We conducted a genome-wide association study on 1843 subjects to identify genetic polymorphisms associated with cellular immunity after rubella vaccination. We identified single-nucleotide polymorphisms in the Wilms tumor gene WT1 that was significantly associated with interindividual differences in subject rubella-specific interleukin 6 secretion.
Keywords: rubella, measles-mumps-rubella vaccine, rubella virus, polymorphisms, genetic, genome-wide association study, IL-6, immunity, SNP
Abstract
Rubella vaccination induces widely variable immune responses in vaccine recipients. While rubella vaccination is effective at inducing immunity to rubella infection in most subjects, up to 5% of individuals do not achieve or maintain long-term protective immunity. To expand upon our previous work identifying genetic polymorphisms that are associated with these interindividual differences in humoral immunity to rubella virus, we performed a genome-wide association study in a large cohort of 1843 subjects to discover single-nucleotide polymorphisms (SNPs) associated with rubella virus–specific cellular immune responses. We identified SNPs in the Wilms tumor protein gene (WT1) that were significantly associated (P < 5 × 10–8) with interindividual variations in rubella-specific interleukin 6 secretion from subjects’ peripheral blood mononuclear cells postvaccination. No SNPs were found to be significantly associated with variations in rubella-specific interferon-γ secretion. Our findings demonstrate that genetic polymorphisms in the WT1 gene in subjects of European ancestry are associated with interindividual differences in rubella virus–specific cellular immunity after measles-mumps-rubella II vaccination.
Rubella virus (RV) is an RNA virus that typically causes mild disease; however, rubella infection can cause miscarriage, fetal death, or fetal defects in up to 90% of pregnancies when rubella is contracted immediately prior to or during the first 10 weeks of gestation [1]. Development and use of rubella vaccine—a live, attenuated strain of RV—has led to a decrease in the worldwide incidence of rubella and congenital rubella syndrome, and elimination of rubella in the Americas [2]. However, rubella outbreaks continue to occur in both industrialized and nonindustrialized countries in Europe, Africa, Asia, and Oceania [3].
In the United States, the rubella vaccine strain virus RA27/3 is administered as a component of the measles-mumps-rubella II (MMR-II) vaccine in a 2-dose series. Rubella vaccine induces the development of protective immunity against rubella disease in a majority of vaccinees [4]; however, immune responses to rubella vaccine are highly variable, and up to 5% of individuals do not reach or maintain protective antibody levels (>10 IU/mL) [5, 6].
Interindividual heterogeneity in vaccine responses limits vaccine effectiveness at the population level. A fully vaccinated, yet rubella-vulnerable, subpopulation that nonetheless believes it is protected presents a risk for rubella outbreaks even in highly vaccinated populations, putting women of childbearing age at risk of rubella infection. There is evidence that much of this interindividual variation is due to genetic differences, as more than half of subjects who are antibody negative after a first rubella vaccination remain negative or fail to maintain long-term protective immunity after a second dose [7]. Studying the genetic basis of vaccine-response heterogeneity can provide information to improve vaccine effectiveness, avoid adverse events, improve public health, and inform a personalized vaccination regimen based on individuals’ genetic profiles [8, 9].
Our group previously identified genetic variations in human leukocyte antigen (HLA) loci and polymorphisms in immune response genes that are associated with rubella-specific antibody titers in vaccinated subjects [10–14]. In particular, HLA-DPB1 allelic variants were found to be associated with variations in neutralizing antibody titers both in candidate gene studies and in a genome-wide association study (GWAS) on 897 European ancestry children and young adults [10, 14, 15]. Here, we extend our previous work with a GWAS that examines genetic associations with rubella-specific cellular responses in a combined cohort of 1843 subjects recruited at 2 different geographical locations in the United States.
METHODS
The study population and laboratory methods described herein are similar or identical to those published in our previous studies [14, 16–20].
