Abstract
Background.
Crucial gaps in our understanding of Plasmodium vivax reticulocyte invasion and protective immunity have hampered development of vivax vaccines. P. vivax exclusively invades reticulocytes that is mediated by the P. vivax reticulocyte-binding proteins (PvRBPs) specifically PvRBP2c and PvRBP1a. Vivax infections in Duffy-null individuals have suggested the evolution of alternate invasion pathways that may be mediated by the PvRBPs. Thus, PvRBPs appear as potential targets for efficacious P. vivax neutralization. However, there are limited data validating their vaccine efficacy. In the absence of vivax invasion assays, binding-inhibitory activity of antibodies has been reported to be associated with protection and a measure of vaccine potential.
Methods.
–based analysis was performed of the PvRBP reticulocyte-binding properties and binding-inhibitory activity of specific anti-PvRBP2c/PvRBP1a human antibodies.
Results.
PvRBP2c and PvRBP1a displayed a distinct reticulocyte-binding specificity, and their specific reticulocyte-binding domains were mapped within their N-terminal regions. Importantly, naturally acquired antibodies against the reticulocyte-binding domains efficaciously blocked reticulocyte binding of native PvRBPs, suggesting that the human immune system produced functional binding-inhibitory antibodies through exposure to vivax malaria.
Conclusions.
Reticulocyte-binding domains of PvRBP2c/PvRBP1a are targets of naturally acquired binding-inhibitory antibodies, substantiating their promise as candidate antigens against which vaccine-inducible immunity could potentially be boosted through natural infections.
Keywords: vivax malaria, reticulocyte-binding proteins, binding-inhibitory antibodies, naturally acquired immunity, vaccines.
Summary
The reticulocyte-specific binding domains of Plasmodium vivax proteins, PvRBP2c and PvRBP1a are targets of naturally acquired binding-inhibitory human antibodies, thus substantiating their promise as candidate antigens for the development of novel P. vivax malaria vaccines.
Plasmodium vivax malaria has remained largely neglected despite its being geographically more widespread than falciparum malaria and responsible for enormous morbidity across the tropics [1–4]. To eliminate malaria, it is crucial to develop effective intervention strategies to counter P. vivax, and in this regard a potent vaccine would be a highly effective tool in combating the disease. An encouraging aspect for vaccine development is that malaria induces naturally acquired immunity (NAI) that confers disease protection [5, 6]. NAI is known to restrict blood-stage parasite densities leading to lower incidences of clinical and severe malaria [5, 6]. A good understanding of NAI could provide key insights on the immune correlates of protection, which would immensely benefit vaccine development. Acquisition of NAI against P. vivax occurs more rapidly than for Plasmodium falciparum, irrespective of the transmission intensity [5]. Mechanisms underlying this rapid NAI acquisition remains poorly understood and warrant further investigations. In this regard, humoral responses against blood-stage antigens constitute a critical component of NAI against vivax malaria [5, 6]. Therefore, parasite proteins involved in erythrocyte invasion are potential targets of protective immunity that need in-depth validation for their development as vaccine candidates.
Unlike P. falciparum, P. vivax primarily invades only reticulocytes (young immature erythrocytes), and the molecular basis underlying P. vivax reticulocyte invasion is not well understood [3, 7]. The central dogma of P. vivax merozoite invasion has been its dependence on the essential interaction between the P. vivax Duffy-binding protein (PvDBP) and its receptor, Duffy antigen receptor for chemokines (DARC) [8, 9], which does not account for reticulocyte specificity because DARC is present on both normocytes and reticulocytes. P. vivax reticulocyte specificity (Belem strain) was first reported to be mediated by 2 P. vixax reticulocyte-binding proteins (PvRBPs), PvRBP1a and PvRBP2c [10]. Thereafter, the P. vivax SalI genome revealed a PvRBP family of 11 members (Supplementary Figure 1), of which 2 are pseudogenes [11]. Reports of vivax infection in Duffy-null individuals strongly suggest that P. vivax has evolved Duffy-independent, redundant invasion pathways [12–14]. It is thus critical to elucidate the molecular basis of P. vivax merozoite invasion. PvRBPs are implicated to play crucial roles in mediating reticulocyte specificity and may play a role in Duffy-independent invasion. Therefore, PvRBPs are attractive candidate antigens for the development of vivax vaccines and warrant further validation.
The lack of P. vivax in vitro culture has deferred in-depth dissection of vivax antigens [3, 7]. In the interim, PvRBP homologues known as reticulocyte binding-like homologous (PfRH) proteins were discovered in P. falciparum, where they function as key determinants of erythrocyte invasion [8, 15, 16], and are considered promising vaccine candidates [17–22]. Among the PvRBP family, only native PvRBP1a and PvRBP2c have been demonstrated to specifically bind reticulocytes [10]. However, a functional analysis of the specific reticulocyte-binding domains of PvRBP1a/PvRBP2c correlating with the specific binding activity of the native parasite proteins is lacking. Furthermore, to our knowledge, no previous study has evaluated the generation of functional binding-inhibitory PvRBP antibodies by the human immune system in response to natural vivax infections.
In the present study, we have screened the reticulocyte-specific binding of the PvRBP2c/PvRBP1a proteins and mapped the constructs exhibiting the maximum binding efficiency that correlated with that of native parasite proteins. Importantly, we have demonstrated that PvRBP-specific human antibodies against these receptor-binding domains potently inhibit the reticulocyte-specific binding of the native PvRBP2c/PvRBP1a parasite proteins. In the absence of P. vivax invasion inhibition assays, binding inhibition is an important surrogate for evaluating vaccine potential of vivax antigens [23]. This is the first report to demonstrate the functionality of naturally acquired human antibodies targeting PvRBPs, which has major implications for the development of vivax malaria vaccines.
MATERIALS AND METHODS
Sample Collection and Ethics Approvals
P. vivax–infected blood samples were collected from the Balaghat district (Madhya Pradesh) by the National Institute of Research in Tribal Health (NIRTH) [24] and at the clinics of the All India Institute of Medical Sciences (AIIMS) [25]. Cross-sectional active door-to-door fever surveys were carried in the study villages of Balaghat (Supplementary Text) [24]. Finger-prick blood smears were collected by NIRTH from all patients with fever or history of fever within the past 14 days. In addition, human subjects from the North Indian belt attending the clinics at AIIMS were screened for P. vivax infections.
Peripheral blood samples were collected from human subjects via finger prick after informed consent. P. vivax infections were diagnosed using a rapid diagnostic test (SD Bioline Malaria Antigen Pf/Pv, Bio Standard Diagnostics, India) and confirmed microscopically by examination of Giemsa-stained thin blood smears. Blood samples (2–5 mL) were collected from 66 P. vivax–positive subjects identified between the NIRTH and AIIMS studies, with the primary inclusion criterion being a positive rapid diagnostic test results confirmed microscopically. Patients with positive results were treated according to the drug policy of the National Vector Borne Disease Control Programme. Infected P. vivax parasites and human plasma samples for our studies were obtained from this source of 66 human blood samples. All approvals were obtained from the institutional ethical review committees, according to the guidelines of the Indian Council of Medical Research (Government of India).
