HCV is readily transmitted among people who inject drugs. Contrary to epidemiological findings implicating drug preparation paraphernalia, HCV is more likely transmitted through passage of HCV from a contaminated syringe into a second syringe rather than retention in paraphernalia.
Keywords: harm reduction, hepatitis C virus, injection drug use, drug paraphernalia, syringes
Abstract
Background
Controlling hepatitis C virus (HCV) transmission among people who inject drugs (PWID) has focused on preventing sharing syringes and drug preparation paraphernalia, but it is unclear whether HCV incidence linked to sharing paraphernalia reflects contamination of the paraphernalia or syringe-mediated contamination when drugs are shared.
Methods
In experiments designed to replicate real-world injection practices when drugs are shared, the residual contents of HCV-contaminated syringes with detachable or fixed needled were passed through the “cookers” and filters used by PWID in preparing drugs for injection and then introduced into a second syringe. All items were tested for the presence of infectious HCV using a chimeric HCV with a luciferase gene.
Results
Hepatitis C virus could not be recovered from cookers regardless of input syringe type or cooker design. Recovery was higher when comparing detachable needles to fixed needles for residue in input syringes (73.8% vs 0%), filters (15.4% vs 1.4%), and receptive syringes (93.8% vs 45.7%).
Conclusions
Our results, consistent with the hypothesis that sharing paraphernalia does not directly result in HCV transmission but is a surrogate for transmissions resulting from sharing drugs, have important implications for HCV prevention efforts and programs that provide education and safe injection supplies for PWID populations.
Hepatitis C virus (HCV) is among the most common viral infections in the world and is especially common among people who inject drugs (PWID). Prevalence in some populations of PWID is near universal and rarely less than 30% [1, 2]. As with human immunodeficiency virus (HIV), HCV is transmitted within populations of PWID primarily through unsafe injection practices, but unlike HIV, incidence of HCV is often not reduced by increasing access to sterile syringes [3, 4]. This has led researchers and public health officials to explore whether other elements involved in the preparation and injection of illicit drugs play a role in HCV transmission [5, 6]. This hypothesis has been tested, exploring transmission roles for the “cookers” used to dissolve drug for injection, the filters (also referred to as “cottons”) used to filter dissolved drugs, and the water used to prepare drugs or rinse syringes. At least 4 studies have found epidemiological evidence that although HCV incidence was not associated with sharing syringes, it was associated with sharing other materials used to prepare drugs and apportion them among 2 or more individuals. In the first study, from Seattle, sharing cottons or filters as a single risk factor was significantly associated with HCV incidence with an adjusted risk ratio of 5.9 (95% confidence interval, 1.1–31.7) [7]. In the second study, from Chicago, both sharing cookers and sharing filters were associated with HCV incidence, and in multivariate analysis sharing cookers was associated with incidence with an adjusted hazard ratio of 3.5 (95% confidence interval, 1.3–9.9) [8]. In the third study, from Wales, sharing any injection equipment was associated with incidence with an adjusted risk ratio of 12.7 (95% confidence interval, 1.62–99.6), whereas sharing syringes only was not significantly associated [9]. In the fourth study, from Montreal, receipt and reuse of filters was marginally associated with HCV incidence with an adjusted hazard ratio of 2.15 (95% confidence interval, 0.99–4.67) [10]. Even in some studies in which HCV incidence was associated with sharing syringes, collective use of other materials remained a significant contributor to incidence [11–13]. These findings dovetail with previous studies of risk behaviors for HIV [14, 15], and they have led syringe access and other harm reduction programs that serve PWID to include the provision of clean cookers and filters and sterile water among their prevention supplies. However, to date, there have been no epidemiologic reports linking such provision with lowered HCV incidence [16].
There is an alternative hypothesis to explain the association of sharing of cookers and filters with HCV incidence. Observations by ethnographers studying drug injection behaviors have found that sharing of cookers and filters occurs when 2 or more people share the same package(s) of drugs [17–19]. Studies of PWID that have focused on this issue confirm that drug sharing is common among PWID [20–24]. These findings suggest it may not be HCV in shared cookers and filters that leads to transmission, but instead that this kind of sharing is a surrogate for situations in which HCV-discordant injectors share drugs. In this scenario, injectors collectively prepare drugs. If a contaminated syringe was used to add water, dissolve, and apportion the drug, then some of the contents of the contaminated syringe would pass through the cooker and filter and into syringe of the uninfected person. In such a case, the cookers and filters may not even harbor infectious virus, and the distribution of clean ones and warnings about sharing them may have little or no impact on HCV incidence.
