This study shows that the immune response elicited by a Japanese encephalitis chimeric virus vaccine (JE-CV) booster in JE-CV–primed children in the Philippines induces long-lasting protection, and further supports the benefit of a booster irrespective of when primary vaccination was administered.
Keywords: JE-CV, IMOJEV, live attenuated Japanese encephalitis vaccine, Philippines, Japanese encephalitis
Abstract
We assessed antibody persistence following booster vaccination with a Japanese encephalitis chimeric virus vaccine (JE-CV; IMOJEV) in JE-CV–primed children. In an open phase 3 trial, 349 children in the Philippines, who received JE-CV 2 years previously, received a booster dose. JE neutralizing antibody titers were assessed (50% plaque reduction neutralization test) annually for up to 5 years after booster vaccination. Seroprotection rates (percentage of children with titers ≥10 [1/dil]) and geometric mean titers (GMTs) were, respectively, 98.2% and 161 after 5 years. JE-CV booster induced long-lasting anamnestic immune response in JE-CV–primed children, with high seroprotection rates and GMTs over the accepted threshold for serological protection (10 [1/dil]).
Clinical Trials Registration
Japanese encephalitis (JE) circulates throughout the Philippines where it is an important cause of encephalitis, with similar epidemiological characteristics to other endemic countries in Asia [1]. There were 1032 suspected cases in the country between 2011 and 2014, and of 497 cases that were tested, 73 were confirmed JE positive [1]. Higher numbers have since been reported: 133 confirmed cases nationwide as of 26 August 2017 [2]. There is currently no JE immunization program in the Philippines, though a subnational vaccination campaign is planned for 2018, followed by routine introduction nationally [3]. Elsewhere in Asia, the introduction of JE vaccination (with inactivated mouse brain–derived JE vaccine [MBDV]) into the Thai national immunization program in 1990 led to a significant reduction in encephalitis cases, from 1500–2500 annually between the 1970s and 1980s to 297–418 annually between 2002 and 2008 [4].
The JE chimeric virus vaccine (JE-CV) is licensed for the prevention of JE in children and adults in 14 countries. The vaccine was launched in the Philippines in 2015 for use in individuals aged ≥9 months. A booster dose 12–24 months after primary vaccination is recommended in pediatric populations to confer long-term protection; however, a robust immune response is observed even if the booster is administered after a 5-year interval [5, 6]. We previously reported the immunogenicity and safety of a single JE-CV dose in JE vaccination–naive toddlers (12–18 months) and a booster vaccination 2 years later [5, 7]. Here, we report antibody responses 5 years later.
METHODS
Study Design and Participants
We assessed JE antibody persistence for up to 5 years following a booster dose of JE-CV (IMOJEV, Sanofi Pasteur, Mérieux Biological Products Co, Ltd, Thailand) in children aged 36–42 months who received a single JE-CV dose 2 years earlier as part of a randomized, controlled phase 3 immunogenicity and safety trial undertaken in Thailand and the Philippines [7]. The composition and manufacture of JE-CV has been described previously [8]. Children vaccinated with JE-CV at 3 sites in the Philippines were subsequently recruited into this study and vaccinated with a JE-CV booster dose as part of a larger open- label, controlled phase 3 trial comparing the immunogenicity and safety of the booster dose with a single JE-CV primary vaccination dose and another control vaccine (Varicella). The study methodology and initial assessments were presented elsewhere, for up to 1 year (Y1) postvaccination (booster group) and up to day 28 (D28) for the 2 control groups [5]. Only participants who received a JE-CV booster were included in the current long-term follow-up assessment.
The study was carried out in accordance with the Declaration of Helsinki and the International Conference on Harmonisation guidelines for Good Clinical Practice. The trial protocol was approved by the institutional/ethical review boards of the Research Institute for Tropical Medicine in the Philippines. Signed informed consent was received from the parents or participant’s legally acceptable representatives.
Procedures and Assessments
Blood samples for immunogenicity assessment were taken at the first study visit (V01) before receipt of the booster JE-CV dose (day 0 [D0]), D7 and D28 after, and then annually from Y1 through to Y5 as described previously [5]. A physical examination was performed and axillary temperature measured at all yearly follow-up visits. Participants’ parents or legally acceptable representative were instructed to inform the study site if participants developed severe (grade 3) fever. Serious adverse events (SAEs) considered related to vaccination as assessed by the study investigators and deaths were reported over the 5-year follow-up period.