Study Participants
We analyzed a cohort of 1843 healthy subjects ranging in age from 11 to 39 years. These subjects were recruited in 2 independent cohorts: a Rochester cohort (n = 962) and a San Diego cohort (n = 881). The clinical and demographic characteristics of these cohorts have been previously published [14, 16–20]. The institutional review boards of the Mayo Clinic (Rochester, Minnesota) and the Naval Health Research Center (San Diego, California) approved the study, and written informed consent was obtained from each subject (ie, from age-appropriate participants and the parents of all children who participated in the study).
Cellular Cytokine Secretion Assays
We measured cellular immunity to RV by stimulating subject peripheral blood mononuclear cell (PBMC) cultures with RV and measuring interleukin 6 (IL-6) and interferon gamma (IFN-γ) secretion in culture supernatants by enzyme-linked immunosorbent assay (ELISA), as previously described [21, 22]. In brief, cryopreserved PBMCs were thawed and cultured in RPMI 1640 (Invitrogen) supplemented with 5% FCS (HyClone) and antibiotics. PBMCs were cultured with live W-Therien RV with a multiplicity of infection of 5 (gift from Dr Teryl Frey, Georgia State University), in triplicate alongside mock-infected and phytohemagglutinin-stimulated (5 ug/mL) controls. Culture supernatants were harvested at 24 hours (IL-6) or 48 hours (IFN-γ) and frozen for cytokine testing. ELISA assays for IL-6 and IFN-γ were performed on the collected supernatants using commercial kits (BD Pharmingen) following the manufacturer’s protocol. The limit of detection for both cytokines was 4.7 pg/mL.
Genotyping
The genome-wide single-nucleotide polymorphism (SNP) typing was performed using the Infinium Omni 1M-Quad SNP array (Illumina, San Diego, California) for the Rochester cohort and Illumina Infinium HumanHap550v3_A or HumanHap650Yv3 BeadChip arrays for the San Diego cohort. To combine genotypes across the different platforms, we imputed genotypes using the 1000 Genomes cosmopolitan samples as a reference, using SHAPEIT [23] to phase the data and IMPUTE2 [24] to complete the imputation. We ran the pooled analysis using SNPs with an imputation dosage allele r2 of at least 0.3 and a minor allele frequency of at least 0.01.
Statistical Analysis
Full details of the analysis are included in the Supplementary Materials. Major ancestry groups and corresponding population stratification eigenvectors were established by platform. Because sample size was insufficient for Asian ancestry (N = 97), we restricted analyses to European ancestry and African-American ancestry groups. For the Rochester cohort, time from vaccination to enrollment, principal components to adjust for population stratification, and assay batch information were used as adjusting covariates. For the San Diego cohort, the date of sample acquisition and assay batch information were used as adjusting covariates. The rubella-specific secreted cytokines IL-6 and IFN-γ were transformed by normal quantiles of the difference of the mean stimulated and mean unstimulated values. Adjusted traits were then computed by regressing out the effects of potential confounders within each of the 2 cohorts. The adjusted traits were then combined across cohorts to maximize power. The adjusted traits were used to evaluate associations with SNPs, both within ancestral categories of European and African American and combined ancestral categories. Following standard practice, we used a GWAS significance threshold of P ≤ 5 × 10–8 [25, 26]. Because the cohort was heavily weighted toward European ancestry, we present GWAS results for both the overall cohort and the European ancestry subset only.
RESULTS
The final combined cohort used for the GWAS was 1843 subjects, of whom 202 (11%) were classified as African American and 1641 (89%) as of European ancestry (see Supplementary Materials for details on genetic ancestry classification). Nine hundred sixty-two subjects were recruited in Rochester, Minnesota, and 881 subjects were recruited in San Diego, California. Of the subjects, 36.6% were female and 63.4% were male, which is due to the inclusion of a predominantly male military subcohort (the San Diego cohort). The average age of subjects in the study was 18.8 years, with a range of 11–39 years.