We set out to test these 2 competing but not mutually exclusive possibilities. We have developed a microculture assay for HCV propagation in tissue culture that allows us to replicate real-world injection practices in the laboratory and measure the amount and duration of infectivity of HCV in the small volumes found in contaminated syringes [25]. Basing our laboratory procedure on field observations, we prepared syringes to contain the residual amounts of HCV-infected liquid left in syringes after a completed injection and used these contaminated syringes to simulate the preparation of the next injection. In this process, the syringe introduced water to dissolve the drug in a cooker, and the drug solution is drawn up through filters and apportioned to 2 syringes. The contents of the syringes, the cookers, and filters were then entered into the microculture system. This report describes the recovery of infectious HCV from a set of experiments, revealing the ability of each of the items involved in drug preparation and injection to harbor and potentially transmit infectious HCV.
METHODS
Virus and Cells
The experiments described in this report rely on an in vitro microculture system that uses as input virus a chimeric genotype 2a full-length J6/JFH virus that has been modified by the insertion of a Gaussia princeps luciferase gene inserted between the p7 and NS2 genes [26–28]. This chimeric Jc1/GLuc2A virus replicates to high copy number in the human hepatoma Huh-7.5 cell line [29]. Previous analysis of sensitivity of the microassay demonstrated that the production of luciferase was strictly linear over a more than 5 log range and directly proportional to the production of infectious virions from <10 to approximately 106 tissue culture infectious doses per milliliter [TCID50/mL] [25, 30].
Experimental Procedures
Preparation of Hepatitis C Virus-Contaminated Syringes
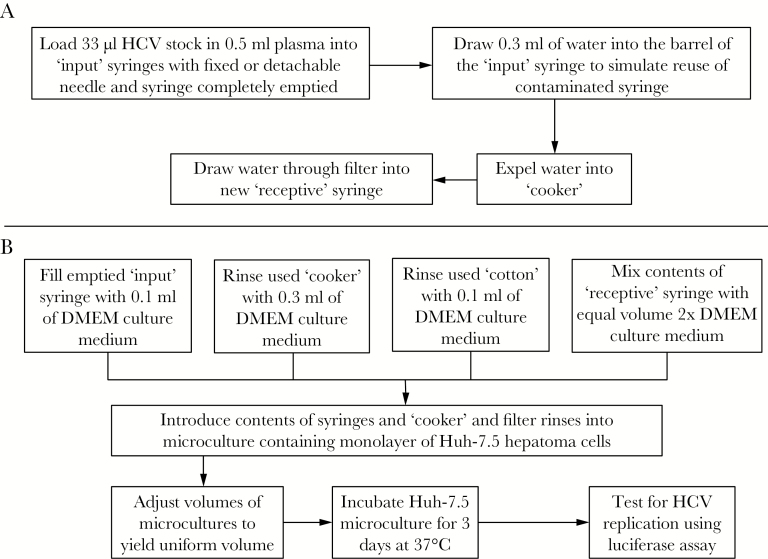
We sought to replicate, as closely as possible, the injection scenarios that occur when individuals prepare drugs for injection starting with shared packages (“bags” or “balloons”) of solid drugs. The risk for HCV transmission occurs when the syringe used for adding water to dissolve the drug or apportion the drug once dissolved has been previously used by an individual with an active HCV infection. The practice that produces syringes with the greatest likelihood of HCV contamination is when injectors “boot” [31]. In this common practice, after the drug is injected and needle is still in the vein, the person draws back on the plunger to introduce a little blood into the syringe and reinjects. Injectors describe “booting” as an attempt to get the last remaining drug from the syringe into the vein. When the plunger is depressed after booting, the residual contents of syringe consist almost entirely of blood that is contaminated with HCV if the person doing this is infected. This represents a worst-case scenario, upper bound for HCV contamination within used syringes in that no attempt has been made to disinfect the syringe before it is reused. We replicated this process by loading “input” syringes with the chimeric HCV at a concentration equal to 10000 TCID50 (Figure 1A). Previous analysis suggests that this concentration is equivalent to 106 copies of HCV viral ribonucleic acid [32], which is considered a high viral load in chronically infected patients.
Figure 1.
Flow diagram of experiments to determine the recovery of infectious hepatitis C virus (HCV) from drug preparation and injection paraphernalia. (A) The process of producing the syringes and paraphernalia tested in this study is depicted, starting with syringes contaminated with HCV replicating the situation when a syringe previously used by an individual actively infected with HCV is then used to add water to dissolve drugs for injection. The water from the “input” syringes is then passed through a “cooker” and filter and into a “receptive” syringe. (B) The process for testing for potentially infectious HCV in each of the drug preparation and injection items is depicted. The volume of liquid tested is made uniform for all 4 items, and HCV replication after 3 days in microculture is measured according to the procedure described in the Methods and in Paintsil et al [30]. Abbreviation: DMEM, Dulbecco’s modified Eagle’s medium.