JE-CV neutralizing antibody titers were measured with the 50% plaque reduction neutralization test (PRNT50; Focus Diagnostics Inc) using the JE-CV virus as the challenge virus. The lower limit of quantitation for the test was 10 [1/dil], corresponding to the accepted threshold for seropositivity and the correlate of protection against JE [9]. No serological assays were performed during the follow-up to detect subclinical infections.
Statistical Methods
The study sample was a subset of participants (maximum 400) from the previous trial in 3 selected sites in the Philippines, who received a single dose of JE-CV and were eligible for a JE-CV booster dose [5]. All analyses were descriptive, without hypothesis testing. Geometric mean titers (GMTs) and 95% confidence intervals (CIs) were calculated on log10 titers (assuming the log10-transformed titers followed a normal distribution) using the usual calculation for normal distribution, and then antilog transformations were applied to provide the results on the original scale. Proportions and their 95% CIs were calculated using the exact binomial distribution. The Kaplan–Meier method was used to estimate the proportion of participants maintaining antibody titers ≥10 from D28 postbooster. Participants with missing titers at a scheduled visit and who were found to be seroprotected at the next visit were assumed to have remained seroprotected, but those subsequently found not to be seroprotected were assumed to have lost seroprotection prior to the previous visit with the missing titer value. Participants who completed or withdrew from the study while seroprotected were censored at the time of their last visit. An additional time-to-event analysis was undertaken with interval-censor data, estimated using the methods of So et al, based on the expectation-maximization algorithm [10]. Immunogenicity analyses were performed on the full analysis set (FAS), which included all participants present at the first study visit and who received a dose of JE-CV.
RESULTS
Study Population
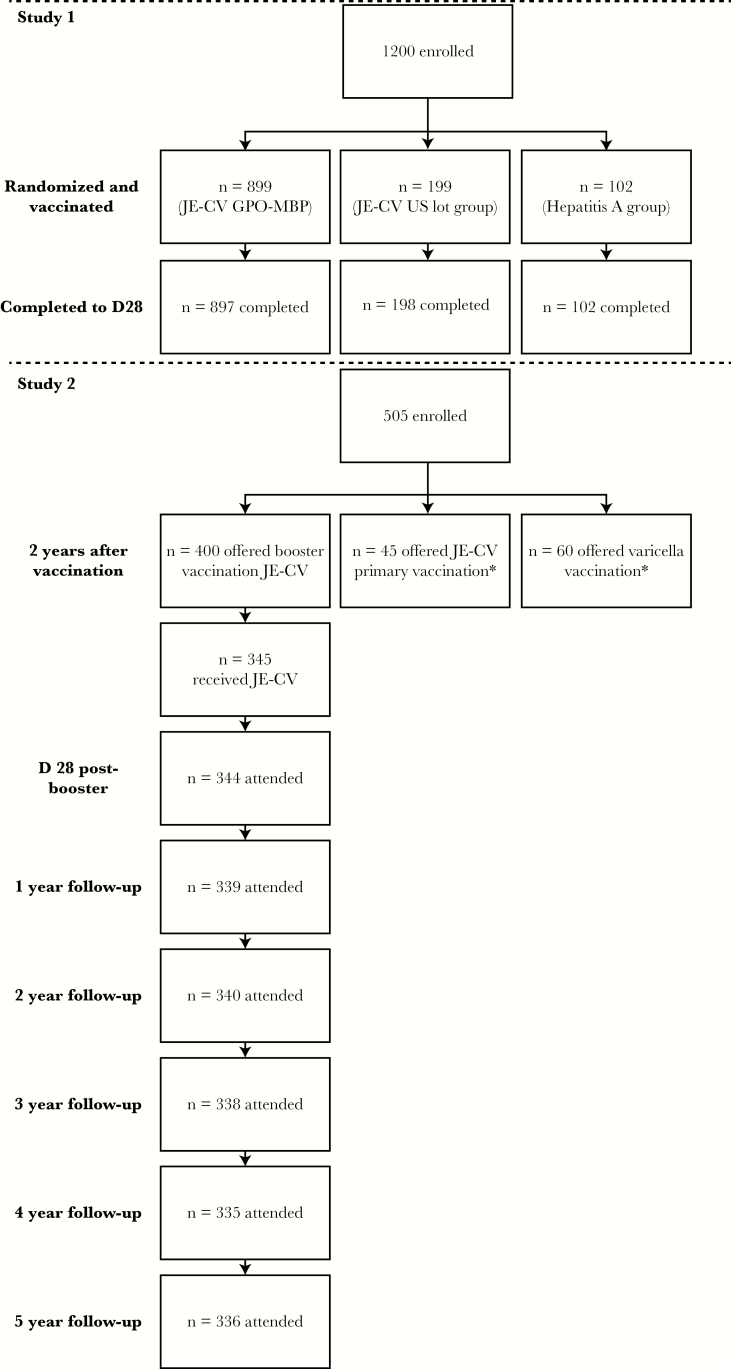
Of the 349 children previously vaccinated with a single dose of JE-CV enrolled into this study, 345 received the second dose of JE-CV; 4 were excluded from further participation because they had a history of febrile convulsions/seizures. Baseline characteristics of the participating children were presented previously [5]. All children were Asian, had a mean age of 39.6 (standard deviation [SD], 1.71) months and a body mass index of 15.3 (SD, 1.75) kg/m2, with a similar proportion of males and females. Overall, 339, 340, 338, 335, and 336 children attended the Y1, Y2, Y3, Y4, and Y5 follow-up visits, respectively, and had blood samples taken. Reasons for discontinuations in the first year were 3 voluntary withdrawals not due to an AE and 2 losses to follow-up; 1 child did not attend the Y1 follow-up visit but continued with the rest of the study. Children who discontinued between Y2 and Y5 visits were lost to follow-up (Figure 1).