Production of Recombinant Proteins
Codon-optimized genes encoding PvRBP2c/PvRBP1a constructs (Gene Art; Life Technologies) were subcloned in the pET24b expression vector (Novagen). Recombinant proteins were expressed in Escherichia coli and purified to homogeneity, as described in the Supplementary Methods. Generation of antibodies [17], preparation of P. vivax lysate and culture supernatant [10], enzyme-linked immunosorbent assay (ELISA) [26] and polymerase chain reaction confirmation of P. vivax are described in the Supplementary Text.
Affinity Purification of Antigen-Specific Human Antibodies
Affinity purification of human antibodies was performed from ELISA-positive high-responder plasma samples as reported elsewhere [26, 27]. Briefly, recombinant proteins were coupled with cyanogen bromide–activated sepharose (Sigma) and pooled plasma was applied over these affinity columns. Elution was performed using 0.1 mol/L glycine (pH 2.7), and the pH was adjusted with 1 mol/L Tris-HCl (pH 9.0). Eluates were pooled and concentrated using centrifugal filters (Millipore; molecular weight cutoff, 10 kDa), followed by buffer exchange with phosphate-buffered saline (PBS; pH 7.4). Affinity purification was validated with ELISA, which demonstrated that the purified antibodies were enriched compared with individual high-responder plasma samples. Cross-reactivity of the affinity-purified antibodies was analyzed with ELISA.
Reticulocyte Binding and Inhibition Assay
Reticulocyte enrichment [28, 29] and enzymatic treatment [30] were performed as reported elsewhere (described in the Supplementary Text). Reticulocyte binding of native PvRBPs (culture supernatant) and recombinant RBPs (rRBPs) were determined using 2 methods described elsewhere, the overlay assay followed by immunoblotting [18] and a dual-color flow cytometry–based assay [31]. Briefly, culture supernatant or recombinant proteins were incubated with enriched reticulocytes (magnetic sorting or Percoll gradient; Supplementary Figure 2). Suspension was laid over dibutyl phthalate and centrifuged. Cell-bound proteins were eluted by salt and analyzed by means of immunoblotting.
For the FACS assay, enriched reticulocytes (magnetic sorting) were incubated with recombinant protein or culture supernatant, washed with PBS, and further incubated with anti-PvRBP antibodies. Samples were washed and incubated with anti-mice or anti-rabbit allophycocyanin-conjugated secondary antibody (Molecular Probes), followed by incubation with thiazole orange, as reported elsewhere [31]. A total of 100 000 total events were acquired per sample with a FACSCanto system (Becton Dickinson). Unstained and thiazole orange–stained cells defined the normocyte and reticulocyte populations, respectively [31]. For binding inhibition experiments, recombinant protein/culture supernatant and enriched reticulocytes were incubated at 37°C in the presence of rat or human antibodies. Bound proteins were detected with FACS, as described above.
Statistical Analysis
Statistical analyses were performed with GraphPad Prism software, version 5.01 (GraphPad Software). Nonparametric statistical tests were used to analyze data. Medians for the 2 groups were compared using t tests, and differences were considered statistically significant at P < .05.
RESULTS
Production of Recombinant Proteins and Generation of Specific Antibodies
Sequence analysis of 8 P. vivax worldwide strains showed that while PvRBP2c exhibited numerous antigenic polymorphisms, PvRBP1a was highly conserved (Supplementary Text, alignments 1–2). Sequence alignments of PvRBP2c and PvRBP1a with their phylogenetically close RBL family members showed a homology with their erythrocyte-binding domains (Supplementary Text, alignments 3–6), which framed the basis for mapping the putative reticulocyte-specific binding domains. These conserved regions of the 2 PvRBPs were selected for recombinant protein production (Figure 1A).
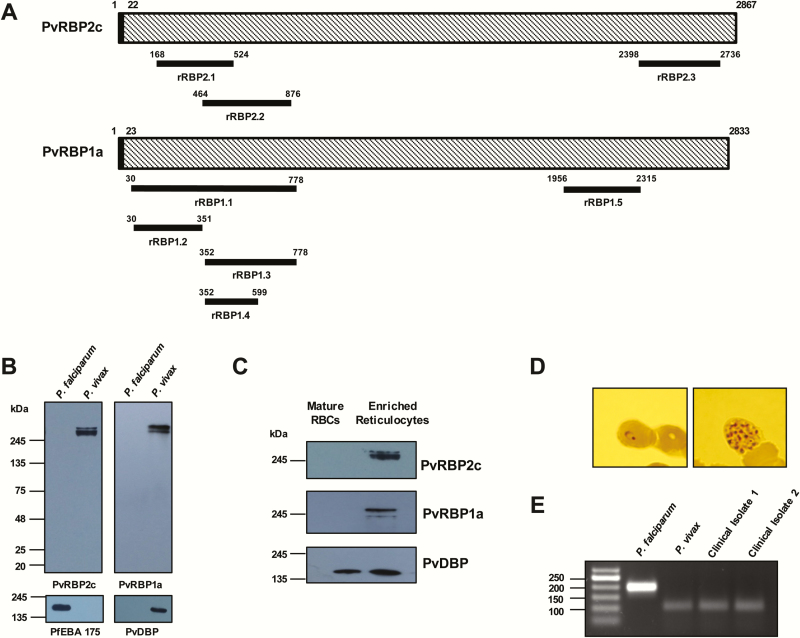
Figure 1.
Structure, expression, and reticulocyte-binding activity of the native Plasmodium vivax reticulocyte-binding proteins (PvRBPs). A, Schematic diagram of the PvRBP2c and PvRBP1a parasite proteins, highlighting the regions against which the recombinant proteins have been produced (black bars). B, Native PvRBP2c and PvRBP1a parasite proteins were detected by means of immunoblotting only in the P. vivax protein extracts by the specific anti-rRBP2.2 and anti-rRBP1.1 mice antibodies. Expression of native PfEBA-175 was detected only in the Plasmodium falciparum extract, confirming no cross-contamination of the P. vivax–infected samples with P. falciparum. Native P. vivax Duffy-binding protein (PvDBP) parasite protein was detected only in the P. vivax extract, confirming the specificity of the vivax sample. C, Binding of native PvRBP2c and PvRBP1a from the P. vivax culture supernatant was detected only with enriched reticulocytes and not with normal erythrocytes (red blood cells [RBCs]). Native PvDBP from the same culture supernatant bound with the same set of normal erythrocytes and enriched reticulocytes. Reticulocytes were enriched over a Percoll cushion. D, Blood film of the P. vivax–infected sample showing the P. vivax ring-stage and schizont-stage parasites. E, Identities of the P. vivax–infected samples were confirmed by means of nested polymerase chain reaction (PCR) amplification of 18S ribosomal RNA sequences, using primers specific for P. vivax and P. falciparum. The specific PCR-amplified DNA fragments from P. vivax and P. falciparum correspond to 100 and 200 base pairs (bp), respectively. Two representative P. vivax–infected clinical isolates are shown that correspond to the 100-bp size, confirming their identity. Abbreviations: PfEBA-175, P. falciparum erythrocyte binding antigen 175; rRBP, recombinant RBP.