Passage of the Contents of Contaminated Syringes Through Drug Preparation and Injection Paraphernalia
Once contaminated syringes were prepared, we performed experiments to test whether HCV could be recovered from (1) a contaminated input syringe after it had been used as a measuring device to add water for dissolving drugs, (2) from drug preparation paraphernalia (cooker and filter), and (3) the “receptive” syringe that would subsequently inject the dissolved drug. The procedure is depicted in Figure 1B. In brief, water was introduced into the barrel of a contaminated input syringe and expelled into a cooker, and the water was drawn up into a receptive syringe through a cotton filter. The input syringe, cooker, and filter were rinsed with tissue culture medium and introduced into the microculture assay. The water drawn into the second syringe was combined with an equal volume of double-strength medium and introduced into the microculture assay.
This experimental protocol was performed on 10 syringes per experiment, and each experiment was repeated at least 3 times. In each experiment, 2 types of syringes were compared: (1) 1-cc insulin syringes with fixed 27-gauge, half-inch long needles and (2) 1-cc tuberculin syringes with detachable 27-gauge, half-inch long needles. Experiments also compared 2 different types of cookers: both resemble the screw tops of soda bottles, but one is ridged and the other smooth, which is the type generally distributed by harm reduction programs.
A second set of experiments was conducted based on ethnographers’ observations that filters are occasionally saved, pooled, and “beaten” to extract whatever drug residue remains, and the recovered drug is then injected [20, 33, 34]. Such an injection contains material from multiple filters, increasing the likelihood that HCV harbored in filters could yield infectious HCV. We tested 2 scenarios of filter pooling. In the first, 10 filters prepared on the same day as in Figure 1 were combined, beaten, and extracted material was introduced into the microculture assay. However, a more realistic scenario is that pooled filters are stored for periods of time before their contents are extracted. In this experiment, 10 filters stored for up to10 days were combined, beaten and the extracted material was introduced into the microculture assay.
Analysis of Microculture Assay Results
For the experiments conducted as part of this report, data are presented both as the percentage of syringes, cookers, and filters producing luciferase at levels consistent with viral replication and the mean relative luciferase (relative light units [RLU]) for the positive samples only. Confidence intervals around means and proportions are reported using standard statistics, except for the calculation of confidence intervals when the proportion of tests yielded zero positive results, which was derived using the method of Louis [35]. Pairwise comparisons of recovery frequencies used standard χ2 with the Fisher exact probability testing.
RESULTS
We used 2 types of syringes—with fixed or detachable needles—because these differ in the amount of fluid retained when the plunger is fully depressed [36–38]. The detachable needle-syringe combination not only retains more fluid but also harbors infectious HCV for much longer periods [25]. We also tested 2 types of metal cookers and single or pooled filters. We have divided the presentation of our results into 3 sections: experiment no. 1 using single filters, experiment no. 2 in which filters were pooled, and a section that combines compatible elements of the 2 experiments.
Experiment No. 1: Hepatitis C Virus Recovery Using Single Filters
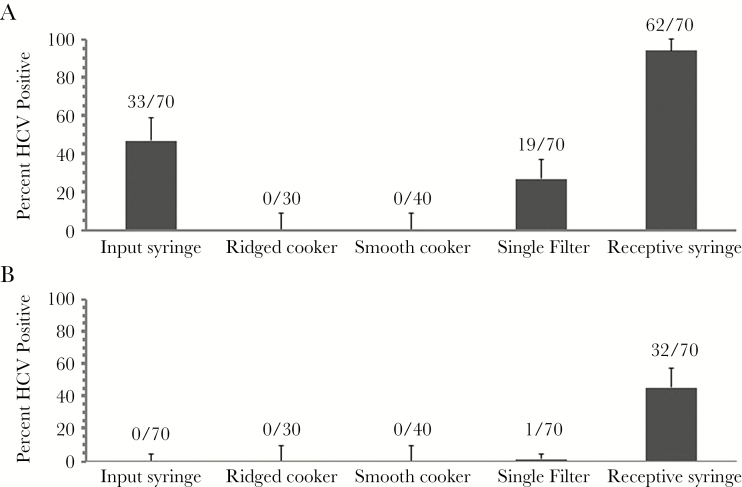
The protocol was run 4 times with 10 replicates per run with the ridged cooker and 3 times with 10 replicates per run with the smooth cooker. Thus, a total of 70 sets of injection equipment were tested for the recovery of infectious HCV for each type of syringe in this part of the experiment. The results, depicted in Figure 2, revealed that HCV was recovered more often when using the detachable needle/syringe combinations. When we tested for infectious HCV remaining inside the input syringes that were used to deliver water into the cooker, we failed to recover HCV from the syringes with fixed needles (0 of 70), but we did from 61.4% (43 of 70) of the syringes with detachable needles. This finding is consistent with past studies [25, 39], and the difference was statistically significant (P ≈ 1.57 × 10−17 using the Fisher exact test). However, virus was successfully passed from both types of syringes to new syringes but at a lower frequency for syringes with fixed needles. Hepatitis C virus was recovered from 94.3% (66 of 70) of syringes when using syringes with detachable needles and 45.7% (32 of 70) of syringes when using syringes with syringes with detachable needles. This difference was statistically significant (P ≈ 1.65 × 10−10 using the Fisher exact test).