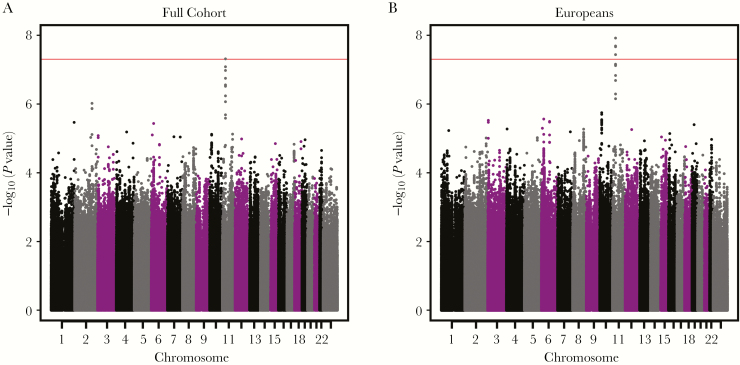
Our combined-cohort GWAS demonstrated a single group of SNPs in linkage disequilibrium (LD) on chromosome 11 with genome-wide significant and suggestive associations with variations in rubella-specific IL-6 secretion (Figure 1A). The most significant SNP (rs4986811; P = 4.87 × 10–8) was in high LD with a cluster of suggestive SNPs in high LD in the same genomic region. To investigate if a particular genetic ancestry in our cohort was responsible for this significant hit, we also conducted parallel GWAS analyses in the European ancestry subjects alone (Figure 1B) and African American subjects alone (not shown).
Figure 1.
Genome-wide Manhattan plots of single-nucleotide polymorphisms associated with rubella-specific cellular interleukin 6 secretion in all subjects (A) and European ancestry subjects only (B).
No evidence of significant association for rs4986811 or any other SNP across the genome was found in the analysis based on the 202 African American subjects (P = 0.46), although we had limited power with this smaller sample size. Subsetting our cohort to the European ancestry subjects, however, increased the strength of the statistical association of SNPs in this chromosome 11 region and expanded the list of significant SNPs to include 3 additional polymorphisms in high LD with rs4986811 (Table 1). To estimate the impact of the SNP rs4986811, we calculated the estimated percentage of variation explained (model r2) by rs4986811 to be 1.9% for the European ancestry subjects.
Table 1.
Single-Nucleotide Polymorphisms Associated With Variations in Rubella-Specific Interleukin 6 Secretion in Subjects of European Ancestry
| SNP | Chromosome | Gene | Reference Base | Substituted Base | Typea | P Value |
|---|---|---|---|---|---|---|
| rs4986811 b | 11 | WT1 | T | G | Intron | 1.22 × 10–8 |
| rs5030172 b | 11 | WT1 | G | C | Intron | 2.07 × 10–8 |
| rs5030157 b | 11 | WT1 | C | T | Intron | 2.18 × 10–8 |
| rs5030166 b | 11 | WT1 | G | T | Intron | 3.69 × 10–8 |
| rs11031780b,c | 11 | WT1 | C | G | Intron | 7.00 × 10–8 |
| rs7936152b | 11 | WT1 | G | A | Intron | 7.17 × 10–8 |
| rs11031781b | 11 | WT1 | G | A | Intron | 7.18 × 10–8 |
| rs11031779b,c | 11 | WT1 | C | T | Intron | 7.68 × 10–8 |
| rs2234582b | 11 | WT1 | C | A | Synonymous | 1.49 × 10–7 |
| rs72893517b | 11 | WT1 | G | C | Intron | 2.08 × 10–7 |
| rs12293603b | 11 | WT1 | C | A | Intron | 5.13 × 10–7 |
| rs5030180b | 11 | WT1 | G | A | Intron | 7.06 × 10–7 |
Abbreviations: SNP, single-nucleotide polymorphism.
Bold indicates P ≤ 5 × 10−8.
aPredicted function.
bIndicates an SNP identified in a WT1 transcript variant known to be a target of nonsense mediated decay (NMD).
cIndicates a predicted transcription factor binding site (Ensembl).