PvRBP recombinant proteins—rRBP2.1 (residues 168–524), rRBP2.2 (residues 464–876), and rRBP2.3 (residues 2398–2736) of PvRBP2c (Figure 1A) and rRBP1.1 (residues 30–778) and rRBP1.5 (residues 1956–2315) of PvRBP1a (Figure 1A)—were expressed in E. coli with a C-terminal His-tag. To dissect the minimal reticulocyte-binding region within the 82-kDa rRBP1.1 protein, we produced 3 fragments within this protein: rRBP1.2 (residues 30–351), rRBP1.3 (residues 352–778), and rRBP1.4 (residues 352–599) (Figure 1A). The proteins were purified to homogeneity (Supplementary Figure 3), and their identities were confirmed using mass spectrometry (Supplementary Table 1). Specific antibodies were raised in rats and mice against the PvRBP and PvDBP recombinant proteins with end-point titers in the range of 1:320000 to 1:640000 (Supplementary Figures 4–7).
Reticulocyte Specific Binding of Native PvRBP2c and PvRBP1a
We confirmed the specificity of the PvRBP2c/1a antibodies by immunoblotting using P. vivax and P. falciparum parasite lysates. rRBP1.1 and rRBP2.2 mice antibodies detected native vivax proteins in the range of approximately 280 kDa (Figure 1B), consistent with previous reports [10]. Both antibodies exhibited P. vivax specificity, as they failed to cross-react with P. falciparum proteins (Figure 1B). Similarly, P. falciparum erythrocyte binding antigen 175 (PfEBA-175) and PvDBP antibodies detected only their respective parasite proteins, with no cross-reactivity (Figure 1B). P. vivax field samples were confirmed with microscopy and polymerase chain reaction using specific 18S ribosomal RNA primers (Figure 1D and E), as reported elsewhere [32].
The specificity of the native parasite proteins detected by the PvRBP antibodies was further confirmed by binding assays, as reported elsewhere [10]. Native PvRBPs were obtained from culture supernatants of single-cycle matured P. vivax–infected human erythrocytes (Supplementary Text). Native P. vivax proteins (~250 kDa) detected with the PvRBP2c/PvRBP1a antibodies failed to bind mature erythrocytes (Figure 1C) but specifically bound enriched reticulocytes (Figure 1C), thus confirming them as native PvRBPs, consistent with the previous report [10]. PvDBP bound both mature erythrocytes and enriched reticulocytes (Figure 1C), as both express DARC. Our results confirm the reticulocyte-specific binding of the native PvRBP2c/PvRBP1a proteins and the specificity of our PvRBP antibodies.
Mapping the Reticulocyte-Binding Domain of PvRBP2c
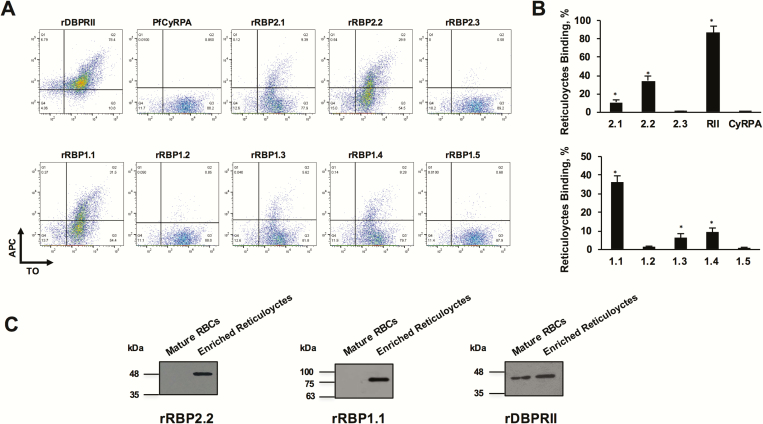
We investigated the reticulocyte-specific binding activity of 2 N-terminal regions of PvRBP2c, rRBP2.1 (residues 168–524) and rRBP2.2 (residues 464–876), which are homologous with the erythrocyte-binding domains of PfRH2 and PkNBPXa, respectively (Supplementary Text, alignments 3 and 4). Reticulocyte binding of the PvRBP2c proteins was analyzed with FACS (Figure 2) [33]. As reported elsewhere, rDBPRII (PvDBP receptor–binding domain) bound both reticulocytes and normocytes [34], whereas the non-binding protein, P. falciparum cysteine-rich protective antigen (PfCyRPA) protein [21] failed to exhibit binding (Figure 2A and B). rRBP2.2 exhibited significantly greater reticulocyte-binding activity (34%) than rRBP2.1 (10%) (Figure 2A). rRBP2.3 (C-terminal fragment) exhibited poor reticulocyte-binding activity (0.96%). Results for 3 independent experiments are depicted in the bar graphs (Figure 2B). rRBP2.2 specifically bound enriched reticulocytes and failed to bind unenriched normocytes (Figure 2C). Thus, we demonstrated for the first time that the 413 residue rRBP2.2 region (Lys464-Ile876) constituted the reticulocyte-binding domain of native PvRBP2c.
Figure 2.
Flow cytometry–based reticulocyte-binding activity of Plasmodium vivax reticulocyte-binding protein (PvRBP) 2c and PvRBP1a recombinant fragments. (A) Flow cytometry dot plots showing the binding of rRBP2.1, rRBP2.2, rRBP2.3, rDBPRII, and PfCyRPA (top) and rRBP1.1, rRBP1.2, rRBP1.3, rRBP1.4, rRBP1.5 (bottom) with enriched reticulocytes (thiazole orange [TO] positive). Reticulocyte binding was detected using the antibodies raised against the respective recombinant proteins followed by a secondary allophycocyanin (APC)–conjugated monoclonal antibody. Of the 3 PvRBP2c proteins, reticulocyte binding was observed for rRBP2.2 and rRBP2.1, with no binding observed for rRBP2.3. Of the 5 PvRBP1a protein constructs, reticulocyte binding was observed for rRBP1.1, rRBP1.3, and rRBP1.4, with no binding reported for rRBP1.2 and rRBP1.5. Binding of rDBPRII and PfCyRPA was analyzed as a positive and negative controls, respectively. B, Bar charts depicting the percentage reticulocyte binding of rRBP2.1, rRBP2.2, rRBP2.3, rDBPRII, PfCyRPA (top) and rRBP1.1, rRBP1.2, rRBP1.3, rRBP1.4, and rRBP1.5 (bottom). Error bars represent standard errors of the mean for 3 independent repeats. *P < .001. C, Binding of recombinant rRBP2.2 and rRBP1.1 was detected only with enriched reticulocytes and not with normal erythrocytes (red blood cells [RBCs]. rDBPRII bound with the same set of normal erythrocytes and enriched reticulocytes. Reticulocytes were enriched by magnetic sorting. Abbreviations: PfCyRPA, Plasmodium falciparum cysteine-rich protective antigen; rDBPRII, recombinant Duffy binding protein region II; rRBP, recombinant RBP.