Figure 2.
Recovery of infectious hepatitis C virus (HCV) from drug preparation and injection paraphernalia. Syringes were loaded with HCV in plasma and the plunger fully depressed. Water was then drawn into the barrel to simulate the use of a contaminated syringe when preparing drugs for a subsequent injection. (A) Recovery from input syringes, “cookers”, filters, and receptive syringes when the input syringes had detachable needles. (B) Recovery from input syringes, cookers, filters, and receptive syringes when the input syringes had fixed needles.
Recovery of HCV from the drug preparation paraphernalia (cookers and filter) was lower than recovery from syringes. Hepatitis C virus was recovered from 19 of 70 filters (27.1%) when the input syringe adding the water had a detachable needle but only 1 of 70 (1.4%) than when the input syringe had a fixed needled. This difference was statistically significant (P = 4.5 × 10−5 using the Fisher exact test). No infectious HCV was recovered from cookers regardless of the type of syringe introducing the contaminated water or the design of the cooker.
In addition to measuring the proportion of items yielding infectious HCV, we quantified the titer of the virus recovered, in terms of relative luciferase transduction activity and expressed at RLU, for those cases in which the RLU exceeded the threshold considered sufficient to indicate active HCV replication (approximately 1000 RLU, based on twice the average negative baseline). In the case of both input syringes and receptive syringes, the average titer was higher when the input into the experiments came from syringes with detachable needles than from syringes with fixed needles.
Experiment No. 2: Hepatitis C Virus Recovery Using Pooled Filters
As noted in the introduction, people may, on occasions when drugs or funds to buy drugs are not available, pool filters and try to extract drugs from them. We replicated this process by passing water from HCV contaminated input syringes through filters, pooling them, and collecting and testing the material extracted from the pool for the presence of infectious HCV. We prepared pools of 1, 3, 6, and 10 filters using syringes with detachable needles as the input syringes. In addition, because drug injectors often collect and store filters for future extraction, we conducted a time course in which the potentially contaminated filters were extracted immediately (day 0) or stored for 1, 3, or 7 days at room temperature before extraction. Each condition in the time course was tested on 20 replicates on 3 separate occasions.
Filter pooling data are presented in Table 1. On day 0, only a small percentage (1 of 60, 1.7%) of single filters yielded HCV when the input came from syringes with fixed needles, consistent with the data from Experiment no. 1. Increasing the number of filters in the pool increased the proportion, but, even with a pool of 10 filters, only 3 of 60 (5%) of the pools yielded HCV that replicated in culture. We were unable to recover HCV from any of the filter pools once the filters were stored.
Table 1.
Experiment No. 2: Recovery of HCV from Pooled Filtersa
| Days of Storage | Item | Filter Pool Size | Number (%) Positive | Average RLU | Standard Deviation |
|---|---|---|---|---|---|
| 0 | Input syringe | – | 53 (88.3%) | 6758 | 5175 |
| Receptive syringe | – | 60 (100%) | 27 480 | 10 290 | |
| 1 | 2 (3.3%) | 5200 | 2674 | ||
| Filters | 3 | 2 (3.3%) | 2589 | 1576 | |
| 6 | 3 (5.0%) | 2038 | 1064 | ||
| 10 | 3 (5.0%) | 13 424 | 4816 | ||
| 1 | Input syringe | – | 29 (48.3%) | 3236 | 2336 |
| Receptive syringe | – | 60 (100%) | 14,892 | 4276 | |
| Filters | 1–10 | 0 | |||
| 3 | Input syringe | – | 9 (15%) | 1187 | 202 |
| Receptive syringe | – | 60 (100%) | 2893 | 1135 | |
| Filters | 1–10 | 0 | |||
| 7 | Input syringe | – | 0 | 2249 | 408 |
| Receptive syringe | – | 60 (100%) | |||
| Filters | 1–10 | 0 |
Abbreviations: HCV, hepatitis C virus; RLU, relative luciferase activity.
aVirus proliferation from the contents recovered from input syringes with detachable needles, receptive syringes, and filters was measured by determining the RLU from the luciferase gene inserted into the chimeric HCV. For each condition listed below, 60 replications were run, and the average RLU was calculated only for those cases in which the RLU exceeded the threshold indicative of active HCV replication.
