Abstract
The emergence and spread of human immunodeficiency virus (HIV) drug resistance from antiretroviral roll-out programs remain a threat to long-term control of the HIV-AIDS epidemic in low- and middle-income countries (LMICs). The patterns of drug resistance and factors driving emergence of resistance are complex and multifactorial. The key drivers of drug resistance in LMICs are reviewed here, and recommendations are made to limit their influence on antiretroviral therapy efficacy.
Keywords: HIV-1 drug resistance, HIV subtype, antiretroviral therapy failure
The last 20 years has seen a rapid expansion of antiretroviral (ARV) access, with approximately 18 million people receiving antiretroviral therapy (ART) as of 2016 [1]. In low- and middle-income countries (LMICs), first-line treatment regimens generally include 1 nonnucleoside reverse transcriptase inhibitor (NNRTI) and 2 nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs), with second-line regimens substituting a boosted protease inhibitor (PI) for the NNRTI and alternate NRTIs [2]. Factors related to specific ART regimens, prior drug exposure, the timing of ART initiation, and viral subtypes in LMICs are important for the development of resistance to commonly used drugs. This article reviews these factors with special emphasis on viral subtypes.
PRETREATMENT HUMAN IMMUNODEFICIENCY VIRUS DRUG RESISTANCE
In LMICs, pretreatment drug resistance is rising and ranges 1%–12.3% in different regions [3]. A meta-analysis of sequences from 50870 individuals initiating therapy found that pretreatment, especially NNRTI, resistance is increasing in sub-Saharan Africa [4]. There were 4 NNRTI mutations—K101E, K103N, Y181C, and G190A—that accounted for >80% of the pretreatment resistance across all HIV subtypes. In addition, 16 NRTI mutations accounted for >69% of the pretreatment resistance, but pretreatment resistance linked to PIs was low [3]. Similarly, in the Pan African Studies to Evaluate Resistance cohort, 5% of individuals starting standard first-line treatment had pretreatment resistance [5]. Importantly, individuals with pretreatment resistance to the regimen prescribed were more likely to fail therapy compared with those with no resistance (odds ratio [OR], 2.13; 95% CI, 1.44–3.14; P < .0001). Single-dose nevirapine (sdNVP) for prevention of mother-to-child transmission of HIV may result in pretreatment drug resistance. The Optimal Combination Therapy after Nevirapine Exposure study examined outcomes following first-line treatment in women with or without sdNVP exposure before starting a nevirapine (NVP)–based first-line regimen [6, 7]. The frequency of NNRTI resistance prior to treatment initiation was 45% in the sdNVP-exposed group and 18% in the group that had no prior sdNVP exposure, and ART failure was more likely in the women with NNRTI resistance and prior sdNVP exposure. Similarly, prior exposure to RTV or indinavir increases PI cross-resistance and probability of failure and drug resistance from lopinavir/ritonavir (LPV/r) in both children and adults [8], and prior exposure to integrase strand transfer inhibitor exposure increases the chance of failure and drug resistance to dolutegravir [9]. Pretreatment resistance can fade over time and form minority variants, which can have an impact on outcome (covered in separate articles in this supplement). These findings highlight the need for cost-effective strategies to assess drug resistance prior to treatment initiation [10] or to change the initial treatment strategy [11].
TIMING OF TREATMENT INITIATION AND TREATMENT MONITORING
Individuals who initiate treatment with CD4 cell counts >500 cells/mm3 have better first-line outcomes compared with those with CD4 cell counts <350 cells/mm3 [12]. Several lines of evidence support the notion that delayed therapy is associated with higher viral diversity and increased viral failure [13, 14]. Cohen et al [14, 15] found that there was an increased chance of experiencing viral failure on rilpivirine (RPV) and efavirenz (EFV) when starting treatment with a viral load >100000 RNA copies/mL. A combined analysis of the TMC278 against HIV, in a once-daily regimen versusefavirenz and efficacy comparison in treatment-naive, HIV-infected subjects of TMC278 and efavirenz studies found that there was a 4.4% difference in response rate in individuals with a starting viral load of 100000 RNA copies/mL versus 500000 RNA copies/mL and an 8% increase when the starting viral load was >500000 RNA copies/mL [16]. The World Health Organization (WHO) has recommended treatment for all HIV-infected individuals, but uptake of this recommendation is incomplete, resulting in delayed initiation of ART [17].
