithms and quality assurance program to support interpretation of HIVDR data generated by next-generation sequencing. As integrase inhibitors become more widely used, capacity for and standardization of integrase inhibitor resistance testing must be realized.
Incident HIV is possible despite adherence to PrEP when individuals are infected with emtricitabine-resistant virus, tenofovir-resistant virus, or both [42]. As PrEP programs are expanded, surveillance of resistance mutations that affect the efficacy of PrEP and that may also compromise the efficacy of first-line ART is needed. There is also a need to expand the knowledge about HIVDR in children and adolescents, pregnant and breastfeeding women, and key populations and develop effective interventions to reduce it. It is similarly critical to ascertain the clinical relevance of resistant mutations present as low-abundance variants and of isolated nucleoside reverse-transcriptase inhibitor mutations on the response to dolutegravir-containing regimens.
WHO aim to convene stakeholders to develop a coordinated research agenda to ensure a shared approach and vision and that resources are directed in a collaborative manner to areas of public health importance.
Through global commitment and collective action, significant progress has been made in the development of, provision of, and access to ARVs and quality treatment services to people living with HIV. Renewed attention to high-quality ART service delivery and high-impact interventions based on HIVDR data is crucial to ensure that the potential threat of HIVDR never derails the universal goal of ending AIDS. WHO renews its commitment to guide and facilitate national and global responses to HIVDR and will adapt to the changing face of the epidemic and critical issues as they arise.
Notes
Acknowledgments. We acknowledge the support of the Bill and Melinda Gates Foundation in the development of the Global Action Plan on HIV Drug Resistance.
Disclaimer. S. B., M. D., and G. H. are WHO employees; the views and conclusions expressed in this paper are those of the authors and do not necessarily represent the official position of the World Health Organization.
Supplement sponsorship. This work is part of a supplement sponsored by the National Institute of Allergy and Infectious Disease, NIH, and the Centers for Disease Control and Prevention.
Potential conflicts of interest. All authors: no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. United Nations General Assembly. Political Declaration on HIV and AIDS: On the Fast Track to Accelerate the Fight Against HIV and to End the AIDS Epidemic by 2030. Geneva Switzerland: UNAIDS; 2016. [Google Scholar]
- 2. Phillips AN, Stover J, Cambiano V et al. . Impact of HIV drug resistance on HIV/AIDS–Associated mortality, new infections, and antiretroviral therapy program costs in sub-Saharan Africa. J Infect Dis 2017; 215:1362–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. UNAIDS. How AIDS changed everything—MDG6: 15 years, 15 lessons of hope from the AIDS responsehttps://issuu.com/unaids/docs/mdg6report_no-annexes_en?reader3=1.
- 4. UNAIDS, WHO. Global AIDS Monitoring. Geneva, Switzerland: UNAIDS, 2017. [Google Scholar]
- 5. Ávila-Ríos S, García-Morales C, Matías-Florentino M et al. . Pretreatment HIV-drug resistance in Mexico and its impact on the effectiveness of first-line antiretroviral therapy: a nationally representative 2015 WHO survey. Lancet HIV 2016; 3:e579–91. [DOI] [PubMed] [Google Scholar]
- 6. Borroto-Esoda K, Waters JM, Bae AS et al. . Baseline genotype as a predictor of virological failure to emtricitabine or stavudine in combination with didanosine and efavirenz. AIDS Res Hum Retroviruses 2007; 23:988–95. [DOI] [PubMed] [Google Scholar]