Mapping the Reticulocyte Binding Domain of PvRBP1a
We analyzed the reticulocyte-specific binding of 4 fragments spanning the 749 amino acid PvRBP1a N-terminal region, which are homologous with the erythrocyte-binding domains of PfRH4 and PfRH1 (Supplementary Text, alignments 5 and 6). rRBP1.1 exhibited the most potent reticulocyte-specific binding activity (37%) (Figure 2A and B). rRBP1.2 and rRBP1.5 failed to bind reticulocytes (Figure 2A and B), and rRBP1.3 and rRBP1.4 exhibited weaker reticulocyte-binding activity (10%–13%) than rRBP1.1 (Figure 2A and B). Thus, large variation in the binding was observed between rRBP1.1 and its smaller fragments. Like PfRH4, the minimal binding domain of PvRBP1a has been mapped to the 261 amino acid region (352–599) (rRBP1.4). Although the upstream region (residues 30–351) is unable to bind reticulocytes independently, it contributes significantly to the potent reticulocyte-binding activity of rRBP1.1. Thus, efficient reticulocyte-binding activity of PvRBP1a lies within the 749-residue rRBP1.1 region, which specifically bound enriched reticulocytes and failed to bind unenriched normocytes (Figure 2C).
Reticulocyte Binding Specificities of PvRBP2c and PvRBP1a
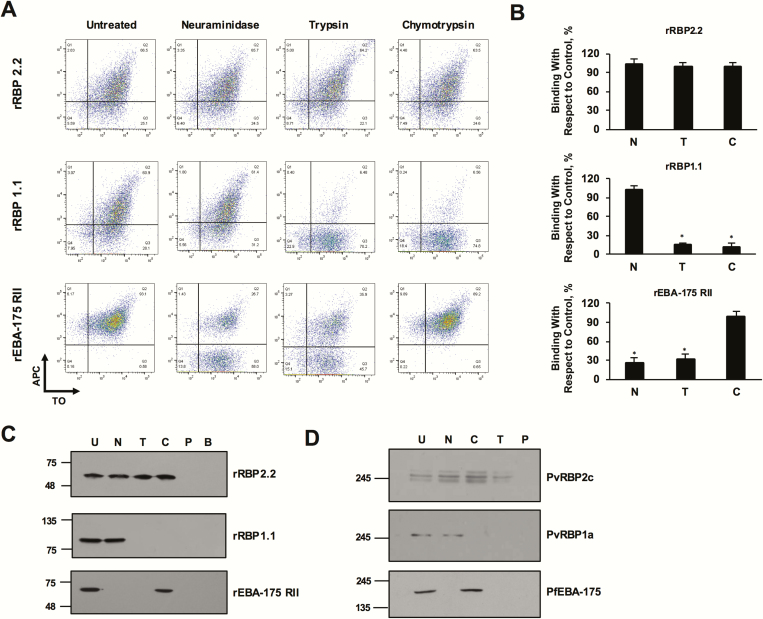
The nature of the reticulocyte receptor that binds with PvRBP2c/PvRBP1a was determined by analyzing the binding specificity of both recombinant and native parasite proteins with enzymatically treated reticulocytes. rRBP1.1 bound neuraminidase-treated reticulocytes (sialic acid depleted) but failed to bind trypsin- or chymotrypsin-treated reticulocytes, which was detected with FACS (Figure 3A and B) and immunoblotting (Figure 3C). However, rRBP2.2 bound all 3 enzymatically treated reticulocytes (Figure 3A–C). The reticulocyte-binding specificity of native PvRBP2c/PvRBP1a parasite proteins was analyzed from culture supernatants (Figure 3D). Native PvRBP2c and PvRBP1a exhibited binding specificities that appeared to match those of rRBP2.2 and rRBP1.1, respectively (Figure 3A–D). As a control for enzymatic treatments, the binding of PfEBA-175 was analyzed (Figure 3A–D). No protein was detected when PBS was incubated with enriched reticulocytes, confirming no cross-contamination of any reticulocyte protein (Figure 3C and D). Furthermore, no protein was detected when recombinant rRBP2.2/rRBP1.1/rEBA-175RII was incubated with the magnetic beads (Figure 3C).
Figure 3.
Reticulocyte-binding specificity of rRBP2.2 and rRBP1.1 recombinant proteins. A, plots depicting rRBP2.2-binding reticulocytes in a neuraminidase-, chymotrypsin-, and trypsin-resistant manner. rRBP1.1 binding with reticulocytes (thiazole orange [TO] positive) was neuraminidase (N) resistant but sensitive to chymotrypsin (C) and trypsin (T). rEBA-175RII (recombinant receptor–binding domain of PfEBA-175) was analyzed as a control with the same set of enzymatically treated reticulocytes. rPfEBA-175RII bound untreated and chymotrypsin-treated cells but had reduced binding to the neuraminidase- and trypsin-treated cells. APC, allophycocyanin. B, Bar charts showing the percentage reticulocyte binding of rRBP2.2, rRBP21.1, and rEBA-175RII. Error bars represent standard errors of the mean for 3 independent repeats. *P < .001. C, Immunoblots depicting the binding of rRBP2.2, rRBP1.1, and rPfEBA-175RII to enzyme-treated reticulocytes. No protein was detected when cells were incubated with phosphate-buffered saline or when the magnetic beads were incubated with the proteins. B, CD71 magnetic beads control; U, untreated; C, chymotrypsin treated; N, neuraminidase treated; trypsin, P, phosphate-buffered saline control; T, trypsin treated. D, Immunoblots depicting the binding of native Plasmodium vivax reticulocyte-binding protein (PvRBP) 2c, PvRBP1a, and PfEBA-175 with the enzyme-treated reticulocytes. Reticulocytes were enriched by magnetic sorting. Abbreviations: PfEBA-175, Plasmodium falciparum erythrocyte binding antigen 175; rRBP, recombinant RBP.
