BCG vaccination nonspecifically reduces mortality in infants. Using data from three randomized trials, we find that BCG at birth reduces mortality in boys already within the first week of immunization, whereas girls experience the largest effect 2–4 weeks after immunization.
Keywords: Bacille Calmette-Guérin vaccine, heterologous immunity, nonspecific effects of vaccines, sex differences, neonatal mortality
Abstract
Background
Three randomized trials (RCTs) in low-weight (<2.5 kg) infants have shown that Bacille Calmette-Guérin (BCG) vaccine nonspecifically reduces all-cause mortality in the neonatal period.
Methods
Using data from 3 RCTs of early BCG (n = 6583) we examined potential sex differences in the timing of the mortality reduction in the neonatal period, presenting metaestimates of the main outcome mortality rate ratios (MRR) for BCG-vaccinated and controls.
Results
Among controls, boys had a particularly high mortality during the first week after randomization: male–female MRR 2.71 (95% CI, 1.70–4.50). During the first week, BCG had a marked beneficial effect for boys, reducing mortality 3-fold (MRR [BCG/no BCG] = 0.36 [0.20–0.67]). In weeks 2–4 the effect waned for boys (MRR = 0.91 [0.51–1.69]). In girls, the pattern was opposite with a limited effect in the first week (MRR = 0.85 [0.46–1.54]), but a significant reduction in weeks 2–4 (MRR = 0.56 [0.31–1.00]). This was consistent in all 3 trials. Verbal autopsies linked early benefit to fewer sepsis-related deaths among BCG-vaccinated boys.
Discussion
The marked reduction in mortality in the days after BCG vaccination in boys emphasizes the importance of providing BCG soon after birth.
Trial registration numbers
ClinicalTrials.gov (NCT00146302) and ClinicalTrials.gov (NCT00625482).
Observational studies have suggested that Bacille Calmette-Guérin (BCG) vaccine is associated with lower all-cause mortality in addition to protecting against tuberculosis [1, 2]. We have called this phenomenon the nonspecific effects of BCG.
In many low-income countries, including Guinea-Bissau, BCG is postponed for low-birth-weight (<2500 g) neonates until they have attained a weight of 2500 g. It was therefore possible to conduct randomized trials (RCTs) among low-weight (LW) infants to test the hypothesis that BCG at birth reduced infant mortality by 25%. Between 2002 and 2008, we conducted 2 RCTs of early BCG showing a nonsignificant reduction of 21% in infant mortality, but a significant reduction of 48% in neonatal mortality [3]. Because the first 2 trials had infant mortality as the primary outcome, we subsequently conducted a third RCT (2008–2013) with neonatal mortality as the main outcome. A combined analysis of all 3 trials showed significant reductions of 38% in neonatal mortality and 16% in infant mortality [4]. The 3 trials supported consistently that BCG has strong beneficial effects on mortality in the first month of life.
While analyzing the first 2 RCTs we observed that the beneficial effects of BCG had different timing for boys and girls. Before publishing, we awaited the results of the third trial, which has now been completed [4]. Here, we present the sex-differential time trend in the effect of BCG on neonatal mortality in the 3 RCTs conducted in LW infants in Guinea-Bissau.
METHODS
Background
The Bandim Health Project (BHP) maintains a health and demographic surveillance system in Bissau, the capital of Guinea-Bissau. The first LW trial began in November 2002 (ClinicalTrials.gov, NCT00146302) [3]. All LW children from the city of Bissau born at the maternity ward of the national hospital, as well as LW children coming for vaccination to the health centers in the study area, were invited to participate in the trial. In November 2004, we discovered that there were faulty randomization procedures at the main hospital and the trial was stopped. The randomization procedures occurred without error at the health centers (Trial 1) [5]. The trial was reorganized and new randomization and control procedures were introduced. Therefore, from November 2004 to February 2008, recruitment to a second RCT took place at the maternity ward at the national hospital and at the 3 health centers in the study area (Trial 2) [3]. In 2008, a third RCT was initiated to test the hypothesis that early BCG reduces neonatal mortality by 45% (Trial 3, ClinicalTrials.gov, NCT00625482). Similar to Trials 1 and 2, recruitment to Trial 3 took place at the national hospital and the 3 health centers in the study area as well as 3 private hospitals. Initially, Trial 3 included only girls; based on growth data we initially thought that BCG was particularly beneficial for girls, but when Trial 2 found that early BCG vaccination also benefitted boys, we applied for ethical permission to enroll boys in Trial 3 as well. Hence, from May 2010, Trial 3 was expanded to include also boys.