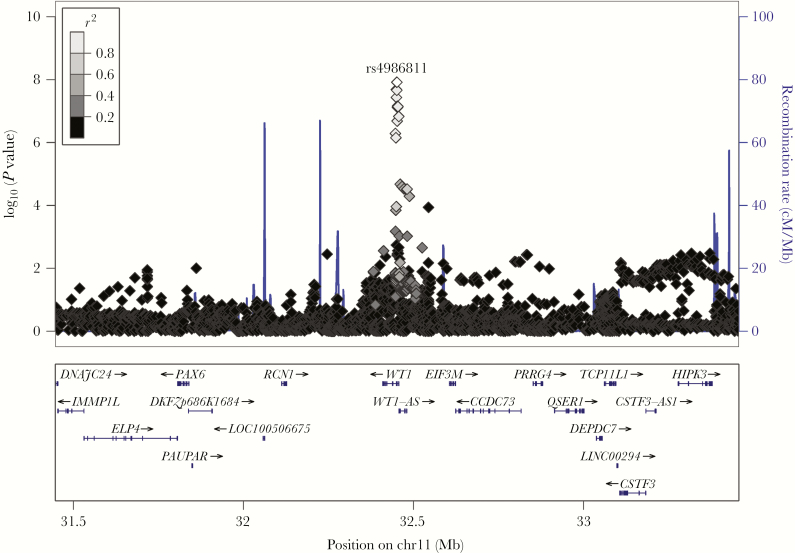
Figure 2 illustrates the SNPs in LD with rs4986811 in the European ancestry subset of our cohort. Twelve SNPs in high LD clustered in the 5ʹ intronic regions of WT1, the gene encoding Wilms tumor 1 protein. The genomic location of these SNPs also puts them in the 5ʹ upstream region of WT1-AS, a short noncoding RNA that shares a promoter region with the WT1 gene.
Figure 2.
LocusZoom plot of significant chromosome 11 single-nucleotide polymorphisms (SNPs) associated with rubella virus–specific interleukin 6 secretion in subjects of European ancestry. The LocusZoom plot visually depicts 12 SNPs in high linkage disequilibrium clustered near the WT1 gene.
We also performed a GWAS to identify genetic associations with RV-specific IFN-γ secretion, yet found no significant SNPs across the genome (ie, all P values >2 × 10–6).
DISCUSSION
In a large cohort of rubella-vaccinated individuals, we identified SNPs in 2 gene regions that influence RV-specific cellular immunity. Four intronic SNPs in high LD (r2 ≥ 0.8) in the WT1 gene region on chromosome 11 were found to be significantly associated (P < 5 × 10–8) with RV-specific IL-6 secretion. Seven additional suggestive SNPs in the WT1 gene region were found in high LD with these 4 significant SNPs (Table 1). The estimated percentage of heritable variation in response explained by the most significant SNP, rs4986811, was estimated to be 1.9%. This effect size is high for a single SNP; it is comparable to or larger than the most significant effects seen for single SNPs in large-scale studies of complex traits such as physical height, Crohn disease, schizophrenia, and various cancers [27, 28].
While not typically associated with immunity, the WT1 gene has many well-characterized functions. WT1 plays important roles in mammalian embryonic development [29], and mutations in the WT1 gene are found in a variety of genetic syndromes associated with genital, optical, olfactory, and renal defects [30, 31]. WT1 also functions as a tumor suppressor gene; alterations in WT1 are associated with the eponymous Wilms tumor, a nephroblastoma [32], as well as other cancers such as leukemia [32], pancreatic cancer [33], breast cancer [34], lung cancer [35], and colorectal cancer [36].
The WT1 gene spans 50 kb and has 10 exons. Alternative splicing of 2 exons, in addition to alternate translation initiation sites and RNA editing mechanisms, leads to at least 24 possible isoforms of the WT1 protein [29]. Different isoforms appear to have distinct functions [29, 37], and some isoforms bind to and/or antagonize the function of other WT1 isoforms [38, 39]. The ratios of splice variants appear to be differentially regulated between tissues and stages of development, and these ratios affect WT1 function; WT1 mutations that cause alterations in these isoform ratios through introduction of defective alternate splicing or triggering of nonsense-mediated decay are associated with the development of the associated genetic syndromes [31, 39, 40].