Reticulocyte Binding Inhibitory Activity of PvRBP Specific Rat Antibodies
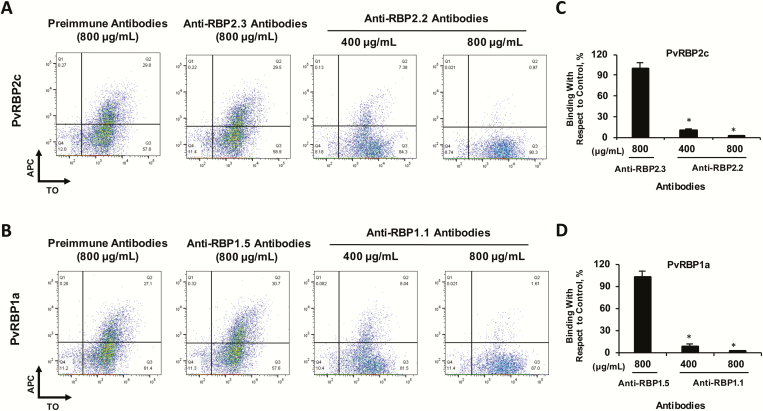
To evaluate the binding-inhibitory potential of the PvRBP2c/PvRBP1a specific antibodies against the respective native PvRBPs, we performed a binding assay in which the culture supernatant was incubated with enriched reticulocytes in the presence of purified anti-PvRBP rat antibodies (total immunoglobulin G [IgG]) and the native protein binding was analyzed with FACS (Figure 4). In the presence of the preimmune antibodies (800 µg/mL), 35% reticulocytes were individually bound with either PvRBP2c or PvRBP1a (Figure 4A and B). This reticulocyte-binding efficiency for both native proteins in the presence of anti-PvRBP rat antibodies was significantly reduced, by 74%–76% (400 µg/mL) and 95%–97% (800 µg/mL) (Figure 4A–D). Purified IgG against the non-binders (rRBP2.3, rRBP1.5) did not exhibit any binding inhibition (Figure 4A and B). Thus, antibodies raised against the specific reticulocyte-binding domains of PvRBP2c/PvRBP1a potently neutralized the binding activity of their respective native PvRBP parasite proteins.
Figure 4.
Plasmodium vivax reticulocyte-binding protein (PvRBP) rat antibodies block reticulocyte binding of the native PvRBP parasite proteins. Flow cytometry dot plots depicting the inhibition of binding of native PvRBP2c (A) and PvRBP1a (B) in the presence of the respective anti-rRBP2.2/1.1 total rat immunoglobulin G (IgG; 400 and 800 µg/mL). APC, allophycocyanin; TO, thiazole orange. C, D, Bar charts showing the percentage of reticulocyte-binding inhibition of native PvRBP2c (C) and PvRBP1a (D) by anti-PvRBP IgG compared with preimmune rat IgG. The reticulocyte binding of the native PvRBP2c and PvRBP1a parasite proteins was potently blocked by the rRBP2.2 and rRBP1.1 rat antibodies (total IgG). As controls, reticulocyte binding of native PvRBP2c/PvRBP1a was analyzed in the presence of total rat IgG against the non-binders, rRBP2.3 and rRBP1.5. Anti-rat rRBP2.3/rRBP1.5 IgG failed to block the binding of native PvRBP2c and PvRBP1a proteins. Reticulocytes were enriched by magnetic sorting. *P < .001. Abbreviation: rRBP, recombinant RBP.
Detection of Naturally Acquired Humoral Immune Responses Against the PvRBPs
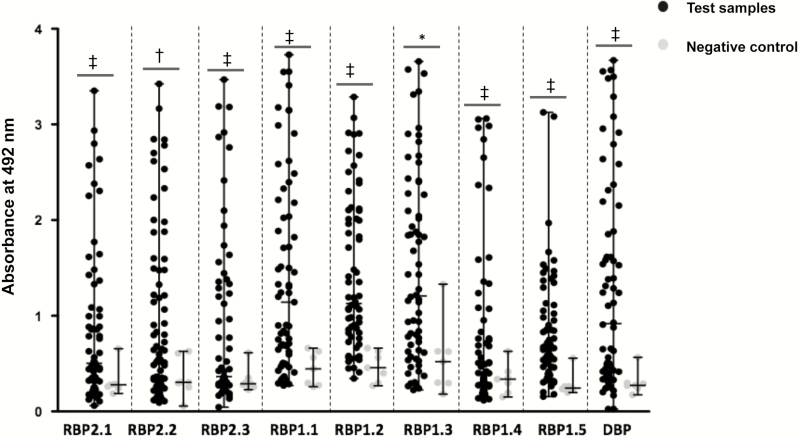
Antibody responses against different PvRBP2c and PvRBP1a recombinant proteins were analyzed among the 66 plasma samples by ELISA (Figure 5). Seven healthy plasma samples from non–malaria-endemic regions with no history of malaria infections were used as controls. High antibody responses against the PvRBP1a/PvRBP2c recombinant proteins were observed among the human plasma samples from endemic regions, compared with the controls (Figure 5), suggesting that naturally acquired human antibodies were raised against PvRBP2c and PvRBP1a. Human antibody responses against rRBP1.4 and rRBP1.5 were significantly lower than those against rRBP1.1 (Figure 5), suggesting that the entire 749–amino acid N-terminal region of PvRBP1a is immunodominant and exposed to the human immune system during vivax infections. Thus, naturally acquired human antibodies raised against P. vivax infections specifically bound our recombinant proteins, confirming their conformational integrity.
Figure 5.
Detection of naturally acquired human antibody responses against Plasmodium vivax reticulocyte-binding protein (PvRBP) 2c, and PvRBP1a, and P. vivax Duffy-binding protein (PvDBP). Antibody responses were studied among 66 P. vivax–infected human plasma samples (dilution 1:200) against 9 recombinant proteins (8 PvRBP and 1 PvDBP). Seven healthy plasma samples from non–malaria-endemic regions with no history of malaria infections were used as controls. Bars indicate medians with ranges. *P < .05; †P < .01; ‡P < .001.
Binding Inhibitory Activity of Naturally Acquired PvRBP Human Antibodies
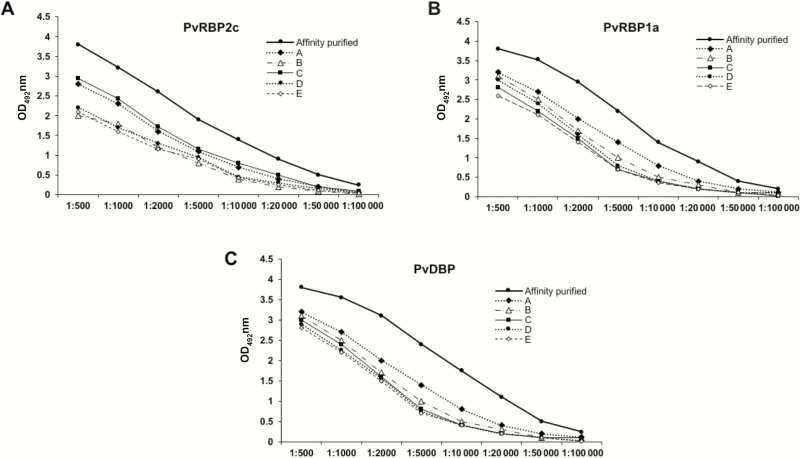
Binding inhibition has been reported to correlate with protective malaria immunity [23]. We thus tested the functional ability of affinity-purified human antibodies to inhibit the reticulocyte binding of the native and recombinant PvRBP2c/PvRBP1a proteins. Antigen-specific human antibodies against PvRBP2c, PvRBP1a, and PvDBPII were affinity purified from pooled human plasma samples (Figure 6), as reported elsewhere [26, 27]. Antibody enrichment was reflected by the significantly higher optical density values of the antigen-specific, affinity-purified human IgG compared with that of the highest-responding individual plasma samples (Figure 6). The affinity-purified antibodies reacted only with their respective antigens and did not cross-react with the other control antigens (Supplementary Figure 8).