Study Design and Randomization
Trials 1, 2, and 3 were identical in design. Hence, the present study uses data from all 3 trials. The inclusion and follow-up procedures have been described in detail elsewhere [3–5]. In brief, LW children were identified at discharge from the hospital or at their first health center contact, and they were randomly assigned to early BCG (intervention group) or the usual delayed BCG (control group). Eligible participants were children with a weight less than 2500 g at the time of enrolment. Children, who had major malformations, were overtly sick, or who had received the BCG vaccine prior to enrolment were excluded from the study. Mothers/guardians of eligible children were informed about the study in the local language, Creole, and received a written explanation in the official language, Portuguese. If they wished to participate, we asked the mother or guardian to sign the consent form with a signature or fingerprint. The children were randomized (1:1) in blocks of 24. To illustrate for the mother/guardian that it was chance that decided what intervention the child would receive, the mother/guardian was asked to draw a lot from a bag containing 24 envelopes. In Trials 1 and 2, pairs of twins were randomized to the same treatment to eliminate potential confusion of the individual twins; in Trial 3, only same-sex twins were randomized to the same treatment.
Children in the intervention group received BCG intradermally (0.05 mL BCG Vaccine SSI; Statens Serum Institut, Copenhagen, Denmark). Control children were treated according to local practice and received BCG vaccine later when they attended a health center and had attained a weight of 2500 g or at 6 weeks of age, when infants were to receive the first doses of diphtheria, pertussis, and tetanus (DTP) and oral poliovirus (OPV) vaccines. All children received a birth dose of OPV (OPV0) at inclusion except for 99 children included between 15 October and 9 December 2007 when OPV was lacking in Guinea-Bissau.
From May 2005 to January 2008 (Trial 2), we also studied the effect of vitamin A supplementation (VAS) at birth in a 2-by-2 factorial design. The children were randomly assigned to early BCG or delayed BCG as well as vitamin A supplementation or placebo. Because there was no interaction in mortality between the 2 interventions [3], the effects of BCG and VAS were reported separately [6].
Children living outside the BHP study area were driven home by a field team. The team drew a map of the house, recorded global positioning system (GPS) coordinates, and took a photograph of the house and the mother to ensure that the team would be able to identify the child at subsequent visits. The children were visited at home after 3 days and at 2, 6, and 12 months of age to assess the status of the child. Children from the study area were identified via their unique study number and followed both by the routine visits by the BHP assistants and at the study specific visits at 3 days and at 2, 6, and 12 months of age.
Verbal Autopsy Procedures
Verbal autopsies were conducted to determine probable causes of death. During Trial 1 and 2, a medical practitioner visited the home 3 months after the child had died. The medical practitioner registered the events leading up to the death of the child, and assigned a cause of death based on this information. In Trial 3, a field assistant trained in performing verbal autopsies visited the home 3 months after the child had died. The field assistant recorded events leading up to the death of the child. Based on this information, the medical practitioner, who had assigned the causes of death in Trial 1 and 2, assigned the causes of death in Trial 3. The medical practitioner was unaware of the intervention allocation.
Statistical Analysis
Cox proportional hazards models with time since randomization as the underlying time variable were used to compare mortality rates between the intervention and the control group, providing mortality rate ratios (MRR). Children entered the analyses at the day of randomization and exited at death or at the end of the neonatal period (28 days of age). Children who moved before 28 days of age were censored. We used robust 95% confidence intervals (CI) to adjust the standard errors for the lifetime dependence between twins. Time from randomization to 28 days was divided into intervals, and within these intervals we analyzed the effect of early BCG. Because most deaths occurred in the first week after randomization, we divided it into 2 intervals of 0–3 days and 4–7 days after randomization; the remaining time of the neonatal period was analyzed in weekly intervals. We allowed a different baseline hazard for each of the 3 trials. When there were 0 deaths in the intervention group (MRR = 0), CIs were obtained by a profile likelihood method, inverting results of the likelihood ratio test into a CI. We evaluated the proportional-hazards assumption by log-log plots and Schoenfeld residuals. Kaplan-Meier curves with time since randomization as the underlying time were drawn to display the cumulative mortality between the intervention and control group for Trials 1–3 combined and for Trials 2 and 3 separately (Trial 1 had too few deaths for graphic presentation
). All analyses were performed separately for boys and girls because the focus of the present study was to determine whether the effect of BCG differed by sex.
Ethics
The Guinean Ministry of Health’s Research Coordination Committee approved all trials and we received consultative approval from the Danish Central Ethical Committee for all trials. Children enrolled in the study had access to free consultations and essential drugs at the health centers in the BHP study area.
Participant Involvement
No participants were actively involved in setting the research question or the outcome measures, nor were they involved in the analysis, interpretation, and writing of the results. The participants are thanked in the acknowledgments. Our findings from the study have been shared with the Ministry of Health in Guinea-Bissau, to inform policy.