WT1 contains 4 zinc finger regions, allowing it to function as an EGR1-like transcription factor [41] with targets that include growth factor genes, growth factor receptor genes, transcription factor genes, and genes coding for extracellular, secreted proteins [29]. Different WT1 isoforms have been demonstrated to activate transcription to different extents [39]. The effects of WT1 include influences on immune signaling molecules; for example, WT1 has been shown to repress the IRF8 promoter [42, 43] and interact with and enhance the function of STAT3 [44, 45]. WT1 also binds directly with the IL-10 promoter and induces IL-10 expression, and this WT1 binding was demonstrated to be necessary for tumor necrosis factor-α–induced interleukin 10 (IL-10) stimulation in macrophages [46]. Interestingly, different naturally occurring WT1 isoforms induced different levels of IL-10 gene expression, indicating a potential mechanism by which SNPs in this gene may affect downstream cellular immunity to antigens such as rubella vaccine.
The effects of WT1 isoforms are widespread. WT1 proteins are known self-antigens [29]. WT1 binds directly with a host of other proteins, including transcription factors and proto-oncogenes [29, 47]. Some isoforms of WT1 have nuclear localization signals, and may also function as posttranscriptional regulators [48, 49]. Additionally, all major isoforms of WT1 induce an array of microRNAs that are capable of suppressing transcription of various genes [50]. Finally, the long noncoding transcript WT1-AS may also have functions such as regulating WT1 expression and promoting cell apoptosis via the JAK/STAT signaling pathway [51]. Because many of our significant SNPs are in the upstream regions of both the WT1 and WT1-AS genes, the impact of these SNPs on WT1 expression, splicing, and interactions with transcription factors and immune signaling molecules may be complex.
The previously identified effects of WT1 polymorphisms are widespread. Future studies should examine the effect of our identified significant SNPs on rubella vaccine–induced immunity. Such studies could include investigation of WT1 expression and isoform/transcript ratios between SNP variants, transcription factor activity of SNP variants, and the ability of these variants to interact with the IRF and JAK/STAT signaling pathways. As IL-6 secretion has previously been associated with adverse events (AEs) after vaccination against other pathogens [52], studies on the nature of AEs after viral vaccination and any relationship of AEs with WT1 polymorphisms may also be of public health interest.
The strengths of our study include a well-characterized viral vaccine and a well-defined cohort that is larger and more diverse than previous efforts [14]. Our cohort consists predominantly of subjects of European descent, improving statistical power for identifying rubella vaccine–associated SNPs typically found in European populations. However, our findings may not be valid for groups with other genetic ancestries. Further studies that more closely examine these effects in a more diverse cohort would meaningfully expand our knowledge of the immunogenetics of human responses to rubella vaccine.
Future work is being designed to measure the effect of the WT1 SNP variants on WT1 protein expression, ratio of WT1 protein isoforms, and effects on the development and maintenance of cellular immunity. These functional studies may lead to identification of the mechanism behind the effect of WT1 variants on rubella-specific cellular immunity after rubella vaccination. This information would contribute to our understanding of the genetic basis of vaccine response heterogeneity, which may lead to better optimization of rubella vaccines that induce robust adaptive immune responses across diverse populations, or offer pathways toward the development of novel vaccine candidates.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH).
Financial support. Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the NIH (award number R37AI048793, which recently received an NIH MERIT award, and award number R01AI033144).