Figure 6.
Analysis of the affinity-purified antibodies against Plasmodium vivax reticulocyte-binding protein (PvRBP) 2c (A), PvRBP1a (B), and P. vivax Duffy-binding protein (PvDBP) proteins (C). The enrichment of antigen-specific immunoglobulin Gs (IgGs) against rRBP2.2, rRBP1.1 and rDBPII were analyzed with enzyme-linked immunosorbent assay (ELISA). The ELISA results are plotted as optical density (OD) at 492 nm (y-axis) to compare concentrations of affinity-purified PvRBP2c (A), PvRBP1a (B), and PvDBP antibodies (C) and the 5 highest responders (plasma samples A–E). Antibody enrichment is reflected by the significantly higher OD values of the antigen-specific IgG in the affinity-purified samples compared with the highest responding individual plasma samples A-E. Abbreviations: DBP, Duffy binding protein; rRBP, recombinant RBP.
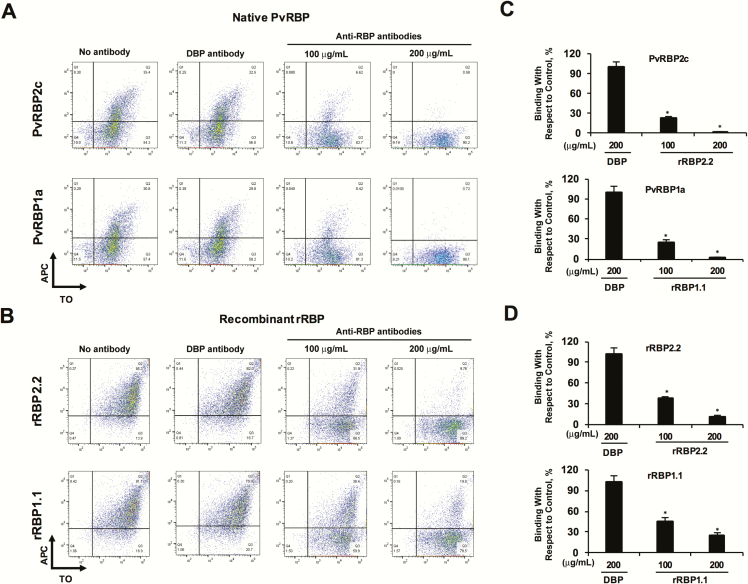
Owing to limitations of affinity-purified human antibodies, we performed the binding inhibition assays against 4 PvRBPs (2 native, 2 recombinant) at only 2 concentrations. PvRBP1.1- and PvRBP2.2-specific human antibodies inhibited the reticulocyte binding of the respective parasite proteins by 72%–80% (100 µg/mL) (Figure 7A and C), which was augmented at 200 µg/mL such that the reticulocyte binding of the native PvRBPs was almost completely inhibited (Figure 7A and C). Similar results were observed for rRBP2.2 and rRBP1.1. The binding inhibition of the recombinant proteins by the human antibodies was 56%–60% at 100 µg/mL, which further increased to 76%–89% at 200 µg/mL (Figure 7B and D). PvDBP-specific human antibodies had no effect on the binding of the PvRBP2c/PvRBP1a parasite proteins (Figure 7A and C) or recombinant rRBP2.2/rRBP1.1 (Figure 7B and D). Thus, our results clearly demonstrate that affinity-purified anti-PvRBP2c and anti-PvRBP1a human antibodies exhibited a specific binding-inhibitory activity.
Figure 7.
Purified Plasmodium vivax reticulocyte-binding protein (PvRBP)–specific human antibodies block reticulocyte binding of the native and recombinant PvRBPs. A, B, Flow cytometry dot plots depicting the inhibition of binding of native PvRBP parasite (A) proteins and rRBP2.2/rRBP1.1 (B) proteins in the presence of the respective anti-PvRBP2c/anti-PvRBP1a human immunoglobulin G (IgG; 100 and 200 µg/mL, respectively). APC, allophycocyanin; TO, thiazole orange. C, D, Bar charts showing the percentage inhibition of reticulocyte binding of native PvRBPs (C) and rRBPs (D) by the anti-PvRBP/P. vivax Duffy-binding protein (PvDBP) IgG compared with binding in the absence of IgG. The reticulocyte binding of the native PvRBP2c/PvRBP1a and recombinant rRBP2.2/rRBP1.1 were potently blocked by the respective PvRBP2c/PvRBP1a-specific human IgGs. As controls, reticulocyte binding of PvRBP2c/PvRBP1a was analyzed in the presence of PvDBP-specific purified human antibodies. Anti-PvDBP human IgG failed to block the reticulocyte binding of native PvRBP2c and PvRBP1a proteins. Reticulocytes were enriched by magnetic sorting. *P < .001. Abbreviation: rRBP, recombinant RBP.
DISCUSSION
P. vivax merozoite invasion was believed to be completely dependent on the essential PvDBP-DARC molecular interaction; thus, vivax vaccine development efforts have focused on PvDBP. However, reports of vivax infections in Duffy-null individuals [12–14], which could not be attributed to PvDBP polymorphisms [32], suggest the evolution of alternate invasion pathways. The significance of PvRBPs has risen as potential mediators of these alternate invasion pathways [14, 32]. Thus, it is evident that P. vivax neutralization would require targeting multiple antigens, including PvDBP and PvRBPs. Precise inhibitory targets within the PvRBP antigens remain unknown.
The discovery of PvRBPs (PvRBP1a/PvRBP2c) provided an important lead toward understanding the molecular mechanisms underlying P. vivax reticulocyte invasion [10]. Owing to the lack of P. vivax in vitro culture, it has been challenging to screen the vaccine potential of vivax antigens, including PvRBPs. In this regard, binding-inhibitory PvDBP antibodies have been reported to be associated with malaria protection [23]. Therefore, binding inhibition (ie, the ability of antibodies to block the reticulocyte binding of key P. vivax adhesins) is of prime significance for assessing the vaccine potential of vivax antigens.