- 7. Clutter DS, Fessel WJ, Rhee SY et al. . Response to therapy in antiretroviral therapy-naive patients with isolated nonnucleoside reverse transcriptase inhibitor-associated transmitted drug resistance. J Acquir Immune Defic Syndr 2016; 72:171–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hong SY, Jonas A, DeKlerk M et al. . Population-based surveillance of HIV drug resistance emerging on treatment and associated factors at sentinel antiretroviral therapy sites in Namibia. J Acquir Immune Defic Syndr 2015; 68:463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bannister WP, Cozzi-Lepri A, Clotet B et al. ; EuroSIDA Study Group Transmitted drug resistant HIV-1 and association with virologic and CD4 cell count response to combination antiretroviral therapy in the EuroSIDA Study. J Acquir Immune Defic Syndr 2008; 48:324–33. [DOI] [PubMed] [Google Scholar]
- 10. Chaix ML, Desquilbet L, Descamps D et al. ; French PRIMO Cohort Study Group (ANRS CO 06); French ANRS AC11 Resistance Study Group Response to HAART in French patients with resistant HIV-1 treated at primary infection: ANRS Resistance Network. Antivir Ther 2007; 12:1305–10. [PubMed] [Google Scholar]
- 11. Hamers RL, Schuurman R, Sigaloff KC et al. ; PharmAccess African Studies to Evaluate Resistance (PASER) Investigators Effect of pretreatment HIV-1 drug resistance on immunological, virological, and drug-resistance outcomes of first-line antiretroviral treatment in sub-Saharan Africa: a multicentre cohort study. Lancet Infect Dis 2012; 12:307–17. [DOI] [PubMed] [Google Scholar]
- 12. Kityo C, Boerma RS, Sigaloff KCE et al. . Pretreatment HIV drug resistance results in virological failure and accumulation of additional resistance mutations in Ugandan children. J Antimicrob Chemother 2017. doi:10.1093/jac/dkx188. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Phanuphak P, Sirivichayakul S, Jiamsakul A et al. . Transmitted drug resistance and antiretroviral treatment outcomes in non-subtype B HIV-1-infected patients in South East Asia. J Acquir Immune Defic Syndr 2014; 66:74–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee GQ, Bangsberg DR, Muzoora C et al. . Prevalence and virologic consequences of transmitted HIV-1 drug resistance in Uganda. AIDS Res Hum Retroviruses 2014; 30:896–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zu Knyphausen F, Scheufele R, Kücherer C et al. . First line treatment response in patients with transmitted HIV drug resistance and well defined time point of HIV infection: updated results from the German HIV-1 seroconverter study. PLoS One 2014; 9:e95956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lockman S, Hughes MD, McIntyre J et al. ; OCTANE A5208 Study Team Antiretroviral therapies in women after single-dose nevirapine exposure. N Engl J Med 2010; 363:1499–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Crowell CS, Maiga AI, Sylla M et al. . High rates of baseline drug resistance and virologic failure among ART naïve HIV-infected children in Mali. Pediatr Infect Dis J 2017. doi:10.1097/INF.0000000000001575. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Boerma RS, Boender TS, Sigaloff KC et al. . High levels of pre-treatment HIV drug resistance and treatment failure in Nigerian children. J Int AIDS Soc 2016; 19:21140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kantor R, Smeaton L, Vardhanabhuti S et al. ; AIDS Clinical Trials Group (ACTG) A5175 Study Team Pretreatment HIV drug resistance and HIV-1 subtype C are independently associated with virologic failure: results from the multinational PEARLS (ACTG A5175) clinical trial. Clin Infect Dis 2015; 60:1541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kuritzkes DR, Lalama CM, Ribaudo HJ et al. . Preexisting resistance to nonnucleoside reverse-transcriptase inhibitors predicts virologic failure of an efavirenz-based regimen in treatment-naive HIV-1-infected subjects. J Infect Dis 2008; 197:867–70. [DOI] [PubMed] [Google Scholar]
- 21. Wittkop L, Günthard HF, de Wolf F et al. ; EuroCoord-CHAIN study group Effect of transmitted drug resistance on virological and immunological response to initial combination antiretroviral therapy for HIV (EuroCoord-CHAIN joint project): a European multicohort study. Lancet Infect Dis 2011; 11:363–71. [DOI] [PubMed] [Google Scholar]