Potential conflicts of interest. G. A. P. is the chair of a safety evaluation committee for novel investigational vaccine trials being conducted by Merck Research Laboratories; and offers consultative advice on vaccine development to Merck & Co Inc, Avianax, Dynavax, Novartis Vaccines and Therapeutics, Seqirus, Protein Sciences, and Adjuvance Technologies. G. A. P. and I. G. O. hold 3 patents related to vaccinia and measles peptide research. R. B. K. has received funding from Merck Research Laboratories to study waning immunity to mumps vaccine. These activities and this research have been reviewed by the Mayo Clinic Conflict of Interest Review Board and are conducted in compliance with Mayo Clinic conflict of interest policies. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Miller E, Cradock-Watson JE, Pollock TM. Consequences of confirmed maternal rubella at successive stages of pregnancy. Lancet 1982; 2:781–4. [DOI] [PubMed] [Google Scholar]
- 2. Kirby T. Rubella is eliminated from the Americas. Lancet Infect Dis 2015; 15:768–9. [DOI] [PubMed] [Google Scholar]
- 3. Lambert N, Strebel P, Orenstein W, Icenogle J, Poland GA. Rubella. Lancet 2015; 385:2297–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Freestone DS, Reynolds GM, McKinnon JA, Prydie J. Vaccination of schoolgirls against rubella. Assessment of serological status and a comparative trial of Wistar RA 27/3 and Cendehill strain live attenuated rubella vaccines in 13-year-old schoolgirls in Dudley. Br J Prev Soc Med 1975; 29:258–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. LeBaron CW, Forghani B, Matter L et al. . Persistence of rubella antibodies after 2 doses of measles-mumps-rubella vaccine. J Infect Dis 2009; 200:888–99. [DOI] [PubMed] [Google Scholar]
- 6. Lambert ND, Haralambieva IH, Ovsyannikova IG, Larrabee BR, Pankratz VS, Poland GA. Characterization of humoral and cellular immunity to rubella vaccine in four distinct cohorts. Immunol Res 2014; 58:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Terada K, Itoh Y, Wakabayashi T et al. . Rubella specific cell-mediated and humoral immunity following vaccination in college students with low antibody titers. Vaccine 2015; 33:6093–8. [DOI] [PubMed] [Google Scholar]
- 8. Poland GA, Kennedy RB, Ovsyannikova IG. Vaccinomics and personalized vaccinology: is science leading us toward a new path of directed vaccine development and discovery?PLoS Pathog 2011; 7:e1002344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Klein SL, Poland GA. Personalized vaccinology: one size and dose might not fit both sexes. Vaccine 2013; 31:2599–600. [DOI] [PubMed] [Google Scholar]
- 10. Ovsyannikova IG, Jacobson RM, Vierkant RA, O’Byrne MM, Poland GA. Replication of rubella vaccine population genetic studies: validation of HLA genotype and humoral response associations. Vaccine 2009; 27:6926–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dhiman N, Haralambieva IH, Kennedy RB et al. . SNP/haplotype associations in cytokine and cytokine receptor genes and immunity to rubella vaccine. Immunogenetics 2010; 62:197–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ovsyannikova IG, Haralambieva IH, Dhiman N et al. . Polymorphisms in the vitamin A receptor and innate immunity genes influence the antibody response to rubella vaccination. J Infect Dis 2010; 201:207–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Haralambieva IH, Dhiman N, Ovsyannikova IG et al. . 2ʹ-5ʹ-Oligoadenylate synthetase single-nucleotide polymorphisms and haplotypes are associated with variations in immune responses to rubella vaccine. Hum Immunol 2010; 71:383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lambert ND, Haralambieva IH, Kennedy RB, Ovsyannikova IG, Pankratz VS, Poland GA. Polymorphisms in HLA-DPB1 are associated with differences in rubella virus-specific humoral immunity after vaccination. J Infect Dis 2015; 211:898–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kennedy RB, Ovsyannikova IG, Vierkant RA, Jacobson RM, Poland GA. Effect of human leukocyte antigen homozygosity on rubella vaccine-induced humoral and cell-mediated immune responses. Hum Immunol 2010; 71:128–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Haralambieva