RESULTS
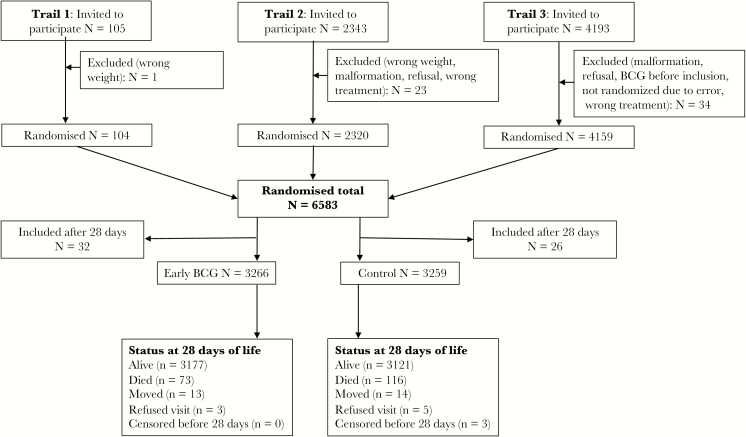
In Trial 1, 105 children were included and 1 child was excluded (Figure 1). In Trial 2, 2343 children were invited to participate and 23 were excluded. In Trial 3, 4193 children were invited and 34 were excluded. A total of 58 children were enrolled after the neonatal period and were not included in the present analysis. We therefore analyzed 6525 children and among them 189 deaths occurred during the first 28 days of life. At baseline, the randomization groups were comparable in each of the 3 trials [3–5]. In the combined population of the 3 trials, the intervention and control groups were comparable when stratified by sex (Supplementary Table 1). The median age of inclusion was 2 days (10–90 percentiles: 2–10 days) in all 3 trials.
Figure 1.
Children enrolled in 3 trials of early BCG effects on neonatal mortality in Guinea-Bissau 2002–2013. Note: The flow of children in each trial has been described in detail elsewhere [3–5]. Abbreviation: BCG, Bacille Calmette-Guérin.
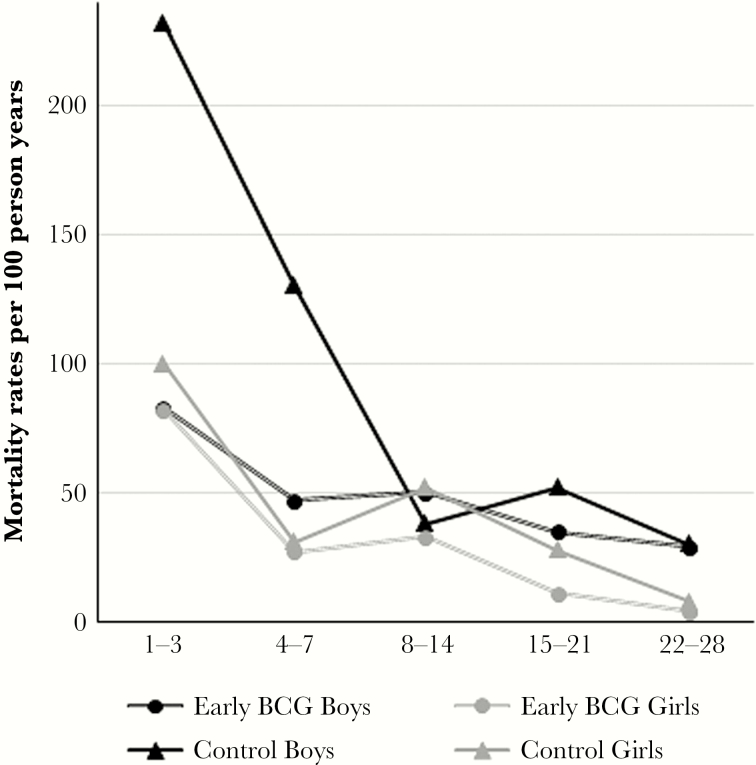
For both sexes, the highest mortality rate was seen in the first 3 days after enrolment (Table 1, Figure 2). The mortality rate was much higher for control boys than for control girls particularly in the first 7 days after enrolment, the male-female MRR being 2.71 (95% CI, 1.64–4.50). After the first week, the MRR comparing boys and girls was reduced to 1.25 (95% CI, 0.70–2.22) (data not shown).
Table 1.