A major driver of the development of HIV drug resistance is the way treatment is monitored. Plasma HIV RNA (viral load) monitoring scale-up is incomplete (reviewed in this issue), and clinical and immunological indicators are often used to determine treatment success. Multiple studies show that the longer an individual is left on a failing regimen the more complex the resistance profile [18], with 1 study showing accumulation of drug-resistance mutations at a rate of 1.45 per year after first virologic failure, resulting in declining drug susceptibility after continued failure [19]. Table 1 shows the progression of resistance as viral load monitoring thresholds are made less stringent or not used at all and the time to switching increases.
Table 1.
Comparison of Large Resistance Datasets Showing Higher Resistance Mutation Frequency and Complex Resistance Mutations Profiles as Individuals Are Left on a Failing Regimen for Longer Time
| Site | Malawi Lilongwe [60] |
South Africa Cape Town [61] |
South Africa Johannesburg [31] |
South Africa Durban [62] |
South Africa CIPRA-SA [63] |
|---|---|---|---|---|---|
| Sample size | 96 | 110 | 226 | 115 | 67 |
| Clinical sites | 1 | 1 | 2 | 2 | 2 |
| Switch criteria | Clinical or immunological | HIV RNA >5000 copies/mL | HIV RNA >5000 or 1000 copies/mL | HIV RNA >1000 copies/mL | HIV RNA >1000 copies/mL |
| Frequency of monitoring | Not applicable | 6 monthly HIV RNA and CD4+ T cell | 6 monthly HIV RNA and CD4+ T cell | 6 monthly HIV RNA and CD4+ T cell | 3 monthly HIV RNA and CD4+ T cell |
| % with failure and resistance | 95% | 85% | 83% | 83.5% | 82% |
| M184V/I | 81% | 78% | 72% | 64.3% | 67.2% |
| NNRTI | 93% | 86% | 78% | Unknown | 75% |
| K103N | 28% | 55% | 38% | 51% | 50% |
| V106M | 7% | 31% | 17% | 19% | 14% |
| >3 TAMS | 44% | 23% | 11% | 32.2% | 1.5% |
| K65R | 19% | 9% | 4.5% | 2.6% | 3% |
| Q151M | 19% | Not reported | 2.5% | 0.9% | 0% |
| NRTI+NNRTI | 91% | 83% | 73% | 64.3% | 63% |
Abbreviations: 3TC, lamivudine; AZT, zidovudine; d4T, stavudine; EFV, efavirenz; FTC, emtricitabine; NNRTI, nonnucleoside reverse transcriptase inhibitor; NVP, nevirapine; TAMs, thymidine analog mutations; TDF, tenofovir.
ANTIRETROVIRAL THERAPY ADHERENCE
Adherence to ART is a key contributor to treatment success, and the “partially” adherent individual is the most vulnerable to developing resistance [20]. Inadequate medication exposure leads to selection pressure, which increases the chance of developing resistance. Intermittent adherence of ARVs with different half-lives results in periods of monotherapy and consequent development of resistance. Suboptimal dosing in growing children and poor tolerance of specific ARVs are important factors in inadequate exposure [21, 22]. There is no consensus on the optimal way to measure adherence and ARV exposure. Self-reported and clinic-based pill counts are poor indicators of adherence compared with electronic drug monitoring, therapeutic drug monitoring, and pharmacy-based calculations [23]. Measures to improve adherence have focused on both patient and ARV factors. Sex and age have been closely linked to treatment success, with females and those of increased age having better adherence and less resistance. Studies of specific adherence support measures have yielded conflicting results, and studies are ongoing to test a variety of electronic interventions [24, 25]. In studies of second-line ART regimens, participants entering with a high level of resistance to first-line treatment had better outcome compared with those with no resistance, possibly indicating that better adherence to first-line treatment was a marker of second-line treatment adherence and success [26–28]. Fixed-dose, single tablet regimens have been shown to improve adherence over multipill regimens and also reduce the development of resistance [29], and long-acting formulations may provide an added advantage in the future. Developing tools that monitor and aid adherence is an active area of research, clearly needed to control HIV drug resistance levels in LMICs.
ANTIRETROVIRAL THERAPY REGIMEN
Certain medication combinations rather than their individual components may also increase the risk of failure and drug resistance. Tang et al [30] provided interesting evidence that the risk sum of a regimen is more than its parts. Specifically, K65R emerged frequently with tenofovir (TDF)/lamivudine (3TC)/NVP, less frequently with TDF/emtricitabine (FTC)/NVP or TDF/3TC/EFV, and rarely with TDF/FTC/EFV. Thus, all first-line regimens, despite consisting of ARVs from the same drug classes, are not equal, and important drug–drug and mutational interactions that drive success or failure need to be better understood. Monitoring the virologic outcomes following roll-out of related but different regimens should be performed at the population level to identify subtler differences in regimen efficacy that may be missed in smaller clinical trials.