IH, Ovsyannikova IG, Kennedy RB et al. . Associations between single nucleotide polymorphisms and haplotypes in cytokine and cytokine receptor genes and immunity to measles vaccination. Vaccine 2011; 29:7883–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Haralambieva IH, Ovsyannikova IG, Umlauf BJ et al. . Genetic polymorphisms in host antiviral genes: associations with humoral and cellular immunity to measles vaccine. Vaccine 2011; 29:8988–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kennedy RB, Ovsyannikova IG, Haralambieva IH et al. . Multigenic control of measles vaccine immunity mediated by polymorphisms in measles receptor, innate pathway, and cytokine genes. Vaccine 2012; 30:2159–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kennedy RB, Ovsyannikova IG, Pankratz VS, Haralambieva IH, Vierkant RA, Poland GA. Genome-wide analysis of polymorphisms associated with cytokine responses in smallpox vaccine recipients. Hum Genet 2012; 131:1403–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ovsyannikova IG, Haralambieva IH, Vierkant RA, O’Byrne MM, Jacobson RM, Poland GA. The association of CD46, SLAM and CD209 cellular receptor gene SNPs with variations in measles vaccine-induced immune responses: a replication study and examination of novel polymorphisms. Hum Hered 2011; 72:206–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ovsyannikova IG, Ryan JE, Vierkant RA et al. . Influence of host genetic variation on rubella-specific T cell cytokine responses following rubella vaccination. Vaccine 2009; 27:3359–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dhiman N, Haralambieva IH, Vierkant RA et al. . Predominant inflammatory cytokine secretion pattern in response to two doses of live rubella vaccine in healthy vaccinees. Cytokine 2010; 50:24–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Delaneau O, Zagury JF, Marchini J. Improved whole-chromosome phasing for disease and population genetic studies. Nat Methods 2013; 10:5–6. [DOI] [PubMed] [Google Scholar]
- 24. Howie BN, Donnelly P, Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet 2009; 5:e1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pe’er I, Yelensky R, Altshuler D, Daly MJ. Estimation of the multiple testing burden for genomewide association studies of nearly all common variants. Genet Epidemiol 2008; 32:381–5. [DOI] [PubMed] [Google Scholar]
- 26. Manolio TA. Genomewide association studies and assessment of the risk of disease. N Engl J Med 2010; 363:166–76. [DOI] [PubMed] [Google Scholar]
- 27. Park JH, Wacholder S, Gail MH et al. . Estimation of effect size distribution from genome-wide association studies and implications for future discoveries. Nat Genet 2010; 42:570–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Franke B, Stein JL, Ripke S et al. . Schizophrenia Working Group of the Psychiatric Genomics Consortium; ENIGMA Consortium Genetic influences on schizophrenia and subcortical brain volumes: large-scale proof of concept. Nat Neurosci 2016; 19:420–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Scharnhorst V, van der Eb AJ, Jochemsen AG. WT1 proteins: functions in growth and differentiation. Gene 2001; 273:141–61. [DOI] [PubMed] [Google Scholar]
- 30. Pelletier J, Bruening W, Kashtan CE et al. . Germline mutations in the Wilms’ tumor suppressor gene are associated with abnormal urogenital development in Denys-Drash syndrome. Cell 1991; 67:437–47. [DOI] [PubMed] [Google Scholar]
- 31. Klamt B, Koziell A, Poulat F et al. . Frasier syndrome is caused by defective alternative splicing of WT1 leading to an altered ratio of WT1 +/-KTS splice isoforms. Hum Mol Genet 1998; 7:709–14. [DOI] [PubMed] [Google Scholar]
- 32. Charlton J, Pritchard-Jones K. WT1 mutation in childhood cancer. Methods Mol Biol 2016; 1467:1–14. [DOI] [PubMed] [Google Scholar]
- 33. Koido S, Okamoto M, Shimodaira S, Sugiyama H. Wilms’ tumor 1 (WT1)-targeted cancer vaccines to extend survival for patients with pancreatic cancer. Immunotherapy 2016; 8:1309–20. [DOI] [PubMed] [Google Scholar]