Mortality Rate Ratios (MRR) for Different Intervals of Time Since Randomization to Early BCG or Control Group, Stratified by Sex
| Boys | Girls | ||||||
|---|---|---|---|---|---|---|---|
| Mortality per 100/PY (deaths/PY) | MRR for Early BCG versus Control (95% CI) | Mortality per 100/PY (deaths/ PY) | MRR for Early BCG versus Control (95% CI) | ||||
| Early BCG Group | Control Group | Early BCG Group | Control Group | ||||
| Divided into small intervals | 1–3 days | 83 (8/9.6) | 232 (22/9.5) | 0.36 (0.16–0.80) | 82 (14/17.1) | 100 (17/17.1) | 0.84 (0.41–1.73) |
| 4–7 days | 47 (6/12.7) | 130 (16/12.4) | 0.37 (0.14–0.94) | 27 (6/22.6) | 31 (7/22.5) | 0.85 (0.29–2.53) | |
| 8–14 days | 50 (10/21.5) | 38 (8/21.1) | 1.23 (0.49–3.11) | 33 (13/38.8) | 52 (20/38.6) | 0.64 (0.32–1.29) | |
| 15–21 days | 35 (7/20.0) | 52 (10/19.4) | 0.68 (0.26–1.79) | 11 (4/36.2) | 28 (10/35.7) | 0.39 (0.12–1.26) | |
| 22–28 days | 36 (4/13.4) | 37 (4/13.2) | 0.82 (0.19–3.50) | 4 (1/24.7) | 8 (2/24.4) | 0.50 (0.05–5.45) | |
| Divided into larger intervals | 1–7 days | 63 (14/22.2) | 174 (38/21.9) | 0.36 (0.20–0.67) | 51 (20/39.7) | 61 (24/39.6) | 0.84 (0.46–1.54) |
| 8–28 days | 38 (21/55.1) | 41 (22/53.8) | 0.91 (0.51–1.69) | 18 (18/99.7) | 32 (32/98.7) | 0.56 (0.31–1.00) | |
Estimates with P < .05 are highlighted in bold.
Abbreviations: BCG, Bacille Calmette-Guérin; PY, person years.
Figure 2.
Mortality rates split into time intervals since randomization, presented by sex and randomization groups. Abbreviation: BCG, Bacille Calmette-Guérin.
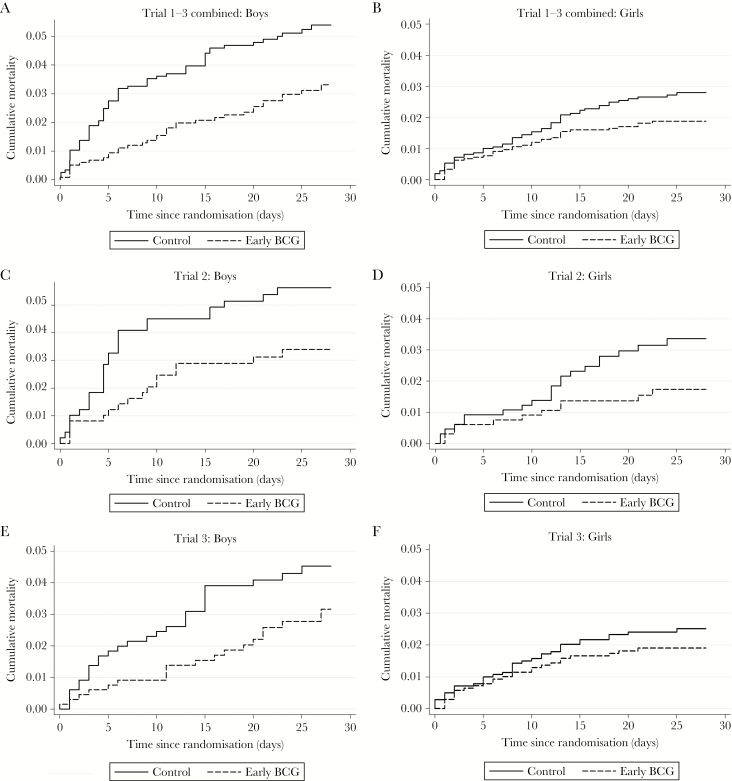
The mortality curves for the 2 randomization groups (early BCG and control) for all trials combined and individually for Trials 2 and 3 are depicted in Figure 3. The effect of BCG was consistently different for boys and girls for Trials 2 and 3 individually and for all 3 trials combined. Trial 1 had too few deaths to be presented graphically, although a strong early effect for boys was also observed in this trial (Table 2). Hence, the more rapidly occurring reduction in mortality after BCG for boys compared with girls was present in all 3 trials (Figure 3 and Table 2).
Figure 3.
Mortality curves for Trials 1, 2, and 3 combined and separately for Trial 2 and Trial 3. Note: It was not possible to calculate a separate mortality curve stratified by sex for Trial 1 due to few deaths within the neonatal period (n = 2 girls, n = 6 boys). Abbreviation: BCG, Bacille Calmette-Guérin.