HUMAN IMMUNODEFICIENCY VIRUS SUBTYPE
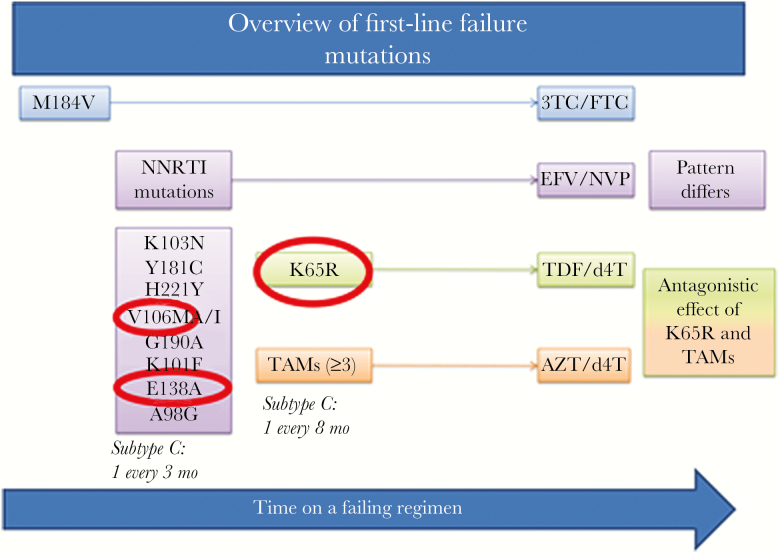
During first-line ART, the mutational signature that develops is linked both to the type of ART prescribed and the HIV subtype (Figure 1). Each of these influences is described herein.
Figure 1.
Overview of mutations linked to first-line failure. Generally, the first mutation to develop is the M184V mutation linked to 3TC/FTC exposure, followed concurrently by nonnucleoside reverse transcriptase inhibitor mutations at a rate of an additional 1 mutation for every 3 months on a failing regimen and nucleoside/nucleotide reverse transcriptase inhibitor (NRTI) mutations. The NRTI mutation patterns depends on the NRTI used. Mutations circled in red have been associated with subtype C. Abbreviations: 3TC, lamivudine; AZT, zidovudine; d4T, stavudine; EFV, efavirenz; FTC, emtricitabine; NNRTI, nonnucleoside reverse transcriptase inhibitor; NVP, nevirapine; TAMs, thymidine analog mutations; TDF, tenofovir.
Stavudine and Tenofovir
In HIV subtype C, an increase in frequency of K65R after stavudine (d4T) exposure [31] or TDF exposure [32] has been observed. Unlike subtype B, for which there was no K65R observed when individuals were exposed to d4T, in subtype C, 4.5% of individuals experiencing viral failure after first-line treatment in South Africa had the K65R mutation [31]. When d4T was replaced by TDF, very high levels of K65R (69%) were observed in this subtype. More frequent development of the K65R mutation may be a result of subtype C nucleotide sequence difference, and/or a delay in treatment switch, and/or combination of d4T and TDF treatment [33]. Analysis of sequences from the SELECT study, made up of predominantly subtypes C, D, and A1, found that K65R occurred in 22% (n = 107) of participants, and when divided by NRTI was 2% (n = 5) in those treated with zidovudine (AZT)/d4T; 70% (n = 63) in those treated with TDF; and 38% (n = 39) in those treated with both TDF and AZT/d4T [34]. Association of mutation by subtype in the SELECT study was confounded by small non-subtype C numbers; however, other studies have found K65R to occur significantly more in subtype C than in other subtypes [35, 36].
Nevirapine versus Efavirenz
The mutational signatures of NVP and EFV differ even though there is a considerable amount of cross-resistance between the 2 ARVs. Efavirenz selects a wider range of mutations compared with NVP, and the mutations selected by both NNRTIs are influenced by subtype [31, 37]. For example, the Y181C mutation is more commonly selected by NVP compared with EFV, and the subtype C–specific V106M mutation [38, 39] is more often selected by EFV (34%) than NVP (2%) [31]. K103N occurs at a greater frequency and in higher levels in women with subtypes C and D rather than subtype A [40]. Sluis-Cremer et al [41] reported that E138A naturally occurs in subtype C compared with subtype B and that E138K or E138Q are more common in treatment-experienced subtype C sequences (1.0% and 1.1%, respectively) than in subtype B sequences (0.3% and 0.6%, respectively). Phenotyping showed that E138A/K/Q in subtype C decreased RPV susceptibility 2.9-, 5.8-, and 5.4-fold, respectively. These observations suggest that E138A could impact treatment or prevention strategies that contain NNRTIs in geographic areas where subtype C infection is prevalent; further investigation is required. The different mutation profiles caused by either NVP or EFV usage and subtype may impact the success of second-generation NNRTIs (eg, etravirine) in future third-line or salvage regimens.