In contrast, we were able to recover HCV from a proportion of the input syringes, beginning with 88.3% (53 of 60) without storage, but within 1 week of storage none of the input syringes yielded replicating HCV. In contrast, all receptive syringes, which contained the contaminated material that had passed through the filters, yielded replicating HCV at all times of storage over the week-long time course.
Combined Results of Experiment No. 1 and No. 2
We combined comparable conditions in the 2 sets of experiments, and the results are summarized in Table 2. This allowed us to increase to 130 the total number of input syringes with detachable needles, cookers to which the contents of detachable needles were added, single filters through which the material passed, and receptive syringes. We found that 96 of 130 (73.8%) input syringes, 20 of 130 filters (15.4%), and 122 (93.8%) of the receptive syringes yielded infectious HCV, whereas none of the cookers yielded infectious HCV.
Table 2.
Quantification of HCV Recovery in Experiment No. 1 and No. 2 From Drug Injection and Preparation Paraphernaliaa
| Input Syringe | Paraphernalia Item | Positive Tests (%) | Average RLU | Standard Deviation |
|---|---|---|---|---|
| Syringe with detachable needle | Input syringe | 96 (73.8%) | 6727 | 5045 |
| Syringe with detachable needle | Filter | 20 (15.4%) | 6494 | 2781 |
| Syringe with detachable needle | Receptive syringe | 122 (93.8%) | 25257 | 11732 |
| Syringe with attached needle | Input syringe | 0 | – | – |
| Syringe with attached needle | Filter | 1 (1.4%) | 1580 | – |
| Syringe with attached needle | Receptive syringe | 32 (45.7%) | 4986 | 3850 |
Abbreviations: HCV, hepatitis C virus; RLU, relative luciferase activity.
aThe level of viral proliferation from HCV recovered from items used to prepare and inject drugs was measured by determining the RLU from the luciferase gene inserted into the chimeric HCV used in these experiments and calculated only for those cases in which the RLU exceeded the threshold indicative of active HCV replication. For conditions with fixed needles, the data came from 130 replications; for conditions with fixed needles, the data came from 70 replications.
DISCUSSION
Assignment of causality requires validating a set of assumptions; such a set of 9 criteria, set forth by Hill [40], includes biological gradient, plausibility, and experiment. Although the prior set of epidemiological studies [7–10] found strong correlations between the sharing of drug preparation paraphernalia and HCV incidence and established temporality and consistency, those studies could not differentiate between the 2 alternativeexplanations as to how sharing of paraphernalia other than needles or syringes produced HCV transmission. We have now provided biological evidence that the more compelling explanation for the association is that sharing of objects associated with the preparation but not the actual injection of drugs is a surrogate for shared injections in which the virus is introduced from a contaminated syringe. We have produced evidence that we can “follow the blood”, and the HCV from a contaminated input syringe ends up in a second receptive syringe, leaving less virus behind in the input syringe and little or no virus in drug preparation paraphernalia.
The conclusion is reinforced if we focus our attention only on the data from syringes with fixed needles, syringes routinely used for the injection of insulin. This style of syringe is most commonly used by PWID in the United States and Canada and is the type overwhelmingly available in pharmacies and supplied by syringe exchange programs. The experimental procedures we describe in this report are equivalent to rinsing the input syringe with water, and, as past work has shown, a single rinse of this type of syringe can greatly reduce the HCV recovery [41]. So it is not surprising that our recovery of HCV from input syringes with fixed needles was negligible. Furthermore, work over the past decade by a group of researchers, including us, has highlighted the increased risk of bloodborne virus transmission that results from using syringes with detachable rather than fixed needles [25, 36, 37, 39, 42, 43].
Our findings on the retention of HCV in filters differ somewhat from that produced from similar laboratory simulations by Doerrbecker et al [44]. In their experiments, they passed 800 µL of fluid containing approximately 105 TCID50/mL through filters. This is far in excess of the 2 to 100 µL of HCV-contaminated fluid that might remain inside a used syringe that is being reused to prepare drugs for injection [25, 36–38]. Although we recovered an average of 23107 ± 13 870 RLU from receptive syringes using inputs from syringes with detachable needles, inputs of 105 TCID50/mL would yield >2 × 106 RLU in our microculture system [25]. Therefore, the experimental protocol of Doerrbecker et al [44] used HCV inputs that are 2 orders of magnitude higher than would occur under “real-world” conditions in which drugs are prepared, shared, and injected, whereas our experimental procedure more closely replicates those conditions.