Figure 1. .
Disposition of participants. *Feroldi et al [5]. Abbreviations: D, day; GPO-MBP, Government Pharmaceutical Organization–Mérieux Biological Products; JE-CV, Japanese encephalitis chimeric virus vaccine; n, number of participants; US, United States.
Persistence of the Immune Response
As previously reported, all children assessed at D28 who received the second dose of JE-CV were seroprotected [7] (Table 1). Seroprotection persisted for up to Y5 in almost all children (98.2% [95% CI, 96.2%–99.3%]). Only 6 children had neutralizing antibody titers <10 at Y5 (FAS, Table 1).
Table 1.
Seroprotectiona Rates Pre- and Post-booster Vaccination with Japanese Encephalitis Chimeric Virus Vaccine up to Year 5 in the Full Analysis Set (N = 345)
| Timepoint | No. | Seroprotection Rate, % (95% CI) |
|---|---|---|
| Pre-vaccination (D0)b | 345 | 80.3 (75.7–84.4) |
| D7b | 345 | 96.2 (93.6–98.0) |
| D28b | 344 | 100.0 (98.9–100.0) |
| Y1b | 339 | 99.4 (97.9–99.9) |
| Y2 | 340 | 98.8 (97.0–99.7) |
| Y3 | 338 | 99.1 (97.4–99.8) |
| Y4 | 335 | 98.2 (96.1–99.3) |
| Y5 | 336 | 98.2 (96.2–99.3) |
Abbreviations: CI, confidence interval; D, day post–Japanese encephalitis chimeric virus vaccine booster unless otherwise stated; No., number of participants with available data; Y, year post–Japanese encephalitis chimeric virus vaccine booster.
aJapanese encephalitis virus 50% plaque reduction neutralization test titers ≥10.
bPreviously published data for seroprotection prior to booster vaccination are shown for these time points [5].
As previously shown [5], JE-CV neutralizing antibody GMTs increased between D0 and D28 after the second JE-CV dose (GMTs for FAS, 39.3 [95% CI, 33.7–45.8] at D0, 233 [95% CI, 193–281] at D7, and 2259 [95% CI, 1930–2645] at D28), decreasing to 596 (95% CI, 502–708) at Y1. GMTs declined more gradually throughout the rest of the study to 161 (95% CI, 141–184) at Y5 (Supplementary Figure 1).
The Kaplan–Meier estimate for remaining seroprotected from D28 to Y5 was 96.5% (95% CI, 93.8%–98.0%); interval-censored data yielded identical results.
Safety
There were no SAEs considered related to vaccination or deaths reported during the 5-year follow-up period. Additionally, no signs or symptoms of JE were reported.
DISCUSSION
We previously showed that a single JE-CV dose was associated with strong immunogenicity in JE vaccination–naive children (seroconversion rate after 28 days, 95%) [7]. In addition, a JE-CV booster 2 years later in JE-CV–primed children elicited a strong anamnestic antibody response [5]. In the current analysis, this response persisted in almost all children for up to 5 years; neutralizing antibody GMTs remained above the threshold for seroprotection in 98.2% of children. These results are consistent with long-term persistence of neutralizing antibody responses following JE-CV booster vaccination in children who received primary vaccination with MBDV in Thailand [11].