Phenotypic Analysis
Two studies have examined the impact of HIV-1 subtype C on phenotypic resistance and genotype interpretation algorithms that were developed based on subtype B datasets [42–44]. These results showed that although the phenotypic scores were concordant between subtype B and C for NVP, EFV, and 3TC, there were differences in TDF, RPV, and ETR, and resistance was misclassified in 17%, 30%, and 30%, respectively, of isolates that showed phenotypic susceptibility despite resistance predicted by algorithms. This discrepancy may result from the presence of compensatory and/or epistatic mutations in reverse transcriptase that maintain or increase susceptibility to ETR, RPV, and TDF.
Second-line antiretroviral therapy regimen
WHO guidelines recommend either LPV/r or atazanavir/ritonavir (ATV/r) with 2 NRTIs as second-line therapy. Resistance testing after first-line failure has been found to be cost effective [45, 46], supporting concerns about unnecessary switches to more expensive regimens. Cost effectiveness was achieved at a test cost of <$100 and was dependent on the prevalence of wild-type virus and timely response to test results. Three large treatment studies (SELECT, SECOND-LINE, and EARNEST [26, 27, 47]) in both high-income countries and LMICs found that using 1 or 2 new ARV classes (2 NRTI + PI vs PI + integrase inhibitor) had equivalent outcomes. More important, NRTI mutations at start of treatment initiation were strongly associated with better outcome versus not having NRTI mutations [26, 27, 47]. Tailoring NRTI selection against baseline resistance data did not improve outcome [28]. Possible explanations include better adherence in individuals failing first-line treatment with resistance. Alternatively, the current phenotyping and genotyping algorithms may not appropriately account for residual activity of NRTIs despite resistance mutations or for combination therapy and therefore overestimate resistance to NRTIs when used in combination with protease inhibitors.
The EARNEST study also demonstrated inferior virologic suppression rates for LPV/r monotherapy after 12 weeks of induction therapy with LPV/r and raltegravir, accompanied by high rates of LPV/r resistance [28]. Four small studies of dolutegravir (DTG) monotherapy, used for regimen simplification after virologic suppression, have demonstrated high rates of DTG resistance after virologic failure [48]. These studies, as well as others yielding similar data after darunavir/ritonavir (DRV/r) monotherapy, discourage the use of monotherapy for second-line ART and for regimen simplification [49, 50].
Studies show that the level of protease resistance that develops is low after LPV/r treatment failure [51, 52]; however, the level of drug resistance is increasing (22%) as second-line regimens are rolled out on a larger scale [53, 54]. There are minimal data on ATV/r resistance in LMICs, but it is known that mutations develop more easily during ATV exposure compared with LPV [55]. Depending on subtype (subtype B, C, or CRF02_AG) and PI (nelfinavir, LPV, ATV), the effect of a polymorphism at codon 36 of protease has been found to have a differential effect on both drug susceptibility and the viral replication capacity [56]. There are multiple other protease polymorphisms across different subtypes that may increase the likelihood of additional protease mutations and thus result in higher levels of resistance [56]. In an LPV/r monotherapy study, it was found that subtype AG and G were linked to lower PI susceptibility and subsequent response to treatment [57]. Overall, resistance to PIs is complex, and ongoing work is elucidating mutations in both the gag [58] and env gene [59] that may be associated with treatment failure and subtype.
CONCLUSIONS
The development of resistance is a major hindrance to successful treatment programs in LMICs. Multiple factors can affect the emergence of resistance during therapy, including the presence of pretreatment resistance; the timing of treatment initiation; HIV subtype; ARVs used in first-and second-line ART; whether viral load monitoring is performed, along with the schedules for monitoring; and medication adherence. Several unanswered questions still exist, however. How will mutations selected by new ARVs (DTG, capsid inhibitors, and others) alter resistance and cross-resistance patterns? Will new ARVs select mutations that affect outcomes differently across subtypes? Can genotypic results accurately predict virologic outcomes in all subtypes, or will different subtype-specific mutations require region-specific interpretation and guidelines? What are the consequences of mutations that develop outside the gene targeted by an ARV; for example, mutations in gag and gp41 that may affect resistance to protease inhibitors? These unanswered questions highlight the need for ongoing research in the field of HIV drug resistance.