Our findings on the stability of virus over time in the experiments pooling filters found rapid attenuation of infectivity in the input syringes and filters. This is consistent with our previous findings when titers of HCV are initially low and with findings on the duration of infectivity noted by Doerrbecker et al [25, 45]. A study by Ciesek et al [32], similar to that of Doerrrbecker et al [45], used an amount of HCV that was 10-fold higher than we did, so the results are not directly comparable.
As noted by Glass et al [46], once causality in the public health realm is firmly established, appropriate interventions can follow. In this case, it should lead us to reconsider policies, widely adapted by syringe exchange and other harm reduction programs, to provide clean cookers and filters along with sterile syringes when attempting to reduce the transmission of HCV. At a minimum, our findings should compel programs that serve PWID to focus more on the process of drug preparation and injection and less on the preparation paraphernalia. Going further, programs may want to reconsider expanding scarce resources to provide supplies that will do little or nothing to prevent HCV transmission. Given the usual situation of limited financial resources facing syringe exchange and related harm reduction programs, spending money on objects that can have little impact on disease transmission should come to be viewed as profligate. Money spent on cookers and filters would be better spent on giving away more syringes. Because HCV and HIV transmission are more likely if the syringe has a detachable rather than a fixed needle, efforts should focus on providing more syringes with fixed needles. An alternative for people who need syringes with detachable needles is to develop and market reduced dead space syringes [39, 43].
One additional way to improve bloodborne virus prevention efforts is providing guidance and materials to reduce the chances of using contaminated syringes to prepare or apportion drug. One such piece of drug preparation material could be syringes without accompanying needles that could be used to introduce water into cookers or apportion dissolved drugs. Lacking a needle, such a syringe is unlikely to become contaminated with HCV unless the water source itself was contaminated. Provision of sterile water supplies and training to minimize the commingling of water sources used to prepare drugs and rinse used syringes will do more than the provision of cookers and filters to prevent HCV transmission.
There are 3 significant limitations to our work. First, we are using an in vitro system that is strongly parallel but not identical to the real-world situation, most notably in that it uses a chimeric virus derived from a genotype 2a virus that may not reflect survival and infectivity characteristics of the viruses passed among PWID or when patient-derived viruses are tested in culture. Second, the predominant genotypes among PWID worldwide are genotypes 1 and 3, so it would be useful to replicate our findings with viruses of these genotypes should they become available. If we were to validate our findings using genotype 1 and 3 viruses, it would strengthen the argument about refocusing prevention messaging and the provision of drug preparation and injection supplies more on needles and syringes and less on cookers and filters. Finally, our experiments are replications, reduced realities of real-world situations that are contingent on a host of interacting drug, set, and setting variables [47–50]. Although we have tried to select and replicate a worst-case scenario, we cannot describe the full range of HCV transmission risk that injectors experience.
CONCLUSIONS
Our studies reinforce the need for expanded education efforts and further environmental interventions, such as upscaling distribution of syringes with fixed needles or with reduced dead space to decrease the likelihood for HCV transmission among PWID. These syringes retain less fluid than syringes with detachable needles, and hence less HCV should the person using the syringe be actively infected, and, as previously shown, HCV infectivity persists for a shorter time [25]. Furthermore, as the current study demonstrates, there is less likelihood that shared drug preparation paraphernalia will harbor infectious virus. Given all these benefits, we would advise syringe access and harm reduction education programs to emphasize the distribution of insulin-type syringes with fixed needles and de-emphasize and not expend limited program resources on the distribution of cookers and filters.