- 34. Silberstein GB, Van Horn K, Strickland P, Roberts CT Jr, Daniel CW. Altered expression of the WT1 Wilms tumor suppressor gene in human breast cancer. Proc Natl Acad Sci U S A 1997; 94:8132–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Oji Y, Miyoshi S, Maeda H et al. . Overexpression of the Wilms’ tumor gene WT1 in de novo lung cancers. Int J Cancer 2002; 100:297–303. [DOI] [PubMed] [Google Scholar]
- 36. Sangkhathat S, Maneechay W, Chaiyapan W, Kanngern S, Boonpipattanapong T. Association of Wilms’ tumor 1 gene single-nucleotide polymorphism rs16754 with colorectal cancer. Mol Clin Oncol 2015; 3:1401–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hewitt SM, Fraizer GC, Wu YJ, Rauscher FJ 3rd, Saunders GF. Differential function of Wilms’ tumor gene WT1 splice isoforms in transcriptional regulation. J Biol Chem 1996; 271:8588–92. [DOI] [PubMed] [Google Scholar]
- 38. Tatsumi N, Hojo N, Sakamoto H et al. . Identification of a novel C-terminal truncated WT1 isoform with antagonistic effects against major WT1 isoforms. PLoS One 2015; 10:e0130578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Reddy JC, Morris JC, Wang J et al. . WT1-mediated transcriptional activation is inhibited by dominant negative mutant proteins. J Biol Chem 1995; 270:10878–84. [DOI] [PubMed] [Google Scholar]
- 40. Gessler M, Poustka A, Cavenee W, Neve RL, Orkin SH, Bruns GA. Homozygous deletion in Wilms tumours of a zinc-finger gene identified by chromosome jumping. Nature 1990; 343:774–8. [DOI] [PubMed] [Google Scholar]
- 41. Bharathavikru R, Dudnakova T. Methods to identify and validate WT1-RNA interaction. Methods Mol Biol 2016; 1467:197–209. [DOI] [PubMed] [Google Scholar]
- 42. Montano G, Ullmark T, Jernmark-Nilsson H et al. . The hematopoietic tumor suppressor interferon regulatory factor 8 (IRF8) is upregulated by the antimetabolite cytarabine in leukemic cells involving the zinc finger protein ZNF224, acting as a cofactor of the Wilms’ tumor gene 1 (WT1) protein. Leuk Res 2016; 40:60–7. [DOI] [PubMed] [Google Scholar]
- 43. Vidovic K, Svensson E, Nilsson B et al. . Wilms’ tumor gene 1 protein represses the expression of the tumor suppressor interferon regulatory factor 8 in human hematopoietic progenitors and in leukemic cells. Leukemia 2010; 24:992–1000. [DOI] [PubMed] [Google Scholar]
- 44. Rong Y, Cheng L, Ning H et al. . Wilms’ tumor 1 and signal transducers and activators of transcription 3 synergistically promote cell proliferation: a possible mechanism in sporadic Wilms’ tumor. Cancer Res 2006; 66:8049–57. [DOI] [PubMed] [Google Scholar]
- 45. Xu C, Wu C, Xia Y et al. . WT1 promotes cell proliferation in non-small cell lung cancer cell lines through up-regulating cyclin D1 and p-pRb in vitro and in vivo. PLoS One 2013; 8:e68837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sciesielski LK, Kirschner KM, Scholz H, Persson AB. Wilms’ tumor protein Wt1 regulates the interleukin-10 (IL-10) gene. FEBS Lett 2010; 584:4665–71. [DOI] [PubMed] [Google Scholar]
- 47. Bharathavikru R, von Kriegsheim A. WT1-associated protein-protein interaction networks. Methods Mol Biol 2016; 1467:189–96. [DOI] [PubMed] [Google Scholar]
- 48. Davies RC, Calvio C, Bratt E, Larsson SH, Lamond AI, Hastie ND. WT1 interacts with the splicing factor U2AF65 in an isoform-dependent manner and can be incorporated into spliceosomes. Genes Dev 1998; 12:3217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ladomery MR, Slight J, Mc Ghee S, Hastie ND. Presence of WT1, the Wilm’s tumor suppressor gene product, in nuclear poly(A)(+) ribonucleoprotein. J Biol Chem 1999; 274:36520–6. [DOI] [PubMed] [Google Scholar]
- 50. Akpa MM, Iglesias D, Chu L et al. . Wilms tumor suppressor, WT1, cooperates with microRNA-26a and microRNA-101 to suppress translation of the polycomb protein, EZH2, in mesenchymal stem cells. J Biol Chem 2016; 291:3785–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lv L, Chen G, Zhou J, Li J, Gong J. WT1-AS promotes cell apoptosis in hepatocellular carcinoma through down-regulating of WT1. J Exp Clin Cancer Res 2015; 34:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bae HG, Domingo C, Tenorio A et al. . Immune response during adverse events after 17D-derived yellow fever vaccination in Europe. J Infect Dis 2008; 197:1577–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.