Our study examines the functional ability of naturally acquired human antibodies against the receptor-binding domains of the PvRBPs to inhibit the specific reticulocyte binding of the native parasite proteins, PvRBP2c and PvRBP1a [10]. We have demonstrated the receptor-binding specificity of the native PvRBP2c and PvRBP1a parasite proteins, which was sialic acid independent. However, trypsin/chymotrypsin abolished PvRBP1a binding with no effect on PvRBP2c binding, suggesting that only the PvRBP1a receptor was trypsin/chymotrypsin sensitive. Thus, the distinct binding specificity of the PvRBPs suggests that 2 binding sites, on the same or on different receptors, may be involved in reticulocyte invasion. The identity of the reticulocyte-specific receptors remains a major gap in vivax biology.
We have mapped the receptor-binding domains of both PvRBP2c (rRBP2.2) and PvRBP1a (rRBP1.1), which exhibit potent reticulocyte-binding activity with a specificity that appears to match that of native PvRBPs. We compared 3 PvRBP2c constructs and demonstrated that rRBP2.2 (residues 464–876) exhibited the most potent reticulocyte-binding activity. This is the first report for the identification of the reticulocyte-binding domain of PvRBP2c. A study reported last year noted the binding activity of several PvRBP recombinant proteins with no direct data on PvRBP2c [35]. The percentage of reticulocyte binding reported for the recombinant constructs was modestly low, with no correlation shown with the binding of native PvRBPs [35]. Similarly, we analyzed 5 PvRBP1a constructs and showed that rRBP1.1 (residues 30–778) exhibited the highest reticulocyte-binding activity. Also last year, a 248–amino acid region of PvRBP1a/1b homologous with the PfRH4 binding region was reported to bind reticulocytes [36], which is consistent with our results. However, we show that the binding of this 248–amino acid stretch was significantly weaker than that of the 749–amino acid rRBP1.1 construct, which suggests that the larger region favors a stronger interaction with its receptor.
Previous reports produced recombinant reticulocyte-binding proteins with N-terminal His-tags [35, 36], whereas our study has produced C-terminal His-tag proteins. It is plausible that the different His-tag locations may influence binding efficiency or specificity. To address this possibility, we demonstrated that the recombinant rRBP2.2, rRBP1.1 bound reticulocytes with a specificity that appears similar to that of native PvRBPs in their monomeric form. Native PvRBP1a/PvRBP2c have been suggested to be part of homogeneous or heterogeneous protein complexes [10, 37]. Thus, further studies are crucial to investigate the potential protein-protein interactions involving PvRBP2c/PvRBP1a and their effect on the reticulocyte-binding specificity during merozoite invasion.
Importantly, we demonstrated that specific human antibodies against the reticulocyte-binding domains of the 2 PvRBPs raised through natural P. vivax exposure efficiently block the binding of native parasite proteins. To the best of our knowledge, ours is the first report demonstrating the functional binding-inhibitory activity of naturally acquired human antibodies targeting any PvRBP. Binding-inhibitory antibodies suggest that the recombinant proteins had a structure resembling the native 3-D configuration, in which the critical binding epitopes were exposed in the correct conformation. PvRBP1a/PvRBP2c elicited strong humoral responses among humans residing in endemic areas, consistent with findings of previous studies [35, 36, 38]. Significantly, affinity-purified naturally acquired PvRBP2c/PvRBP1a-specific human antibodies potently inhibited the reticulocyte-binding activity of the respective native proteins.
We have thus demonstrated that the human immune system generates functional binding-inhibitory antibodies against the reticulocyte-binding domains of the PvRBPs, which, like PvDBP antibodies [23], may have the potential to neutralize P. vivax. Therefore, PvRBPs seem to be promising candidate antigens for vivax vaccines. Importantly, PvRBPs are immunogenic in humans, suggesting that immunity induced by a PvRBP-based vaccine could further be boosted through natural exposure, potentially leading to an augmentation in immunity and protection against vivax malaria.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Author contributions. Conception and design of experiments: D. G. Performance of experiments: E. D. G, G. A., H. S., and K. C. Data analysis: E. D. G, G. A., H. S., Y. D. S., and D. G. Contribution of reagents, materials, and analysis tools: P. K. B, N. S., Y. D. S., and D. G. Drafting the manuscript: E. D. G., G. A., H. S., and D. G.
Acknowledgments. We are grateful to Bruce Russell (National University of Singapore; now University of Otago, Dunedin, New Zealand), Laurent Renia (Singapore Immunology Network), and Benoit Malleret (Singapore Immunology Network) for helpful discussions during the project. We are thankful to Asis Das (BITS Pilani) for the P. vivax genomic DNA, Lee Hall (National Institutes of Health) for the EBA-175 antibodies, Virander S. Chauhan for critically reviewing the manuscript, Inderjeet Kaur (International Centre for Genetic Engineering and Biotechnology) for help with liquid chromatography–mass spectrometry, and Rakesh Singh and Ashok Das (International Centre for Genetic Engineering and Biotechnology animal facility) for technical assistance in the animal immunizations.