Financial support. This work was funded by grant 1R01DA030420 from the National Institute on Drug Abuse.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Klevens RM, Hu DJ, Jiles R, Holmberg SD. Evolving epidemiology of hepatitis C virus in the United States. Clin Infect Dis 2012; 55(Suppl 1):S3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nelson PK, Mathers BM, Cowie B et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet 2011; 378:571–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jordan A, Des Jarlais DC, Arasteh K et al. Incidence and prevalence of hepatitis C virus infection among persons who inject drugs in New York City: 2006–2013. Drug Alcohol Depend 2015; 152:194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Judd A, Hutchinson S, Wadd S et al. Prevalence of, and risk factors for, hepatitis C virus infection among recent initiates to injecting in London and Glasgow: cross sectional analysis. J Viral Hepat 2005; 12:655–62. [DOI] [PubMed] [Google Scholar]
- 5. Koester S, Booth RE, Zhang Y. The prevalence of additional injection-related HIV risk behaviors among injection drug users. J Acquir Immune Defic Syndr Hum Retrovirol 1996; 12:202–7. [DOI] [PubMed] [Google Scholar]
- 6. Koester SK, Booth RE, Wiebel W. The risk of HIV transmission from sharing water, drug-mixing containers and cotton filters among intravenous drug users. Intl J Drug Policy 1990; 1:28–30. [Google Scholar]
- 7. Hagan H, Thiede H, Weiss NS et al. Sharing of drug preparation equipment as a risk factor for hepatitis C. Am J Public Health 2001; 91:42–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thorpe LE, Ouellet LJ, Hershow R et al. Risk of hepatitis C virus infection among young adult injection drug users who share injection equipment. Am J Epidemiol 2002; 155:645–53. [DOI] [PubMed] [Google Scholar]
- 9. Craine N, Hickman M, Parry JV et al. Incidence of hepatitis C in drug injectors: the role of homelessness, opiate substitution treatment, equipment sharing, and community size. Epidemiol Infect 2009; 137:1255–65. [DOI] [PubMed] [Google Scholar]
- 10. Roy E, Arruda N, Leclerc P et al. Injection of drug residue as a potential risk factor for HCV acquisition among Montréal young injection drug users. Drug Alcohol Depend 2012; 126:246–50. [DOI] [PubMed] [Google Scholar]
- 11. Hahn JA, Page-Shafer K, Lum PJ et al. Hepatitis C virus seroconversion among young injection drug users: relationships and risks. J Infect Dis 2002; 186:1558–64. [DOI] [PubMed] [Google Scholar]
- 12. Pouget ER, Hagan H, Des jarlais DC. Meta-analysis of hepatitis C seroconversion in relation to shared syringes and drug preparation equipment. Addiction 2011; 107:1057–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Palmateer N, Hutchinson S, McAllister G et al. Risk of transmission associated with sharing drug injecting paraphernalia: analysis of recent hepatitis C virus (HCV) infection using cross-sectional survey data. J Viral Hepat 2014; 21:25–32. [DOI] [PubMed] [Google Scholar]
- 14. Grund JP, Kaplan CD, Adriaans NF, Blanken P. Drug sharing and HIV transmission risks: the practice of frontloading in the Dutch injecting drug user population. J Psychoactive Drugs 1991; 23:1–10. [DOI] [PubMed] [Google Scholar]
- 15. Jose B, Friedman SR, Neaigus A et al. Syringe-mediated drug-sharing (backloading): a new risk factor for HIV among injecting drug users. AIDS 1993; 7:1653–60. [DOI] [PubMed] [Google Scholar]
- 16. Gillies M, Palmateer N, Hutchinson S et al. The provision of non-needle/syringe drug injecting paraphernalia in the primary prevention of HCV among IDU: a systematic review. BMC Public Health 2010; 10:721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Grund JP. Drug Use as a Social Ritual: Functionality, Symbolism, and Determinants of Self-Regulation. Rotterdam, the Netherlands: Addiction Research Institute, Erasmus Universiteit, 1993. [Google Scholar]
- 18. Colón H, Finlinson A, Robles RR et al. Joint drug purchases and drug preparation risk behaviors among Puerto Rican injection drug users. AIDS Behav 2000; 5:85–96. [Google Scholar]
- 19. Koester S, Heimer R, Barón AE et al. Re: “Risk of hepatitis C virus among young adult injection drug users who share injection equipment”. Am J Epidemiol 2003; 157:376; author reply 376–8. [DOI] [PubMed] [Google Scholar]
- 20. Koester S, Glanz J, Barón A. Drug sharing among heroin networks: implications for HIV and hepatitis B and C prevention. AIDS Behav 2005; 9:27–39. [DOI] [PubMed] [Google Scholar]
- 21. Lalander P. Hooked on Heroin: Drugs and Drifters in a Globalized World. Oxford, UK: Berg Publishers, 2007. [Google Scholar]
- 22. Wilkins L, Bissell P, Meier PS. Risky injecting practices associated with snowballing: a qualitative study. Drug Alcohol Rev 2010; 29:256–62. [DOI] [PubMed] [Google Scholar]
- 23. Zule WA. Risk and reciprocity: HIV and the injection drug user. J Psychoactive Drugs 2012; 24:243–9. [DOI] [PubMed] [Google Scholar]