The immune response elicited by the JE-CV booster does not appear to be dependent on the JE vaccine administered during primary vaccination, nor on the interval between primary and booster. The magnitude of response after JE-CV booster in our study was similar to JE-CV booster responses observed in other studies following primary vaccination with JE-CV or other JE vaccines, even when the booster was administered up to 5 years later [12, 13]. Thus, a long-lasting JE-CV booster antibody response would be expected irrespective of the previous JE vaccine. Furthermore, compared with a single primary dose of JE-CV, the booster provides higher residual seroprotection rate at Y5 [11]. A single primary dose of JE-CV in JE vaccine–naive toddlers gave seroprotection rates of 58.8% (95% CI, 50.9%–66.4%) at Y5 in the FAS [11], compared with 98.2% (95% CI, 96.2%–99.3%) 5 years after booster in our study. The lower seroprotection rates following primary vaccination support a timely JE-CV booster dose in maintaining high seroprotection rates among children.
The JE-CV booster response observed in our study by D28 was >10-fold higher than the response 28 days following primary vaccination with JE-CV (GMT, 214 [95% CI, 168–271]) [7]. Similarly, the D28 response following JE-CV booster in MBDV–primed children (GMT, 2634) was approximately 10-fold higher than the JE-CV primary response in a separate cohort of JE vaccine–naive toddlers (GMT, 281) [13]. Five years following the JE-CV booster in our study, the GMTs decreased to 161. Previously reported GMTs 5 years following JE-CV booster in MBDV–primed children decreased to 252 [11], whereas much lower GMTs were reported in 2 separate studies 5 years after a single JE-CV primary vaccination dose in JE vaccine–naive toddlers (GMTs, 63.4 [6] and 63.8 [FAS, 26.7] [6, 11, 13]), albeit above the threshold for protection in the majority of children [6, 11].
In JE-CV–primed children, who subsequently became seronegative prior to boosting, a JE-CV booster elicited an anamnestic antibody response with GMTs quickly rising to level much higher than the initial response [5]. Therefore, it is reasonable to assume that those few participants who become seronegative over the longer term following a primary and/or booster vaccination would be able to mount an anamnestic response to natural infection that may be sufficiently quick to confer protection from disease. Similar to yellow fever vaccination, it is therefore conceivable that a single dose of JE-CV might confer lifelong protection against symptomatic JE disease based on this rapid anamnestic response, even in participants in whom the antibody titers drop below the threshold for protection.
Previous analyses suggest that the JE virus may be undergoing a genotype shift in Southeast Asia [14]. The neutralization of wild-type JE virus isolates from the main 4 genotypes circulating in Southeast Asia and India has been evaluated using serum samples from children up to 5 years after receiving a JE-CV booster. Neutralizing GMTs against wild-type isolates were found to follow a pattern over time similar to that observed with the JE-CV virus in our study, with rates of seroprotection against wild-type viruses remaining high and similar for the different genotypes tested, including genotypes isolated in Thailand and in Asia between 1997 and 2005 [15]. Thus, seroprotection across JE genotypes exists.
It is not clear to what extent exposure to wild-type JE contributed to antibody persistence in the absence of an appropriate comparator. Although no JE disease was reported in our study, we cannot exclude the possibility that asymptomatic infections by wild-type JE virus, or a different, cross-reactive flavivirus, contributed to the results. To our knowledge, such boosting of JE antibody responses by a putative cross-reactive flavivirus has not been confirmed. It remains to be established whether wild-type symptomatic and asymptomatic infections contribute differentially to the persistence of JE neutralizing antibodies. In addition, the decay in vaccine-induced antibody titers may not be quantitatively similar to the decay of JE antibodies acquired following wild-type infection.
In conclusion, our findings show that the immune response elicited by a JE-CV booster dose in JE-CV–primed children induces long-lasting protection, further supporting the benefit of a JE-CV booster irrespective of the interval between primary and booster vaccination.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the infants and their parents for their participation. Authors also acknowledge the investigational staff at the Research Institute for Tropical Medicine (RITM), Muntinlupa City, Philippines and the Health Centers in Alabang, Buli, and Cupang for their contribution to the organization of the study. Authors thank the following Sanofi Pasteur employees: Karen Privat for clinical program management, Mae Ann Verdan for study monitoring and logistics, Christel Guillaume for data management, Mark Boaz and Matthew Bonaparte for laboratory analyses, Agnès Machmer for her support in writing the study report; and Jean-Sébastien Persico for coordination of manuscript development. The manuscript was prepared with the assistance of Richard Glover (inScience Communications, Springer Healthcare, Chester, United Kingdom), funded by Sanofi Pasteur.