Table 2.
Mortality Rate Ratios (MRR) in Time Intervals (0–7 days and 8–28 days) Since Randomization, Stratified by Sex and Trial
| Boys | Girls | |||||
|---|---|---|---|---|---|---|
| Mortality per 100/PY (deaths/PY) | MRR for Early BCG versus CONTROL (95% CI) | Mortality per 100/PY (deaths/PY) | MRR for Early BCG versus CONTROL (95% CI) | |||
| Early BCG Group | Control Group | Early BCG Group | Control Group | |||
| Trial 1 | ||||||
| 1–7 days | 0 (0/0.4) | 1130 (4/0.4) | 0 (0–0.55) | 225 (1/0.4) | 174 (1/0.6) | 1.29 (0.08–20.28) |
| 8–28 days | 93 (1/10.7) | 142 (1/7.1) | 0.36 (0.02–7.00) | 0 (0/9.6) | 0 (0/12.3) | … |
| Trial 2 | ||||||
| 1–7 days | 86 (8/9.3) | 218 (20/9.2) | 0.40 (0.17–0.90) | 40 (5/12.6) | 56 (7/12.4) | 0.70 (0.22–2.22) |
| 8–28 days | 35 (8/21.6) | 37 (7/21.6) | 1.14 (0.38–3.91) | 20 (6/30.2) | 48 (14/29.5) | 0.42 (0.16–1.09) |
| Trial 3 | ||||||
| 1–7 days | 48 (6/12.5) | 113 (14/12.3) | 0.42 (0.16–1.10) | 53 (14/26.6) | 60 (16/26.6) | 0.84 (0.41–1.73) |
| 8–28 days | 41 (12/29.2) | 49 (14/28.4) | 0.83 (0.39–1.80) | 19 (12/62.9) | 28 (18/62.4) | 0.66 (0.31–1.40) |
Estimates with P < .05 are highlighted in bold.
Abbreviations: BCG, Bacille Calmette-Guérin; PY, person years.
When analyzing all 3 trials combined, during the first 7 days after enrolment BCG versus control was associated with a significant reduction in mortality (1–3 days: MRR = 0.36 [95% CI, 0.16–0.80] and 4–7 days: MRR = 0.37 [95% CI, 0.14–0.94]) for boys (Table 1). In girls, there was only a very small effect of BCG at 1–3 days (MRR = 0.84 [95% CI, 0.41–1.73]) and 4–7 days (MRR = 0.85 [95% CI, 0.29–2.53]). This resulted in an interaction between sex and BCG in the first 7 days (P = .05 for interaction). In the following 3 weeks, boys had only a limited response to BCG (MRR = 0.91 [95% CI, 0.51–1.69]); however, girls had a significant 44% reduction in mortality from day 8 to 28 after birth (MRR: 0.56 [95% CI, 0.31–1.00]). Hence, the effect of BCG in boys and girls was significantly different in the first 1–7 days compared to the subsequent 8–28 days (P = .03 for 3-way interaction). We also tested other cut points from day 1 to 14 to verify that the 3-way interaction between BCG, sex, and time interval was not a chance observation (Supplementary Table 2). For all cut points in the first 14 days after enrolment, we observed the pattern of a stronger beneficial effect for boys in the first time interval and a stronger effect for girls in the second time interval. However, the strongest differential effect was seen for cut points between 4 and 10 days after enrolment and the most significant 3-way interaction was seen at 6 days after enrolment (P = .02 for interaction). When stratifying by trial, the same sex-differential time course effect of BCG was evident in all 3 trials (Table 2).
We had verbal autopsy information on cause of death from 95% (179/189) of the deaths that occurred in the neonatal period (Table 3). Sepsis was the cause of 50% of the deaths (90/179), other infections accounted for 19% (34/179) and noninfectious causes were 31% (55/179). For both sexes combined, BCG reduced sepsis-related mortality by 43% (MRR = 0.57 [95% CI, 0.36–0.91]) and other infections by 52% (MRR = 0.48 [95% CI, 0.21–1.08]), whereas the effect was limited for noninfectious causes (MRR = 0.89 [95% CI, 0.50–1.58]). Among boys, there was a significant reduction in sepsis-related deaths at 1–28 days (MRR = 0.51 [95% CI, 0.26–0.95]); however, this occurred most strongly in the first week (MRR = 0.37 [95% CI, 0.14–0.88]); for girls the reduction in sepsis-related deaths was strongest in weeks 2–4 after enrolment (MRR = 0.46 [95% CI, 0.14–1.29]).
Table 3.