Disclaimer. The content of this publication does not necessarily reflect the views of policies of the Department of Health and Human Services, nor does mention of trade name, commercial products, or organizations imply endorsement by the US government.
Financial support. J. W. M. and C. L. W. are supported by a grant from the AIDS Clinical Trials Group Network (ACTG) to the University of Pittsburgh Virology Specialty Laboratory and Lancet Laboratories funded by the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH) (UM1 AI106701). J. W. M. is also funded from the National Cancer Institute, NIH, under contract HHSN261200800001E.
Supplement sponsorship. This work is part of a supplement sponsored by the National Institute of Allergy and Infectious Disease, NIH, and the Centers for Disease Control and Prevention.
Potential conflicts of interest. C. L. W. has received honoraria from AbbVie, Janssen Pharmaceutical, MSD, and Abbott Molecular and was a consultant for Celera Diagnostics. C. L. W. is employed by Lancet Laboratories, a pathology partnership servicing Africa. J. W. M. is a consultant for Gilead Sciences, has received grant support from Gilead Sciences and Janssen Pharmaceuticals, and owns shares of Co-Crystal Pharma, Inc. This paper was written by C. G. and J. E. F. in their capacity as NIH employees, but the views expressed in this paper do not necessarily represent those of the NIH. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. UNAIDS. Global AIDS update http://www.unaids.org/sites/default/files/media_asset/global-AIDS-update-2016_en.pdf. Accessed 8 July 2017.
- 2. World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection recommendations for a public health approach, 2nd ed. http://www.who.int/hiv/pub/arv/arv-2016/en/. Accessed 8 July 2017.
- 3. Rhee SY, Blanco JL, Jordan MR et al. . Geographic and temporal trends in the molecular epidemiology and genetic mechanisms of transmitted HIV-1 drug resistance: an individual-patient- and sequence-level meta-analysis. PLoS Med 2015; 12:e1001810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gupta RK, Jordan MR, Sultan BJ et al. . Global trends in antiretroviral resistance in treatment-naive individuals with HIV after rollout of antiretroviral treatment in resource-limited settings: a global collaborative study and meta-regression analysis. Lancet 2012; 380:1250–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hamers RL, Wallis CL, Kityo C et al. ; PharmAccess African Studies to Evaluate Resistance (PASER) HIV-1 drug resistance in antiretroviral-naive individuals in sub-Saharan Africa after rollout of antiretroviral therapy: a multicentre observational study. Lancet Infect Dis 2011; 11:750–9. [DOI] [PubMed] [Google Scholar]
- 6. Boltz VF, Bao Y, Lockman S et al. ; OCTANE/A5208 Team Low-frequency nevirapine (NVP)–resistant HIV-1 variants are not associated with failure of antiretroviral therapy in women without prior exposure to single-dose NVP. J Infect Dis 2014; 209:703–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boltz VF, Zheng Y, Lockman S et al. . Role of low-frequency HIV-1 variants in failure of nevirapine-containing antiviral therapy in women previously exposed to single-dose nevirapine. Proc Natl Acad Sci U S A 2011; 108:9202–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Feucht UD, Rossouw T, Van Dyk G, Forsyth B, Kruger M. Consequences of prior use of full-dose ritonavir as single protease inhibitor as part of combination antiretroviral regimens on the future therapy choices in HIV-1–infected children. Pediatr Infect Dis J 2014; 33:e53–9. [DOI] [PubMed] [Google Scholar]
- 9. Castagna A, Ferrara M, Galli L, Comi L. Long-term efficacy of dolutegravir 50MG BID in INI-resistant failing HIV-1 subjects (CROI abstract 460). Top Antivir Med 2017; 25:983. [Google Scholar]
- 10. Rhee SY, Jordan MR, Raizes E et al. . HIV-1 drug resistance mutations: potential applications for point-of-care genotypic resistance testing. PLoS One 2015; 10:e0145772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nichols BE, Sigaloff KC, Kityo C et al. . Increasing the use of second-line therapy is a cost-effective approach to prevent the spread of drug-resistant HIV: a mathematical modelling study. J Int AIDS Soc 2014; 17:19164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lundgren JD, Babiker AG, Gordin F et al. . Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015; 373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Leth F, Andrews S, Grinsztejn B et al. ; 2NN study group The effect of baseline CD4 cell count and HIV-1 viral load on the efficacy and safety of nevirapine or efavirenz-based first-line HAART. AIDS 2005; 19:463–71. [DOI] [PubMed] [Google Scholar]