- 22. Mackie NE, Dunn DT, Dolling D et al. ; UK HIV Drug Resistance Database; UK CHIC study The impact of HIV-1 reverse transcriptase polymorphisms on responses to first-line nonnucleoside reverse transcriptase inhibitor-based therapy in HIV-1–infected adults. AIDS 2013; 27:2245–53. [DOI] [PubMed] [Google Scholar]
- 23. Shet A, Neogi U, Kumarasamy N, DeCosta A, Shastri S, Rewari BB. Virological efficacy with first-line antiretroviral treatment in India: predictors of viral failure and evidence of viral resuppression. Trop Med Int Health 2015; 20:1462–72. [DOI] [PubMed] [Google Scholar]
- 24. Boender TS, Hoenderboom BM, Sigaloff KC et al. . Pretreatment HIV drug resistance increases regimen switches in sub-Saharan Africa. Clin Infect Dis 2015; 61:1749–58. [DOI] [PubMed] [Google Scholar]
- 25. Lai CC, Hung CC, Chen MY et al. . Trends of transmitted drug resistance of HIV-1 and its impact on treatment response to first-line antiretroviral therapy in Taiwan. J Antimicrob Chemother 2012; 67:1254–60. [DOI] [PubMed] [Google Scholar]
- 26. World Health Organization. Guidelines on the Public Health Response to Pretreatment HIV Drug Resistance. Geneva, Switzerland: World Health Organization;2017. [Google Scholar]
- 27. World Health Organization. HIV Drug Resistance Report 2017. Geneva, Switzerland: World Health Organization; 2017. [Google Scholar]
- 28. World Health Organization. Global Action Plan on HIV Drug Resistance, 2017–2021. Geneva, Switzerland: World Health Organization; 2017. [Google Scholar]
- 29. World Health Organization. Global Action Plan on Antimicrobial Resistance. Geneva, Switzerland: World Health Organization; 2015. [DOI] [PubMed] [Google Scholar]
- 30. World Health Organization. Global Action Plan on Antimicrobial Resistance. Geneva, Switzerland: World Health Assembly Resolution; Sixty-Eighth World Health Assembly; 2015. [Google Scholar]
- 31. PHIA Project. Malawi Population-Based HIV Impact Assessment. http://phia.icap.columbia.edu/wp-content/uploads/2016/09/MALAWI-Factsheet.FIN_.pdf [Google Scholar]
- 32. PHIA Project. Zimbabwe Population-Based HIV Impact Assessment. http://phia.icap.columbia.edu/wp-content/uploads/2016/11/ZIMBABWE-Factsheet.FIN_.pdf. [Google Scholar]
- 33. PHIA Project. Swaziland HIV Incidence Measurement Survey 2: A Population-Based HIV Impact Assessment. Preliminary findings . http://phia.icap.columbia.edu/shims2-summary-sheet-preliminary-findings-july-2017/. [Google Scholar]
- 34. PHIA Project. Zimbabwe Population-Based HIV Impact Assessment. http://phia.icap.columbia.edu/wp-content/uploads/2016/09/ZAMBIA-Factsheet.FIN_.pdf. [Google Scholar]
- 35. World Health Organization. Consolidated Strategic Information Guidelines for HIV in the Health Sector: HIV Strategic Information for Impact. Geneva, Switzerland: World Health Organization; 2015. [PubMed] [Google Scholar]
- 36. World Health Organization. HIV Drug Resistance Surveillance Guidance: 2015 Update. Geneva, Switzerlaned: World Health Organization; 2015. [Google Scholar]
- 37. World Health Organization. Global report on early warning indicators of HIV drug resistance: technical report. Geneva, Switzerland: World Health Organization; 2016. [Google Scholar]
- 38. World Health Organization. Consolidated Guidelines on Person-Centred HIV Patient Monitoring and Case Surveillance. Geneva, Switzerland: World Health Organization; 2017. [Google Scholar]
- 39. St-Jean M, Harrigan PR, Sereda P, Montaner J, Lima VD. An assessment of the relationship between the World Health Organization HIV drug resistance early warning indicators and HIV drug resistance acquisition. HIV Med 2017; 18:342–53. [DOI] [PubMed] [Google Scholar]
- 40. World Health Organization. WHO HIV Drug Resistance Report 2012. Geneva, Switzerland: World Health Organization;2012. [Google Scholar]
- 41. World Health Organization. HIV Drug Resistance Laboratory Strategy. http://www.who.int/hiv/topics/drugresistance/laboratory/en/. [Google Scholar]
- 42. Knox DC, Anderson PL, Harrigan PR, Tan DH. Multidrug-resistant HIV-1 infection despite preexposure prophylaxis. N Engl J Med 2017; 376:501–2. [DOI] [PubMed] [Google Scholar]