Mortality Rate Ratios (MRR) of Causes of Deaths Stratified by Sex, Presented as the Complete Period or Split into Time Intervals since Randomization
| Boys | Girls | |||||
|---|---|---|---|---|---|---|
| Causes of Deaths | Early BCG | Control | MRR (BCG/Control) | Early BCG | Control | MRR (BCG/Control) |
| N Deaths/PY | N Deaths/PY | |||||
| 1–28 days after randomization a | ||||||
| Sepsis | 16/77.3 | 31/75.7 | 0.50 (0.27–0.92) | 17/139.5 | 26/138.4 | 0.66 (0.36–1.21) |
| Other infectionsb | 7/77.3 | 14/75.7 | 0.49 (0.20–1.22) | 4/139.5 | 10/138.4 | 0.40 (0.12–1.27) |
| Noninfectionsc | 11/77.3 | 13/75.7 | 0.82 (0.37–1.81) | 15/139.5 | 15/138.4 | 1.00 (0.48–2.08) |
| Time intervals since randomization a | ||||||
| 1–7 days after randomization | ||||||
| Sepsis | 8/22.2 | 21/21.9 | 0.37 (0.17–0.84) | 11/17.1 | 13/17.1 | 0.86 (0.38–1.91) |
| Other infectionsb | 2/22.2 | 8/21.9 | 0.25 (0.05–1.16) | 2/17.1 | 5/17.1 | 0.40 (0.08–2.06) |
| Noninfectionsc | 3/22.2 | 7/21.9 | 0.42 (0.11–1.64) | 6/17.1 | 4/17.1 | 1.51 (0.43–5.37) |
| 8–28 days after randomization | ||||||
| Sepsis | 8/55.1 | 10/53.8 | 0.76 (0.29–1.96) | 6/99.7 | 13/98.7 | 0.46 (0.17–1.20) |
| Other infectionsb | 5/55.1 | 6/53.8 | 0.82 (0.25–2.68) | 2/99.7 | 5/98.7 | 0.39 (0.08–2.03) |
| Noninfectionsc | 8/55.1 | 6/53.8 | 1.27 (0.44–3.62) | 9/99.7 | 11/98.7 | 0.81 (0.32–2.02) |
Estimates with P < .05 are highlighted in bold.
Abbreviations: BCG, Bacille Calmette-Guérin; PY, person years.
10 verbal autopsies were not conducted due to the respective families having moved outside the catchment area before the verbal autopsy visit.
The category ‘Other infections’ consists of deaths due to fever, respiratory infections, gastrointestinal infections, infections in the umbilical cord, malaria, or HIV.
The category ‘Noninfections’ consists of deaths due to prematurity, anemia, congenital problems, kernicterus, sudden infant death syndrome, gastrointestinal hemorrhage, convulsions, or bleeding.
DISCUSSION
Using data from 3 RCTs of early BCG, the present analysis found a rapidly occurring protective effect on mortality in boys; the effect was strongest for boys in the first week after receiving BCG, whereas there was little or no effect in the remainder of the neonatal period. This pattern differed significantly from that seen in girls, who had the strongest beneficial effect in weeks 2–4.
Strength and Weaknesses
The time course of the protective effect of BCG on mortality has not been examined previously. All 3 RCTs showed a strong early beneficial effect of BCG in boys, a pattern different from that seen in girls.
The loss to follow-up was very low and only 0.6% of the children did not have complete follow-up in the neonatal period. Therefore, we do not believe that deaths were underreported in any group. The randomization was not blinded to the trial team, as the vaccinations were registered on the children’s health cards. This was done to ensure that control mothers would return to the health centers to get their children vaccinated with BCG when they had gained weight. The staff members working on the 3 trials were not involved in providing health care for study children, and health care providers at the hospital and health centers were not aware of the trials. It is therefore unlikely that lack of blinding affected subsequent treatment.
Biological Mechanisms
The rapid protective effect of BCG vaccination on all-cause mortality in humans was first described in connection with the present trials [3–5]. A study in adult mice found that BCG administered 24 hour prior to challenge with Klebsiella pneumoniae significantly improved survival, and a protective effect was also seen when BCG was administered 1 hour after the challenge [7]. The rapid onset of these effects suggests that innate immune mechanisms are involved. The innate immune system can be stimulated nonspecifically, resulting in increased protection against unrelated infections, a phenomenon that has been called trained innate immunity [8]. Innate training effects of BCG has recently been demonstrated in healthy adult volunteers, in whom BCG was associated with increased proinflammatory in vitro cytokine responses to unrelated pathogens 2 weeks and 3 months after vaccination. Mechanistic studies showed that this was due to epigenetic reprogramming of monocytes [9]. In severe combined immune deficiency (SCID) mice, lacking an adaptive immune system, BCG was associated with no mortality after challenge with a Candida infection which was otherwise lethal in nonvaccinated mice [9]. These effects were not analyzed by sex. We have recently shown, in an immunological study of a subgroup of the infants enrolled in Trial 3, that BCG was associated with increased proinflammatory in vitro cytokine responses to innate agonists 4 weeks after vaccination [10], consistent with the findings from the above mechanistic study in adults. Analyzed by sex, the effect estimates were larger in girls than in boys for most cytokine responses. The stronger immunological effect in girls 4 weeks after early BCG is consistent with the findings from the present study that 2–4 weeks after BCG vaccination there is a greater beneficial effect in females than males.