- 14. Cohen CJ, Molina JM, Cahn P et al. . Efficacy and safety of rilpivirine (TMC278) versus efavirenz at 48 weeks in treatment-naive HIV-1-infected patients: pooled results from the phase 3 double-blind randomized ECHO and THRIVE Trials. J Acquir Immune Defic Syndr (1999) 2012; 60:33–42. [DOI] [PubMed] [Google Scholar]
- 15. Cohen CJ, Andrade-Villanueva J, Clotet B et al. ; THRIVE study group Rilpivirine versus efavirenz with two background nucleoside or nucleotide reverse transcriptase inhibitors in treatment-naive adults infected with HIV-1 (THRIVE): a phase 3, randomised, non-inferiority trial. Lancet 2011; 378:229–37. [DOI] [PubMed] [Google Scholar]
- 16. Cohen CJ, Molina JM, Cassetti I et al. ; ECHO, THRIVE study groups Week 96 efficacy and safety of rilpivirine in treatment-naive, HIV-1 patients in two phase III randomized trials. AIDS 2013; 27:939–50. [DOI] [PubMed] [Google Scholar]
- 17. Siedner MJ, Ng CK, Bassett IV, Katz IT, Bangsberg DR, Tsai AC. Trends in CD4 count at presentation to care and treatment initiation in sub-Saharan Africa, 2002–2013: a meta-analysis. Clin Infect Dis 2015; 60:1120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sigaloff KC, Hamers RL, Wallis CL et al. . Unnecessary antiretroviral treatment switches and accumulation of HIV resistance mutations; two arguments for viral load monitoring in Africa. J Acquir Immune Defic Syndr (1999) 2011; 58:23–31. [DOI] [PubMed] [Google Scholar]
- 19. Boender TS, Kityo CM, Boerma RS et al. . Accumulation of HIV-1 drug resistance after continued virological failure on first-line ART in adults and children in sub-Saharan Africa. J Antimicrob Chemother 2016; 71:2918–27. [DOI] [PubMed] [Google Scholar]
- 20. Walsh JC, Pozniak AL, Nelson MR, Mandalia S, Gazzard BG. Virologic rebound on HAART in the context of low treatment adherence is associated with a low prevalence of antiretroviral drug resistance. J Acquir Immune Defic Syndr (1999) 2002; 30:278–87. [DOI] [PubMed] [Google Scholar]
- 21. Parienti JJ, Das-Douglas M, Massari V et al. . Not all missed doses are the same: sustained NNRTI treatment interruptions predict HIV rebound at low-to-moderate adherence levels. PLoS One 2008; 3:e2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Parienti JJ, Massari V, Descamps D et al. . Predictors of virologic failure and resistance in HIV-infected patients treated with nevirapine- or efavirenz-based antiretroviral therapy. Clin Infect Dis 2004; 38:1311–6. [DOI] [PubMed] [Google Scholar]
- 23. Orrell C, Cohen K, Leisegang R, Bangsberg DR, Wood R, Maartens G. Comparison of six methods to estimate adherence in an ART-naïve cohort in a resource-poor setting: which best predicts virological and resistance outcomes? AIDS Res Ther 2017; 14:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gross R, Zheng L, La Rosa A et al. ; ACTG 5234 team Partner-based adherence intervention for second-line antiretroviral therapy (ACTG A5234): a multinational randomised trial. Lancet HIV 2015; 2:e12–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gross R, Bellamy SL, Chapman J et al. . Managed problem solving for antiretroviral therapy adherence: a randomized trial. JAMA Intern Med 2013; 173:300–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. La Rosa AM, Harrison LJ, Taiwo B et al. ; ACTG A5273 Study Group Raltegravir in second-line antiretroviral therapy in resource-limited settings (SELECT): a randomised, phase 3, non-inferiority study. Lancet HIV 2016; 3:e247–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Paton NI, Kityo C, Hoppe A et al. ; EARNEST Trial Team Assessment of second-line antiretroviral regimens for HIV therapy in Africa. N Engl J Med 2014; 371:234–47. [DOI] [PubMed] [Google Scholar]
- 28. Boyd MA, Moore CL, Molina JM et al. ; SECOND-LINE study group Baseline HIV-1 resistance, virological outcomes, and emergent resistance in the SECOND-LINE trial: an exploratory analysis. Lancet HIV 2015; 2:e42–51. [DOI] [PubMed] [Google Scholar]