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This work was supported by Grand Challenges Canada (grant RS 163-01); the Department of Biotechnology (DBT) (GLUE grant BT/PR14918/MED/15/60/2010, DBT Ramalingaswami fellowship to D. G., and DBT fellowship to H. S.); and the University Grants Commission (fellowships to G. A. and K. C.).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. World Malaria Report. 2015. [Google Scholar]
- 2. Gething PW, Elyazar IR, Moyes CL, et al. A long neglected world malaria map: Plasmodium vivax endemicity in 2010. PLoS Negl Trop Dis 2012; 6:e1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mueller I, Galinski MR, Baird JK, et al. Key gaps in the knowledge of Plasmodium vivax, a neglected human malaria parasite. Lancet Infect Dis 2009; 9:555–66. [DOI] [PubMed] [Google Scholar]
- 4. Tanwar GS, Khatri PC, Sengar GS, et al. Clinical profiles of 13 children with Plasmodium vivax cerebral malaria. Ann Trop Paediatr 2011; 31:351–6. [DOI] [PubMed] [Google Scholar]
- 5. Mueller I, Galinski MR, Tsuboi T, Arevalo-Herrera M, Collins WE, King CL. Natural acquisition of immunity to Plasmodium vivax: epidemiological observations and potential targets. Adv Parasitol 2013; 81:77–131. [DOI] [PubMed] [Google Scholar]
- 6. Doolan DL, Dobaño C, Baird JK. Acquired immunity to malaria. Clin Microbiol Rev 2009; 22:13–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Galinski MR, Barnwell JW. Plasmodium vivax: who cares? Malar J 2008; 7:S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gaur D, Chitnis CE. Molecular interactions and signaling mechanisms during erythrocyte invasion by malaria parasites. Curr Opin Microbiol 2011; 14:422–8. [DOI] [PubMed] [Google Scholar]
- 9. Miller LH, Mason SJ, Clyde DF, McGinniss MH. The resistance factor to Plasmodium vivax in blacks: the Duffy-blood-group genotype, FyFy. N Engl J Med 1976; 295:302–4. [DOI] [PubMed] [Google Scholar]
- 10. Galinski MR, Medina CC, Ingravallo P, Barnwell JW. A reticulocyte-binding protein complex of Plasmodium vivax merozoites. Cell 1992; 69:1213–26. [DOI] [PubMed] [Google Scholar]
- 11. Carlton JM, Adams JH, Silva JC, et al. Comparative genomics of the neglected human malaria parasite Plasmodium vivax. Nature 2008; 455:757–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ménard D, Barnadas C, Bouchier C, et al. Plasmodium vivax clinical malaria is commonly observed in Duffy-negative Malagasy people. Proc Natl Acad Sci U S A 2010; 107:5967–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carvalho TA, Queiroz MG, Cardoso GL, et al. Plasmodium vivax infection in Anajás, State of Pará: no differential resistance profile among Duffy-negative and Duffy-positive individuals. Malar J 2012; 11:430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mendes C, Dias F, Figueiredo J, et al. Duffy negative antigen is no longer a barrier to Plasmodium vivax—molecular evidences from the African West Coast (Angola and Equatorial Guinea). PLoS Negl Trop Dis 2011; 5:e1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gaur D, Mayer DC, Miller LH. Parasite ligand-host receptor interactions during invasion of erythrocytes by Plasmodium merozoites. Int J Parasitol 2004; 34:1413–29. [DOI] [PubMed] [Google Scholar]
- 16. Cowman AF, Berry D, Baum J. The cellular and molecular basis for malaria parasite invasion of the human red blood cell. J Cell Biol 2012; 198:961–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sahar T, Reddy KS, Bharadwaj M, et al. Plasmodium falciparum reticulocyte binding-like homologue protein 2 (PfRH2) is a key adhesive molecule involved in erythrocyte invasion. PLoS One 2011; 6:e17102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gaur D, Singh S, Singh S, Jiang L, Diouf A, Miller LH. Recombinant Plasmodium falciparum reticulocyte homology protein 4 binds to erythrocytes and blocks invasion. Proc Natl Acad Sci U S A 2007; 104:17789–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gao X, Yeo KP, Aw SS, et al. Antibodies targeting the PfRH1 binding domain inhibit invasion of Plasmodium falciparum merozoites. PLoS Pathog 2008; 4:e1000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hayton K, Gaur D, Liu A, et al. Erythrocyte binding protein PfRH5 polymorphisms determine species-specific pathways of Plasmodium falciparum invasion. Cell Host Microbe 2008; 4:40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reddy KS, Amlabu E, Pandey AK, Mitra P, Chauhan VS, Gaur D. Multiprotein complex between the GPI-anchored CyRPA with PfRH5 and PfRipr is crucial for Plasmodium falciparum erythrocyte invasion. Proc Natl Acad Sci U S A 2015; 112:1179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gaur D, Chitnis CE, Chauhan VS. Molecular basis of erythrocyte invasion by Plasmodium merozoites. In: Gaur D, Chitnis C, Chauhan VS, eds. Advances in malaria research. Hoboken, NJ, USA: John Wiley & Sons; 2017:33–86. [Google Scholar]
- 23. King CL, Michon P, Shakri AR, et al. Naturally acquired Duffy-binding protein-specific binding inhibitory antibodies confer protection from blood-stage Plasmodium vivax infection. Proc Natl Acad Sci U S A 2008; 105:8363–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Singh N, Chand SK, Bharti PK, et al. Dynamics of forest malaria transmission in Balaghat district, Madhya Pradesh, India. PLoS One 2013; 8:e73730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zeeshan M, Tyagi K, Sharma YD. CD4+ T cell response correlates with naturally acquired antibodies against Plasmodium vivax tryptophan-rich antigens. Infect Immun 2015; 83:2018–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Reiling L, Richards JS, Fowkes FJ, et al. The Plasmodium falciparum erythrocyte invasion ligand Pfrh4 as a target of functional and protective human antibodies against malaria. PLoS One 2012; 7:e45253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Patel SD, Ahouidi AD, Bei AK, et al. Plasmodium falciparum merozoite surface antigen, PfRH5, elicits detectable levels of invasion-inhibiting antibodies in humans. J Infect Dis 2013; 208:1679–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Roobsoong W, Tharinjaroen CS, Rachaphaew N, et al. Improvement of culture conditions for long-term in vitro culture of Plasmodium vivax. Malar J 2015; 14:297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Russell B, Suwanarusk R, Borlon C, et al. A reliable ex vivo invasion assay of human reticulocytes by Plasmodium vivax. Blood 2011; 118:e74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pandey AK, Reddy KS, Sahar T, et al. Identification of a potent combination of key Plasmodium falciparum merozoite antigens that elicit strain-transcending parasite-neutralizing antibodies. Infect Immun 2013; 81:441–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tran TM, Moreno A, Yazdani SS, Chitnis CE, Barnwell JW, Galinski MR. Detection of a Plasmodium vivax erythrocyte binding protein by flow cytometry. Cytometry A 2005; 63:59–66. [DOI] [PubMed] [Google Scholar]
- 32. Gunalan K, Lo E, Hostetler JB, et al. Role of Plasmodium vivax Duffy-binding protein 1 in invasion of Duffy-null Africans. Proc Natl Acad Sci U S A 2016; 113:6271–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cho JS, Russell B, Kosasaivee V, et al. Unambiguous determination of Plasmodium vivax reticulocyte invasion by flow cytometry. Int J Parasitol 2016; 46:31–9. [DOI] [PubMed] [Google Scholar]
- 34. Grimberg BT, Udomsangpetch R, Xainli J, et al. Plasmodium vivax invasion of human erythrocytes inhibited by antibodies directed against the Duffy binding protein. PLoS Med 2007; 4:e337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. França CT, He WQ, Gruszczyk J, et al. Plasmodium vivax reticulocyte binding proteins are key targets of naturally acquired immunity in young Papua New Guinean children. PLoS Negl Trop Dis 2016; 10:e0005014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Han JH, Lee SK, Wang B, et al. Identification of a reticulocyte-specific binding domain of Plasmodium vivax reticulocyte-binding protein 1 that is homologous to the PfRh4 erythrocyte-binding domain. Sci Rep 2016; 6:26993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Galinski MR, Barnwell JW. Plasmodium vivax: Merozoites, invasion of reticulocytes and considerations for malaria vaccine development. Parasitol Today 1996; 12:20–9. [DOI] [PubMed] [Google Scholar]
- 38. Tran TM, Oliveira-Ferreira J, Moreno A, et al. Comparison of IgG reactivities to Plasmodium vivax merozoite invasion antigens in a Brazilian Amazon population. Am J Trop Med Hyg 2005; 73:244–55. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.