In the first week after vaccination, BCG decreased the high mortality in boys to a level comparable to that of girls. The sex-differential and time-dependent effects were particularly evident for sepsis-related deaths: BCG decreased sepsis-related mortality in boys within the first week after vaccination, with no immediate effect in girls, whereas a protective effect against sepsis-related mortality in girls was seen after the first week. It is well known that newborn boys experience infection-related disease and death more frequently than girls [11, 12]. The dissimilarity may arise from inherent differences in the immune function between boys and girls. However, very few studies have compared immune function in newborn boys and girls. High levels of interleukin-1 (IL-1) receptor antagonist in umbilical cord blood from preterm infants were associated with adverse outcome in girls but not in boys [13]. After uncomplicated pregnancies, cord blood responses to lipopolysaccharide stimulation were higher in boys than in girls for IL-1β and IL-6, but not tumor necrosis factor-alpha (TNF-α) [14]. The proportion of CD4 T cells in cord blood is higher and the CD4 and CD8 T cells express more Fas in girls compared with boys [15], indicative of lower apoptotic potential in boys. Although such sex-differences in early life immune status have been reported, we do not know whether they are related to the sex-differential timing of the beneficial effect of BCG.
Implications
Even though the World Health Organization (WHO) recommends BCG at birth to all infants, BCG is often administrated much later. Less than 50% receive BCG during the neonatal period in Africa [16]. There is no focus on delivering BCG very early and there is an inherent flaw in the health infrastructure to provide BCG early; for example, many maternity wards do not provide BCG before discharge and though WHO recommend home visits to newborn children these visits do not include vaccinations. Furthermore, there has been a counterproductive emphasis on reducing wastage of vaccine doses; for BCG, this implies that a 20-dose vial is not opened for just 1 child and BCG is often given only on specific days to assure that at least 10–12 children can be vaccinated [17]. Furthermore, some countries do not to give BCG at birth to LW infants. If BCG can substantially reduce neonatal mortality, it is important that WHO strongly encourage countries to follow their recommendations and provide BCG to all children immediately after birth, including LW children. Early administration of BCG should be part of the delivery services because that is where the first contact occurs for many children in low-income countries. Because boys have much higher mortality than girls in the first week of life, it is particularly important that boys receive BCG at birth. Receiving a BCG vaccine at birth might reduce the early mortality among boys to the same level as that of girls. Given the efficacy of BCG in reducing neonatal mortality it would be cost effective to open a vial of BCG even if only 1 child was present.
WHO and other policy makers should take this rapidly occurring effect of BCG into account when designing vaccination schedules. For example, if a new tuberculosis vaccine is introduced to replace BCG, the new vaccine should be evaluated not only for specific protection against tuberculosis but also for its nonspecific effect on neonatal mortality. The best option might well be to introduce a future tuberculosis vaccine for its effect against tuberculosis and maintain BCG at birth as an early “immune training” vaccine.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Transparency declaration. The first author affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted and that any discrepancies from the study as planned have been explained.
Author contributions. P. A. and C. S. B. conceived and designed the BCG trials. S. B. S., I. M., and K. J. J. supervised the field data collection. S. B. S. analyzed the data. H. R. supervised the data analysis. S. B. S. wrote the first draft of the manuscript. All authors contributed to the final version of the manuscript.
Acknowledgments. We thank the mothers and children who participated in the study.
Financial support. Trials 1 and 2 were supported by the European Union (grant number ICA4-CT-2002-10053), the March of Dimes, and the Danish National Research Foundation. Trial 3 was supported by an European Research Council (ERC) Starting Grant (grant number ERC-2009-StG-243149) that also funds S. B. S. and C. S. B. The Danish National Research Foundation (grant number DNRF108) supports the Research Center for Vitamins and Vaccines. P. A. held a research professorship grant from the Novo Nordisk Foundation. The work on nonspecific effects of vaccines has been supported by the Danish Council for Development Research, Ministry of Foreign Affairs, Denmark (grant number 104.Dan.8.f.), and European Union FP7 support for OPTIMUNISE (grant number Health-F3-2011-261375).