- 29. van Galen KA, Nellen JF, Nieuwkerk PT. The effect on treatment adherence of administering drugs as fixed-dose combinations versus as separate pills: systematic review and meta-analysis. AIDS Res Treat 2014; 2014:967073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tang MW, Rhee SY, Bertagnolio S et al. . Nucleoside reverse transcriptase inhibitor resistance mutations associated with first-line stavudine-containing antiretroviral therapy: programmatic implications for countries phasing out stavudine. J Infect Dis 2013; 207suppl 2:S70–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wallis CL, Mellors JW, Venter WD, Sanne I, Stevens W. Varied patterns of HIV-1 drug resistance on failing first-line antiretroviral therapy in South Africa. J Acquir Immune Defic Syndr (1999) 2010; 53:480–4. [DOI] [PubMed] [Google Scholar]
- 32. Sunpath H, Wu B, Gordon M et al. . High rate of K65R for antiretroviral therapy-naive patients with subtype C HIV infection failing a tenofovir-containing first-line regimen. AIDS 2012; 26:1679–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Invernizzi CF, Coutsinos D, Oliveira M et al. . The preferential selection of K65R in HIV-1 subtype C is attenuated by nucleotide polymorphisms at thymidine analogue mutation sites. J Antimicrob Chemother 2013; 68:2192–6. [DOI] [PubMed] [Google Scholar]
- 34. Harrison L, La Rosa A, Viana RV et al. . Is resistance testing of value after first-line ART failure in resource-limited settings? Global Antivi J 2016; 12:37. [Google Scholar]
- 35. Skhosana L, Steegen K, Bronze M et al. . High prevalence of the K65R mutation in HIV-1 subtype C infected patients failing tenofovir-based first-line regimens in South Africa. PLoS One 2015; 10:e0118145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Smit E, White E, Clark D et al. . An association between K65R and HIV-1 subtype C viruses in patients treated with multiple NRTIs. J Antimicrob Chemother 2017. Jul 1; 72(7):2075–82. doi: 10.1093/jac/dkx091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wallis CL, Aga E, Ribaudo H et al. ; A5230 team Drug susceptibility and resistance mutations after first-line failure in resource limited settings. Clin Infect Dis 2014; 59:706–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brenner B, Turner D, Oliveira M et al. . A V106M mutation in HIV-1 clade C viruses exposed to efavirenz confers cross-resistance to non-nucleoside reverse transcriptase inhibitors. AIDS 2003; 17:F1–5. [DOI] [PubMed] [Google Scholar]
- 39. Morris L, Pillay C, Chezzi C et al. . Low frequency of the V106M mutation among HIV-1 subtype C-infected pregnant women exposed to nevirapine. AIDS 2003; 17:1698–700. [DOI] [PubMed] [Google Scholar]
- 40. Flys TS, Chen S, Jones DC et al. . Quantitative analysis of HIV-1 variants with the K103N resistance mutation after single-dose nevirapine in women with HIV-1 subtypes A, C, and D. J Acquir Immune Defic Syndr (1999) 2006; 42:610–3. [DOI] [PubMed] [Google Scholar]
- 41. Sluis-Cremer N, Jordan MR, Huber K et al. . E138A in HIV-1 reverse transcriptase is more common in subtype C than B: implications for rilpivirine use in resource-limited settings. Antiviral Res 2014; 107:31–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Derache A, Wallis CL, Vardhanabhuti S, Bartlett J, Kumarasamy N, Katzenstein D. Phenotype, genotype, and drug resistance in subtype C HIV-1 infection. J Infect Dis 2016; 213:250–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Penrose K, Wallis C, Scoulos-Hanson M, Viana R, Mellors J, Parikh U High prevalence of cross-resistance to rilpivirine in subtype C HIV-1 isolates from first-line ART failures in South Africa [abstract P22-04]. In: Abstract Book: HIV Research for Prevention 2014 AIDS Vaccine, Microbicide and ARV-based Prevention Science. (Cape Town, South Africa). [Google Scholar]
- 44. Penrose KJ, Wallis CL, Brumme CJ et al. . Frequent cross-resistance to dapivirine in HIV-1 subtype C–infected individuals after first-line antiretroviral therapy failure in South Africa. Antimicrob Agents Chemother 2017; 61:e01805–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Phillips A, Cambiano V, Nakagawa F et al. . Cost-effectiveness of HIV drug resistance testing to inform switching to second line antiretroviral therapy in low income settings. PLoS One 2014; 9:e109148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Levison JH, Wood R, Scott CA et al. . The clinical and economic impact of genotype testing at first-line antiretroviral therapy failure for HIV-infected patients in South Africa. Clin Infect Dis 2013; 56:587–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Amin J, Boyd MA, Kumarasamy N et al. . Raltegravir non-inferior to nucleoside based regimens in second-line therapy with lopinavir/ritonavir over 96 weeks: a randomised open label study for the treatment of HIV-1 infection. PLoS One 2015; 10:e0118228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gallant J, Sugarman J. Dolutegravir monotherapy: when should clinical practice be clinical research? Antivir Ther 2017; 22:93–5. [DOI] [PubMed] [Google Scholar]