- 24. Heimer R, Barbour R, Palacios WR et al. Associations between injection risk and community disadvantage among suburban injection drug users in southwestern Connecticut, USA. AIDS Behav 2014; 18:452–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Paintsil E, He H, Peters C et al. Survival of hepatitis C virus in syringes: implication for transmission among injection drug users. J Infect Dis 2010; 202:984–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lindenbach BD, Meuleman P, Ploss A et al. Cell culture-grown hepatitis C virus is infectious in vivo and can be recultured in vitro. Proc Natl Acad Sci U S A 2006; 103:3805–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lindenbach BD, Evans MJ, Syder AJ et al. Complete replication of hepatitis C virus in cell culture. Science 2005; 309:623–6. [DOI] [PubMed] [Google Scholar]
- 28. Phan T, Beran RK, Peters C et al. Hepatitis C virus NS2 protein contributes to virus particle assembly via opposing epistatic interactions with the E1-E2 glycoprotein and NS3-NS4A enzyme complexes. J Virol 2009; 83:8379–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Blight KJ, McKeating JA, Rice CM. Highly permissive cell lines for subgenomic and genomic hepatitis C virus RNA replication. J Virol 2002; 76:13001–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Paintsil E, Binka M, Patel A et al. Hepatitis C virus maintains infectivity for weeks after drying on inanimate surfaces at room temperature: implications for risks of transmission. J Infect Dis 2014; 209:1205–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Koester S. Following the blood: syringe reuse leads to blood-borne virus transmission among injection drug users. J Acquir Immune Defic Syndr Human Retrovirol 1998; 18(Suppl 1):S139–40. [DOI] [PubMed] [Google Scholar]
- 32. Ciesek S, Friesland M, Steinmann J et al. How stable is the hepatitis C virus (HCV)? Environmental stability of HCV and its susceptibility to chemical biocides. J Infect Dis 2010; 201:1859–66. [DOI] [PubMed] [Google Scholar]
- 33. Roy E, Arruda N, Bourgois P. The growing popularity of prescription opioid injection in downtown Montréal: new challenges for harm reduction. Subst Use Misuse 2011; 46:1142–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Otiashvili D, Latypov A, Kirtadze I et al. Drug preparation, injection, and sharing practices in Tajikistan: a qualitative study in Kulob and Khorog. Subst Abuse Treat Prev Policy 2016; 11:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Louis TA. Confidence intervals for a binomial parameter after observing no successes. Amer Statist 1981; 35:154. [Google Scholar]
- 36. Abdala N, Stephens PC, Griffith BP, Heimer R. Survival of HIV-1 in syringes. J Acquir Immune Defic Syndr Hum Retrovirol 1999; 20:73–80. [DOI] [PubMed] [Google Scholar]
- 37. Zule WA, Bobashev G. High dead-space syringes and the risk of HIV and HCV infection among injecting drug users. Drug Alcohol Depend 2009; 100:204–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Oramasionwu CU, Bailey SC, Moore HN et al. Dead space in over-the-counter syringes: the implications for HIV and HCV transmission. Int J Drug Policy 2015; 26:1282–4. [DOI] [PubMed] [Google Scholar]
- 39. Binka M, Paintsil E, Patel A et al. Survival of hepatitis C virus in syringes is dependent on the design of the syringe-needle and dead space volume. PLoS One 2015; 10:1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hill AB. The environment and disease: association or causation?Proc R Soc Med 1965; 58:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Binka M, Paintsil E, Patel A et al. Disinfection of syringes contaminated with hepatitis C virus by rinsing with household products. Open Forum Infect Dis 2015; 2(1): ofv017. doi: 10.1093/ofid/ofv017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. CIccacrone D. Saying goodbye to high-dead-space syringes. Intl J Drug Policy 2013; 24:15–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zule WA, Cross HE, Stover J, Pretorius C. Are major reductions in new HIV infections possible with people who inject drugs? The case for low dead-space syringes in highly affected countries. Int J Drug Policy 2013; 24:1–7. [DOI] [PubMed] [Google Scholar]
- 44. Doerrbecker J, Behrendt P, Mateu-Gelabert P et al. Transmission of hepatitis C virus among people who inject drugs: viral stability and association with drug preparation equipment. J Infect Dis 2013; 207:281–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Doerrbecker J, Friesland M, Ciesek S et al. Inactivation and survival of hepatitis C virus on inanimate surfaces. J Infect Dis 2011; 204:1830–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Glass TA, Goodman SN, Hernán MA, Samet JM. Causal inference in public health. Annu Rev Public Health 2013; 34:61–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zinberg NE. Drug, Set and Setting: The Basis for Controlled Intoxicant Use. New Haven Yale University Press, 1984. [Google Scholar]
- 48. Rhodes T, Singer M, Bourgois P et al. The social structural production of HIV risk among injecting drug users. Soc Sci Med 2005; 61:1026–44. [DOI] [PubMed] [Google Scholar]
- 49. Rhodes T. Risk environments and drug harms: a social science for harm reduction approach. Int J Drug Policy 2009; 20:193–201. [DOI] [PubMed] [Google Scholar]
- 50. Grund JP. Control, can we have some? Towards a transdisciplinary ecological perspective of drug consumption and addiction. In: Zuffa G, Ronconi S, eds. Drugs and Control Guidelines for Self-Regulation—A Blueprint for People Who Use Drugs and Drug Service Professionals. Rome, Italy: Ediesse, 2017. [Google Scholar]