Financial support. This study was supported by Sanofi Pasteur. This manuscript was prepared with the assistance of Springer Healthcare, funded by Sanofi Pasteur.
Potential conflicts of interest. The study sponsor participated in operational aspects of the study, including data collection and statistical analyses. A. B., T. M. L., D. C., C. M., and E. F. are employees of Sanofi Pasteur. M. R. C., an employee of RITM, Philippines, received funding from Sanofi Pasteur to conduct the study. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Lopez AL, Aldaba JG, Roque VG Jr et al. Epidemiology of Japanese encephalitis in the Philippines: a systematic review. PLoS Negl Trop Dis 2015; 9:e0003630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rappler. DOH warns vs mosquito bites after 9 deaths from Japanese encephalitis https://www.rappler.com/nation/181371-doh-warning-mosquito-bites-japanese-encephalitis. Accessed 20 September 2017.
- 3. Heffelfinger JD, Li X, Batmunkh N et al. Japanese encephalitis surveillance and immunization—Asia and Western Pacific Regions, 2016. Morb Mortal Wkly Rep 2017; 66:579–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang H, Liang G. Epidemiology of Japanese encephalitis: past, present, and future prospects. Ther Clin Risk Manag 2015; 11:435–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Feroldi E, Capeding MR, Boaz M, Gailhardou S, Meric C, Bouckenooghe A. Memory immune response and safety of a booster dose of Japanese encephalitis chimeric virus vaccine (JE-CV) in JE-CV-primed children. Hum Vaccin Immunother 2013; 9:889–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kosalaraksa P, Watanaveeradej V, Pancharoen C, Capeding MR, Feroldi E, Bouckenooghe A. Long-term immunogenicity of a single dose of Japanese encephalitis chimeric virus vaccine in toddlers and booster response 5 years after primary immunization. Pediatr Infect Dis J 2017; 36:e108–13. [DOI] [PubMed] [Google Scholar]
- 7. Feroldi E, Pancharoen C, Kosalaraksa P et al. Single-dose, live-attenuated Japanese encephalitis vaccine in children aged 12-18 months: randomized, controlled phase 3 immunogenicity and safety trial. Hum Vaccin Immunother 2012; 8:929–37. [DOI] [PubMed] [Google Scholar]
- 8. Chambers TJ, Nestorowicz A, Mason PW, Rice CM. Yellow fever/Japanese encephalitis chimeric viruses: construction and biological properties. J Virol 1999; 73:3095–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hombach J, Solomon T, Kurane I, Jacobson J, Wood D. Report on a WHO consultation on immunological endpoints for evaluation of new Japanese encephalitis vaccines, WHO, Geneva, 2–3 September, 2004. Vaccine 2005; 23:5205–11. [DOI] [PubMed] [Google Scholar]
- 10. So Y, Johnston G, Kim SE.. Analyzing interval-censored survival data with SAS software, paper 257–2010. SAS Global Forum 2010. https://support.sas.com/resources/papers/proceedings10/257-2010.pdf. Accessed 12 December 2017. [Google Scholar]
- 11. Chokephaibulkit K, Sirivichayakul C, Thisyakorn U et al. Long-term follow-up of Japanese encephalitis chimeric virus vaccine: immune responses in children. Vaccine 2016; 34:5664–9. [DOI] [PubMed] [Google Scholar]
- 12. Janewongwirot P, Puthanakit T, Anugulruengkitt S et al. Immunogenicity of a Japanese encephalitis chimeric virus vaccine as a booster dose after primary vaccination with SA14-14-2 vaccine in Thai children. Vaccine 2016; 34:5279–83. [DOI] [PubMed] [Google Scholar]
- 13. Chokephaibulkit K, Sirivichayakul C, Thisyakorn U et al. Safety and immunogenicity of a single administration of live-attenuated Japanese encephalitis vaccine in previously primed 2- to 5-year-olds and naive 12- to 24-month-olds: multicenter randomized controlled trial. Pediatr Infect Dis J 2010; 29:1111–7. [DOI] [PubMed] [Google Scholar]
- 14. Pan XL, Liu H, Wang HY et al. Emergence of genotype I of Japanese encephalitis virus as the dominant genotype in Asia. J Virol 2011; 85:9847–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Feroldi E, Boaz M, Yoksan S et al. Persistence of wild-type Japanese encephalitis virus strains cross-neutralization 5 years after JE-CV immunization. J Infect Dis 2017; 215:221–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.