- 49. Ciaffi L, Koulla-Shiro S, Sawadogo AB et al. . Boosted protease inhibitor monotherapy versus boosted protease inhibitor plus lamivudine dual therapy as second-line maintenance treatment for HIV-1–infected patients in sub-Saharan Africa (ANRS12 286/MOBIDIP): a multicentre, randomised, parallel, open-label, superiority trial. Lancet HIV 2017. Sep; 4(9):e384–92. doi: 10.1016/S2352-3018(17)30069-3. [DOI] [PubMed] [Google Scholar]
- 50. Girard PM, Antinori A, Arribas JR et al. . Week 96 efficacy and safety of darunavir/ritonavir monotherapy vs. darunavir/ritonavir with two nucleoside reverse transcriptase inhibitors in the PROTEA trial. HIV Med 2017; 18:5–12. [DOI] [PubMed] [Google Scholar]
- 51. Ndahimana Jd, Riedel DJ, Muhayimpundu R et al. . HIV drug resistance mutations among patients failing second-line antiretroviral therapy in Rwanda. Antivir Ther 2016; 21:253–9. [DOI] [PubMed] [Google Scholar]
- 52. Wallis CL, Mellors JW, Venter WD, Sanne I, Stevens W. Protease inhibitor resistance is uncommon in HIV-1 subtype C infected patients on failing second-line lopinavir/r-containing antiretroviral therapy in South Africa. AIDS Res Treat 2011; 2011:769627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Inzaule SC, Hamers RL, Mukui I et al. . Emergence of untreatable, multidrug-resistant HIV-1 in patients failing second-line therapy in Kenya. AIDS 2017; 31:1495–8. [DOI] [PubMed] [Google Scholar]
- 54. Boender TS, Hamers RL, Ondoa P et al. . Protease inhibitor resistance in the first 3 years of second-line antiretroviral therapy for HIV-1 in sub-Saharan Africa. J Infect Dis 2016; 214:873–83. [DOI] [PubMed] [Google Scholar]
- 55. Colonno R, Rose R, McLaren C, Thiry A, Parkin N, Friborg J. Identification of I50L as the signature atazanavir (ATV)-resistance mutation in treatment-naive HIV-1–infected patients receiving ATV-containing regimens. J Infect Dis 2004; 189:1802–10. [DOI] [PubMed] [Google Scholar]
- 56. Lisovsky I, Schader SM, Martinez-Cajas JL, Oliveira M, Moisi D, Wainberg MA. HIV-1 protease codon 36 polymorphisms and differential development of resistance to nelfinavir, lopinavir, and atazanavir in different HIV-1 subtypes. Antimicrob Agents Chemother 2010; 54:2878–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sutherland KA, Ghosn J, Gregson J et al. . HIV-1 subtype influences susceptibility and response to monotherapy with the protease inhibitor lopinavir/ritonavir. J Antimicrob Chemother 2015; 70:243–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nijhuis M, van Maarseveen NM, Lastere S et al. . A novel substrate-based HIV-1 protease inhibitor drug resistance mechanism. PLoS Med 2007; 4:e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rabi SA, Laird GM, Durand CM et al. . Multi-step inhibition explains HIV-1 protease inhibitor pharmacodynamics and resistance. J Clin Invest 2013; 123:3848–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hosseinipour MC, van Oosterhout JJ, Weigel R et al. . The public health approach to identify antiretroviral therapy failure: high-level nucleoside reverse transcriptase inhibitor resistance among Malawians failing first-line antiretroviral therapy. AIDS 2009; 23:1127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Orrell C, Walensky RP, Losina E, Pitt J, Freedberg KA, Wood R. HIV type-1 clade C resistance genotypes in treatment-naive patients and after first virological failure in a large community antiretroviral therapy programme. Antivir Ther 2009; 14:523–31. [PMC free article] [PubMed] [Google Scholar]
- 62. Marconi VC, Sunpath H, Lu Z et al. ; South Africa Resistance Cohort Study Team Prevalence of HIV-1 drug resistance after failure of a first highly active antiretroviral therapy regimen in KwaZulu Natal, South Africa. Clin Infect Dis 2008; 46:1589–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wallis CL, Papathanasopolous MA, Fox M et al. ; CIPRA-SA project 1 study team Low rates of nucleoside reverse transcriptase inhibitor resistance in a well-monitored cohort in South Africa on antiretroviral therapy. Antivir Ther 2012; 17:313–20. [DOI] [PMC free article] [PubMed] [Google Scholar]