Potential conflicts of interest. All authors have completed the ICMJE uniform disclosure form at http://www.icmje.org/coi_disclosure.pdf and declare: no support from any organization for the submitted work. The salaries for all authors are administered, but not financed, by SSI, the producer of the BCG vaccine SSI used in the present study; no other relationships or activities reported that could appear to have influenced the submitted work. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Kristensen I, Aaby P, Jensen H. Routine vaccinations and child survival: follow up study in Guinea-Bissau, West Africa. BMJ 2000; 321:1435–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Roth A, Jensen H, Garly ML et al. . Low birth weight infants and Calmette-Guérin bacillus vaccination at birth: community study from Guinea-Bissau. Pediatr Infect Dis J 2004; 23:544–50. [DOI] [PubMed] [Google Scholar]
- 3. Aaby P, Roth A, Ravn H et al. . Randomized trial of BCG vaccination at birth to low-birth-weight children: beneficial nonspecific effects in the neonatal period?J Infect Dis 2011; 204:245–52. [DOI] [PubMed] [Google Scholar]
- 4. Biering-Sorensen S, Aaby P, Lund N et al. . Early BCG-Denmark and neonatal mortality among infants weighing <2500 g: a randomized controlled trial. Clin Infect Dis 2017; 657:1183–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Biering-Sørensen S, Aaby P, Napirna BM et al. . Small randomized trial among low-birth-weight children receiving bacillus Calmette-Guérin vaccination at first health center contact. Pediatr Infect Dis J 2012; 31:306–8. [DOI] [PubMed] [Google Scholar]
- 6. Benn CS, Fisker AB, Napirna BM et al. . Vitamin A supplementation and BCG vaccination at birth in low birthweight neonates: two by two factorial randomised controlled trial. BMJ 2010; 340:c1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chedid L, Parant M, Parant F, Lefrancher P, Choay J, Lederer E. Enhancement of nonspecific immunity to Klebsiella pneumoniae infection by a synthetic immunoadjuvant (N-acetylmuramyl-L-alanyl-D-isoglutamine) and several analogs. Proc Natl Acad Sci U S A 1977; 74:2089–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Netea MG, Quintin J, van der Meer JW. Trained immunity: a memory for innate host defense. Cell Host Microbe 2011; 9:355–61. [DOI] [PubMed] [Google Scholar]
- 9. Kleinnijenhuis J, Quintin J, Preijers F et al. . Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci U S A 2012; 109:17537–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jensen KJ, Larsen N, Biering-Sørensen S et al. . Heterologous immunological effects of early BCG vaccination in low-birth-weight infants in Guinea-Bissau: a randomized-controlled trial. J Infect Dis 2015; 211:956–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Neubauer V, Griesmaier E, Ralser E, Kiechl-Kohlendorfer U. The effect of sex on outcome of preterm infants—a population-based survey. Acta Paediatr 2012; 101:906–11. [DOI] [PubMed] [Google Scholar]
- 12. Watson RS, Carcillo JA, Linde-Zwirble WT, Clermont G, Lidicker J, Angus DC. The epidemiology of severe sepsis in children in the United States. Am J Respir Crit Care Med 2003; 167:695–701. [DOI] [PubMed] [Google Scholar]
- 13. Elsmén E, Ley D, Cilio CM, Hansen-Pupp I, Hellstrom-Westas L. Umbilical cord levels of interleukin-1 receptor antagonist and neonatal outcome. Biol Neonate 2006; 89:220–6. [DOI] [PubMed] [Google Scholar]
- 14. Kim-Fine S, Regnault TR, Lee JS et al. . Male gender promotes an increased inflammatory response to lipopolysaccharide in umbilical vein blood. J Matern Fetal Neonatal Med 2012; 25:2470–4. [DOI] [PubMed] [Google Scholar]
- 15. Wasiluk A, Ratomski K, Wnuczko K et al. . Expression of FasR, Fas-L and Bcl-2 in CD4+ and CD8+ subpopulations of T lymphocytes in the cord blood of healthy full-term newborns, is gender of influence?Adv Med Sci 2009; 54:99–103. [DOI] [PubMed] [Google Scholar]
- 16. Clark A, Sanderson C. Timing of children’s vaccinations in 45 low-income and middle-income countries: an analysis of survey data. Lancet 2009; 373:1543–9. [DOI] [PubMed] [Google Scholar]
- 17. Fisker AB, Hornshøj L, Rodrigues A et al. . Effects of the introduction of new vaccines in Guinea-Bissau on vaccine coverage, vaccine timeliness, and child survival: an observational study. Lancet Glob Health 2014; 2:e478–87. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.